Abstract
Tissue transglutaminase (tgase2) is a multifunctional enzyme that crosslinks proteins but also acts as a G-protein, differential functions regulated by calcium and GTP. In the epithelial cell membrane, we show that manipulation of tgase2 function by monodansylcadaverine or retinoic acid (RA) alters the activity of a membrane-bound protein kinase, nucleoside diphosphate kinase (NDPK, nm23-H1/H2) that is known to control G-protein function. We find that NDPK function is abnormally low in cystic fibrosis but can be restored by RA treatment in vitro. Our data suggest that tgase2 is overexpressed in cystic fibrosis and affects NDPK function.
Structured summary
MINT-7219905, MINT-7219896: tgase2 (uniprotkb:P21980) physically interacts (MI:0914) with NDPK (uniprotkb:P15531) by anti bait coimmunoprecipitation (MI:0006)
Keywords: Fibrosing colonopathy, phosphohistidine, Cross-linking, Cystic fibrosis transmembrane regulator
Abbreviations: NDPK, nucleoside diphosphate kinase; CF, cystic fibrosis; tgase2, transglutaminase 2, tissue transglutaminase; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; CFTR, cystic fibrosis transmembrane conductance regulator; HMW, high molecular weight; HBE, 16HBE14o-; CFBE, CFBE16o-; MDC, monodansylcadaverine; RA, retinoic acid; WT, wild-type; kDa, kiloDaltons; AMPK, AMP-activated protein kinase; cAMP, cyclic adenosine monophosphate
1. Introduction
Cystic fibrosis (CF) results from mutation of the cystic fibrosis transmembrane regulator (CFTR) and leads to widespread organ-damaging fibrosis [1] in pancreas, lung and liver [2]. This suggests that there may be a profibrotic factor endogenous to CF-affected tissues. Tissue transglutaminase (tgase2) is one candidate, that catalyses the formation of ε-γ-glutamyl-lysine crosslinks between glutamine (Q) and lysine (K) residues on proteins in a Ca2+-dependent manner [3]. Normally, this activity is silent inside cells, due to high GTP levels, but crosslinking has nevertheless been implicated in the regulation of tgase2 unrelated enzymatic activity [4] and in the regulation of a protein kinase by oligomerisation [5]. Here, we expand the theme linking tgase2 to kinase function in a disease context.
The regulation of tgase2 activity is not fully understood and a recent paper described disturbed tgase2 in CF [6], finding that tgase2 is both over-expressed and over-active in CF cells. Our laboratory has been investigating the membrane-localised activity of nucleoside diphosphate kinase (nm23, NDPK-H1/H2 isoforms; ∼17 kDa) [7–12], a multifunctional, hexameric enzyme that not only regulates the balance of cellular nucleotides such as ATP/ADP and GTP/GDP but also controls multiple cellular processes [13]. Unexpectedly, during the course of related studies using epithelial membranes derived from sheep tracheal epithelium, we repeatedly observed SDS- and mercaptoethanol-resistant, high molecular weight, NDPK-positive (HMW; ∼51 kDa) bands in membrane samples. These were not always present, suggestive of regulation and given that others had also reported unexpected ladders of high molecular weight NDPK species [14], we speculated that these HMW, SDS-resistant bands could be cross-linked monomers that would otherwise have dissociated under reducing conditions in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Our results suggest that tgase2 can regulate NDPK activity.
2. Materials and methods
SDS-PAGE, immunoblotting, immunoprecipitations and NDPK phosphorylation assays were carried out as previously described [8,12,13]. Tgase2 was purchased from Calbiochem. All other reagents were from Sigma, except for radionuclides, obtained from NEN. Quantification of radioactivity was carried out by electronic autoradiography using an InstantImager (Packard). The anti-NDPK antibody used in this work (Santa Cruz) was raised against nm23-H1, but cross-reacts with nm23-H2; we do not distinguish between these two isoforms. Ovine tracheal samples were prepared as previously described [15].
