Abstract
Introduction
Adult human studies suggest frontal dysfunction associated with chronic marijuana (MJ) use, but due to continued neuromaturation, adult studies may not generalize to adolescents. This study characterized prefrontal cortex (PFC) morphometry in chronic MJ-using adolescents following one month of monitored abstinence.
Methods
Data were collected from MJ users (n=16) and controls (n=16) aged 16-18. Extensive exclusionary criteria included comorbid psychiatric and neurologic disorders. Substance use and anatomical measures were collected after 28 days of monitored abstinence. PFC volumes were ascertained from manual tracing by reliable raters on high-resolution magnetic resonance images.
Results
After controlling for lifetime alcohol use, gender, and intracranial volume, MJ users did not differ from controls in PFC volume. However, marginal group-by-gender interactions were observed (p<.09): female MJ users demonstrated comparatively larger PFC volumes while male MJ users had smaller volumes compared to same-gender controls. Further, group status and total PFC volume interacted in predicting executive functioning (p<.05). Among MJ users, smaller PFC total volume was associated with better executive functioning while the opposite pattern was seen among the controls.
Conclusions
These preliminary results indicate that gender may moderate the relationship between MJ use and PFC morphometry. Given the relationship between larger PFC total volumes and poorer executive functioning among MJ users, female MJ users may be at increased risk for neurocognitive consequences. Future research will measure PFC gray and white matter separately and follow boys and girls over adolescence to examine the influence of MJ use on neurodevelopment.
Keywords: Adolescents, Cannabis, Drug Effects, Executive Functioning, Gender, MRI
INTRODUCTION
Marijuana continues to be the most popular illicit drug used among adolescents. Almost half of 12th graders have used marijuana and 5% use daily (Johnston et al., 2005). Given the high prevalence of marijuana use during adolescence, a time of continued neurodevelopment (e.g. Giedd et al., 1996b; Lenroot and Giedd, 2006; Nagel et al., 2006; Sowell et al., 2004), the impact of chronic use on brain morphometry among teens is of great interest.
Animal and human research have suggested that the prefrontal cortex (PFC), the last brain region to undergo maturation during adolescence (Gogtay et al., 2004; Lenroot and Giedd, 2006; Sowell et al., 2004), may be particularly vulnerable to the effects of heavy marijuana exposure (see Egerton et al., 2006; Loeber, 1999). Recent research has found particularly high cannabinoid receptor type 1 (CB1) density in the PFC region among humans compared to other species (Eggan and Lewis, 2007; Herkenham et al., 1990). In vivo measurement of CB1 binding, completed through simultaneous administration of a high-affinity CB1 radioligand ([18F]MK-9470) and a positron emission tomography brain scan, confirmed higher CB1 density in the PFC in healthy adult humans compared to other species, especially in the orbitofrontal cortex (see Van Laere et al., 2008a for details regarding this novel methodology).
Although onset of marijuana use is typically during adolescence, a time when the brain is still maturing, few brain structure studies have been conducted with adolescent users. Adult human neuroimaging studies have also suggested vulnerability of the PFC to chronic marijuana exposure (Loeber, 1999). In general, fMRI and PET studies have found differential activation in the PFC among MJ users compared to controls, including the dorsolateral and orbitofrontal PFC (Amen and Waugh, 1998; Block et al., 2000; Block et al., 2002; Chang et al., 2006; Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005; Jager et al., 2007; Kanayama et al., 2004; Lundqvist et al., 2001; Volkow et al., 1996). Despite these functional abnormalities, few studies have examined structural brain changes as a result of marijuana exposure during adulthood, and none have specifically examined PFC structure. The existing studies utilizing high-resolution magnetic resonance imaging (MRI) technology have yielded conflicting results. One study found reduced global brain volume, smaller cerebellar vermis, and focal white matter abnormalities in young adult marijuana users with a history of polysubstance abuse (Aasly et al., 1993). However, this may be due to alcohol use in the sample, as alcohol has been independently associated with smaller brain volumes (e.g., Deshmukh et al., 2002; Medina et al., 2008). Matochik and colleagues (Matochik et al., 2005) reported decreased gray matter and increased white matter density in hippocampal regions among young adult marijuana users compared to controls. Yücel and colleagues (2008) found smaller hippocampal and amygdala volumes in chronic marijuana using adults than in non-using controls. In contrast, two studies examining overall white and gray matter volumes (Block et al., 2000) and hippocampal volumes (Tzilos et al., 2005) in adult marijuana users did not find structural abnormalities. These studies conducted with adults cannot necessarily generalize to adolescent marijuana users.
As the PFC continues to refine into late adolescence/early adulthood (e.g., Lenroot and Giedd, 2006), the introduction of exogenous cannabinoids may interrupt its development. This is of great concern given the high prevalence of marijuana use during adolescence, which may place teens at increased risk for neurocognitive deficits (Ehrenreich et al., 1999; Medina et al., 2007a; Pope et al., 2003; Wilson et al., 2000) or psychiatric disorders (Arseneault et al., 2002; Bovasso, 2001; Brook et al., 2002; Cohen, Solowij, & Carr, 2008; Fergusson et al., 2002; Green and Ritter, 2000; Medina et al., 2007b; Patton et al., 2002). Functional neuroimaging studies conducted with adolescents have found abnormal PFC activation patterns among adolescent marijuana users compared to controls in response to go/no-go (Tapert et al., 2007), verbal working memory (Jacobsen et al., 2007) and spatial working memory (Schweinsburg et al., 2005; Schweinsburg et al., 2008) tasks.
From a structural standpoint, our laboratory found that marijuana users did not significantly differ from controls in hippocampal volumes, although correlations between hippocampal size and verbal memory were abnormal when contrasted to non-users (Medina et al., 2007c). A recent study examining white matter integrity, utilizing diffusion tensor imaging, found reduced apparent diffusion coefficient values in the left middle frontal gyrus and posterior cingulate and increased fractional anisotropy values in the left anterior cingulate, right medial frontal gyrus, left precentral gyrus, right inferior parietal, right cingulate gyrus, and left superior frontal gyrus among adolescents and young adults with histories of marijuana use compared to controls (Delisi et al., 2006). Although no structural MRI studies in adolescent marijuana users have focused on the PFC to date, recent findings have reported abnormal N-acetylaspartate/total creatine ratio in the dorsolateral PFC among late adolescent and young adult male marijuana users (Hermann et al., 2007).
