Abstract
Objectives
(1) Describe the clinical characteristics, hospital courses and outcomes of a cohort of children cared for within the Pediatric Emergency Care Applied Research Network (PECARN) who experienced in-hospital cardiac arrest with sustained return of circulation between July 1, 2003 and December 31, 2004, and (2) identify factors associated with hospital mortality in this population. These data are required to prepare a randomized trial of therapeutic hypothermia on neurobehavioral outcomes in children after in-hospital cardiac arrest.
Design
Retrospective cohort study.
Setting
Fifteen children’s hospitals associated with PECARN.
Patients
Patients between one day and 18 years of age who had cardiopulmonary resuscitation (CPR) and received chest compressions for >1 minute, and had a return of circulation for >20 minutes.
Interventions
None.
Measurements and Main Results
A total of 353 patients met entry criteria; 172 (48.7%) survived to hospital discharge. Among survivors, 132 (76.7%) had good neurological outcome documented by Pediatric Cerebral Performance Category scores. After adjustment for age, gender and first documented cardiac arrest rhythm, variables available prior to and during the arrest that were independently associated with increased mortality included pre-existing hematologic, oncologic, or immunologic disorders, genetic or metabolic disorders, presence of an endotracheal tube prior to the arrest, and the use of sodium bicarbonate during the arrest. Variables associated with decreased mortality included post-operative CPR. Extending the time frame to include variables available prior to, during, and within 12 hours following arrest, variables independently associated with increased mortality included the use of calcium during the arrest. Variables associated with decreased mortality included higher minimum blood pH and pupillary responsiveness.
Conclusions
Many factors are associated with hospital mortality among children after in-hospital cardiac arrest and return of circulation. Such factors must be considered when designing a trial of therapeutic hypothermia after cardiac arrest in pediatric patients.
Keywords: cardiac arrest, return of circulation, children, pediatric, cohort study, mortality, outcome, therapeutic hypothermia
INTRODUCTION
Cardiac arrest in childhood is a tragic event often resulting in death or poor neurological outcome. Previous studies have reported survival rates in children ranging from 9–47% after in-hospital cardiac arrest (1–7), and 0–29% after out-of-hospital cardiac arrest (8). Better outcomes after in-hospital compared to out-of-hospital arrest have been attributed to differences in etiology of arrest, and more rapid recognition and treatment by skilled caregivers in the in-hospital setting (9). Since many of these previous studies are small retrospective case series conducted in single hospitals, their findings are often not generalizable (10). In addition, lack of uniformity in case definitions and outcome measures complicates comparison of findings between studies and prevents integration of data in meta-analyses (10).
Notable exceptions to the literature on cardiac arrest in childhood are the recent reports generated from the American Heart Association (AHA) National Registry of Cardiopulmonary Resuscitation (NRCPR) (11–16). The NRCPR is an international registry of in-hospital cardiopulmonary resuscitation (CPR) that includes adult and pediatric patients from over 500 hospitals (17). Patients with out-of-hospital arrests are excluded. Variables included in the NRCPR registry are based on the Utstein Style Guidelines for uniform reporting of cardiac arrest and resuscitation data (18). However, the NRCPR registry contains less extensive and detailed data than that potentially needed to plan and conduct future clinical trials of CPR interventions within specific pediatric research networks.
Therapeutic hypothermia is an intervention that has recently been shown to improve outcome in adults who are comatose after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest (19, 20). AHA guidelines for post-resuscitation support recommend induced hypothermia for such adult patients (21). Benefits of therapeutic hypothermia have also been reported for newborns with hypoxic-ischemic encephalopathy (22). However, the effect of therapeutic hypothermia on outcome after pediatric cardiac arrest has not been studied.
The Pediatric Emergency Care Applied Research Network (PECARN) is a federally-funded multi-institutional emergency medicine network that conducts research on prevention and management of acute illness and injury in children (23, 24). PECARN includes academic, community, urban, rural, general, and children’s hospitals located across the U.S., and provides the necessary infrastructure to carry out randomized controlled trials of CPR interventions in pediatric patients. In order to plan a randomized trial of the effect of therapeutic hypothermia on neurobehavioral outcomes after cardiac arrest, specific data describing the epidemiology of cardiac arrest from PECARN clinical sites are needed. The first objective of this report is to describe patient characteristics, cardiac arrest events, post-arrest hospital courses, and outcomes in a cohort of pediatric patients who received in-hospital CPR for greater than one minute and who had a sustained return of circulation. The second objective is to identify factors most strongly associated with hospital mortality in this population using information available at the time of return of circulation, and within 12 hours following return of circulation. Such factors need to be considered in the design of a trial of therapeutic hypothermia in pediatric patients.
METHODS
A retrospective review of in-hospital cardiac arrest events occurring between July 1, 2003 and December 31, 2004 was conducted across 15 children’s hospitals within the PECARN network. Patients between one day (24 hours) and 18 years of age who had an in-hospital cardiac arrest and return of circulation for at least 20 consecutive minutes were eligible for inclusion. Cardiac arrest was defined as a CPR event with greater than one minute of chest compressions. Return of circulation includes both spontaneous and assisted circulation (e.g. extracorporeal membrane oxygenation (ECMO)). Patients who were cared for in a neonatal intensive care unit, who had cardiac arrest in the operating room as part of a planned cardiac surgical procedure, or who had arrest beginning prior to hospital arrival (out-of-hospital) were excluded. These criteria were selected to identify a cohort of pediatric patients similar to those who would be potentially eligible for a future hypothermia trial. Patients were identified by medical record ICD-9 codes, procedure codes, institutional arrest logs, morbidity and mortality reviews, emergency department records, trauma records, Pediatric Risk of Mortality (PRISM) scores (25), and other site specific mechanisms. If a patient experienced more than one cardiac arrest during the study period, only the first arrest meeting eligibility criteria was included. The study was approved and a waiver of informed consent granted by the Institutional Review Board at each site.
The PECARN Central Data Management and Coordinating Center (CDMCC) at the University of Utah trained investigators and data abstractors at each site to review patient records and collect data. Training included review of a manual of operations, teleconferences, and comparative coding of hypothetical patient records. During data collection, a sample of nearly 20% of records coded by data abstractors was reviewed by the site investigators for 27 key data fields. Overall accuracy was >96%. Data fields reviewed by the site investigator that did not match with those of the abstractor were flagged for investigator review and resolution. All data were double-entered into a secure, encrypted Internet site and electronically submitted to the CDMCC. The CDMCC performed a secondary review to ensure data quality, and site investigators were queried to resolve data discrepancies.
Data collected included (1) patients’ baseline clinical characteristics; (2) cardiac arrest event characteristics such as location and timing, first and subsequent documented cardiac rhythms, and monitoring devices and interventions used prior to and during the arrest; (3) etiology of cardiac arrest; (4) hospital course such as use of ECMO and therapeutic hypothermia, and the occurrence of subsequent arrests and seizures; (5) physiologic and laboratory data such as pupillary reflexes, minimum and maximum body temperature, blood pH, and glucose concentration, and maximum lactate concentration in the first 12 hours post-arrest; (6) Pediatric Cerebral Performance Category (PCPC) (26) scores prior to cardiac arrest and at hospital discharge; and (7) survival to hospital discharge. In addition, dates and times of important clinical events were recorded and related time intervals determined. These intervals included the time from arrest to initiation of CPR, first epinephrine dose, first defibrillation attempt, ECMO, therapeutic hypothermia, as well as the durations of CPR, and PICU and hospital stay.