3. Results
3.1. NDPK forms HMW species in ovine epithelial membranes
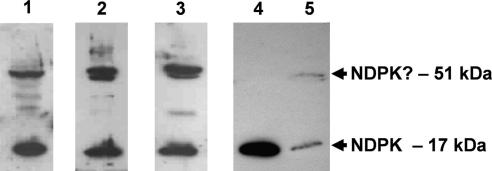
Immunoblots of cytosolic and membrane protein from ovine airway epithelium probed for NDPK show that there is a HMW form (Fig. 1, lanes 1−3) that is only detected in the membrane compartment (Fig. 1, lanes 4+5: equal protein loading from cytosol and membrane, respectively). These unexpected bands corresponded to trimers of the 17 kDa NDPK monomer (Fig. 1, lanes 1–3). HMW bands were sometimes also observed in membranes from human epithelial cell lines (data not shown) and this led us to investigate the relationship between tgase2 and NDPK in human airway cells.
Fig. 1.
NDPK immunoblots of ovine airway epithelial membrane protein separated on SDS-PAGE, showing consistent appearance of 51 kDa HMW bands in three independent preparations (lanes 1–3). Equivalent loadings of cytosolic (lane 4) and membrane (lane 5) protein showed that the HMW species is absent from the cytosolic fraction. The antibody used and the membrane preparation protocol are described in the methods for this and subsequent figures.
3.2. Tgase2 is elevated in a human CF cell model
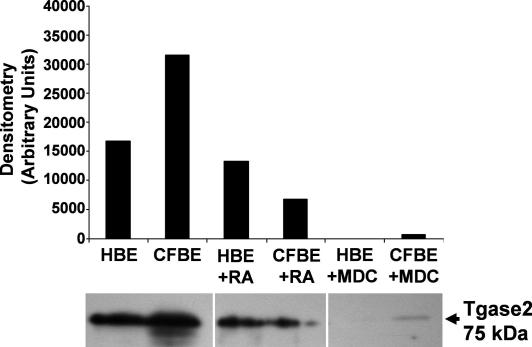
If tgase2 dysfunction is associated with the pathophysiology of CF [6], tgase2 should be present in the affected tissues. Immunoblots show that tgase2 is present but there is nearly twice the level in cell membranes derived from CFBE cells, a human bronchial CF cell line expressing the ΔF508-CFTR when compared to the wild-type (WT) equivalent (HBE) (Fig. 2, lanes 1+2). Tgase2 activity can be inhibited by monodansylcadaverine (MDC) and cellular levels of tgase2 are strongly induced by retinoic acid (RA); these reagents are commonly used to manipulate tgase2 activity. Surprisingly RA treatment (5 μM, 5 days) reduced tgase2 in CFBE membranes three-fold to below that found in untreated WT HBE membranes (Fig. 2, compare lanes 4 and 1). The inhibitory effect of RA on HBE cell membrane tgase2 levels was more modest (lanes 3 and 1). By contrast, MDC treatment (50 μM, 5 days) of the cells dramatically reduced membrane-bound tgase2, but maintained the differential between WT and CF such that some residual tgase2 was detectable in CF but not wild-type cell membranes (Fig. 2, lanes 5+6).
Fig. 2.
Immunoblot and densitometric quantification of tgase2 (mouse monoclonal antibody cocktail, Neomarkers MS-300-p, 1:1000) in HBE and CFBE membranes isolated from cells pretreated with retinoic acid (RA, 5 μM, 5 days) and monodansylcadaverine (MDC, 50 μM, 5 days). Identical membrane protein loading (20 μg) was present in each lane.
3.3. NDPK co-immunoprecipitates with tgase2
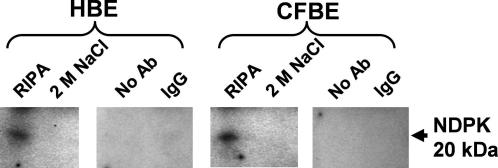
Anti-tgase2 immunoprecipitates from HBE and CFBE membranes were probed for associated NDPK. Fig. 3 demonstrates that NDPK precipitates with tgase2 in both cell types and that the interaction is only abolished by an additional wash in 2 M NaCl suggesting tight association. Control precipitates with no antibody or irrelevant IgG were negative for NDPK.
Fig. 3.