During adolescence, gender may moderate the effects of marijuana on the brain (Benes et al., 1994; Block et al., 2000; Giedd, 2004; Huttenlocher, 1979; Huttenlocher and Dabholkar, 1997; Spear, 2000). In healthy adolescents, on average, females' PFC gray matter volumes peak one to two years earlier than males (Giedd et al., 1996b). In contrast to prior studies of white matter neuromaturation suggesting increased myelination of the PFC among female adolescents (e.g., Giedd et al., 1999; Pfefferbaum et al., 1994; Reiss et al., 1996), our laboratory found that, among females, PFC white matter volume actually decreased from age 15-18, while males' PFC white matter remained relatively stable (Nagel et al., 2006). Given these gender differences in neuromaturation, chronic endogenous cannabinoid exposure may have disparate effects on PFC structure and function for boys and girls. Indeed, gender has been shown to moderate the relationship between neurocognitive functioning and drug effects in young adult polydrug (including marijuana) users (Bolla et al., 1998), young adult and adolescent alcohol users (Caldwell et al., 2005; Medina et al., 2008), and young adult marijuana users (Pope et al., 1997). In addition, our laboratory has reported a relationship between decreased white matter volume and depressive symptoms that was primarily driven by female adolescent marijuana users (Medina et al., 2007b).
As noted above, although animal and adult human studies have suggested marijuana-induced PFC alterations, no studies of adolescents have yet examined marijuana's effect on PFC morphometry and executive functioning, which is subserved by the PFC. Identifying whether boys and girls demonstrate different neurocognitive consequences of marijuana use may help clarify whether sex hormones and the endogenous cannabinoid system influence PFC neurodevelopment. The goal of the current study was to determine whether gender moderates the relationship between chronic marijuana use, PFC volume, and executive functioning in adolescent marijuana users without comorbid psychiatric disorders after approximately one month of abstinence.
METHODS
Participants
Adolescents were recruited from local high schools and colleges via flyers and advertisements. To assess eligibility, a comprehensive telephone screen was administered to both adolescents and parents/guardians. Inclusion criteria required that youth were age 16-18, fluent in English, and had a parent or legal guardian available to consent and provide historical data. Exclusionary criteria were: history of chronic medical illness, neurological condition (e.g., meningitis, migraine), or head trauma with loss of consciousness >2 minutes; history of DSM-IV Axis I disorder (other than substance use disorder) or use of psychoactive medications; prenatal alcohol (≥4 drinks in a day or ≥7 drinks in a week) or drug exposure; complicated delivery or premature birth (<33 weeks gestation); learning disability or mental retardation; first-degree relative with history of bipolar I or psychotic disorders; left-handedness; non-correctable vision, colorblindness or hearing impairments; and MRI contraindications. If at any time during the 28-day monitored abstinence period preceding the brain scan, a participant reported or tested positive for any substance use, he/she was excluded from study and not included in any analyses.
All participants and their parents/guardians (if teen was a minor) underwent written informed consent (written assent for minors) in accordance with the UCSD Human Research Protections Program. Teens were classified as a marijuana user (“MJ-user”) or as a drug-free (“control”) group. MJ-users had >60 lifetime marijuana experiences; past month marijuana use; <25 lifetime uses of drugs other than marijuana, alcohol, or nicotine; and did not meet Cahalan criteria for heavy drinking status (Cahalan et al., 1969). Controls had <5 lifetime experiences with marijuana (none in the past month), no previous use of any other drug except nicotine or alcohol, and did not meet criteria for heavy drinking status.
Measures
Detailed Screening Interview
The computerized NIMH Diagnostic Interview Schedule for Children (C-DISC-4.0; (Shaffer et al., 2000)) was administered to exclude participants with major psychiatric disorders, including DSM-IV Axis I mood, anxiety, attention deficit hyperactivity and conduct disorders. Parallel modules of the computerized Diagnostic Interview Schedule (C-DIS-IV;(Robins et al., 1996) were used for 18-year-olds. The Structured Clinical Interview (SCI) measured psychosocial functioning, activities, last menstruation (for females), health history, and handedness, and family history of psychiatric and substance use disorders was assessed (Rice et al., 1995).
Adolescents were administered the Customary Drinking and Drug Use Record (CDDR) to assess lifetime and past 3-month marijuana, alcohol, nicotine, and other drug use, withdrawal symptoms, DSM-IV abuse and dependence criteria, and substance-related life problems (Brown et al., 1998; Stewart and Brown, 1995). The modified Timeline Followback (TLFB; Sobell and Sobell, 1992) was administered to obtain detailed information regarding type, quantity, and frequency of substance use during the month prior to the monitored abstinence period. Teens were asked about frequency of use for each of the following drugs: marijuana, alcohol, nicotine, stimulants (cocaine, amphetamine, methamphetamine, MDMA/ecstasy), opiates (heroin, narcotic pain relievers other than as prescribed), dissociatives/hallucinogens (PCP, mushrooms, LSD, ketamine), sedatives (GHB, barbiturates, benzodiazepines), and misuse of other prescription or over-the-counter medications.
Parent Interview
If the teen continued to be eligible, their parent or guardian underwent a detailed screening interview using the parent version of the SCI, including information on prenatal/infant development, childhood behavior, age of developmental milestones, parental socioeconomic status (Hollingshead, 1965), family history of psychiatric and substance use disorders (Rice et al., 1995), and youth medical and psychiatric history. Parents/guardians were also administered the parent version of the C-DISC-4.0 and the TLFB on youth behaviors.
Executive Functioning
Participants completed a neuropsychological evaluation. As the focus of the current study was on the relationship between PFC morphometry and executive functioning, a composite variable from a principal components analysis comprised of executive functioning variables (Medina et al., 2007a) was calculated using scores from the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) Verbal Fluency total correct, Tower total achievement score, and Tower error scores.
Procedures
Eligible teens were scheduled to begin a monitored abstinence protocol, followed by neuropsychological testing and structural brain imaging. Adolescents were monitored with supervised urine and Breathalyzer tests every 3-4 days for 4 weeks. Youths with positive urine samples or breath alcohol concentrations (Intoximeter AlcoSensor IV, St. Louis, MO) or who appeared intoxicated were offered the option of restarting the toxicology screen procedure at a later time or to discontinue the study. If toxicology results indicated cessation and maintenance of abstinence, the adolescent completed the research battery between Day 23 and 27. Youth who did not maintain abstinence were discontinued and compensated for their time. Upon completion of the study, youth and parents/guardians received financial compensation for participation.