Variable definitions were based on Utstein Style Guidelines (18). Weekends were defined as Friday 11:00 PM to Monday 6:59 AM, and nights as 11:00 PM to 6:59 AM. Post-operative CPR was defined as the provision of CPR after a surgical operation and prior to discharge from the hospitalization in which the operation occurred. PCPC scores measure degree of cognitive function and range from 1 to 6 where 1 = normal, 2 = mild disability, 3 = moderate disability, 4 = severe disability, 5 = coma or vegetative state, and 6 = brain death (26). Good neurological outcome was defined as PCPC score of 1 or 2 at hospital discharge, or no change in score from pre-arrest to hospital discharge.
Statistical Analyses
Analyses were restricted to patients having full data on relevant variables. Each variable was described for survivors and non-survivors using counts and percentages for categorical variables and the median and interquartile range (IQR) (25th – 75th percentile) for continuous variables. The association of each variable with hospital mortality was examined using Chi-square or Fisher's exact tests for categorical variables and the Wilcoxon rank-sum test for continuous variables. The Cochran-Armitage test for trend was used for ordered categorical variables.
Logistic regression was used to identify variables that were independently associated with hospital mortality. First, the univariate association of each variable with mortality was examined, as described above. All variables with p < 0.25 were eligible for inclusion in the logistic regression model. In addition, the decision was made a priori to include patient age, gender and first documented cardiac arrest rhythm in the model regardless of statistical significance. Forward stepwise selection was next applied to this pool of potential predictors in order to obtain a final model. The criteria for variable selection were a significance level to enter of 0.05 and significance level to stay of 0.10. Patient weight was not included in the model because of its strong correlation with age. Maximum pH was not included because minimum pH was considered to be the more appropriate variable and both were highly associated with outcome. Maximum lactate was not included because of the large number of missing values.
Three final logistic regression models were built. The first model included only variables available before and during the arrest. The second model additionally evaluated variables collected in the first 12 hours post-arrest. Finally, because patients placed on ECMO in the first 12 hours post-arrest are likely to have a different mortality risk, a third model excluded ECMO patients. Adjusted odds ratios and 95% confidence intervals were calculated for each model. The c-statistic, or area under the receiving operating characteristic curve, is also reported (27). All analyses were conducted in SAS version 9.1 (SAS Institute Inc., North Carolina).
RESULTS
Three hundred and fifty-three patients (n=353) had an in-hospital CPR event with chest compressions for greater than one minute, and return of circulation for at least 20 minutes. Of these, 172 (48.7%) survived to hospital discharge. Baseline patient characteristics are shown in Table 1. Younger age and lower body weight were associated with survival. Survivors were more likely to have pre-existing congenital heart disease whereas non-survivors were more likely to have pre-existing hematologic, oncologic or immunologic disorders, genetic or metabolic disorders, and renal disorders.
Table 1.
Patient Characteristics and Relationship to Hospital Mortalitya
| Survivors (N=172) |
Non-survivors (N=181) |
P-Valueb | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Age (years) | 0.7 (0.1, 3.7) | 1.3 (0.3, 8.7) | 0.01 |
| Weight (kg) | 5.8 (3.6, 17.3) | 9.1 (4.0, 27.9) | 0.02 |
| n (percent) | n (percent) | ||
| Age category (Utstein) | 0.01 | ||
| 0 to 30 days | 37 (21.5) | 26 (14.4) | |
| 31 days to < 1 year | 63 (36.6) | 58 (32.0) | |
| 1 year - < 3 years | 24 (14.0) | 26 (14.4) | |
| 3 years - < 8 years | 19 (11.0) | 23 (12.7) | |
| 8 years to < 14 years | 16 (9.3) | 26 (14.4) | |
| 14 years to < 19 years | 13 (7.6) | 22 (12.2) | |
| Gender (male) | 102 (59.3) | 100 (55.6) | 0.48 |
| Race | 0.62 | ||
| White | 83 (48.3) | 85 (47.0) | |
| Black | 47 (27.3) | 44 (24.3) | |
| Other / Unknown | 42 (24.4) | 52 (28.7) | |
| Ethnicity | 0.58 | ||
| Hispanic | 15 (8.7) | 11 (6.1) | |
| Not Hispanic | 58 (33.7) | 59 (32.6) | |
| Unknown | 99 (57.6) | 111 (61.3) | |
| Insurance type | 0.72 | ||
| Commercial | 96 (57.8) | 92 (53.8) | |
| Medicaid | 59 (35.5) | 68 (39.8) | |
| Other insurance | 11 (6.6) | 11 (6.4) | |
| Any chronic pre-existing condition | 151 (87.8) | 159 (87.8) | 0.99 |
| Specific chronic pre-existing conditionsc | |||
| Prenatal conditions or complications | 22 (12.8) | 24 (13.3) | 0.90 |
| Lung or airway disease | 50 (29.1) | 44 (24.3) | 0.31 |
| Congenital heart disease | 96 (55.8) | 80 (44.2) | 0.03 |
| Acquired heart disease | 20 (11.6) | 23 (12.7) | 0.76 |
| Hematologic, oncologic or immunologic | 15 (8.7) | 41 (22.7) | < 0.01 |
| Gastrointestinal | 32 (18.6) | 43 (23.8) | 0.24 |
| Genetic/metabolic | 19 (11.0) | 35 (19.3) | 0.03 |
| Endocrine | 6 (3.5) | 6 (3.3) | 0.93 |
| Renal | 14 (8.1) | 31 (17.1) | 0.01 |
| Neurological | 43 (25.0) | 39 (21.5) | 0.44 |
Unavailable (missing) values were excluded from calculations of percentages and summary statistics for the following variables: weight (5), gender (1), insurance type (16).
For comparison between survivors and non-survivors, Chi-square or Fisher’s exact was used for categorical variables, Wilcoxon rank-sum test was used for continuous variables, and the Cochran-Armitage trend test was used for the ordered age categorical variable.
For chronic pre-existing conditions, a condition was assumed to be not present unless specifically noted otherwise.
Cardiac arrest event characteristics are shown in Table 2. Survivors were more likely to have received CPR during the daytime and post-operatively. Non-survivors were more likely to have an endotracheal tube prior to arrest, and to have received sodium bicarbonate, calcium and vasopressin during the arrest. Non-survivors received a greater number of epinephrine doses than survivors (Figure 1). First documented cardiac rhythm was not significantly associated with survival (Table 2). Of those with bradycardia as the first documented rhythm (N=173), 28 (16.2%) subsequently developed asystole, 17 (9.8%) ventricular fibrillation or tachycardia, and 4 (2.3%) pulseless electrical activity (PEA).
Table 2.