Immunoprecipitation from HBE and CFBE membranes using a specific anti-tgase2 antibody followed by immunoblotting for NDPK. The ‘IP’ sample in each case has been washed several times and represents NDPK bound to tgase. Control lanes show no antibody and irrelevant IgG samples. See Refs. [7–12] for methods.
3.4. NDPK activity can be altered by exogenous tgase2, but only if it originates from cytosol
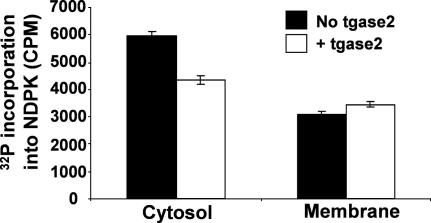
We incubated membrane and cytosolic fractions from HBE cells with exogenous tgase2, and then tested for NDPK activity. Membrane extracts were unaffected by tgase2, but cytosolic NDPK activity was reduced (Fig. 4). This is consistent with the idea that membrane-associated NDPK is already cross-linked or in some way resistant to the actions of transglutaminase for example by lying within a multiprotein complex involving the CFTR ion channel and AMP-activated protein kinase [16,17], so no change is observed.
Fig. 4.
16HBE14o cytosolic or membrane extracts were incubated with exogenous tgase2 (1 μg, 4 h) and NDPK activity measured by its phosphorylation (for methods, see Ref. [13]). Filled columns show data from samples without tgase2, white columns with tgase2. n = 3 ± S.E.M.
3.5. NDPK activity is affected by tgase2 modulation
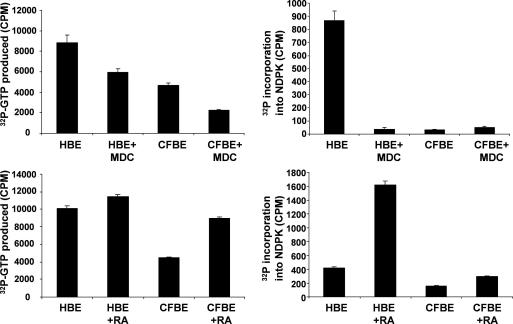
For unknown reasons, NDPK is dysfunctional in CF epithelium [18]. We investigated whether the RA ‘normalisation’ of tgase2 levels would result in restoration of NDPK function in CF cells. We found that both the histidine autophosphorylation [10] and the transferase activity of NDPK measured as GTP production [7,8] were restored to WT levels after a five-day treatment of CFBE cells with 5 μM RA (Fig. 5, bottom panels). MDC treatment also inhibited WT NDPK activity to similar levels to those found in CF (Fig. 5, top panels).
Fig. 5.
Data showing NDPK phosphorylation (left) and phosphotransferase activity (right) in HBE and CFBE cell lines and the effect of treatment with RA (bottom) and MDC (top). For methods, see Ref. [13]. n = 3 ± S.E.M.
4. Discussion
Thus, we have shown that NDPK can exist as a multimeric, SDS-resistant complex and that manipulation of tgase2 alters NDPK function. The difference in NDPK activity between CF membranes and wild-type membranes can be normalised. Our data also demonstrate that a fraction of cellular tgase2 co-immunoprecipitates with NDPK, suggesting that they are associated proteins.
Tgase2 is identical to the G-protein Gαh [19], implicating [GTP] in its regulation. One of the key roles of NDPK is maintenance of GTP levels local to G-proteins at the membrane [20]. Ca2+ and GTP are antagonistic in their regulation of tgase2 activity; Ca2+ is stimulatory, GTP inhibitory. Its transglutaminase or GTPase activities are reported to predominate, depending on its subcellular localisation to cytosol or membrane, respectively [21]. A disturbed tgase2-NDPK axis could have major implications, e.g. in the regulatory connection between tgase2 and the ras oncogene [22], which affects NDPK activity [23]. CFTR itself is regulated by unidentified G-proteins [24,25] and is also reported to be regulated by calcium via unknown pathways [26]. Tgase2 has been reported to directly regulate adenylyl cyclase and, therefore, [27,28] is known to be both activated and induced by cyclic adenosine monophosphate (cAMP) [29], the major activator of CFTR. We note that NDPK is also regulated by cAMP [30].