High-resolution anatomical magnetic resonance images were collected for all teens on a 1.5 Tesla GE Signa LX system using a sagittally acquired inversion recovery prepared T1-weighted 3D spiral fast spin echo sequence (TR = 2000 ms, TE = 16 ms, FOV = 240 mm, voxel dimensions = 0.9375 × 0.9375 × 1.328 mm, 128 continuous slices, acquisition time = 8:36) (Wong, 2000). Each participant's high resolution anatomical image was AC-PC aligned and skull-stripped using a combination of a hybrid watershed and deformable surface semi-automated skull-stripping program (Segonne et al., 2004), and manual editing to remove any non-brain matter and CSF beyond the dura matter. Intracranial volume (ICV) was determined from this hand-edited skull-stripped brain, and PFC regions of interest (ROI) were defined manually utilizing the below described protocol in AFNI (Cox, 1996) by raters blind to participant characteristics who attained high levels of inter-rater reliability (intraclass correlation coefficients >.90) prior to data collection.
Data Processing
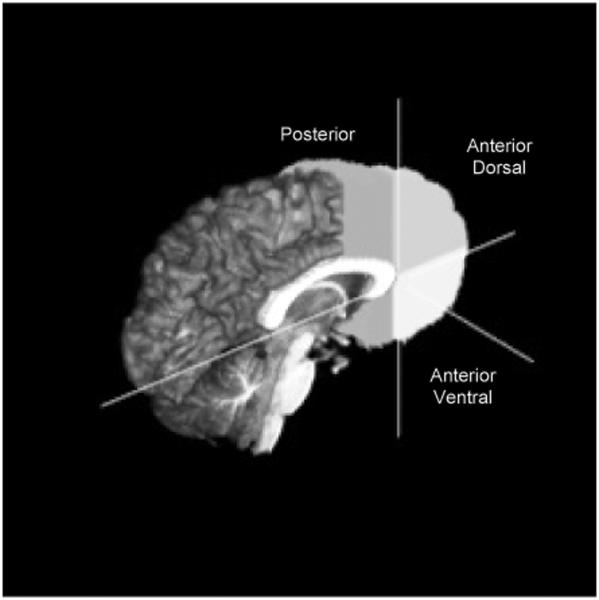
All PFC ROIs were manually delineated on coronal 3D images (perpendicular to the AC-PC plane). The PFC ROI protocol (Medina et al., 2008) was a modified version based on Nagel and colleagues (2006). The posterior PFC ROI included all cortical area (gray matter, white matter, and sulcal CSF) anterior to the anterior commissure and posterior to the anterior edge of the genu, and excluded the corpus callosum, optic tracts, insula, subcortical regions (caudate, putamen, globus palladus, internal capsule), and lateral ventricles. The anterior PFC ROIs (dorsal and ventral) included all cortical areas anterior to the anterior edge of the genu. The anterior dorsal PFC ROI included all cortex superior to the midline of the most anterior portion of the genu, while the anterior ventral PFC ROI included cortex inferior to the midline of the genu. Tracing continued until the most anterior slice where cortex was still visible and these ROIs excluded the lateral ventricles and the corpus callosum (see Figure I).
Figure I.
3D sagittal view of prefrontal cortex (PFC) boundary delineation.
For each ROI, white matter was segmented from gray and sulcal CSF compartments by processing skull-stripped T1 images with the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain's FAST automated segmentation tool (Zhang et al., 2001). Based on visual inspection of each subject, it was found that the automated segmentation process did not adequately separate gray matter from sulcal CSF, but correctly identified white matter (WM) within each of the ROIs. To calculate regional WM volume, each PFC ROI mask was applied to the WM compartment. All total and WM volumes were analyzed as a ratio to overall ICV to control for individual variability in brain size (Giedd et al., 1996b).
Data Analysis
ANOVAs and chi-square tests compared groups on important demographic and drug use variables. Bivariate correlations between drug use variables, executive functioning, and PFC volumes were run. Interpretations of statistical significance were made if p<.05.
To assess the relationships between group status, gender-by-group interactions, and PFC volumes, ordinary least squares multiple regressions were run (N = 32) with each of the eight PFC variables (total, anterior dorsal, anterior ventral, and posterior volumes; total WM, anterior dorsal WM, anterior ventral WM, and posterior WM volumes). The first step entered the following independent variables: gender, lifetime alcohol use, and group status (MJ-user vs. control). An interaction between group and gender was entered on the second step. As a follow-up analysis, regressions were run to assess the relationship between PFC volumes, group, and PFC-by-group interactions in predicting executive functioning after controlling for gender and lifetime alcohol use.
To improve power to detect a medium effect size in a relatively small sample, alpha was set at .10 for statistical significance in both analyses (primary analysis: medium effect size f2=.15, N=32, numerator df=1, predictors=4; power=.69; follow-up analysis: f2=.15, N=32, numerator df=1, predictors=5; power=.68, respectively) (Faul et al., 2007).
RESULTS
Descriptive Comparisons
ANOVAs and chi-squares assessed whether MJ-users and controls differed demographically (n=16/group). Groups differed in ethnic identification [x2(4)=10.18, p=.04]: MJ-users were 75% Caucasian, 13% multiple ethnicities, 6% Pacific Islander, and 6% “other,” while controls were 63% Caucasian and 37% Asian-American (see Table I). The MJ-users and controls did not differ on age [average=18.0 years, range 16.0-18.9; F(1,31)=.12, p=.74]; WRAT-3 (Wilkinson, 1993) Reading standard score [MJ-users 106.7 mean±6.4 SD, range 98-119; controls 106.0±8.6, range 85-116; F(1,31)=.14, p=.71], or gender composition [MJ-users 4 females, 12 males; controls 6 females, 10 males; x2(1)=.52, p=.45]. The MJ-users and controls did not significantly differ in ICV [F(1,31)=.15, p=.71] or PFC/ICV volumes (see Table I for raw values by group and gender).
Table I.
