1. Introduction
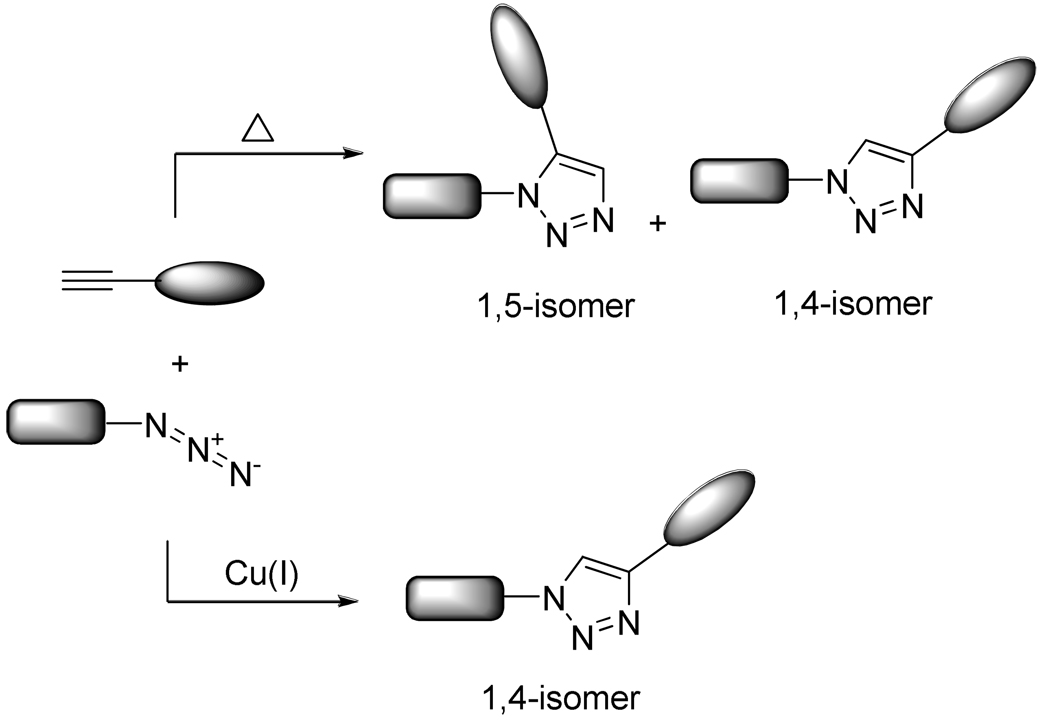
Pioneered by Huisgen in the 1960’s1, the 1,3-dipolar cycloaddition reaction between acetylenes and azides was brought back into focus by Sharpless and others2 when they developed the concept of “click chemistry”. This approach, based on the joining of smaller units mimics the approach used by nature to generate substances. This concept takes advantage of reactions that are modular, wide in scope, stereospecific, high yielding, and generate only non-offensive by-products to efficiently access new useful compounds. Moreover, to be completely “click”, the process must involve simple reaction conditions, readily available starting materials and reagents, the use of no solvent, or a benign or easily removable solvent.3 At first, the classical Huisgen 1,3-dipolar cycloaddition did not fall into the above definition, but the discovery of copper (I) salts catalyzing the reaction first by Medal and then by Sharpless4 allowed chemists to evolve from harsh reaction conditions that lead to a mixture of 1,4- and 1,5- regio-isomers to a regioselective reaction which can be performed at room temperature in very short reaction times (Scheme 1). The Cu alkyne-azide cycloaddition (CuAAC) fit so well into the above definition that it has become almost synonymous of “click chemistry” itself.
Scheme 1.