The data in Fig. 4 suggest that the activity of NDPK is specifically held in check in the membrane by drugs known to regulate tgase2. Interestingly, suitable Q/K residues for cross-linking exist in close proximity at interfaces between NDPK monomers [31] and K31 and Q111 are conserved in the ubiquitous NDPK-H1 and H2 isoforms. There is only one report linking tgase2 activity with CF. Maiuri et al. [6] showed recently that tgase2 activity is elevated in CF tissues and that PPARγ is a substrate for this activity. Here we suggest that NDPK is a further probable substrate for this excess cross-linking activity, which might explain our earlier observation of NDPK dysfunction in CF membranes [18].
We have recently reported a complex relationship between membrane-local NDPK, its co-precipitating partner AMP-activated kinase, GTP and the differential phosphorylation of NDPK itself on histidine and serine residues. The latter was promoted by the presence of GTP [13]. Since the GTP produced by NDPK could also regulate tgase2 activity, this relationship could form a tight feedback loop that could control the membrane-localised metabolic environment. In this context, it may be pertinent that membrane-bound, epithelial NDPK can interact with the metabolic sensor AMP-activated protein kinase (AMPK) [13], which in turn is known to bind CFTR. Our observations on RA are unexpected in that they normalise NDPK function in CF whilst reducing tgase2 in the membrane. Others, whilst looking for ubiquitinated NDPK, observed curious ladders of NDPK high molecular weight species when they were expecting the usual smear of NDPK suggestive of poly-ubiquitination [14]. Whatever the mechanism, our observed normalisation of tgase2 levels with RA treatment, coupled with the elevation of NDPK activity, indicates that RA (or tgase2 inhibitors) could be promising therapies for CF. Inhalation of aerosolised RA has already been tested as a therapy for lung cancer [32] and as an alternative means of administering supplemental vitamin A [33] making this route a viable approach to treat the CF lung. Thus two independent studies concur that tgase2 is overexpressed and is likely to be over-active in CF cells. Further investigation of tgase2 regulation is warranted in CF because fibrotic lung destruction is a major cause of CF morbidity.
Acknowledgements
This work was funded by the Wellcome Trust grant numbers 079965/Z/06/Z, 069150/Z/02/Z and 061003/Z/00/Z.
References
- 1.Serban D.E., Florescu P., Miu N. Fibrosing colonopathy revealing cystic fibrosis in a neonate before any pancreatic enzyme supplementation. J Pediatr. Gastroenterol. Nutr. 2002;35:356–359. doi: 10.1097/00005176-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Lewindon P.J., Pereira T.N., Hoskins A.C., Bridle K.R., Williamson R.M., Shepherd R.W., Ramm G.A. The role of hepatic stellate cells and transforming growth factor-β1 in cystic fibrosis liver disease. Am. J. Pathol. 2002;160:1705–1715. doi: 10.1016/s0002-9440(10)61117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin M., Casadio R., Bergamini C.M. Transglutaminases: nature’s biological glues. Biochem. J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang K.C., Gray C.D., Sivasubramanian N., Im M.J. Interaction site of GTP binding Gh (transglutaminase II) with phospholipase C. J. Biol. Chem. 1995;270:27058–27062. doi: 10.1074/jbc.270.45.27058. [DOI] [PubMed] [Google Scholar]
- 5.Hebert S.S., Daviau A., Grondin G., Latreille M., Aubin R.A., Blouin R. The mixed lineage kinase DLK is oligomerized by tissue transglutaminase during apoptosis. J. Biol. Chem. 2000;275:32482–32490. doi: 10.1074/jbc.M006528200. [DOI] [PubMed] [Google Scholar]