Demographic, substance use, and PFC volume measures by group and gender
| Female MJ Users (n=4) % or M±SD | Female Controls (n=6) % or M±SD | Male MJ Users (n=12) % or M±SD | Male Controls (n=10) % or M±SD | |
|---|---|---|---|---|
| Ethnicity (Caucasian) * | 75% | 83% | 75% | 50% |
| Age | 18.2±0.6 | 18.5±0.5 | 18.1±0.8 | 17.7±1.1 |
| Lifetime alcohol use (episodes) * | 301±132 | 32±64 | 158±124 | 17±37 |
| Lifetime marijuana use (episodes) * | 515±392 | 0±.8 | 463±236 | 0.8±1.6 |
| ICV (cc3) | 1427±119 | 1363±118 | 1515±100 | 1544±101 |
| Total PFC (cc3) | 329±27 | 304±38 | 345±22 | 363±29 |
| Total PFC /ICV (cc3) | .2306 ±.0031 | .2227 ±.0109 | .2282 ±.0111 | .2354 ±.0162 |
| Anterior dorsal PFC /ICV (cc3) | .0677±.0036 | .0703 ±.0084 | .0687 ±.0084 | .0699 ±.0102 |
| Anterior ventral PFC /ICV (cc3) | .0557±.0005 | .0502 ±.0046 | .0559 ±.0058 | .0582 ±.0091 |
| Posterior PFC /ICV (cc3) | .1072±.0057 | .1021 ±.0084 | .1036 ±.0130 | .1072 ±.0135 |
| Total PFC WM /ICV (cc3) | .0662±.0019 | .0665 ±.0099 | .0682±.0033 | .0714 ±.0058 |
| Anterior dorsal PFC WM /ICV (cc3) | .0158±.0010 | .0174 ±.0039 | .0172 ±.0027 | .0178 ±.0038 |
| Anterior ventral PFC WM /ICV (cc3) | .0135±.0006 | .0124 ±.0033 | .0138 ±.0014 | .0150 ±.0030 |
| Posterior PFC WM /ICV (cc3) | .0369±.0022 | .0367 ±.0058 | .0372 ±.0050 | .0385 ±.0049 |
Notes: PFC=Prefrontal cortex volume; ICV=Intracranial Volume; WM=White matter. There were no within-gender differences by group in ICV.
p<.05 between MJ users vs. controls.
Abstinence was monitored with urine toxicology screens and Breathalyzer tests that occurred for a minimum of 23 days, and in all, participants were abstinent from all drugs for at least 30 days (light to moderate alcohol use may have occurred; participants with self-reported binge drinking or biological evidence of alcohol use during this time were excluded). MJ-users reported more lifetime episodes of MJ use than controls [MJ-users 475.6±268.5, range 60-1000; controls 0.6±1.4, range 0-5; F(1,31)=50.0, p=.0001]. On average, MJ-users had used marijuana for 3.4 years (±1.7, range= 0.8-6.7), and had more lifetime episodes of alcohol intake than controls [MJ-users 194.5±136.8, range 20-420; controls 22.6±46.9, range 0-160; F(1,31)=22.6, p=.0001] (see Table 1). No control had used any drug besides alcohol or marijuana, but MJ-users had used other drugs an average of 6.9 times in their lives [±8.6, range 0-25; F(1,31)=10.42, p=.003]. The average abstinence from any alcohol use for MJ-users was 44 days (±61, range=9-270 days) and 132 days (±130, range=30-365 days) for controls. Average abstinence from drugs for the MJ-users was 107 days (±33, range=30-300 days).
Because the study examined gender-by-group interactions, drug use variables were examined between the male and female MJ users (see Table I for values by gender and group); they did not differ in MJ dependence symptoms, duration of regular MJ use, lifetime uses of other illicit drugs, recent MJ use, lifetime MJ use, years of regular drinking, length of abstinence from either alcohol or other drugs, or ICV. However, female MJ users (M=301±132) had marginally more lifetime drinking episodes compared to male MJ users (M=158±123) [F(1,15)=3.87, p=.07] and greater alcohol dependence symptoms [female MJ users M=3.5±1.3; male MJ users M=1.4±1.3; F(1,15)=7.6, p=.02]. Control females and males did not differ on any substance use variable.
Correlations
See Table II for bivariate relationships by group. Among controls, greater alcohol use (recent use and number of alcohol dependence symptoms) was marginally associated with smaller posterior PFC volumes (p<.09) and significantly associated with smaller posterior PFC WM volumes (p<.05). Recent alcohol use, lifetime alcohol use, and number of dependence symptoms were also significantly associated with poorer executive functioning (p<.05). Finally, superior executive functioning was associated with larger total PFC volumes (p<.05).
Table II.
Correlations between drug use, executive functioning, and PFC variables by group.
| Marijuana Users (n=16)† | |||||||
|---|---|---|---|---|---|---|---|
| Recent MJ Use | Lifetime MJ Use | #MJ Symptoms | Recent Alc Use | Lifetime Alc Use | #Alc Symptoms | Exec Func | |
| Total PFC/ICV | .03 | .12 | -.24 | -.20 | -.13 | -.10 | -.53* |
| Anterior dorsal PFC/ICV | -.07 | .01 | -.36 | -.12 | .06 | -.43 | -.06 |
| Anterior ventral PFC/ICV | .60* | .34 | -.09 | -.24 | .06 | -.17 | .16 |
| Posterior PFC/ICV | -.19 | -.04 | .06 | .01 | -.12 | .27 | -.47 |
| Total PFC WM/ICV | -.10 | -.05 | -.32 | .01 | -.25 | -.14 | -.12 |
| Anterior dorsal PFC WM/ICV | -.04 | .02 | -.39 | -.08 | .01 | -.48 | .12 |
| Anterior ventral PFC WM/ICV | .56* | .21 | -.10 | -.19 | -.11 | -.17 | .39 |
| Posterior PFC WM/ICV | .23 | -.10 | .02 | .10 | -.15 | .22 | -.26 |
| Executive functioning | .23 | -.11 | -.23 | -.08 | -.09 | -.07 | - |
| Controls (n=16) | |||||||
|---|---|---|---|---|---|---|---|
| Recent MJ Use | Lifetime MJ Use | # MJ Symptoms | Recent Alc Use | Lifetime Alc Use | # Alc Symptoms | Exec Func | |
| Total PFC/ICV | - | - | - | -.35 | -.16 | -.30 | .22 |
| Anterior dorsal PFC/ICV | - | - | - | .31 | .30 | .36 | -.20 |
| Anterior ventral PFC/ICV | - | - | - | -.22 | -.11 | -.19 | .35 |
| Posterior PFC/ICV | - | - | - | -.48 | -.32 | -.47 | .17 |
| Total PFC WM/ICV | - | - | - | -.40 | -.41 | -.37 | .55* |
| Anterior dorsal PFC/ICV | - | - | - | .10 | -.01 | .14 | .17 |
| Anterior ventral PFC WM/ICV | - | - | - | -.21 | -.20 | -.19 | .48 |
| Posterior PFC WM/ICV | - | - | - | -.53* | -.48 | -.53* | .39 |
| Executive functioning | - | - | - | -.59* | -.52* | -.59* | - |
Notes: MJ=marijuana; Alc=alcohol; # MJ/Alc Symptoms= denotes the number of DSM-IV marijuana or alcohol abuse or dependence symptoms met; Recent MJ/Alc Use= past 3 month in joints (MJ) or standard drinks (alcohol); Exec Func= Executive functioning composite score; PFC=Prefrontal cortex; ICV=Intracranial Volume; WM=White matter.