1,3-Dipolar Cycloaddition Between Azides and Alkynes
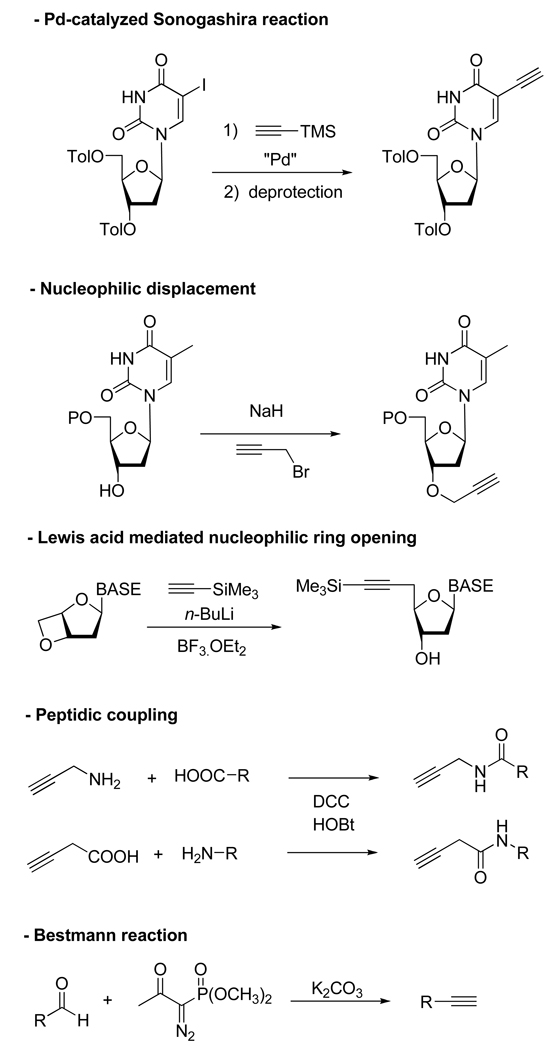
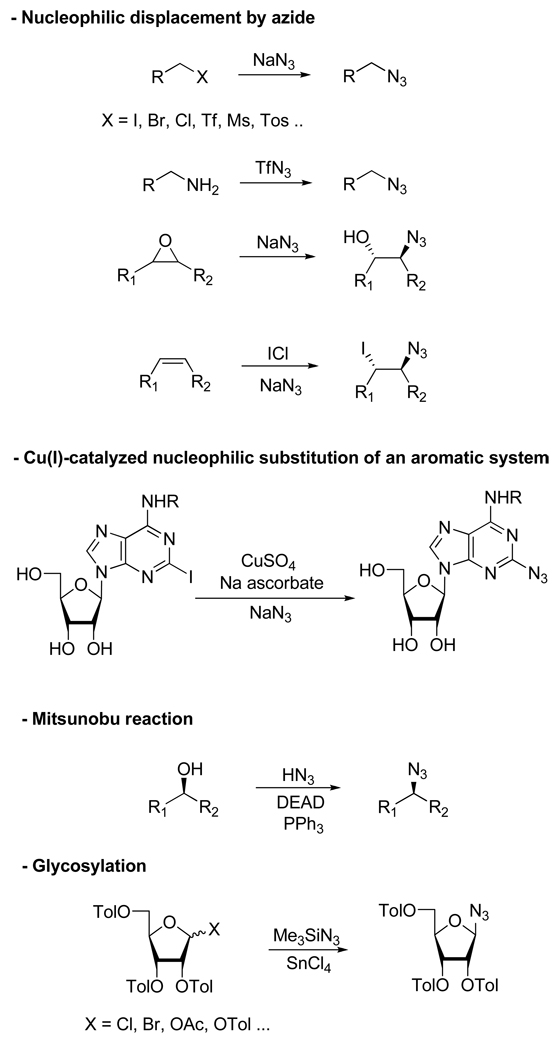
Indeed, CuAAC proceeds in a variety of solvents including aqueous media which, combined with the relative innocuousness of the reactants, render it bio-compatible. Compared to other metal-catalyzed reactions, the use of Cu(I) presents the major advantages of being inexpensive and easy to handle (Most of the protocols involve the reduction of stable sources of Cu(II) such as CuSO4 with sodium salts or the comproportionation of Cu(II)/Cu(0) species). In addition, the fact that both alkyne and azide functional groups can be incorporated into a wide range of compounds by several very general methods might also help explain the widespread use of this reaction (Scheme 2 and Scheme 3).5 All these attributes, combined with the potentially favorable physicochemical properties of the resulting triazoles, has propelled the Cu(I)-catalyzed Huisgen cycloaddition to be one of the most popular and efficient reactions within the concept of click chemistry; and as a result, a burst in the number of publications on the topic has occurred in a last few years.
Scheme 2.
Most Common Methods for the Introduction of a Terminal Alkyne
Scheme 3.
Most Common Methods for the Introduction of an Azide Functional Group
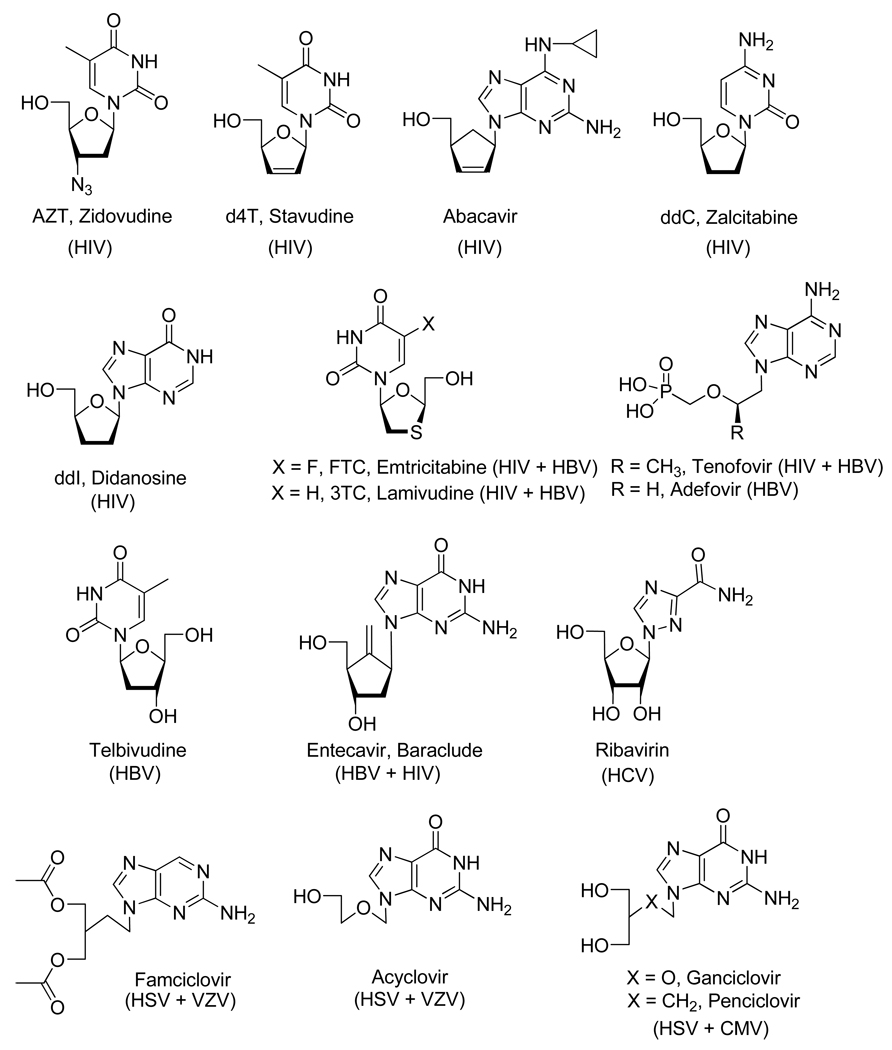
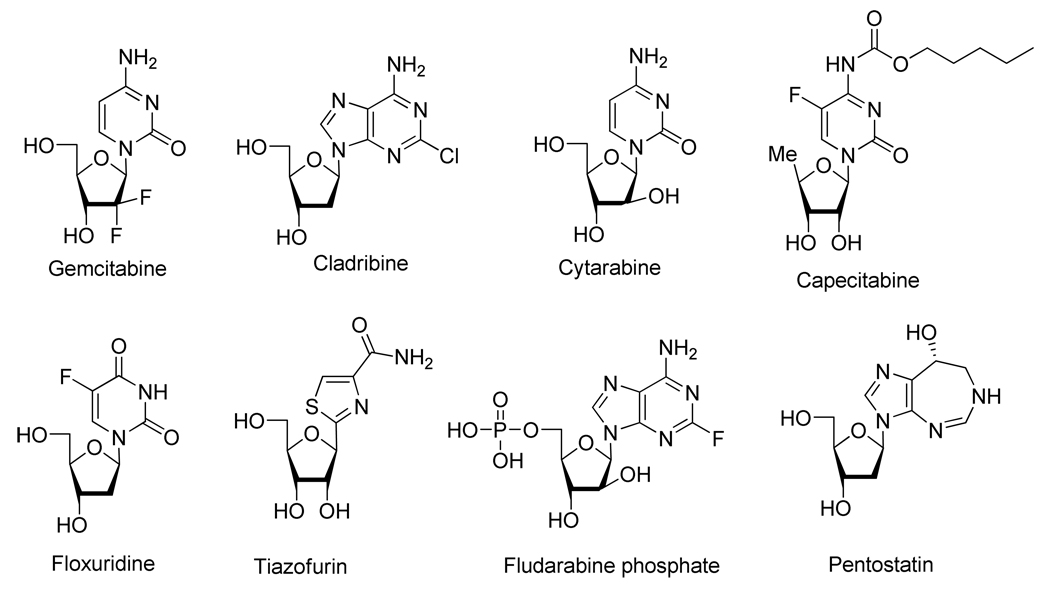
