Abstract
Adhesion of circulating tumour cells to the blood vessel endothelium is a critical step in cancer metastasis. We show in this study that galectin-3, whose concentration is greatly increased in the circulation of cancer patients, increases cancer cell adhesion to macro- and micro-vascular endothelial cells under static and flow conditions, increases trans-endothelial invasion and decreases the latency of experimental metastasis in athymic mice. These effects of galectin-3 are shown to be a consequence of its interaction with cancer-associated MUC1, which breaks the “protective shield” of the cell surface MUC1 by causing MUC1 polarization leading to exposure of smaller cell surface adhesion molecules/ligands including CD44 and ligand(s) for E-Selectin. Thus, the interaction in the bloodstream of cancer patients between circulating galectin-3 and cancer cells expressing MUC1 bearing the galectin-3-ligand TF (Galβ1,3GalNAc-) promote metastasis. This provides insight into the molecular regulation of metastasis and has important implications for the development of novel therapeutic strategies for prevention of metastasis.
Keywords: circulating galectin-3, MUC1, cell adhesion, metastasis, glycosylation
Introduction
One of the critical steps in cancer metastasis is the adhesion of disseminating tumour cells to the blood vessel endothelium in distant organs. This process is thought to be regulated by the mechanical properties of the cancer cells and also by the specific expression of various adhesion molecules and/or ligands to adhesion molecules on the surface of cancer cells and endothelial cells (1).
MUC1 is a large transmembrane mucin protein that is expressed on the apical surface of most secretory epithelia. The extracellular domain of MUC1 consists of variable numbers of 20-amino acid tandem repeat peptides (VNTR) that are heavily glycosylated (up to 50% of the molecule weight) with complex O-glycans (2). The expression of MUC1 is increased up to 10-fold in epithelial cancers (3) and this increased MUC1 expression is associated with high metastatic potential and poor prognosis (4, 5). The cancer-associated MUC1 loses its apical membrane polarization, becoming expressed over the entire cell surface (6,7) and shows reduced expression of complex O-glycans and increased expression of short oligosaccharides such as GalNAcα- (Tn antigen), sialylated GalNAcα- (sialyl-Tn) and Galβ1,3GalNAcα-, the oncofetal Thomsen-Friedenreich, T or TF antigen (8). The TF antigen, which is concealed by more extensive glycosylation and sialylation in normal epithelium, is revealed on about 90% of human epithelial cancer cells (9). Cancer-associated MUC1 and the cancer-associated high molecular weight splice variant of the adhesion molecule CD44v6 (10) are probably the major cell surface glycoproteins that carry the unsubstituted TF antigen but the secreted mucin, MUC2, has also been shown to carry the unsubstituted TF in LSLiM6 human colon cancer cells (4). In gastric and colorectal adenocarcinomas, MUC1 is the predominant carrier of TF (11, 12) and the expression of TF on MUC1 correlates with increased pTNM staging, histologic grading, and unfavourable prognosis (13, 14).
By virtue of its huge size and length (protruded over 10 times higher above the cell surface than the typical cell surface molecules), over-expression of MUC1 promotes tumour cell release from primary tumour sites by inhibiting E-Cadherin-mediated cell-cell and integrin-mediated cancer-matrix interactions (6, 7). Binding of cell surface MUC1 by ICAM-1 increases cancer cell interaction with B lymphocytes (15), fibroblasts (16) and endothelial cells (17) in cell culture under static conditions. Cell surface MUC1 is also involved in signal transduction via interaction of its cytoplasmic tail with important intracellular signalling proteins such as p53 and β-catenin and suppresses cellular apoptosis in response to DNA damage (18, 19).
Galectin-3 is a galactoside-binding protein that is expressed in many cell types (20) and is found inside cells, extracellularly (but still cell surface-associated) and in the circulation. Intracellular galectin-3 is an apoptosis inhibitor and mRNA splicing promoter whilst extracellular cell surface-associated galectin-3 acts as an adhesion molecule during cell-cell interactions and is associated with metastasis (20, 21). For example, galectin-3 expressed on the surface of breast cancer cells as well as on endothelial cells promotes adhesion of the breast cancer cells to endothelium by interaction with cancer-associated TF antigen expressed by unknown cell surface molecules (22, 23). Concentrations of circulating galectin-3 are markedly increased in the sera of cancer patients and patients with metastatic disease have higher concentrations of circulating galectin-3 than those with localized tumours (24-26).
Recently we reported that MUC1 is a natural ligand of galectin-3 in human cancer cells and that binding of recombinant galectin-3 to cancer-associated MUC1, via the TF antigen, induces MUC1 cell surface polarization and increases the adhesion of human epithelial cells to human umbilical vein endothelial cells under static cell culture conditions (27). Previous investigations have indicated that the great size and length of MUC1 allows it to form a protective shield on the cell surface and inhibit cell-cell and cell-matrix interactions (6, 7). We therefore suggested that interaction between circulating galectin-3 and cancer-associated MUC1 in the bloodstream of cancer patients may break the “protective shield” of MUC1 and reveal smaller cell surface adhesion molecules/ligands that enhance cancer-endothelial adhesion and hence promote metastasis (27).
The present study provides in vitro and in vivo evidence that strongly supports this hypothesis. It shows that over-expression of cell surface MUC1 is associated with reduced cancer cell-endothelial adhesion under static and flow conditions and with decreased cancer cell trans-endothelial invasion and increased survival of athymic nude mice intravenously inoculated with malignant melanoma cells. These effects are shown to be mediated by interaction of cell surface MUC1 with recombinant galectin-3 at pathologically-relevant concentrations that causes polarisation of MUC1 thus revealing cell surface adhesion molecules/ligands including CD44 and ligand(s) for E-Selectin.
Materials and Methods
Materials
Recombinant full-length human galectin-3 and monoclonal antibodies (mAb) against human CD44H (BBA10) and E-Selectin (BBA16) were from R&D Systems (Abingdon, UK). B27.29 anti-MUC1 mAb was kindly provided by Dr. Mark Reddish (Biomira Inc, Canada). Non-Enzymatic Cell Dissociation Solution (NECDS) was from Sigma. Vybrant DiO Cell-labelling Solutions were from Molecular Probes (Cambridge, UK).