Correlations are consistent for both male and female MJ users.
p<.05
In contrast, weak relationships were observed between alcohol use variables and PFC volumes among the MJ users; although there were marginal negative relationships between number of alcohol dependence symptoms and anterior dorsal PFC total and WM volumes (ps<.09). Increased recent MJ use was associated with larger anterior ventral PFC total and WM volumes (ps<.05).
Executive functioning did not correlate with drug use among the MJ users. Finally, relative to controls, the opposite relationship was observed between PFC volume and executive functioning; among the MJ users, superior executive functioning was associated with smaller total PFC volumes (p<.05). Relationships between PFC volume and executive functioning were similar for males and females, therefore correlations for all marijuana users are reported here.
Multivariate Relationships: Predicting PFC Volumes
Group effects
After controlling for gender and lifetime alcohol use, group status (MJ users vs. controls) was not associated with any of the PFC volumes. Calculated effect sizes for the addition of MJ group status to the model were all small (f2 range .00-.03; Cohen, 1988).
Group x gender interactions
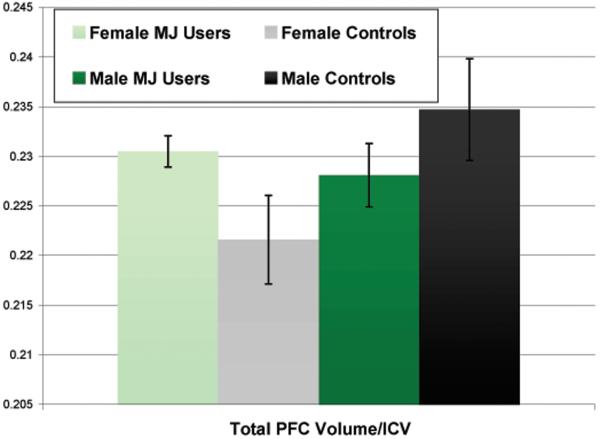
After controlling for group, gender, and lifetime alcohol use, gender and group interacted in predicting total PFC volume/ICV [beta = -.68, p < .09]. This interaction had a medium effect size (f2= .11) while the interactions between gender and group and the other measures of PFC structure were small (f2 range .006-.08) (Cohen, 1988; Faul et al., 2007). Visual inspection of the data revealed that female MJ users demonstrated larger PFC total volumes compared to female controls, while male MJ users had smaller PFC total volumes compared to male controls (see Figure II).
Figure II.
Total PFC volume/ICV by group and gender (multivariate interaction p<.09).
Multivariate Relationships: Predicting Executive Functioning
PFC volume effects
After controlling for gender, group, and lifetime alcohol use, larger anterior ventral PFC WM volume predicted superior executive functioning in both groups [beta = .32, p < .09; f2= .13].
Group x PFC volume interactions
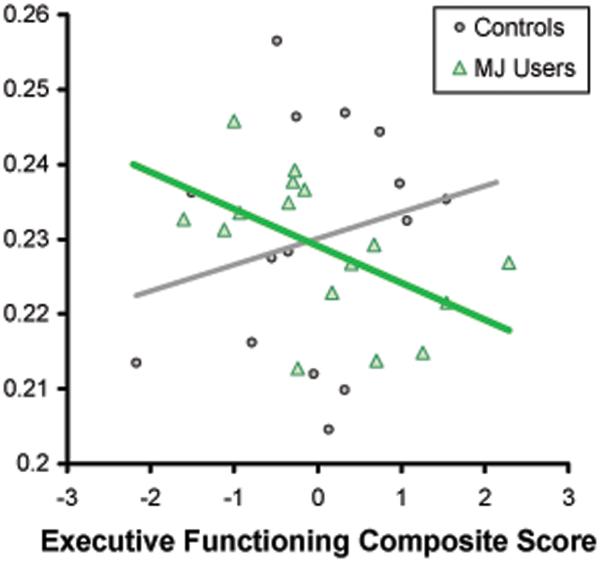
After controlling for group, gender, total PFC volume, and lifetime alcohol use, group and total PFC volume significantly interacted in predicting executive functioning [beta = -.44, p < .05; f2= .13]; inspection of the scatter plots (see Figure 3) and correlations (Table 2) revealed that among the controls, increased total PFC volume was associated with improved executive functioning while the opposite was true for the MJ users. Marginal interactions were also found between posterior PFC volume and group [beta = -.40 p < .09] and posterior PFC WM volume and group [beta = -.40, p < .09] and executive functioning, although the effect sizes were relatively small (f2= .07). Among controls, larger PFC volumes were associated with better executive functioning, while MJ users showed negative relationships between these PFC volumes and executive functioning. The remaining non-significant relationships also had small effect sizes (f2 range .00-.05).
Figure III.
Bivariate scatterplot between the total PFC volume/ICV and executive functioning composite score by group (multivariate interaction p<.05).
DISCUSSION
Marijuana (MJ) is the most commonly used illicit substance among adolescents (Johnston et al., 2005). Moreover, due to continued neurodevelopment (e.g., Giedd et al., 1996b; Sowell et al., 2002), adolescence may be a period of increased risk of MJ-induced neurocognitive deficits (Cha et al., 2006; Medina et al., 2007a). Prior animal (Diana et al., 1998a; Egerton et al., 2006; Jentsch et al., 1997b; Pistis et al., 2002; Verrico et al., 2003) and human (Amen and Waugh, 1998; Block et al., 2000; Block et al., 2002; Chang et al., 2006; Eldreth et al., 2004; Gruber and Yurgelun-Todd, 2005; Jager et al., 2007; Kanayama et al., 2004; Loeber, 1999; Lundqvist et al., 2001; McPartland et al., 2007; Van Laere et al., 2008a; Volkow et al., 1996) research has demonstrated that the prefrontal cortex (PFC) may be particularly vulnerable to MJ effects, and gender differences exist in PFC development and neurocognitive consequences of drug use during adolescence and young adulthood (Medina et al., 2008; Medina et al., 2007b; Pope et al., 1997). Therefore, the goal of the current study was to examine whether group status or interactions between MJ use and gender were associated with PFC volumes in a sample of marijuana using adolescents who demonstrated approximately one month of abstinence.