Cardiac Arrest Event Characteristics and Relationship to Hospital Moralitya
| Survivors (N=172) |
Non-survivors (N=181) |
P-Valueb | |
|---|---|---|---|
| n (percent) | n (percent) | ||
| Location of arrest | 0.51 | ||
| Emergency department | 18 (10.6) | 26 (14.5) | |
| General ward | 24 (14.1) | 19 (10.6) | |
| Intensive care unit | 113 (66.5) | 115 (64.2) | |
| Other | 15 (8.8) | 19 (10.6) | |
| Day of arrest | 0.43 | ||
| Weekday (Mon 7:00 am - Fri 10:59 pm) | 121 (71.2) | 117 (67.2) | |
| Weekend (Fri 11:00 pm - Mon 6:59 am) | 49 (28.8) | 57 (32.8) | |
| Time of arrest | 0.03 | ||
| Day (7:00 am – 10:59 pm) | 131 (77.1) | 116 (66.7) | |
| Night (11:00 pm – 6:59 am) | 39 (22.9) | 58 (33.3) | |
| CPR provided post-operatively | 60 (35.7) | 37 (21.1) | < 0.01 |
| First documented rhythm | 0.34 | ||
| Asystole | 23 (13.4) | 32 (17.7) | |
| Bradycardia | 93 (54.1) | 80 (44.2) | |
| Pulseless electrical activity | 16 (9.3) | 15 (8.3) | |
| Ventricular fibrillation/tachycardia | 16 (9.3) | 19 (10.5) | |
| Other / Unknown | 24 (14.0) | 35 (19.3) | |
| Presence of IV prior to arrest | 160 (93.6) | 164 (90.6) | 0.31 |
| Presence of ET tube prior to arrest | 98 (57.6) | 123 (68.0) | 0.05 |
| Interventions and monitoring devices present prior to arrest | |||
| Central venous catheter | 94 (59.5) | 109 (66.9) | 0.17 |
| Arterial catheter | 73 (46.2) | 89 (54.6) | 0.13 |
| Cardiac monitor | 145 (91.8) | 152 (93.3) | 0.61 |
| Pulse oximeter | 146 (92.4) | 151 (92.6) | 0.94 |
| Defibrillated during arrest | 24 (14.6) | 33 (19.3) | 0.26 |
| Open chest CPR | 13 (7.6) | 12 (6.8) | 0.75 |
| Drugs administered | |||
| Fluid bolus | 67 (42.4) | 72 (40.7) | 0.75 |
| Atropine | 56 (35.4) | 68 (38.4) | 0.57 |
| Sodium Bicarbonate | 78 (49.4) | 125 (70.6) | < 0.01 |
| Calcium | 69 (43.7) | 105 (59.3) | < 0.01 |
| Vasopressin | 3 (1.9) | 15 (8.5) | 0.01 |
| Lidocaine | 15 (9.5) | 18 (10.2) | 0.84 |
| Amiodarone | 7 (4.4) | 12 (6.8) | 0.35 |
| Epinephrine | 134 (80.7) | 159 (90.9) | 0.01 |
| Median (IQR) | Median (IQR) | ||
| Epinephrine doses administered | 2.0 (1.0, 3.0) | 3.0 (1.0, 4.0) | < 0.01 |
Unavailable (missing) values were excluded from calculations of percentages and summary statistics for the following variables: location of arrest (4), day and time of arrest (9), CPR post operative (10), presence of IV (1), presence of ET tube (2), interventions and monitoring devices (32), defibrillated during arrest (18), open chest CPR (6), drugs administered (except epinephrine, 18), epinephrine administered (12).
For comparison between survivors and non-survivors, Chi-square or Fisher's exact was used for categorical variables and Wilcoxon rank-sum test was used for continuous variables.
Figure 1. Patient Mortality By Number of Epinephrine Doses Received.
Data are unadjusted and represent a basic summary of mortality by number of epinephrine doses. The solid line includes all patients. The dashed line excludes patients placed on ECMO in the 12 hours following arrest.
Etiologies of cardiac arrest are shown in Table 3. Survivors were more likely to have respiratory causes of arrest whereas non-survivors were more likely to have trauma and known terminal conditions.
Table 3.
Etiology of Cardiac Arrest and Relationship to Hospital Mortalitya
| Survivors (N=167) |
Non-survivors (N=180) |
P-Valueb | |
|---|---|---|---|
| n (percent) | n (percent) | ||
| Cardiac (not congenital heart disease) | 57 (34.1) | 67 (37.2) | 0.55 |
| Arrhythmia | 25 (15.0) | 17 (9.4) | |
| Hypovolemic shock | 8 (4.8) | 11 (6.1) | |
| Septic shock | 9 (5.4) | 19 (10.6) | |
| Cardiomyopathy | 5 (3.0) | 3 (1.7) | |
| Other | 15 (9.0) | 26 (14.4) | |
| Congenital heart disease | 68 (40.7) | 62 (34.4) | 0.23 |
| Arrhythmia | 30 (18.0) | 39 (21.7) | |
| Low cardiac output | 19 (11.4) | 19 (10.6) | |
| Hypoxemia | 9 (5.4) | 6 (3.3) | |
| During post-op course | 31 (18.6) | 21 (11.7) | |
| Tamponade | 2 (1.2) | 2 (1.1) | |
| Other | 4 (2.4) | 5 (2.8) | |
| Respiratory | 80 (47.9) | 65 (36.1) | 0.03 |
| Endotracheal tube displacement | 10 (6.0) | 9 (5.0) | |
| Respiratory failure | 55 (32.9) | 57 (31.7) | |
| Airway obstruction | 8 (4.8) | 0 (0.0) | |
| Other | 8 (4.8) | 3 (1.7) | |
| Neurologic | 3 (1.8) | 5 (2.8) | 0.54 |
| Drug overdose/Ingestion | 2 (1.2) | 1 (0.6) | 0.61 |
| Trauma | 5 (3.0) | 17 (9.4) | 0.01 |
| Electrolyte imbalance | 10 (6.0) | 20 (11.1) | 0.09 |
| Terminal condition | 2 (1.2) | 10 (5.6) | 0.03 |
Patients could have multiple categories identified for etiology of arrest. There were six patients (5 survivors and 1 non-survivor) who did not have any information documented for etiology of arrest. These patients were excluded from percentage calculations.
Chi-square or Fisher's exact used for comparison between survivors and non-survivors.
Interventions and monitoring devices used during the first 12 hours post-arrest are shown in Table 4. Non-survivors were more likely to have received inotropes and/or vasopressors. In the first 24 hours after the initial cardiac arrest, 28 (16.3%) survivors and 70 (38.7%) non-survivors had one or more subsequent arrests (P < 0.01). Seizures occurred after the initial arrest and prior to hospital discharge in 22 (13.2%) survivors and 28 (15.6%) non-survivors (P = 0.51).
Table 4.