Cell lines
Human colon cancer HT29 and HT29-5F7 cells were obtained and cultured as previously described (27). Human macro-vascular umbilical vein endothelial cells (HUVECs) and micro-vascular lung endothelial cells (HMVEC-Ls) were from Cambrex BioSciences (Wokingham, UK) and were cultured, respectively, in EGM endothelial growth media (EGM Bulletkit) and EGM-2 media (EGM-2 Bulletkit, Cambrex Bio Sciences). Cells that had been passaged less than five times were used in the experiments. MUC1 transfection of human melanoma A375 cells with full length cDNA encoding MUC1 resulted in the MUC1 positive transfectants ACA19+, and the subsequent bulk selection of the MUC1 negative revertants ACA19-, were as described previously (6).
Cancer cell-endothelial adhesion
Cancer cell-endothelial adhesion was performed as previously described (27). At the end of the experiments, the samples were blinded and the fluorescent-labelled cells remaining on the endothelial monolayer were counted in ten randomly selected fields of view (FOV) using fluorescent microscopy (Olympus B51). The ability of the cells to be labelled by the fluorescent dye was different between any two human cell lines tested in our pilot experiment. As comparison of the cell adhesion between different MUC1-expressing cells is an important part of this study, the actual number of the fluorescently-labelled cells adhered to endothelial monolayer, rather than the reading of the fluorescent density, was used as the endpoint.
MUC1 siRNA knock-down
ACA19+ cells were transfected with 100nM MUC1 siRNA or scrambled control non-targeting siRNA (siCONTRO non-targeting siRNA, Dhamacom) for 48 hr at 37°C. The cells were lysed and the expression of MUC1 assessed by MUC1 immunoblotting with the B27.29 anti-MUC1 antibody.
Cell adhesion under flow conditions
HUVECs, cultured in flattened glass capillaries for 24 hr at 37°C to allow the cells to form monolayers as previously described (28), were unstimulated or stimulated with 10 ng/ml TNFα for 24 hr at 37°C prior to the introduction of cancer cells. ACA19+/- cells were incubated with or without 1 μg/ml galectin-3 for 30 min at 37°C. The cells were then perfused through the glass capillaries at a flow rate to deliver 0.05pa shear wall stress. After 4 minutes washing with PBS, the capillaries were video-recorded and the number of adherent cells was quantified and expressed as adherent cells/mm2/106 cells perfused.
Cell surface expressions of E-Selectin and CD44
Cells released with NECDS were fixed with 2% paraformaldehyde for 0.5 hr, blocked with 5% normal goat serum/PBS for 0.5 hr, incubated with antibodies against-E-Selectin or - CD44H for 1 hr at room temperature. After application of fluorescein-conjugated secondary antibody for 0.5 hr, the expression of cell surface E-Selectin or CD44 was analysed by flow cytometry.
Trans-endothelial invasion
HUVECs were cultured in the transwell inserts with 8-μm pore filters (BD Falcon, MA) for 3 days to allow tight formation of cell monolayers. Monolayer integrity was monitored by measuring trans-endothelial electrical resistance (TEER) using a Volt Ohm Meter (EVOM, World Precision Instruments, UK) and monolayers with TEER > 800Ω/cm2 were used for trans-endothelial assessment. Epithelial cells, labelled with DiO, were incubated with or without galectin-3 for 30 min at 37°C before application of the cells to the HUVECs for 16 hr at 37°C. The cells at the upper side of the transwell membrane were removed with a cotton swab and fluorescent cells migrated to the bottom side of the transwell membrane were counted using an Olympus B51 fluorescence microscope.
Metastasis and survival
Eight-week-old female Balb/c athymic (Nu+/Nu+) nude mice were obtained from the Shanghai SLAC Laboratory Animals Co. Ltd (Shanghai Institute for Biological Sciences, Shanghai, China) and maintained and used in accordance with the animal care protocol approved by Shandong University.
Suspensions (5.0×106 cells/ml with PBS) of ACA19+ and ACA19- cells were passed though a 20 μm nylon mesh, incubated with or without galectin-3 (1μg galectin-3 per 0.25×106 cells) in gentle shaking (30-60 rpm/min) for 1 hr at 37°C before injection of the whole cell suspension into the lateral tail vein of the experimental mice. Forty-eight experimental animals were divided randomly into four groups: 11 animals/group were injected with or without galectin-3-pretreated ACA19- and 13 animals/group were injected with or without galectin-3-treated ACA19+ cells. The animals were maintained under standard conditions and observed daily. Two animals per group were randomly picked and sacrificed with anaesthetic by ether at day 20, 40, 60 and 80 (only the two ACA19+ groups at this last time point) and tumour metastasis in lung, liver and kidney was assessed by light microscopy. The remainder of the animals (5 animals per group) were killed when considered by an independent blinded observer to be moribund and the animal survival time from metastasis-associated death was recorded. Immediate dissections of these animals were also performed and metastasis to lung, liver and kidney was examined by light microscopy.
Statistical analysis
Paired or unpaired t test for single comparison, one-way analysis of variance (ANOVA) followed by Newman and Keuls test for multiple comparisons, Chi-Square test, Kaplan-Meier analysis followed by log-rank test (StatsDirect for Windows, StatsDirect Ltd; Sale, UK) were used where appropriate. Differences were considered significant when p<0.05.