The primary finding was that after controlling for alcohol consumption, gender, and intracranial volume, MJ users had similar PFC volumes as controls. However, group-by-gender interactions were observed in the total PFC volume (p<.09; medium effect size f2= .11). It is important to note that although provocative, these preliminary findings should be viewed with caution due to the low sample of females (MJ users n=4, controls n=6). Female MJ users demonstrated comparatively larger and male MJ users had smaller PFC volumes compared to their same-gender controls. Put another way, MJ use may moderate the effects of gender on PFC structure. On average, females' PFC gray matter volume peaks one to two years earlier than males (Giedd et al., 1996b). Given that the adolescents from the current study are on average 18 years old, the girls should have undergone substantial pruning and show smaller PFC volumes compared to males, which is consistent with the findings seen in the healthy non-drug using adolescents in this study. However, this gender difference in PFC volume was different in the MJ users; female MJ users actually had somewhat larger PFC volumes compared to male users. Further, when examining the functional relationship between PFC volume and executive functioning ability, we found that among MJ users, larger PFC total volume was associated with worse executive functioning while the opposite pattern was seen among the controls (p<.05; medium effect size f2= .13). As stated above, because the study included relatively few female MJ users, findings need to be replicated in larger samples.
If replicated, larger PFC volumes in the female MJ users compared to female controls may indicate an interruption in the healthy pruning process. The mechanism underlying a possible disruption in healthy pruning is unknown. Recent animal findings found that astrocytes sparked neurons to release a complement protein, C1q, that in turn tagged neurons with weak synaptic connections; this protein peaked during the adolescent pruning process in rats (Stevens et al., 2007). Notably, exogenous cannabinoid exposure has been shown to affect normal astrocytic function (Bindukumar et al., 2008); therefore, repeated exposure of cannabinoids during adolescence may directly affect the elimination of weak synapses. Chronic exposure may also alter brain-derived neurotrophic factor (BDNF) expression (Berghuis et al., 2005; D'Souza et al., 2008; Jockers-Scherubl et al., 2004), resulting in non-physiological increases in BDNF over time that may interfere with synaptic pruning. Both of these possibilities would lead to inefficient neural networks, which is consistent with our structural findings of larger volumes being associated with poorer executive functioning.
Other mechanisms that may interrupt healthy pruning are also possible. Administration of exogenous cannabinoids in animals has increased mRNA encoding of immediate early gene proteins, including zif-268, c-fos, c-jns, and FosB in the cingulate (Mailleux et al., 1994; Porcella et al., 1998) and PFC (Egerton et al., 2006), which may also alter neuronal functioning and result in morphometric changes over time. Structural abnormalities could also be due to marijuana-induced alteration of neurotransmitters such as dopamine and norepinepherine (Diana et al., 1998a; Egerton et al., 2006; Oropeza et al., 2007; Pistis et al., 2002; Verrico et al., 2003), an effect that has been associated with poorer executive functioning in animals (Jentsch et al., 1997a), reduced cerebral metabolism and regional blood flow in the PFC (Amen and Waugh, 1998; Block et al., 2002; Lundqvist et al., 2001; Tunving, 1985; Volkow et al., 1996), or vasoconstriction resulting in ischemic changes (Herning et al., 2001).
Possible reasons for gender differences in marijuana effects include differential hormone and receptor distributions that may interact with the cannabinoid system (Ahmed et al., 2008; Coddington, et al., 2007; Emanuele et al., 2001; Kim et al., 2003; Mani et al., 2000; Ogilvie and Rivier, 1997). Among young adults, increased age is associated with higher CB1 density in PFC regions among females, but not males (Van Laere et al., 2008a). Increases in CB1 availability and introduction of chronic MJ use may cause greater disruption of the above outlined cannabis-related neuromodulation in girls. It is also notable that the female MJ users demonstrated marginally higher lifetime drinking episodes and significantly greater alcohol dependence symptoms compared to male MJ users. However, alcohol was statistically controlled for and female MJ users demonstrated larger PFC volumes compared to female controls, which is inconsistent with recent research from our laboratory showing significantly smaller PFC volumes among females with alcohol use disorders compared to female controls (Medina et al., 2008).
Male MJ users had relatively smaller total PFC volumes compared to gender-matched controls, and smaller PFC volumes were associated with improved executive functioning among the MJ users. This may indicate that male MJ users may be less sensitive to the neurocognitive effects of marijuana than females. However, it is important to note that the male MJ users demonstrated an abnormal relationship between PFC volume and executive functioning compared to controls, for whom increased PFC volume was associated with improved executive functioning. The lack of white matter differences in the male MJ users is consistent with DeLisi et al (2006), who found no evidence of reduced white matter integrity among a primarily male (90%) young adult sample of previous marijuana users who began heavy use during adolescence. Although this preliminary evidence suggests that the structural effects of chronic marijuana use on the brain among male adolescents may be subtle compared to females, additional studies combining neuroimaging techniques are necessary. For example, one study reported abnormal NAA/tCR ratios in the dorsolateral PFC among male adolescent and young adult MJ users compared to controls (Hermann et al., 2007). Recently, our group found reduced white matter integrity in fronto-parietal tracks in adolescent marijuana users (Bava et al., under review). Therefore, macromorphic measures of PFC structure may not be sufficient to measure the effects of MJ on PFC development in male adolescents. Further, because the study is cross-sectional and males and females undergo neuromaturation at separate rates, it is possible that the gender differences are not due to gender per se, but instead represents differential trajectories based on stage of neuronal maturation. Male MJ users could show the same patterns as the females, but at different times in adolescence. To further examine the effects of chronic MJ use on PFC integrity, longitudinal studies that investigate the interactions between gender (including hormone levels), CB1 density, markers of PFC integrity (including structure and function), and chronic MJ use during adolescence are needed.
Some methodological limitations of the current study should be considered. Preexisting differences in PFC morphometry and executive functioning (Aytaclar et al., 1999; Nigg et al., 2004) cannot be ruled out in this cross-sectional study. Still, it is of note that the current sample excluded individuals with Axis I comorbid disorders, including conduct disorder, and the groups were matched on family history of SUD, education, SES, and reading ability. The internal consistency of the sample may actually limit generalizalization of results, as the MJ using teens had above average verbal ability, high SES, and were able to complete the challenging month of abstinence protocol. Future studies are needed to examine PFC morphometry in more heterogeneous samples. Second, although comparable to other neuroimaging studies, the sample was relatively small and disproportionately male; therefore, the gender findings were based on a small sample of female users. This limited our power to detect significant differences, although the observed effect sizes were medium (f2=.11 and .13). Because of low power, we did not perform correction for multiple analyses, so the correlation and multivariate analyses need to be replicated in samples with larger numbers of male and female MJ adolescent users. Third, although white matter was reliably separable from gray matter and CSF, consistent parcellation of sulcal CSF from gray matter was not possible. Although we believe the contribution of CSF on these findings are minimal due to hand editing of CSF from the vast majority of the PFC ROIs, we cannot definitively state that it did not have an impact. Therefore, additional research examining gray matter separated from CSF is needed to confirm that the results are driven by gray matter differences. Given the cross-sectional nature of the current study, it is unknown whether continued abstinence from MJ results in neurocognitive recovery or subsequent healthy neurodevelopment among adolescents. Therefore, longitudinal studies are necessary to investigate the long-term trajectory of PFC morphometry and executive functioning in adolescent MJ users.