- 6.Maiuri L. Tissue transglutaminase activation modulates inflammation in cystic fibrosis via PPARg down-regulation. J. Immunol. 2008;180:7697–7705. doi: 10.4049/jimmunol.180.11.7697. [DOI] [PubMed] [Google Scholar]
- 7.Marshall L.J., Muimo R., Riemen C.E., Mehta A. Na+ and K+ regulate the phosphorylation state of nucleoside diphosphate kinase in human airway epithelium. Am. J. Physiol. 1999;276:C109–C119. doi: 10.1152/ajpcell.1999.276.1.C109. [DOI] [PubMed] [Google Scholar]
- 8.Muimo R., Banner S.J., Marshall L.J., Mehta A. Nucleoside diphosphate kinase and Cl-sensitive protein phosphorylation in apical membranes from ovine airway epithelium. Am. J. Respir. Cell Mol. Biol. 1998;18:270–278. doi: 10.1165/ajrcmb.18.2.2850. [DOI] [PubMed] [Google Scholar]
- 9.Muimo R., Crawford R.M., Mehta A. Nucleoside diphosphate kinase A as a controller of AMP-kinase in airway epithelia. J. Bioenerg. Biomembr. 2006;38:181–187. doi: 10.1007/s10863-006-9033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treharne K.J., Riemen C.E., Marshall L.J., Muimo R., Mehta A. Nucleoside diphosphate kinase – a component of the [Na+]- and [Cl−]-sensitive phosphorylation cascade in human and murine airway epithelium. Pflugers. Arch. 2001;443(Suppl. 1):S97–102. doi: 10.1007/s004240100653. [DOI] [PubMed] [Google Scholar]
- 11.Treharne K.J., Crawford R.M., Mehta A. CFTR, chloride concentration and cell volume: could mammalian protein histidine phosphorylation play a latent role? Exp. Physiol. 2006;91:131–139. doi: 10.1113/expphysiol.2005.031823. [DOI] [PubMed] [Google Scholar]
- 12.Treharne K.J., Marshall L.J., Mehta A. A novel chloride-dependent GTP-utilizing protein kinase in plasma membranes from human respiratory epithelium. Am. J. Physiol. 1994;267:L592–L601. doi: 10.1152/ajplung.1994.267.5.L592. [DOI] [PubMed] [Google Scholar]
- 13.Treharne K.J., Best O.G., Mehta A. The phosphorylation status of membrane-bound nucleoside diphosphate kinase in epithelia and the role of AMP. Mol. Cell. Biochem. 2009 doi: 10.1007/s11010-009-0118-1. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochrainer K., Kroismayr R., Baranyi U., Binder B.R., Lipp J. Highly homologous HERC proteins localize to endosomes and exhibit specific interactions with hPLIC and Nm23B. Cell Mol. Life Sci. 2008;65:2105–2117. doi: 10.1007/s00018-008-8148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muimo R., Hornickova Z., Riemen C.E., Gerke V., Matthews H., Mehta A. Histidine phosphorylation of annexin I in airway epithelia. J. Biol. Chem. 2000;275:36632–36636. doi: 10.1074/jbc.M000829200. [DOI] [PubMed] [Google Scholar]
- 16.Kongsuphol P., Cassidy D., Hieke B., Treharne K.J., Schreiber R., Mehta A., Kunzelmann K. Mechanistic insight into control of CFTR by AMPK. J. Biol. Chem. 2008;284:5645–5653. doi: 10.1074/jbc.M806780200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King J.D., Jr., Fitch A.C., Lee J.K., McCane J.E., Mak D.O., Foskett J.K., Hallows K.R. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am. J. Physiol. Cell Physiol. 2009;297:C94–C101. doi: 10.1152/ajpcell.00677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treharne K.J., Mehta A., Muimo R. NDPK, a protein kinase defective in CF, binds to and is regulated by AMPK, a CFTR-associated protein. Pediatric Pulmonol. 2001;S22:240. [Google Scholar]