Post-Arrest Hospital Course (0–12 Hours) and Relationship to Hospital Mortalitya
| Survivors (N=172) |
Non-survivors (N=181) |
P-Valueb | |
|---|---|---|---|
| n (percent) | n (percent) | ||
| ICU interventions and monitoring devices | |||
| Mechanical ventilation | 159 (92.4) | 176 (98.3) | 0.01 |
| Therapeutic hypothermia | 7 (4.1) | 6 (3.3) | 0.71 |
| ECMO | 28 (16.3) | 30 (16.8) | 0.90 |
| Dialysis | 4 (2.3) | 15 (8.4) | 0.01 |
| Central venous catheter | 143 (83.1) | 164 (91.6) | 0.02 |
| Arterial catheter | 130 (75.6) | 151 (84.4) | 0.04 |
| Intraosseous access | 7 (4.1) | 13 (7.3) | 0.20 |
| Peripheral intravenous catheter | 132 (76.7) | 124 (69.3) | 0.12 |
| Intracranial pressure monitor | 2 (1.2) | 7 (3.9) | 0.17 |
| Cardiac monitor | 172 (100.0) | 178 (99.4) | 1.00 |
| Pulse oximeter | 172 (100.0) | 178 (99.4) | 1.00 |
| Drug therapies | |||
| Antiarrhythmics | 33 (19.3) | 33 (18.3) | 0.82 |
| Anticonvulsants | 23 (13.5) | 31 (17.2) | 0.33 |
| Any inotrope or vasopressor | 131 (76.6) | 162 (90.0) | < 0.01 |
| Dopamine | 85 (49.7) | 95 (52.8) | 0.57 |
| Dobutamine | 21 (12.3) | 29 (16.1) | 0.30 |
| Epinephrine | 85 (49.7) | 131 (72.8) | < 0.01 |
| Norepinephrine | 8 (4.7) | 19 (10.6) | 0.04 |
| Milrinone or amrinone | 67 (39.2) | 50 (27.8) | 0.02 |
| Vasopressin | 18 (10.5) | 36 (20.0) | 0.01 |
| Other inotrope or vasopressor | 20 (11.7) | 29 (16.1) | 0.23 |
Unavailable (missing) values were excluded from calculations of percentages and summary statistics. There were two non-survivors with missing data for ICU interventions and monitoring devices (except therapeutic hypothermia which had complete data capture). There was one survivor and one non-survivor with missing data for drug therapies.
Chi-square or Fisher's exact used for comparison between survivors and non-survivors.
Physiologic and laboratory values measured in the first 12 hours post-arrest are shown in Table 5. Survivors had higher body temperatures, higher pH and lower lactate concentrations than non-survivors. Survivors were more likely to have two responsive pupils throughout the first 12 hours post-arrest than non-survivors.
Table 5.
Physiologic and Laboratory Values (0–12 Hours Post Arrest) and Relationship to Hospital Mortalitya
| Survivors (N=172) |
Non-survivors (N=181) |
P-Valueb | |||
|---|---|---|---|---|---|
| N |
Median (IQR) N |
N |
Median (IQR) |
||
| Minimum body temperature, °C | 172 | 35.5 (34.7, 36.3) | 166 | 35.1 (33.4, 36.2) | 0.02 |
| Maximum body temperature, °C | 172 | 37.3 (36.8, 38.0) | 166 | 36.9 (36.1, 37.9) | 0.01 |
| Minimum pH | 158 | 7.26 (7.13, 7.35) | 170 | 7.12 (6.96, 7.29) | < 0.01 |
| Maximum pH | 158 | 7.48 (7.40, 7.55) | 170 | 7.44 (7.32, 7.53) | < 0.01 |
| Maximum lactate, mmol/L | 118 | 4.8 (2.4, 9.6) | 112 | 12.6 (5.9, 18.6) | < 0.01 |
| Minimum glucose, mmol/L | 154 | 6.3 (4.8, 8.8) | 159 | 6.4 (4.4, 10.8) | 0.81 |
| Maximum glucose, mmol/L | 154 | 10.1 (6.5, 14.9) | 159 | 11.3 (6.7, 17.5) | 0.16 |
| N | n (percent) | N | n (percent) | ||
| Two responsive pupils | 158 | 140 (88.6) | 155 | 95 (61.3) | < 0.01 |
Unavailable (missing) values were excluded from calculations of summary statistics.
For comparison between survivors and non-survivors, Chi-square test was used for categorical variables and Wilcoxon rank-sum test was used for continuous variables.
Time intervals between the start of the arrest and various therapeutic modalities or clinical events are shown in Table 6. In the majority of cases (85.7%), initiation of CPR was documented within the first minute of detecting the need for chest compressions; and in 62.3% of cases, administration of the first epinephrine dose was documented within the first two minutes of initiation of chest compressions. Survivors had a shorter duration of CPR than non-survivors.
Table 6.
Time Intervals and Relationship to Hospital Mortalitya
| Survivors (N=172) |
Non-survivors (N=181) |
P-Valueb | |||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | ||
| Interval from arrest to | |||||
| Initiation of CPR (mins) | 168 | 0.0 (0.0, 0.0) | 168 | 0.0 (0.0, 0.0) | 0.84 |
| First epinephrine dose (mins) | 128 | 0.0 (0.0, 3.0) | 148 | 1.0 (0.0, 2.0) | 0.91 |
| First attempted defibrillation (mins)c | 12 | 1.0 (0.0, 2.0) | 12 | 2.0 (0.5, 9.0) | 0.12 |
| Initiation of ECMO (hrs) | 28 | 1.0 (1.0, 2.0) | 30 | 1.0 (1.0, 2.0) | 0.65 |
| Initiation of therapeutic hypothermia (mins) | 5 | 74.0 (33.0, 135.0) | 5 | 105.0 (100.0, 184.0) | 0.55 |
| Duration of CPR (mins) | 167 | 8.0 (3.0, 19.0) | 163 | 13.0 (5.0, 31.0) | < 0.01 |
| Duration of PICU stay (days) | 146 | 15.0 (6.0, 28.0) | 157 | 6.0 (1.0, 27.0) | < 0.01 |
| Duration of hospital stay (days) | 165 | 26.0 (15.0, 48.0) | 170 | 10.0 (2.0, 35.0) | < 0.01 |
Unavailable (missing) values were excluded from calculations of summary statistics.
Wilcoxon rank-sum test used for comparison between survivors and non-survivors.
For those whose first documented rhythm was ventricular fibrillation or ventricular tachycardia.
PCPC scores are shown in Table 7. Pre-arrest PCPC score was not associated with survival. Among survivors that had pre-arrest and discharge PCPC scores available (N=140), 132 (94.3%) had a good neurological outcome (PCPC score of 1 or 2 at hospital discharge or no change from pre-arrest to hospital discharge). Among all survivors (N=172), 132 (76.7%) had a good neurological outcome.
Table 7.
PCPC Scoresa
| Survivors (N=172) |
Non-survivors (N=181) |
P-Valueb | |
|---|---|---|---|
| n (percent) | n (percent) | ||
| Pre-arrest PCPC | |||
| Normal | 100 (69.0) | 87 (62.6) | 0.08 |
| Mild disability | 23 (15.9) | 19 (13.7) | |
| Moderate disability | 15 (10.3) | 20 (14.4) | |
| Severe disability | 6 (4.1) | 10 (7.2) | |
| Vegetative | 1 (0.7) | 3 (2.2) | |
| Hospital d/c PCPC | NA | ||
| Normal | 89 (61.0) | ||
| Mild disability | 27 (18.5) | ||
| Moderate disability | 19 (13.0) | ||
| Severe disability | 11 (7.5) | ||
| Vegetative | 0 (0.0) | ||
| Death | 0 (0.0) | 181 (100) | |
| Change in PCPC | NA | ||
| No change | 124 (88.6) | 0 (0.0) | |
| 1 level | 9 (6.4) | 3 (2.2) | |
| 2 levels | 5 (3.6) | 10 (7.2) | |
| 3 levels | 2 (1.4) | 20 (14.4) | |
| 4 levels | 0 (0.0) | 19 (13.7) | |
| 5 levels | 0 (0.0) | 87 (62.6) |
Unavailable (missing) values were excluded from calculations of percentages and summary statistics for the following variables: pre-arrest PCPC (69), hospital discharge PCPC (26), change in PCPC (74).