In conclusion, the general pattern of results suggests that even after a month of abstinence, adolescent marijuana users demonstrate subtle PFC morphometry abnormalities that may be moderated by gender. No differences in PFC white matter volume were seen between the groups. Female MJ users demonstrated comparatively larger PFC volumes and male MJ users had comparatively smaller PFC volumes compared to their same-gender controls. Unlike controls, increased PFC volume was associated with poorer executive functioning among the MJ users, suggesting that female MJ users may be at increased risk for marijuana-induced PFC abnormalities. Given the preliminary nature of these results, additional preclinical and human studies are needed to examine PFC integrity among male and female adolescents following chronic marijuana exposure. Further studies examining the interaction between the endocannabinoid system and sex hormones on PFC development are also warranted. Given the current findings and prior results showing detrimental cognitive and mood consequences of adolescent marijuana use (Medina et al., 2007a; 2007b), additional research focused on pharmacological interventions in marijuana users are also needed.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants 5R01 DA021182-03, 5R01 AA013419-07, and 5P20 DA024194-020002 (PI: Tapert) and (F32 DA020206, PI: Medina). Funding was also provided by the National Institute of Neurological Disorders and Stroke (PI: Nagel, 7K08 NS052147) and the National Institute on Alcohol Abuse and Alcoholism funded Fellowship (Hanson; PI: Riley, 5T32 AA1352505). Portions of this study were presented at the 2007 meeting of the College on Problems of Drug Dependence, Quebec City, Canada.
Gratitude is expressed to the staff of the Adolescent Brain Imaging Project, to the participants, and to their families.
REFERENCES
- Aasly J, Storsaeter O, Nilsen G, Smevik O, Rinck P. Minor structural brain changes in young drug abusers. A magnetic resonance study. Acta neurologica Scandinavica. 1993;87:210–4. doi: 10.1111/j.1600-0404.1993.tb04103.x. [DOI] [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nature Neuroscience. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. British Medical Journal. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen DG, Waugh M. High resolution brain SPECT imaging of marijuana smokers with AD/HD. Journal of Psychoactive Drugs. 1998;30:209–14. doi: 10.1080/02791072.1998.10399692. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–84. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. PNAS. 2005;102(52):19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Valverde O, Otto M, Michel K, Sastre M, Zimmer A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc Natl Acad Sci U S A. 2005;102:15670–5. doi: 10.1073/pnas.0504640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindukumar B, Mahajan SD, Reynolds JL, Hu Z, Sykes DE, Aalinkeel R, Schwartz SA. Genomic and proteomic analysis of the effects of cannabinoids on normal human astrocytes. Brain Research. 2008;1191:1–11. doi: 10.1016/j.brainres.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RI, O'Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–6. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacology, Biochemistry and Behavior. 2002;72:237–50. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, McCann UD, Ricaurte GA. Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology. 1998;51:1532–1537. doi: 10.1212/wnl.51.6.1532. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered White Matter Microstructure in Adolescent Substance Users. doi: 10.1016/j.pscychresns.2009.04.005. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovasso G. Cannabis abuse as risk factor for depressive symptoms. American Journal of Psychiatry. 2001;158:2033–2037. doi: 10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR. The effects of delta9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. Eur J Pharmacol. 2003;459:139–50. doi: 10.1016/s0014-2999(02)02854-6. [DOI] [PubMed] [Google Scholar]
- Brook D, Brook J, Zhang C, Cohen P, Whiteman M. Drug use and the risk of marjor depressive disorder, alcohol dependence, and substance use disorders. Archives of General Psychiatry. 2002;59:1093–1044. doi: 10.1001/archpsyc.59.11.1039. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Butovsky E, Juknat A, Goncharov I, Elbaz J, Eilam R, Zangen A, Vogel Z. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to THC. Journal of Neurochemistry. 2005;93:802–811. doi: 10.1111/j.1471-4159.2005.03074.x. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and attitudes. Rutgers Center of Alcohol Studies; New Brunswick, NJ: 1969. [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on bold (blood oxygen level dependent) response to spatial working memory. Alcohol and Alcoholism. 2005;40(3):194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:448–55. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington E, Lewis C, Rose JD, Moore FL. Endocannabinoids mediate the effects of acute stress and corticosterone on sex behavior. Endocrinology. 2007;148:493–500. doi: 10.1210/en.2006-0740. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. [Google Scholar]
- Cohen M, Solowij N, Carr V. Cannabis, cannabinoids and schizophrenia: integration of the evidence. Aust N Z J Psychiatry. 2008;42(5):357–68. doi: 10.1080/00048670801961156. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Manual for the Delis-Kaplan Executive Function System (D-KEFS) Psychological Corp; San Antonio, TX: 2001. [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh A, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Clinical signs of cerebellar dysfunction in schizophrenia, alcoholism, and their comorbidity. Schizophr Res. 2002;57:281–91. doi: 10.1016/s0920-9964(01)00300-0. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Gessa GL. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci. 1998a;10:2825–30. doi: 10.1111/j.1460-9568.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci U S A. 1998b;95:10269–73. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. 2006;30:680–95. doi: 10.1016/j.neubiorev.2005.12.002. Epub 2006 Mar 29. [DOI] [PubMed] [Google Scholar]
- Egerton A, Brett RR, Pratt JA. Acute delta9-tetrahydrocannabinol-induced deficits in reversal learning: neural correlates of affective inflexibility. Neuropsychopharmacology. 2005;30:1895–905. doi: 10.1038/sj.npp.1300715. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–91. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Emanuele NV, LaPaglia N, Steiner J, Kirsteins L, Emanuele MA. Effect of chronic ethanol exposure on female rat reproductive cyclicity and hormone secretion. Alcohol Clin Exp Res. 2001;25:1025–9. [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Swain-Campbell M. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97:1123–1135. doi: 10.1046/j.1360-0443.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- Fride E. The endocannabinoid-CB receptor system: Importance for development and in pediatric disease. Neuro Endocrinol Lett. 2004;25:24–30. [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, Vauss YC, Hamburger SD, Rapoport JL. A quantitative MRI study of the corpus callosum in children and adolescents. Brain Res Dev Brain Res. 1996a;91:274–80. doi: 10.1016/0165-3806(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996b;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996c;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;17:17. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BE, Ritter C. Marijuana use and depression. Journal of Health and Social Behavior. 