- 19.Nakaoka H., Perez D.M., Baek K.J., Das T., Husain A., Misono K., Im M.J., Graham R.M. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–1596. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- 20.Hippe H.J., Lutz S., Cuello F., Knorr K., Vogt A., Jakobs K.H., Wieland T., Niroomand F. Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and Gβ subunits. Specific activation of Gs by an NDPK B.Gβγ complex in H10 cells. J. Biol. Chem. 2003;278:7227–7233. doi: 10.1074/jbc.M210305200. [DOI] [PubMed] [Google Scholar]
- 21.Park H., Park E.S., Lee H.S., Yun H.Y., Kwon N.S., Baek K.J. Distinct characteristic of Gh (transglutaminase II) by compartment: GTPase and transglutaminase activities. Biochem. Biophys. Res. Commun. 2001;284:496–500. doi: 10.1006/bbrc.2001.4997. [DOI] [PubMed] [Google Scholar]
- 22.Kosa K., Meyers K., De Luca L.M. Transformation of NIH3T3 cells with ras oncogenes abrogates the retinoic acid induction of tissue transglutaminase. Biochem. Biophys. Res. Commun. 1993;196:1025–1033. doi: 10.1006/bbrc.1993.2354. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J., Tseng Y.H., Kantor J.D., Rhodes C.J., Zetter B.R., Moyers J.S., Kahn C.R. Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor nm23 provides a novel mechanism of GTPase regulation. Proc. Natl. Acad. Sci. USA. 1999;96:14911–14918. doi: 10.1073/pnas.96.26.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy M.M., Quinton P.M. CAMP-independent phosphorylation activation of CFTR by G proteins in native human sweat duct. Am. J. Physiol. Cell Physiol. 2001;280:C604–C613. doi: 10.1152/ajpcell.2001.280.3.C604. [DOI] [PubMed] [Google Scholar]
- 25.Reddy M.M., Sun D., Quinton P.M. Apical heterotrimeric g-proteins activate CFTR in the native sweat duct. J. Membr. Biol. 2001;179:51–61. doi: 10.1007/s002320010036. [DOI] [PubMed] [Google Scholar]
- 26.Namkung W., Lee J.A., Ahn W., Han W., Kwon S.W., Ahn D.S., Kim K.H., Lee M.G. Ca2+ activates cystic fibrosis transmembrane conductance regulator- and Cl-dependent HCO3 transport in pancreatic duct cells. J. Biol. Chem. 2003;278:200–207. doi: 10.1074/jbc.M207199200. [DOI] [PubMed] [Google Scholar]
- 27.Milne D.M., Campbell L.E., Campbell D.G., Meek D.W. P53 is phosphorylated in vitro and in vivo by an ultraviolet radiation-induced protein kinase characteristic of the c-Jun kinase, JNK1. J. Biol. Chem. 1995;270:5511–5518. doi: 10.1074/jbc.270.10.5511. [DOI] [PubMed] [Google Scholar]
- 28.Tucholski J., Johnson G.V. Tissue transglutaminase directly regulates adenylyl cyclase resulting in enhanced cAMP-response element-binding protein (CREB) activation. J. Biol. Chem. 2003;278:26838–26843. doi: 10.1074/jbc.M303683200. [DOI] [PubMed] [Google Scholar]
- 29.Maddox A.M., Haddox M.K. Characteristics of cyclic AMP enhancement of retinoic acid induction of increased transglutaminase activity in HL60 cells. Exp. Cell Biol. 1988;56:49–59. doi: 10.1159/000163462. [DOI] [PubMed] [Google Scholar]
- 30.Anciaux K., Van Dommelen K., Willems R., Roymans D., Slegers H. Inhibition of nucleoside diphosphate kinase (NDPK/nm23) by cAMP analogues. FEBS Lett. 1997;400:75–79. doi: 10.1016/s0014-5793(96)01358-0. [DOI] [PubMed] [Google Scholar]
- 31.Min K. Crystallization and preliminary X-ray crystallographic analysis of human nucleoside diphosphate kinase A. Acta Crystallogr. D Biol. Crystallogr. 2000;56:503–504. [PubMed] [Google Scholar]
- 32.Kohlhaufl M. Inhalation of aerosolized vitamin A: reversibility of metaplasia and dysplasia of human respiratory epithelia – a prospective pilot study. Eur. J. Med. Res. 2002;7:72–78. [PubMed] [Google Scholar]
- 33.Biesalski H., Reifen R., Furst P., Edris M. Retinyl palmitate supplementation by inhalation of an aerosol improves vitamin A status of preschool children in Gondar (Ethiopia) Br. J. Nutr. 1999;82:179–182. [PubMed] [Google Scholar]