Cochran-Armitage trend test used for comparison between survivors and non-survivors.
The results of the three regression models are shown in Table 8. Each model was adjusted for age, gender and first documented rhythm. In Model 1 (variables available prior to and during the arrest), pre-existing hematologic, oncologic, or immunologic disorders, and pre-existing genetic or metabolic disorders; presence of an endotracheal tube prior to the arrest; and the use of sodium bicarbonate during the arrest were independently associated with increased hospital mortality. Post-operative CPR was associated with decreased mortality. In Model 2 (variables available up to 12 hours post-arrest), the use of calcium during the arrest was associated with increased mortality. Higher minimum blood pH and pupillary responsiveness were associated with decreased mortality. In Model 3 (excluding ECMO patients), pre-existing genetic or metabolic disorders, electrolyte imbalance as an etiology of arrest, and longer duration of CPR were associated with increased mortality. Higher minimum blood pH and papillary responsiveness were associated with decreased mortality.
Table 8.
Logistic Regression Models for Hospital Mortalitya
| Model | Variable | Odds Ratio |
95% CI | P-value |
|---|---|---|---|---|
| Model 1b (N=323) |
Pre-existing condition | |||
| Hematologic, oncologic, or immunologic | 2.61 | 1.27–5.35 | 0.01 | |
| Genetic or metabolic | 1.85 | 0.91–3.79 | 0.09 | |
| Presence of ET tube prior to arrest | 1.97 | 1.17–3.31 | 0.01 | |
| CPR during post-operative period | 0.44 | 0.25– 0.76 | <0.01 | |
| Sodium bicarbonate administered during CPR | 2.72 | 1.66–4.48 | <0.01 | |
| Model 2c (N=277) |
Calcium administered during CPR | 2.26 | 1.29–3.96 | <0.01 |
| pH (0.10 unit increase) | 0.77 | 0.67–0.89 | <0.01 | |
| Two responsive pupils | 0.23 | 0.11–0.46 | <0.01 | |
| Model 3d (N=224) |
Pre-existing conditions | |||
| Genetic or metabolic | 2.28 | 1.02–5.13 | 0.05 | |
| Etiology of arrest | ||||
| Electrolyte imbalance | 3.35 | 1.18–9.47 | 0.02 | |
| Duration of CPR (mins) | 1.02 | 1.00–1.03 | 0.05 | |
| pH (0.10 unit change) | 0.80 | 0.68–0.95 | 0.01 | |
| Two responsive pupils | 0.21 | 0.09–0.48 | ≤0.01 |
Odds ratios and 95% confidence intervals are based on multivariable logistic regression controlled for age, gender and first documented rhythm.
Model 1 include variables available prior to and during the arrest. The c-statistic (AUC) for Model 1 is 0.73.
Model 2 includes variables available up to 12 hours post-arrest. The c-statistic for Model 2 is 0.76.
Model 3 excludes ECMO patients. The c-statistic for Model 3 is 0.77.
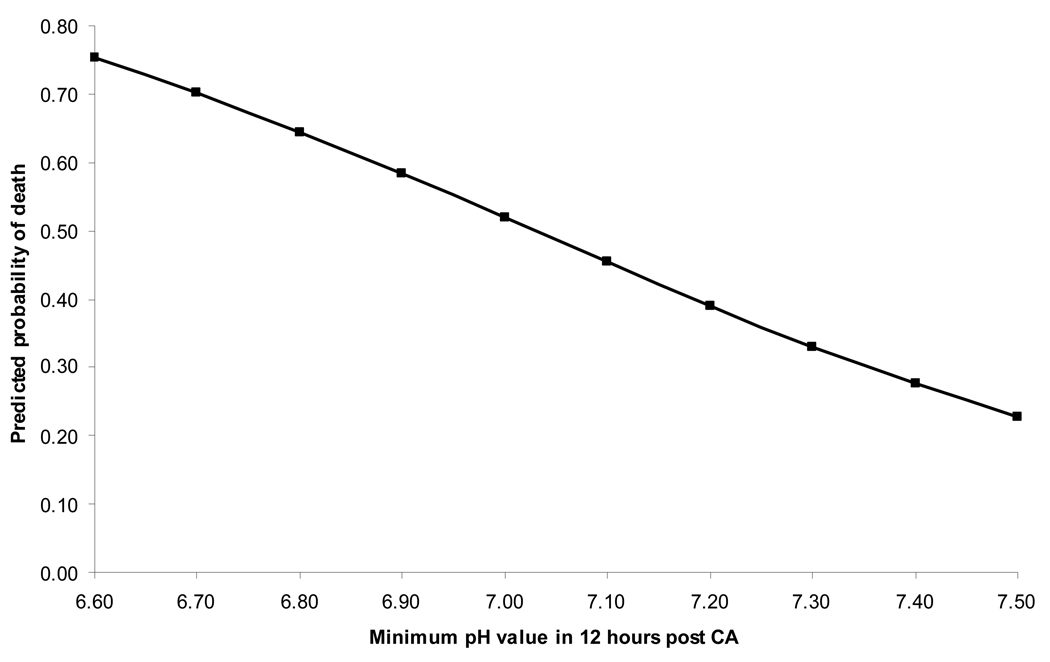
Figure 2 depicts the predicted probability of death based on minimum pH value during the first 12 hours post-arrest, after adjusting for factors described in Model 2. For an average patient (median age and other characteristics as most frequently observed in the population), the predicted probability of mortality was 70% or higher for pH values < 6.70, and 30% or lower for values > 7.35. A pH of 7.03 corresponded to a 50% predicted probability of death.
Figure 2. Probability of Death Based on pH Values.
Predicted probabilities are based on logistic regression Model 2 (including variables prior to, during, and up to 12 hours after cardiac arrest). The predicted probabilities are based on an "average" cardiac arrest patient with median age (0.9 years) and values for all other variables based on most frequently observed in population, ie, male with initial arrest rhythm of bradycardia, calcium administered during CPR, and both pupils responsive during 12 hours post arrest.
DISCUSSION
Our findings describe the clinical characteristics, early hospital course and outcome of a cohort of pediatric patients who experienced an in-hospital CPR event with chest compressions for greater than one minute, and who had a return of circulation for at least 20 minutes. This cohort was identified from 15 PECARN children’s hospitals whose locations are geographically diverse and represent all four U.S. Census Bureau Regions and six of nine Census Bureau Divisions (28). The majority of patients within this cohort had chronic pre-existing conditions and was highly monitored both before and after the cardiac arrest event. Many arrests occurred in the PICU. Initiation of CPR was most often immediate with the first epinephrine dose usually being given within one to two minutes of the start of arrest. The rate of survival to hospital discharge within this cohort who had a sustained return of circulation was 48.7%. Of those who survived to hospital discharge, 76.7% had a documented good neurological outcome (i.e., PCPC score of 1, 2, or no change from pre-arrest). Our cohort’s survival rate is similar to that reported by Nadkarni et al from the NRCPR registry who found that 236 (51.4%) of 459 children with in-hospital cardiac arrest and sustained return of circulation survived to hospital discharge (11). Nadkarni et al reported good neurological outcome at hospital discharge (PCPC score of 1, 2, 3, or no change from pre-arrest) in 65% of pediatric patients who survived in-hospital cardiac arrest. However, in their study, good neurologic outcome was defined to include patients discharged with moderate disability (i.e., PCPC score of 3).