Results
Cancer cell-endothelial adhesion is inhibited by MUC1 expression but increased by MUC1-galectin-3 interaction
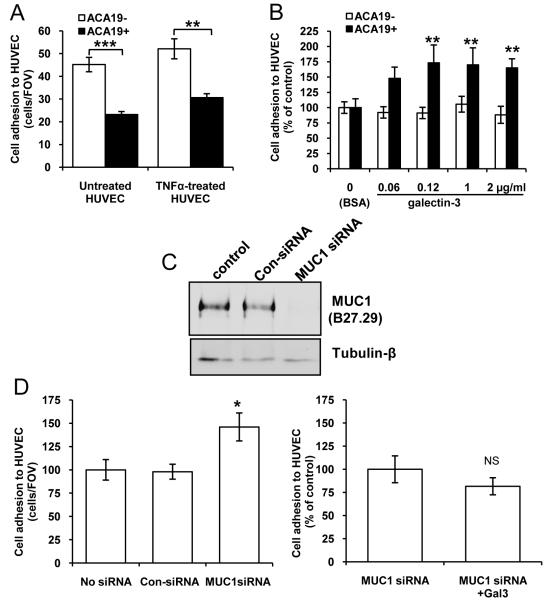
A375 human melanoma cells transfected with MUC1 (ACA19+) showed significantly less adhesion to unstimulated and TNFα-pre-stimulated HUVECs than the MUC1 negative-revertants (ACA19-) (Fig 1A). To determine the effect of circulating galectin-3 on cancer cell adhesion to endothelium, we pre-treated the cells with recombinant galectin-3 at several pathologically-relevant circulating galectin-3 concentrations. Earlier investigation by Iurisci et al (24) has shown that the concentration of circulating galectin-3 increases up to 5-fold in the sera of cancer patients with melanoma, breast and gastrointestinal cancer (in the range of 20 - 950 ng/ml), compared with healthy people. Our own investigation has indicated that the concentrations of circulating galectin-3 in the sera of colorectal cancer patients are over 14-fold higher (up to 5μg/ml) than in healthy people1. We found that pre-treatment of the cells with galectin-3 at concentrations above 100ng/ml resulted in significant increase of ACA19+, but not ACA19-, cell adhesion to HUVECs (Fig 1B). Immuno/lectin blots of ACA19+ and ACA19- cells with B27.29 anti-MUC1 mAb and TF-binding PNA showed that MUC1 in ACA19+ cells is abundantly decorated with TF antigens (Supplementary Fig S1). Suppression of MUC1 expression by siRNA in ACA19+ cells was associated with 47% increased adhesion of the cells to HUVECs and this prevented the increase of cell adhesion in response to galectin-3 (Fig 1C and D). Thus, the presence of extracellular free galectin-3, by its interaction with MUC1, counteracts the anti-adhesive effect of MUC1 expression on cancer cell adhesion.
Figure 1. MUC1 and MUC1-galectin-3 on cancer-HUVEC adhesion under static conditions.
A: ACA19+ adhere less than ACA19- to HUVECs. Data are expressed as mean ± SEM of triplicate determinations from three independent experiments. B: Pre-treatment of the cells with galectin-3 increases ACA19+ but not ACA19- cell adhesion to HUVECs. Data are expressed as mean ± SEM from three independent experiments. C: siRNA MUC1 suppression in ACA19+ cells. Representative blots of three experiments are shown. D: MUC1 suppression increases ACA19+ cell adhesion (left panel) and abolishes galectin-3-induced cell adhesion (right panel). The data are expressed as mean ± SEM from three independent experiments. *p<0.05, **p<0.01, ***p<0.001.
To determine whether MUC1-galectin-3 interaction has similar effects on cell adhesion in cancer cells that naturally express MUC1, we compared the adhesion of human colon cancer HT29 and HT29-5F7 cells in the presence or absence of recombinant galectin-3. HT29-5F7 is a HT29 subline selected by its resistance to 5-fluorouracil that has much greater MUC1 than the parental HT29 cells (29, Fig S2). HT29 cells showed significantly more adhesion to unstimulated and TNF-α-pre-stimulated HUVECs than HT29-5F7 cells (Fig S3A). Pre-treatment of the cells with galectin-3 significantly increased adhesion of HT29-5F7, but not of HT29 cells, to HUVECs and this effect was abolished by the presence of TF-expressing asialofetuin (ASF) (Fig S3).
We also assessed whether galectin-3 may have been secreted into the medium by the cancer cells but found no detectable endogenously-secreted galectin-3 (less than 0.325 ng/ml, the detectable limit of the assay) in the medium during the 1.5 hr assessment period. Thus, the contribution of endogenously-secreted galectin-3 towards recombinant galectin-3-mediated cell adhesion in these assessments is minimal.
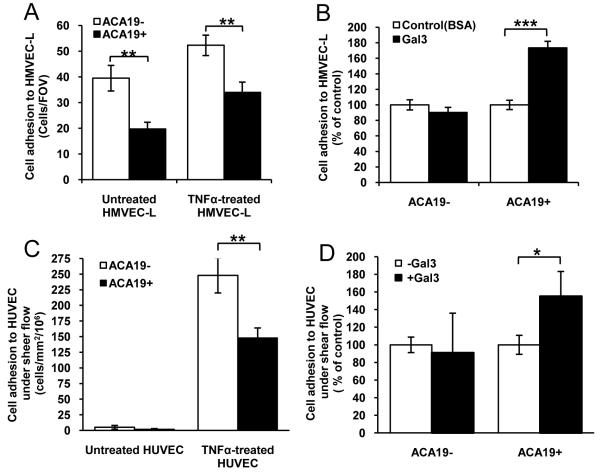
To see whether the effects of MUC1-galectin-3 interaction on cancer cell adhesion to HUVECs also occurs with micro-vascular endothelium, a model that is probably closer to the in vivo situation in metastasis, we analysed cell adhesion to HMVEC-L. Again, ACA19+ showed significantly less adhesion to unstimulated and TNFα-pre-stimulated HMVEC-Ls than the ACA19- cells (Fig 2). Pre-treatment of the cells with galectin-3 (1μg/ml) increased adhesion of ACA19+ but not of ACA19- cells.
Figure 2. MUC1 and MUC1-galectin-3 on cancer-HMVEC-Ls adhesion under static and cancer-HUVECs adhesion under flow conditions.