2000;41:40–49. [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Research Cognitive Brain Research. 2005;23:107–18. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann N Y Acad Sci. 1992;654:19–32. doi: 10.1111/j.1749-6632.1992.tb25953.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, Mann K. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61:1281–9. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Herning RI, Better WE, Tate K, Cadet JL. Marijuana abusers are at increased risk for stroke. Preliminary evidence from cerebrovascular perfusion data. Ann N Y Acad Sci. 2001;939:413–5. doi: 10.1111/j.1749-6632.2001.tb03652.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–70. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17:289–97. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Jentsch J, Andrusiak E, Tran A, Bowers M, Jr., Roth R. Delta9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: Blockade of dopaminergic effects with HA966. Neuropsychopharmacology. 1997a;16:426–432. doi: 10.1016/S0893-133X(97)00018-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Andrusiak E, Tran A, Bowers MB, Jr., Roth RH. Delta 9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: blockade of dopaminergic effects with HA966. Neuropsychopharmacology. 1997b;16:426–32. doi: 10.1016/S0893-133X(97)00018-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Verrico CD, Le D, Roth RH. Repeated exposure to delta 9-tetrahydrocannabinol reduces prefrontal cortical dopamine metabolism in the rat. Neurosci Lett. 1998;246:169–72. doi: 10.1016/s0304-3940(98)00254-7. [DOI] [PubMed] [Google Scholar]
- Jockers-Scherubl MC, Danker-Hopfe H, Mahlberg R, Selig F, Rentzsch J, Schurer F, Lang UE, Hellweb R. Brain-derived neurotrophic factor serum concentrations are increased in drug-naïve schizophrenic patients with chronic cannabis abuse and multiple substance abuse. Neuroscience Letters. 2004;371:79–83. doi: 10.1016/j.neulet.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman HG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2004. National Institute on Drug Abuse; Bethesda, MD: 2005. [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology. 2004;176:239–47. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim HJ, Noh HS, Roh GS, Kang SS, Cho GJ, Park SK, Lee BJ, Choi WS. Suppression by ethanol of male reproductive activity. Brain Res. 2003;989:91–8. doi: 10.1016/s0006-8993(03)03372-9. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Yurgelun-Todd DA. Human neuroimaging of acute and chronic marijuana use: implications for frontocerebellar dysfunction. Human Psychopharmacology: Clinical & Experimental. 1999;14:291–304. [Google Scholar]
- Lundqvist T, Jonsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicology and Teratology. 2001;23:437–43. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Verslype M, Preud'homme X, Vanderhaeghen J. Activation of multiple transcription factor genes by tetraydrocannabinol in rat forebrain. Neuroreport. 1994;5:1265–1268. doi: 10.1097/00001756-199406020-00028. [DOI] [PubMed] [Google Scholar]
- Mani SK, Mitchell A, O'Malley BW. progesterone receptor and dopamine receptors are required in Delta 9-tetrahydrocannabinol modulation of sexual receptivity in female rats. Proc Natl Acad Sci. 2001;98:1249–1254. doi: 10.1073/pnas.031563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152:583–93. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007a;13:807–20. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–94. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007b;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007c;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age-related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;17:1427–31. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin MB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: Cohort study. British Medical Journal. 2002;325:1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Research. 2002;948:155–8. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biological Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and Alcohol Dependence. 2003;69:303–10. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Jacobs A, Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychotherapy & Psychosomatics. 1997;66:179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Porcella A, Gessa GLP, Pani L. 9-Tetrahydrocannabinol increases sequence-specific AP-1 DNA-binding activity and Fos-related antigens in the rat brain. Eur. J. Neurosci. 1998;10:1743–1751. doi: 10.1046/j.1460-9568.1998.00175.x. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule. Version 4.0. 1996. DIS 4.0. [Google Scholar]
- Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–23. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia L, Fernandez-Ruiz JJ, Cebeira M, Ramos JA. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-tetrahydrocannabinol. Pharmacology, Biochemistry and Behavior. 1995;51:731–7. doi: 10.1016/0091-3057(95)00023-p. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–9. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–10. doi: 10.1016/j.drugalcdep.2005.01.009. Epub 2005 Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res 30. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Raye Z, Litten JPA, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press, Inc; Totowa, NJ, US: 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–31. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Learning impairment in the radial-arm maze following prolonged cannabis treatment in rats. Psychopharmacology (Berl) 1982;77:117–23. doi: 10.1007/BF00431932. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology (Berl) 1985;85:436–9. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–83. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunving K. Psychiatric effects of cannabis use. Acta Psychiatrica Scandinavica. 1985;72:209–17. doi: 10.1111/j.1600-0447.1985.tb02597.x. [DOI] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Jr., Yurgelun-Todd DA. Lack of hippocampal volume change in long-term heavy cannabis users. The American Journal on Addictions. 2005;14:64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, de Hoon J, Bormans G. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [(18)F]MK-9470 PET. Neuroimage. 2008a;39:1533–41. doi: 10.1016/j.neuroimage.2007.10.053. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Koole M, Sanabria Bohorquez SM, Goffin K, Guenther I, Belanger MJ, Cote J, Rothenberg P, De Lepeleire I, Grachev ID, Hargreaves RJ, Bormans G, Burns HD. Whole-body biodistribution and radiation dosimetry of the human cannabinoid type-1 receptor ligand 18F-MK-9470 in healthy subjects. J Nucl Med. 2008b;49:439–45. doi: 10.2967/jnumed.107.047290. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Jentsch JD, Roth RH. Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse. 2003;49:61–6. doi: 10.1002/syn.10215. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Research. 1996;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Ali SF, Paule MG, Scallet AC, Slikker W., Jr. Chronic exposure to delta 9-tetrahydrocannabinol fails to irreversibly alter brain cannabinoid receptors. Brain Res. 1991;544:145–9. doi: 10.1016/0006-8993(91)90897-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test-3 Administration Manual. Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. Journal of Addictive Diseases. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Wong EC, Luh WM, Buxton RB, Frank RL. Single slab high resolution 3D whole brain imaging using spiral FSE. Proceedings of the International Society of Magnetic Resonance Medicine. 2000;8:683. [Google Scholar]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]