Variables collected in our study were based on Utstein Style definitions developed for uniform reporting of cardiac arrest and resuscitation data (18). Utstein guidelines define cardiac arrest as the cessation of cardiac mechanical activity as confirmed by the absence of signs of circulation. Absent signs of circulation include the inability to palpate a central pulse, unresponsiveness and apnea. Because of the retrospective nature of our study and the lack of consistent documentation of central pulses in medical records, we defined cardiac arrest as a CPR event with greater than 1 minute of chest compressions. Patients receiving greater than one minute of chest compressions by health professionals were assumed to have absent signs of circulation. Several studies have demonstrated extremely poor diagnostic accuracy of the central pulse check for both lay rescuers and professional healthcare providers (29–32).
Cardiac arrest is a clinical diagnosis and may be present in a child even when the first documented rhythm reveals some form of organized electrical activity (33). Indeed, the most frequent first documented rhythm reported at the time of initiation of chest compressions in 173 (49.0%) of our patients was bradycardia. Many of these patients with initial bradycardia went on to develop rhythms more typically associated with absent pulses. However, many others who were initially bradycardic had no subsequent abnormal rhythms documented. Patients with initial bradycardia received a median of 8 minutes of chest compressions (IQR 3–20 minutes) and a median of two doses of epinephrine (IQR 1–3 doses). It is unlikely that patients with this duration of CPR and epinephrine requirement had effective mechanical activity of the heart. A small proportion of our cohort (4.6%) did not receive mechanical ventilation following the cardiac arrest event. These patients either recovered sufficiently after CPR not to require mechanical ventilation (e.g., ventricular tachycardia or fibrillation that resolved with cardioversion), or died after a 20 minute period of returned circulation without mechanical ventilation (e.g., intubation with hand-bagging prior to PICU transfer).
Our cohort does not include all children who experienced an in-hospital cardiac arrest within participating PECARN sites during the time period of the study. Only those patients who would potentially be eligible for a randomized trial of the effect of therapeutic hypothermia on neurobehavioral outcomes after in-hospital cardiac arrest were included. We excluded cases with brief duration of CPR (less than one minute) even if epinephrine or defibrillation were administered. Such patients may be less likely to have significant neurobehavioral deficits that could be measured in the context and timeframe of a clinical trial. We also excluded cases without return of circulation since these cases would not be eligible for a clinical trial to establish efficacy of therapeutic hypothermia. Comparison of our findings with those from other studies should be careful to account for these differences in inclusion and exclusion criteria.
Previous NRCPR studies have suggested that younger age at the time of cardiac arrest is associated with better outcomes. Meaney et al found that newborns and infants had higher rates of survival to hospital discharge than older children following cardiac arrest in the PICU (13). Nadkarni et al found that children had higher survival rates than adults after in-hospital pulseless cardiac arrest (11). NRCPR studies have also described that first documented pulseless arrest rhythm is associated with survival to hospital discharge (11). In one study that included both adults and children, a first rhythm of ventricular fibrillation or tachycardia provided a survival benefit (11). In another NRCPR study that included only children, Samson et al found that patients with ventricular fibrillation or tachycardia as a first rhythm had similar survival rates as patients with other types of first rhythms (15). Although our logistic regression models controlled for age, gender and first documented arrest rhythm, these variables were not found to be independent predictors of hospital mortality in our cohort. The inclusion of only those with a sustained return of circulation likely influenced our results.
Variables available prior to and during the cardiac arrest event that were independently associated with increased hospital mortality in our cohort included pre-existing hematologic, oncologic, or immunologic disorders, genetic or metabolic disorders, the presence of an endotracheal tube prior to arrest, and the use of sodium bicarbonate during the arrest (see Model 1). Other studies have similarly reported that chronic pre-existing conditions are common among pediatric patients that experience in-hospital cardiac arrest and that such conditions are often predictive of survival (5, 7, 15). Decreased survival after cardiac arrest among patients with malignancy or genetic disorders likely reflects the poor prognoses often associated with these conditions. Similarly, the presence of an endotracheal tube prior to arrest likely reflects the patient’s severity of illness at the time of the arrest. Within our cohort, patients who received sodium bicarbonate had a longer duration of CPR, and were more likely to receive other pharmacologic interventions such as calcium, vasopressin and a greater number of epinephrine doses. Variables that were independently associated with decreased mortality included CPR provided post-operatively. Post-operative CPR was often performed on young infants after surgical repair of congenital heart defects, a situation previously found to be associated with better outcomes (15). A recent report from the NRCPR described time of day and day of week as predictors of pulseless cardiac arrest outcome in adults (34). In our cohort of in-hospital pediatric cardiac arrest with return of circulation, night and weekend arrests were not independently associated with mortality when age, gender and first documented rhythm were controlled.
When extending the time frame of our regression model to include variables available within 12 hours post-arrest, the use of calcium during the arrest was independently associated with increased hospital mortality (see Model 2). In the year 2000, the American Heart Association recommended restricting calcium use during CPR to specific conditions including hypocalcemia, hyperkalemia, hypermagnesemia, and calcium channel blocker overdose (35). Despite this recommendation, calcium was given to 174 (51.9%) of our patients. This finding is similar to that reported by Srinivasan et al who found that calcium was given to 45% of pediatric patients included in the NRCPR registry, and that calcium use was associated with decreased survival and poor neurological recovery (16). In our cohort, higher minimum blood pH and pupillary responsiveness were associated with decreased hospital mortality. Blood pH correlated inversely with lactic acid concentration; higher pH likely reflects better oxygen delivery during CPR. Pupil reactivity has been associated with survival after cardiac arrest in adults (36).
In our final logistic regression model, we excluded patients who received ECMO following in-hospital cardiac arrest. ECMO is increasingly used to restore circulation in patients with cardiac arrest refractory to conventional CPR, but may not be universally available after cardiac arrest at all children’s hospitals. Recent studies report survival rates of 34%–41% in such patients who without ECMO would not be likely to survive (7, 37–40). Excluding ECMO patients from our analysis, variables independently associated with increased hospital mortality included pre-existing genetic or metabolic disorders, electrolyte imbalance, and longer duration of CPR. Higher minimum blood pH and pupillary responsiveness were associated with decreased mortality. These variables are similar to or correlated with those described above in our models that include ECMO patients. Differences likely reflect the smaller sample size and missing data.
Recent reports have suggested that hyperglycemia is associated with increased mortality in heterogeneous PICU populations (41, 42). In our multicenter cohort study that examines a more homogenous group with recent cardiac arrest, peak glucose concentrations in the immediate period 12 hours post-arrest were not associated with mortality. Bernard et al described the use of 12 hours of therapeutic hypothermia in a cohort of adult cardiac arrest patients and found this intervention to be associated with improved outcome and hyperglycemia (19).