A: ACA19+ adhere less than ACA19- cells to HMVEC-Ls. Data expressed as mean ± SEM from three independent experiments. B: Galectin-3 (1μg/ml) increases ACA19+ but not ACA19- adhesion to HMVEC-Ls. Data are expressed as mean ± SEM from two independent experiments. C: ACA19+ adhere less than ACA19- cells to HUVECs under 0.05Pa flow. Data expressed as mean ± SEM from seven independent experiments. D: Galectin-3 (1μg/ml) treatment increases adhesion of ACA19+ but not ACA19- cells to HUVECs under flow. Data expressed as mean ± SEM from five ACA19+ and six ACA19- independent assessments. *p<0.05, **p<0.01, ***p<0.001.
Collectively, these results indicate that MUC1 expression prevents cancer cell adhesion to macro- and micro-vascular endothelium and that MUC1-galectin-3 interaction reduces this protective effect of MUC1.
Cancer cell-endothelial adhesion under flow is inhibited by MUC1 expression but increased by MUC1-galectin-3 interaction
Under shear flow conditions, very few ACA19+ or ACA19- cells showed adhesion to unstimulated HUVECs but their adhesion were markedly increased when HUVECs were pre-treated with TNFα (Fig 2C). Moreover, ACA19+ showed 68% less adhesion than ACA19- cells to TNFα-stimulated HUVECs under such conditions. Pre-treatment of the cells with 1μg/ml galectin-3 resulted in 55% increased adhesion of ACA19+ but not of ACA19- cells (Fig 2D). Thus, the effects of MUC1 expression and galectin-3-MUC1 interaction on cancer-endothelium adhesion seen under static conditions also hold true under flow conditions.
Galectin-3 and B27.29 anti-MUC1 mAb have similar effects on MUC1 cell surface polarization and on cell adhesion
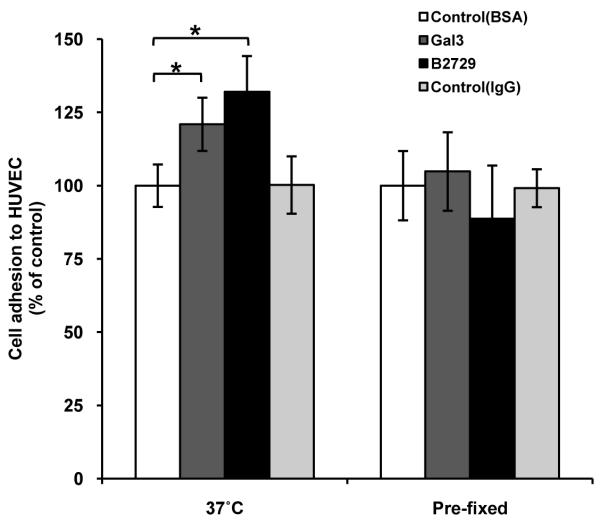
We have previously shown that galectin-3-MUC1 interaction induces change of MUC1 cell surface localization (27). To see whether this change in MUC1 localization is associated with altered cell adhesion to endothelium, we compared MUC1 cell surface clustering in response to galectin-3 with cell adhesion to HUVECs.
It was found that 10% (48/500) ACA19+ cells showed spontaneous clustering of MUC1 on the cell surface as illustrated by discontinuity of MUC1 cell surface staining when cultured in suspension for 1 hour at 37°C. After pre-treatment with galectin-3 (1μg/ml) at 37°C for 1 hr, 40% more (68/500, p<0.05) cells demonstrated MUC1 cell surface polarization than control cells. Introduction of B27.29 anti-MUC1 mAb also resulted in significant increase (128/500, p<0.01) of the number of cells showing MUC1 cell surface polarization. This effect of B27.29 on MUC1 cell surface polarization is in keeping with previous reports that the presence of 214D4 anti-MUC1 mAb, which also recognizes the VNTR region of MUC1, induces MUC1 cell surface polarization in MUC1-transfected human melanoma cells (6). The B27.29 mAb recognizes the PDTRPAP epitope in the VNTR region of MUC1 (30) and NMR analysis has indicated an enhanced binding affinity of B27.29 to MUC1 in the presence of short sugar chains within the VNTR region (31).
It was found that the increases of MUC1 cell surface polarization in response to galectin-3 and to B27.29 mAb are both associated with increased adhesion of ACA19+ cells to HUVECs (Fig 3). Furthermore, introduction of galectin-3 or B27.29 mAb to paraformaldehyde pre-fixed cells, which could not affect the MUC1 cell surface localization, failed to induce cell adhesion to HUVECs compared with the control cells. These results support a direct link of discontinuous cell surface localization of MUC1 and increased cell adhesion in response to galectin-3 and B27.29 mAb.
Figure 3. Galectin-3 and B27.29 anti-MUC1 mAb both induce increased adhesion to HUVECs of live, but not pre-fixed ACA19+ cells, nor ACA19- cells.
Live or paraformaldehyde pre-fixed ACA19+/- cells were pre-treated with 1μg/ml recombinant galectin-3, B27.29 mAb, BSA or control mouse IgG before adhesion to HUVECs. Data expressed as mean ± SEM from four independent experiments. *p<0.05.
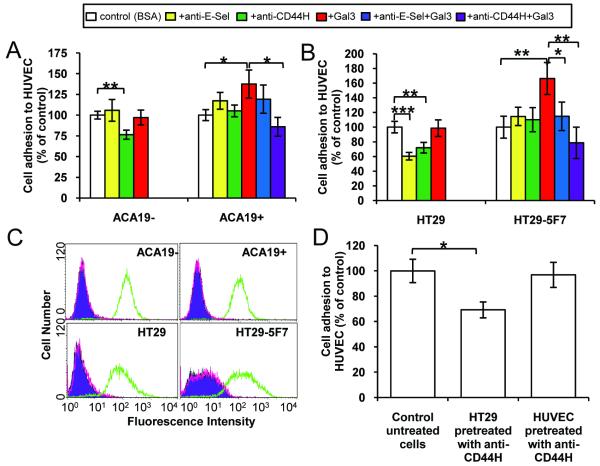
Involvement of cancer associated-CD44 and endothelial-E-Selectin in MUC1-galectin-3-induced cancer-endothelial adhesion
The presence of (25μg/ml) anti-CD44H mAb (BBA10), which recognizes all CD44 isoforms, caused 26% (p<0.05) inhibition of ACA19-, but not ACA19+, adhesion to HUVECs but largely blocked ACA19+ cell adhesion induced by galectin-3 (Fig 4A). The presence of (25μg/ml) anti-E-Selectin antibody, however, failed to block the adhesion of either ACA19- or ACA19+ cells and also showed no significant inhibition (p=0.47) of galectin-3 induced ACA19+ adhesion (Fig 4A). The presence of either anti-CD44H or anti-E-Selectin antibody inhibited HT29 cell adhesion to HUVECs (Fig 4B). Neither of these antibodies at this concentration showed significant inhibition of HT29-5F7 cell adhesion to HUVECs but their presence completely prevented the increase of HT29-5F7 cell adhesion in response to galectin-3.