Findings from this study are important for designing a multicenter trial of therapeutic hypothermia following in-hospital cardiac arrest in pediatric patients. Importantly, the findings provide information about the number of patients available for study across the participating PECARN sites. Estimates of neurological outcomes with PCPC scores allow more accurate power calculations for a hypothermia trial that proposes to use neurobehavioral outcome as a primary outcome measure. Relying on past literature alone, certain groups of patients such as those with sepsis as an etiology of cardiac arrest would likely be excluded from a trial of therapeutic hypothermia because of the extremely high mortality rates reported (4). However, in our in-hospital cardiac arrest cohort, 32.1 % of patients with septic shock as an etiology of arrest survived to hospital discharge justifying their inclusion in a clinical trial. The low reported use of hypothermia in our cohort (< 5%) suggests that clinical equipoise existed for therapeutic hypothermia after pediatric cardiac arrest during the time period of the study. However, this observation must be interpreted with caution since equipoise within the critical care community may change over time. A survey of the pediatric critical care community conducted in 2005 and published in 2006 reported that 95% of respondents would be willing to randomize their cardiac arrest patients to a therapeutic hypothermia trial (43). This finding likely reflects the fact that no trial of therapeutic hypothermia has been conducted in any in-hospital cardiac arrest population, and no trial has been conducted following pediatric cardiac arrest. A trial investigating the use of therapeutic hypothermia in pediatric patients with severe traumatic brain injury was published in 2008 (44). The trial concluded that therapeutic hypothermia does not improve neurological outcome and may increase mortality in children with brain injury (44). These findings may also contribute to equipoise regarding therapeutic hypothermia for pediatric conditions other than traumatic brain injury such as pediatric cardiac arrest.
Limitations of this study include the retrospective nature of case identification and data collection. Missing data occurred when specific variables were not adequately documented in the medical records. Missing data likely accounted for some of the difference in identified predictors of mortality between regression models. In a retrospective study, associations between variables may not represent cause and effect. Our data were collected between July 1, 2003 and December 31, 2004, and some practices such as use of therapeutic hypothermia after CPR may presently occur at different rates. PCPC scores are recommended in the Utstein Guidelines to assess neurological outcome after cardiac arrest. However, PCPC scores provide only a global assessment of neurological status and may fail to detect milder or more specific forms of dysfunction. PCPC scores were frequently missing especially for the younger patients. Strengths of this study include the multicenter participation of a geographically diverse group of PECARN children’s hospitals across the U.S., the extensive training and monitoring of data abstractors, and the detailed data collection performed as part of planning a randomized clinical trial.
CONCLUSIONS
Approximately half of children who experience an in-hospital cardiac arrest event with sustained return of circulation survive to hospital discharge. Among survivors, over three-fourths have good neurological outcomes based on PCPC measurements. Many variables are independently associated with hospital mortality. Future research should evaluate whether any of these associations represent cause and effect. Clinical investigators evaluating the efficacy of new interventions for pediatric cardiac arrest such as therapeutic hypothermia will need to consider these findings in their study designs.
ACKNOWLEDGEMENTS
We acknowledge the efforts of the following individuals participating in PECARN at the time this study was initiated.
PECARN Steering Committee: N. Kuppermann, Chair; E. Alpern, J. Chamberlain, J. M. Dean, M. Gerardi, J. Goepp, M. Gorelick, J. Hoyle, D. Jaffe, C. Johns, N. Levick, P. Mahajan, R. Maio, K. Melville, S. Miller*, D. Monroe, R. Ruddy, R. Stanley, D. Treloar, M. Tunik, A. Walker. MCHB/EMSC liaisons: D. Kavanaugh, H. Park.
Central Data Management and Coordinating Center (CDMCC): M. Dean, R. Holubkov, S. Knight, A. Donaldson.
Data Analysis and Management Subcommittee (DAMS): J. Chamberlain, Chair; M. Brown, H. Corneli, J. Goepp, R. Holubkov, P. Mahajan, K. Melville, E. Stremski, M. Tunik
Grants and Publications Subcommittee (GAPS): M. Gorelick, Chair; E. Alpern, J. M. Dean, G. Foltin, J. Joseph, S. Miller*, F. Moler, R. Stanley, S. Teach
Protocol Concept Review and Development Subcommittee (PCRADS): D. Jaffe, Chair; K. Brown, A. Cooper, J. M. Dean, C. Johns, R. Maio, N. C. Mann, D. Monroe, K. Shaw, D. Teitelbaum, D. Treloar
Quality Assurance Subcommittee (QAS): R. Stanley, Chair; D. Alexander, J. Brown, M. Gerardi, M. Gregor, R. Holubkov, K. Lillis, B. Nordberg, R. Ruddy, M. Shults, A. Walker
Safety and Regulatory Affairs Subcommittee (SRAS): N. Levick, Chair; J. Brennan, J. Brown, J. M. Dean, J. Hoyle, R. Maio, R. Ruddy, W. Schalick, T. Singh, J. Wright
Participating children’s hospital, university affiliation and site investigators are listed below in alphabetical order:
Children's Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio (R. Brilli)
Children's Hospital of Buffalo, SUNY-Buffalo, Buffalo, NY (L. Hernan)
Children's Hospital of Michigan, Wayne State University, Detroit, MI (K. Meert)
Children's Hospital of New York, Columbia University, New York, NY (C. Schleien)
Children's Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA (V. Nadkarni)
Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, PA (R. Clark)
Children's Hospital of Wisconsin, Medical Collage of Wisconsin, Milwaukee, WI (K. Tieves/T. Rice)
Children's National Medical Center, George Washington University, Washington D.C. (H. Dalton)
C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI (F. Moler)
Golisano Children’s Hospital, University of Rochester, Rochester, NY (E. van der Jagt)
Helen DeVos Children's Hospital, Michigan State University, Grand Rapids, MI (R. Hackbarth)
Primary Children’s Medical Center, University of Utah, Salt Lake City, UT (K. Statler)
St. Louis Children's Hospital, Washington University, St. Louis, MO (F. Levy/D. Jaffe)
The Johns Hopkins Hospital, Johns Hospkins University, Baltimore, MD (D. H. Shaffner)
University of California at Davis, Sacramento, CA (R. Pretzlaff)
Project supported by the following federal grants: NIH NICHD R21 HD044955 and R34 HD 050531 (FWM); the Pediatric Emergency Care Applied Research Network (PECARN) is supported by cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008 from the Emergency Medical Services for Children (EMSC) program of the Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services.
Footnotes
Presented in part at Society of Critical Care Medicine’s 36th Critical Care Congress, Orlando, FL.
deceased
REFERENCES
- 1.Ehrlich R, Emmett SM, Rodriguez-Torres RO. Pediatric cardiac resuscitation team: A 6 year study. J Pediatr. 1974;84:152–155. doi: 10.1016/s0022-3476(74)80580-9. [DOI] [PubMed] [Google Scholar]
- 2.Zaritsky A, Nadkarni V, Getson P, et al. CPR in children. Ann Emerg Med. 1987;16:1107–1111. doi: 10.1016/s0196-0644(87)80465-1. [DOI] [PubMed] [Google Scholar]
- 3.Slomin AD, Patel KM, Ruttimann UE, et al. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med. 1997;25:1951–1955. doi: 10.1097/00003246-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Torres A, Pichert CB, Firestone J, et al. Long-term functional outcome of inpatient pediatric cardiopulmonary resuscitation. Pediatr Emerg Care. 1997;13:369–373. doi: 10.1097/00006565-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Reis AG, Nadkarni V, Perondi MB, et al. A prospective investigation into the epidemiology of in-hospital pediatric cardiopulmonary resuscitation using the international Utstein reporting style. Pediatrics. 2002;109:200–209. doi: 10.1542/peds.109.2.200. [DOI] [PubMed] [Google Scholar]
- 6.Tibballs J, Kinney S. A prospective study of outcome of in-patient paediatric cardiopulmonary arrest. Resuscitation. 2006;71:310–318. doi: 10.1016/j.resuscitation.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.de Mos N, van Litsenburg RRL, McCrindle B, et al. Pediatric in-intensive care unit cardiac arrest: Incidence, survival, and predictive factors. Crit Care Med. 2006;34:1209–1215. doi: 10.1097/01.CCM.0000208440.66756.C2. [DOI] [PubMed] [Google Scholar]
- 8.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: An epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Horisberger T, Fischer JE, Fanconi S. One-year survival and neurological outcome after pediatric cardiopulmonary resuscitation. Intensive Care Med. 2002;28:365–368. doi: 10.1007/s00134-001-1188-z. [DOI] [PubMed] [Google Scholar]
- 10.Young KD, Seidel JS. Pediatric cardiopulmonary resuscitation: A collective review. Ann Emerg Med. 1999;33:195–205. doi: 10.1016/s0196-0644(99)70394-x. [DOI] [PubMed] [Google Scholar]
- 11.Nadkarni VM, Larkin GL, Peberdy MA, et al. National Registry of Cardiopulmonary Resuscitation Investigators: First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 12.Donoghue AJ, Nadkarni VM, Elliott M, et al. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators: Effect of hospital characteristics on outcomes from pediatric cardiopulmonary resuscitation: A report from the National Registry of Cardiopulmonary Resuscitation. Pediatrics. 2006;118:995–1001. doi: 10.1542/peds.2006-0453. [DOI] [PubMed] [Google Scholar]
- 13.Meaney PA, Nadkarni VM, Cook EF, et al. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators: Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118:2424–2433. doi: 10.1542/peds.2006-1724. [DOI] [PubMed] [Google Scholar]
- 14.October TW, Schleien CL, Berg RA, et al. National Registry of Cardiopulmonary Resuscitation Investigators: Increasing amiodarone use in cardiopulmonary resuscitation: An analysis of the National Registry of Cardiopulmonary Resuscitation. Crit Care Med. 2008;36:126–130. doi: 10.1097/01.CCM.0000295592.97331.5A. [DOI] [PubMed] [Google Scholar]
- 15.Samson RA, Nadkarni VM, Meaney PA, et al. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med. 2006;354:2328–2339. doi: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan V, Morris MC, Helfaer MA, et al. American Heart Association National Registry of CPR Investigators. Calcium use during in-hospital pediatric cardiopulmonary resuscitation: A report from the National Registry of Cardiopulmonary Resuscitation. Pediatrics. 2008;121:e1144–e1151. doi: 10.1542/peds.2007-1555. [DOI] [PubMed] [Google Scholar]
- 17.National Registry of Cardiopulmonary Resuscitation. [Accessed June 25, 2008]; Available online at: http://www.nrcpr.org.
- 18.Jacobs I, Nadkarni V, et al. The ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes. Cardiac arrest and cardiopulmonary resuscitation outcomes reports: Update and simplification of the Utstein templates for resuscitation registries: A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa. Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 19.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 20.Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 21.Guidelines 2005 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 7.5: Post resuscitation support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2005;112:IV-84–IV-88. [Google Scholar]
- 22.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 23.Pediatric Emergency Care Applied Research Network (PECARN) The Pediatric Emergency Care Applied Research Network (PECARN): Rationale, development, and first steps. Pediatr Emerg Care. 2003;19:185–193. doi: 10.1097/01.pec.0000081245.98249.6e. [DOI] [PubMed] [Google Scholar]
- 24. [Accessed June 25, 2008];Pediatric Emergency Care Applied Research Network. Available online at: http://www.pecarn.org.
- 25.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Fiser DH, Long N, Roberson PK, et al. Relationship of Pediatric Overall Performance Category and Pediatric Cerebral Performance Category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE, Jr, Lee KL, Califf RM, et al. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 28. [Accessed June 25, 2008];Census Regions and Divisions of the United States. Available on line at: http://www.census.gov/geo/www/us_regdiv.pdf.
- 29.Lapostolle F, Le Toumelin P, Agostinucci JM, et al. Basic cardiac life support providers checking the carotid pulse: Performance, degree of conviction, and influencing factors. Acad Emerg Med. 2004;11:878–880. doi: 10.1111/j.1553-2712.2004.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 30.Eberle B, Dick WF, Schneider T, et al. Checking the carotid pulse check: Diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation. 1996;33:107–116. doi: 10.1016/s0300-9572(96)01016-7. [DOI] [PubMed] [Google Scholar]
- 31.Ochoa FJ, Ramalle-Gomara E, Carpintero JM, et al. Competence of health professionals to check the carotid pulse. Resuscitation. 1998;37:173–175. doi: 10.1016/s0300-9572(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 32.Inagawa G, Morimura N, Miwa T, et al. A comparison of five techniques for detecting cardiac activity in infants. Paediatr Anaesth. 2003;13:141–146. doi: 10.1046/j.1460-9592.2003.00970.x. [DOI] [PubMed] [Google Scholar]
- 33.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: The pediatric Utstein style. Ann Emerg Med. 1995;26:487–503. doi: 10.1016/s0196-0644(95)70119-2. [DOI] [PubMed] [Google Scholar]
- 34.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 35.Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 10: pediatric advanced life support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102:I291–I342. [PubMed] [Google Scholar]
- 36.Denton R, Thomas AN. Cardiopulmonary resuscitation: a retrospective review. Anaesthesia. 1997;52:324–327. doi: 10.1111/j.1365-2044.1997.105-az0102.x. [DOI] [PubMed] [Google Scholar]
- 37.Huang SC, Wu ET, Chen YS, Chang CI, et al. Extracorporeal membrane oxygenation rescue for cardiopulmonary resuscitation in pediatric patients. Crit Care Med. 2008;36:1607–1613. doi: 10.1097/CCM.0b013e318170b82b. [DOI] [PubMed] [Google Scholar]
- 38.Tajik M, Cardarelli MG. Extracorporeal membrane oxygenation after cardiac arrest in children: what do we know? Eur J Cardiothorac Surg. 2008;33:409–417. doi: 10.1016/j.ejcts.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Alsoufi B, Al-Radi OO, Nazer RI, et al. Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thorac Cardiovasc Surg. 2007;134:952–959. doi: 10.1016/j.jtcvs.2007.05.054. e2. [DOI] [PubMed] [Google Scholar]
- 40.Thiagarajan RR, Laussen PC, Rycus PT, et al. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693–1700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan V, Spinella PC, Drott HR, et al. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 42.Yung M, Wilkins B, Norton L, et al. Glucose control, organ failure, and mortality in pediatric intensive care. Pediatr Crit Care Med. 2008;9:147–152. doi: 10.1097/PCC.0b013e3181668c22. [DOI] [PubMed] [Google Scholar]
- 43.Haque IU, LaTour MC, Zaritsky AL. Pediatric critical care community survey of knowledge and attitudes toward therapeutic hypothermia in comatose children after cardiac arrest. Pediatr Crit Care Med. 2006;7:7–14. doi: 10.1097/01.pcc.0000192322.45123.80. [DOI] [PubMed] [Google Scholar]
- 44.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]