Figure 4. Involvement of cell surface CD44 and ligand(s) to endothelial-E-Selectin in galectin-3-induced cancer-endothelial adhesion.
A: The presence of 25 μg/ml anti-CD44H, but not anti-E-Selectin, mAb reduces ACA19- cell adhesion and adhesion of ACA19+ cells induced by 1μg/ml galectin-3. Data expressed as mean ± SEM from three independent experiments. B: The presence of 25 μg/ml anti-CD44H or anti-E-Selectin antibody inhibits HT29 and HT29-5F7 cell adhesion and adhesion of HT29-5F7 cells induced by 1μg/ml recombinant galectin-3. Data expressed as mean ± SEM from three independent experiments. C: Cell surface expression of E-Selectin and CD44 are similar between HT29 and HT29-5F7 cells and between ACA19+ and ACA19- cells. Green: CD44, red: E-Selectin, purple: isotype control. D: Pre-treatment of the HT29 cells, but not of the HUVECs, with 25 μg/ml anti-CD44H antibody inhibits HT29 cell adhesion to HUVECs. Data expressed as mean ± SEM from three independent experiments. *p<0.05, **p<0.01, ***p<0.001.
Antibody binding followed by flow cytometry analysis showed similar CD44 cell surface expression and antibody accessibility between HT29 and HT-29-5F7 and between ACA19- and ACA19+ cells (Fig 4C). Thus, the lack of effect of the anti-CD44H antibody on the adhesion of HT29-5F7 and ACA19+ cells to HUVECs is likely due to the inability of cell surface CD44 to gain access to its receptor on HUVECs as a result of functional concealment of cell surface CD44, e.g. by the presence of adjacent MUC1. The inhibition by the anti-CD44H antibody of galectin-3-induced cell adhesion, in which cell surface CD44 is functionally exposed following MUC1 cell surface polarization, supports this conclusion. The relatively modest inhibition of the anti-CD44 antibody at 25 μg/ml on ACA19- adhesion indicates that cell surface CD44 may represent just one of several cell adhesion molecules involved in melanoma-endothelial adhesion.
We found that anti-CD44H antibody pre-treatment of the HT29 cells, but not of the HUVECs, caused a significant inhibition of subsequent HT29 cell adhesion to HUVECs (Fig 4D). This indicates the involvement of HT29-associated, but not HUVEC-associated, CD44 molecules in HT29-endothelial interaction and in galectin-3-mediated cancer-endothelial adhesion. This is in keeping with earlier reports of a role for cancer-associated CD44 in the initial endothelial adhesion of human prostate, breast and colon cancer cells (32, 33).
Neither HT29/HT29-5F7 nor ACA19-/ACA19+ cells express E-Selectin on their cell surface (Fig 4C). The HT29 cell adhesion to HUVECs and the HT29-5F7 adhesion to HUVECs induced by galectin-3 were, however, inhibited by the presence of the anti-E-Selectin antibody (Fig 4A and B). This suggests the involvement of endothelial-E-Selectin in these cell adhesion events. The different effects of the anti-E-Selectin antibody on HT29/HT29-5F7 and on ACA19-/+ adhesion to HUVECs likely reflect differences in expression of E-Selectin ligands on the surface of HT29 colon and ACA19 melanoma cells.
Cancer cell trans-endothelial invasion is inhibited by MUC1 expression and increased by MUC1-galectin-3 interaction
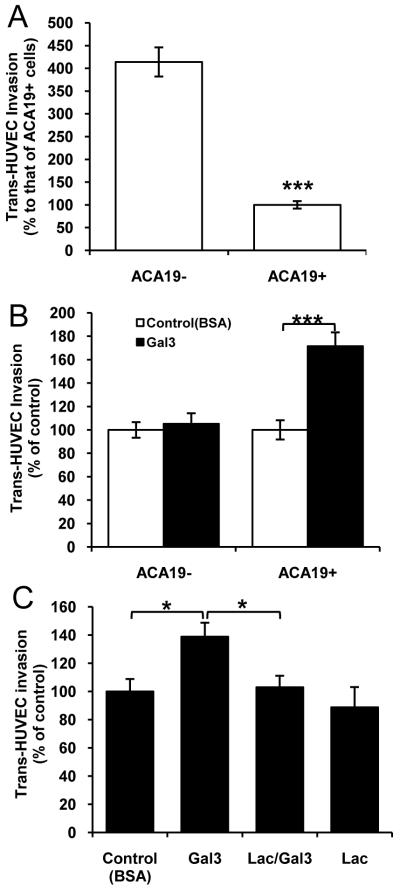
ACA19- showed 46% greater trans-HUVEC invasion than ACA19+ cells (Fig 5A). Pre-treatment of the cells with galectin-3 (1μg/ml) resulted in 64% increased invasion of ACA19+ but not of ACA19- cells (Fig 5B). This effect of galectin-3 was completely prevented by the presence of lactose (Fig 5C).
Figure 5. MUC1 and MUC1-galectin-3 on cancer cell trans-endothelial invasion.
A: ACA19+ show less trans-HUVEC invasion than ACA19- cells. Data expressed as mean ± SEM of triplicate determinations from six independent experiments. B: Galectin-3 (1μ/ml) pre-treatment increases ACA19+ but not ACA19- cell trans-HUVEC invasion. Data expressed as mean ± SEM from six independent experiments. C: Galectin-3-mediated trans-endothelial invasion of ACA19+ is inhibited by the presence of (10 μg/ml) lactose. Data expressed as mean ± SEM from four independent assessments. *p<0.05, ***p<0.0001.
Galectin-3 promotes metastasis of ACA19+ MUC1-expressing cells in vivo
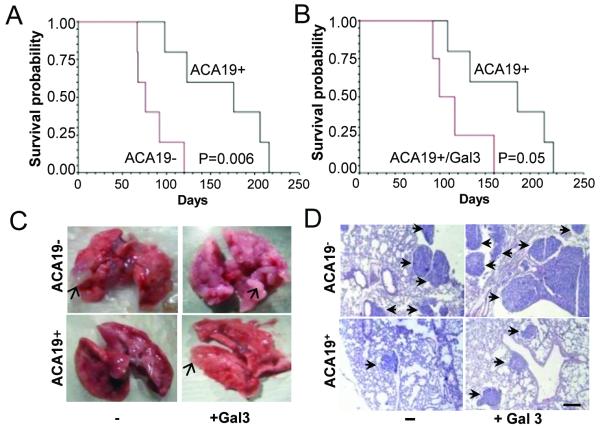
Forty-seven of the 48 experimental animals developed metastasis after inoculation of ACA19+ or ACA19- cells with or without galectin-3 pre-treatment. Metastatic foci were seen in the lungs but not in the other organs. At day 20, several metastatic foci in the edges of the lung lobes appeared in animals injected with ACA19-. Metastatic foci were not visible in those injected with ACA19+ at day 20 but were visible at day 40 (Fig 6 and Table S1). Animals bearing ACA19+ cells survived significantly longer (164 ± 52 days) than those bearing ACA19- cells (85±22 days, p=0.006, Fig 6A). Pre-treatment of ACA19+ cells with galectin-3 resulted in 35% reduction in animal survival (107±31 days, p=0.05) (Fig 6B). These results provide proof of concept that the effects of MUC1 expression and galectin-3-MUC1 interaction on cancer-endothelial adhesion are associated with decreased and increased metastasis, respectively. Pre-treatment of ACA19- cells with galectin-3 resulted in a small but statistically non-significant reduction of animal survival from metastasis-associated death (62 ±12 days, p=0.1) compared to those injected with ACA19- cells alone. This suggests that recombinant/circulating galectin-3 may interact with other cell surface molecules apart from MUC1 and that such interaction, although not affecting endothelial adhesion, may also contribute to metastasis.
Figure 6. MUC1 and MUC1-galectin-3 on ACA19 cell metastasis in athymic mice.
A. Animals injected with ACA19+ cells have markedly increased survival compared with those injected with ACA19- cells. B. Pre-treatment of ACA19+ cells with galectin-3 before injection led to shortened animal survival. C. Macroscopic appearance of representative lungs 40 days after tumour cell injections. Arrows point to metastatic foci. D. H&E staining of representative lung sections from animals 40 days after tumour cell injection. Arrows point to the metastatic lesions. Bar = 200μm.
Discussion
This study shows that over-expression of cell surface MUC1 is associated with reduced cancer cell-endothelial adhesion under static and flow conditions and with decreased cancer cell trans-endothelial invasion and increased survival of athymic nude mice intravenously inoculated with malignant melanoma cells. The interaction of cell surface MUC1 with circulating galectin-3 at pathologically-relevant concentrations reduces the protective effects of MUC1 on cancer cell adhesion, trans-endothelial invasion and metastasis. These effects of galectin-3 are mediated by MUC1 cell surface clustering and the consequent exposure of cell surface adhesion molecules including CD44 and the ligand(s) for endothelial-E-Selectin. Thus, the enhanced molecular interaction between circulating galectin-3 and cancer-associated MUC1 in the bloodstream of cancer patients, occurring as a result of the increased expression of MUC1 by cancer cells, the increased expression of the galectin-3-ligand TF antigen by cancer-associated MUC1 and the increased concentration of circulating galectin-3, all of which are common features in cancer, promotes metastasis.
Cancer cell adhesion to endothelium is a vital step in metastasis and is mediated by a range of adhesion molecules and their ligands, including Selectins and integrins expressed on cancer and on endothelial cells, which in turn are regulated by circulating molecules such as cytokines (1). The inhibitory effect of MUC1 expression and the stimulatory effect of galectin-3-MUC1 interaction on cancer-endothelial adhesion demonstrated in this study suggest that MUC1 cell surface polarization that leads to uncovering the smaller adhesion molecules and/or ligands to adhesion molecules represents an essential first step in the process of cancer cell-endothelial adhesion. Given the variable expression of MUC1 in different cancer cell lines (34), the lack of inhibitory effect of anti-Selectin antibodies on cancer-endothelial adhesion observed in several previous investigations (35) may be, to a large extent, due to the concealment of the cell surface Selectin ligands by MUC1.
MUC1 can carry sialyl-Lewis-related carbohydrate structures that act as ligands for selectins (36). However, an interaction between cancer-associated MUC1 and endothelial-E-selectin, will probably not induce tight cell adhesion, a process that is believed to require the involvement of cell surface integrins as has been well established for leukocyte-endothelial adhesion (37). Tight cancer-endothelial adhesion may only occur after MUC1 cell surface polarization and exposure of integrins and other smaller cell adhesion molecules. Our previous demonstration (27) that MUC1 is absent at the cancer-endothelial contact point supports this.
The protective effect of the MUC1 “shield” and the “de-protective” effect of the galectin-3-TF/MUC1 interaction on cancer cell adhesion provide explanations at the molecular level for several recent clinical and experimental observations related to metastasis, e.g. the correlation between increased apical MUC1 cell surface polarization and increased lymphatic invasion, recurrence rate and lower overall survival in breast cancer patients (38). The correlation of increased concentrations of circulating anti-TF antibodies, which would inhibit galectin-3-mediated TF/MUC1 interactions, with improved prognosis in gastric cancer (39) is also consistent with our model. The association of MUC1 sialylation with a better prognosis in breast cancer (40) is also in keeping with a reduced galectin-3-TF/MUC1 interaction as a consequence of concealment of TF by sialic acid thus inhibiting MUC1-galectin-3 interaction (27). The significant extension of animal survival induced by intraperitoneal co-injection of an anti-TF antibody with metastatic 4T1 breast cancer cells (41) could be the consequence of the blockade of the galectin-3-TF/MUC1 interaction.
Since increased occurrence of the TF glycan is one of the commonest glycosylation changes in cancer (9), this study also highlights the functional importance of cancer-associated changes in cellular glycosylation (42, 43) and indicates a potential for glycan profiling in predicting cancer metastasis and prognosis. Furthermore, as the increased concentrations of circulating galectin-3 in cancer patients are probably produced not only by the tumour cells but also by the peritumoral inflammatory and stromal cells (24), this also reinforces the importance of the tumour microenvironment for metastasis (44, 45).
It should be emphasised that this study focuses on the role of circulating galectin-3 on cancer cell adhesion and metastasis. The functional importance of cancer cell-associated galectin-3 in metastasis is well documented (20, 21). For example, antisense suppression of galectin-3 in metastatic LSLiM6 human colon cancer cells before inoculation of the cells into athymic mice results in reduced liver colonization and metastasis (46) whilst suppression of galectin-3 expression by shRNA in melanoma cells reduces tumour cell invasiveness and capacity to form tube-like structures on collagen, so-called vasculogenic mimicry (47). Similarly, reduction of galectin-3 expression in highly malignant human breast cancer MDA-MB-435 cells leads to loss of serum- and anchorage-independent growth in vitro and tumour growth in nude mice (48).
Thus, galectin-3 released into the bloodstream of cancer patients promotes cancer cell haematogenous dissemination by its interaction with TF-expressing MUC1 on cancer cell surface. This provides insight into the molecular regulation of metastasis and has important implications for the development of therapeutic strategies to prevent metastasis.
Supplementary Material
Acknowledgements
The authors thank Dr. Thecla Lesuffleur (INSERM U560, Lille, France) for the HT29-5F7 cells and Dr. Mark Reddish (Biomira Inc, Edmonton, Canada) for the B27.29 mAb.
This work was supported by Cancer Research UK grant C7596 (to LGY) and North West Cancer Research Fund grant CR777 (to LGY).
Abbreviations
- NECDS
Non-Enzymatic Cell Dissociation Solution
- FOV
field of view
- HMVEC-L
human micro-vascular lung endothelial cells
- HUVEC
human umbilical vein endothelial cells
- TF
Thomsen-Friedenreich (Galβ1,3GalNAc-) antigen
Footnotes
Barrow H, Rhodes JM and Yu LG, Manuscript in preparation.
References
- 1.Miles FL, Pruitt FL, van-Golen KL, Cooper CR. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25:305–24. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 2.Kim YS, Gum, Brockhausen I. Mucin glycoproteins in neoplasia. Glycoconj J. 1996;13:693–707. doi: 10.1007/BF00702333. [DOI] [PubMed] [Google Scholar]
- 3.Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992;17:359–63. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- 4.Bresalier RS, Niv Y, Byrd JC, et al. Mucin production by human colonic carcinoma cells correlates with their metastatic potential in animal models of colon cancer metastasis. J Clin Invest. 1991;87:1037–45. doi: 10.1172/JCI115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106:353–61. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 6.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-Cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–77. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesseling J, wan der walk SW, Vos HJ, Sonnenberg A, Hilkens J. Epithelin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–65. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271:33325–34. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]
- 9.Yu LG. The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconj J. 2007;24:411–20. doi: 10.1007/s10719-007-9034-3. [DOI] [PubMed] [Google Scholar]
- 10.Singh R, Campbell BJ, Yu LG, et al. Cell surface-expressed Thomsen-Friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of CD44. Glycobiology. 2001;11:587–92. doi: 10.1093/glycob/11.7.587. [DOI] [PubMed] [Google Scholar]
- 11.Baldus SE, Hanisch FG, Kotlarek GM, et al. Coexpression of MUC1 mucin peptide core and the Thomsen-Friedenreich antigen in colorectal neoplasms. Cancer. 1998;82:1019–27. doi: 10.1002/(sici)1097-0142(19980315)82:6<1019::aid-cncr3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Baldus SE, Hanisch FG, Monaca E, et al. Immunoreactivity of Thomsen-Friedenreich (TF) antigen in human neoplasms: the importance of carrier-specific glycotope expression on MUC1. Histol Histopathol. 1999;14:1153–8. doi: 10.14670/HH-14.1153. [DOI] [PubMed] [Google Scholar]
- 13.Baldus SE, Wienand JR, Werner JP, et al. Thomsen-Friedenreich antigen presents as a prognostic factor in colorectal carcinoma: A clinicopathologic study of 264 patients. Cancer. 2000;88:1536–43. [PubMed] [Google Scholar]
- 14.Baldus SE, Zirbes TK, Glossmann J, et al. Immunoreactivity of monoclonal antibody BW835 represents a marker of progression and prognosis in early gastric cancer. Oncology. 2001;61:147–55. doi: 10.1159/000055366. [DOI] [PubMed] [Google Scholar]
- 15.McDermott KM, Crocker PR, Harris A, et al. Overexpression of MUC1 reconfigures the binding properties of tumor cells. Int J Cancer. 2001;94:783–91. doi: 10.1002/ijc.1554. [DOI] [PubMed] [Google Scholar]
- 16.Rahn JJ, Shen Q, Mah BK, Hugh JC. MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J Biol Chem. 2004;279:29386–90. doi: 10.1074/jbc.C400010200. [DOI] [PubMed] [Google Scholar]
- 17.Rahn JJ, Chow JW, Horne GJ, et al. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis. 2005;22:475–83. doi: 10.1007/s10585-005-3098-x. [DOI] [PubMed] [Google Scholar]
- 18.Wei X, Xu H, Kufe D. MUC1 oncoprotein regulates p53-responsive gene transcription in the genotoxic stress response. Cancer Cell. 2005;7:167–78. doi: 10.1016/j.ccr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–76. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 21.Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2004;19:543–9. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- 22.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–7. [PubMed] [Google Scholar]
- 23.Khaldoyanidi SK, Glinsky VV, Sikora L, et al. MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by Thomsen-Friedenreich antigen-galectin-3 interactions. J Biol Chem. 2003;278:4127–34. doi: 10.1074/jbc.M209590200. [DOI] [PubMed] [Google Scholar]
- 24.Iurisci I, Tinari N, Natoli C, et al. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–93. [PubMed] [Google Scholar]
- 25.Saussez S, Lorfevre F, Lequeux T, et al. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumour progression and/or responses to therapy. Oral Oncol. 2008;44:86–93. doi: 10.1016/j.oraloncology.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Vereecken P, Zouaoui Boudjeltia K, Debray C, et al. High serum galectin-3 in advanced melanoma: preliminary results. Clin Exp Dermatol. 2006;31:105–9. doi: 10.1111/j.1365-2230.2005.01992.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu LG, Andrews N, Zhao Q, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282:773–81. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh S, Rainger GE, Gale Z, Rahman M, Nash GB. Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-alpha: a basis for local variations in vascular sensitivity to inflammation. Blood. 2003;102:2828–34. doi: 10.1182/blood-2003-01-0080. [DOI] [PubMed] [Google Scholar]
- 29.Leteurtre E, Gouyer V, Rousseau K, et al. Differential mucin expression in colon carcinoma HT-29 clones with variable resistance to 5-fluorouracil and methotrexate. Biol Cell. 2004;96:145–51. doi: 10.1016/j.biolcel.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Schol DJ, Meulenbroek MFA, Snijdewint FGM, et al. ‘Epitope fingerprinting’ using overlapping 20-mer peptides of the MUC1 tandem repeat sequence. Tumor Biol. 1998;19:35–45. doi: 10.1159/000056503. [DOI] [PubMed] [Google Scholar]
- 31.Grinstead JS, Koganty RR, Krantz MJ, Longenecker BM, Campbell AP. Effect of glycosylation on MUC1 humoral immune recognition: NMR studies of MUC1 glycopeptide-antibody interactions. Biochemistry. 2002;41:9946–9961. doi: 10.1021/bi012176z. [DOI] [PubMed] [Google Scholar]
- 32.Draffin JE, McFarlane S, Hill A, et al. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64:5702–11. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- 33.Fujisaki T, Tanaka Y, Fujii K, et al. CD44 stimulation induces integrin-mediated adhesion of colon cancer cell lines to endothelial cells by up-regulation of integrins and c-Met and activation of integrins. Cancer Res. 1999;59:4427–34. [PubMed] [Google Scholar]
- 34.Ligtenberg MJ, Vos HL, Gennissen AM, Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990;265:5573–8. [PubMed] [Google Scholar]
- 35.Krause T, Turner GA. Are Selectins involved in metastasis? Clin Exp Metastasis. 1999;17:181–92. doi: 10.1023/a:1006626500852. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, Baeckström D, Brevinge H, Hansson GC. Secreted MUC1 mucins lacking their cytoplasmic part and carrying sialyl-Lewis a and x epitopes from a tumor cell line and sera of colon carcinoma patients can inhibit HL-60 leukocyte adhesion to E-selectin-expressing endothelial cells. J Cell Biochem. 1996;60:538–49. doi: 10.1002/(SICI)1097-4644(19960315)60:4%3C538::AID-JCB10%3E3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 37.Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–8. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Li YS, Kaneko M, Sakamoto DG, Takeshima Y, Inai K. The Reversed Apical Pattern of MUC1 Expression Is Characteristics of Invasive Micropapillary Carcinoma of the Breast. Breast Cancer. 2006;13:58–63. doi: 10.2325/jbcs.13.58. [DOI] [PubMed] [Google Scholar]
- 39.Kurtenkov O, Klaamas K, Mensdorff-Pouilly S, Miljukhina L, Shljapnikova L, Chuzmarov V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 2007;46:316–23. doi: 10.1080/02841860601055441. [DOI] [PubMed] [Google Scholar]
- 40.Baldus SE, Wienand JR, Werner JP, et al. Expression of MUC1, MUC2 and oligosaccharide epitopes in breast cancer: prognostic significance of a sialylated MUC1 epitope. Int J Oncol. 2005;27:1289–97. [PubMed] [Google Scholar]
- 41.Heimburg J, Yan J, Morey S, Glinskii OV, et al. Inhibition of spontaneous breast cancer metastasis by anti-Thomsen-Friedenreich antigen monoclonal antibody JAA-F11. Neoplasia. 2006;8:939–48. doi: 10.1593/neo.06493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. 1997;14:569–76. doi: 10.1023/a:1018580324971. [DOI] [PubMed] [Google Scholar]
- 43.Gorelik E, Galili U, Raz A. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 2001;20:245–77. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- 44.Albini A, Mirisola V, Pfeffer U. Metastasis signatures: genes regulating tumor-microenvironment interactions predict metastatic behavior. Cancer Metastasis Rev. 2008;27:75–83. doi: 10.1007/s10555-007-9111-x. [DOI] [PubMed] [Google Scholar]
- 45.Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–27. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 46.Bresalier RS, Mazurek N, Sternberg LR, et al. Metastasis of human colon cancer is altered by modifying expression of the beta-galactoside-binding protein galectin 3. Gastroenterology. 1998;115:287–96. doi: 10.1016/s0016-5085(98)70195-7. [DOI] [PubMed] [Google Scholar]
- 47.Mourad-Zeidan AA, Melnikova VO, Wang H, Raz A, Bar-Eli M. Expression profiling of Galectin-3-depleted melanoma cells reveals its major role in melanoma cell plasticity and vasculogenic mimicry. Am J Pathol. 2008;173:1839–52. doi: 10.2353/ajpath.2008.080380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7:661–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.