Over the last 40 years, the development of nucleic acids and nucleoside analogs for medicinal uses have had a marked impact on clinical chemotherapy as applied to antiviral and anticancer treatment. Numerous nucleoside analogs, for instance, were successfully developed for the treatment of human immunodeficiency viruses (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), herpes simplex virus (HSV), cytomegalovirus (CMV) or varicella zoster virus (VZV) (Figure 1) and various cancers (Figure 2).6
Figure 1.
Nucleoside Analogs Used for the Treatment of HIV, HBV, HCV, HSV, CMV and VZV.
Figure 2.
Nucleoside Analogs Used for the Treatment of Various Cancers.
In parallel, studies of the properties of modified oligonucleotides led to the development of unnatural oligomers with huge therapeutic potential, especially for the treatment of diseases characterized by the expression of unwanted genes.7 The structural diversity of active nucleosides as shown in Figure 2 and Figure 3 is the evidence that nucleosides analogs do not need to be close to their natural counterpart to be interesting and that any new structure is worth exploring. Therefore it seems logical that researchers turned their attention toward the possible benefits of innovative and new synthetic approaches such as the CuAAC for the synthesis of base- or sugar-modified nucleosides, nucleosides bioconjugates, and modified oligonucleotides. This review covers the literature up to March 2009 and has deliberately excluded postsynthetic DNA modifications since a review on this topic has recently been published.8
Figure 3.
Actives Five Membered Heterocyclic Based Nucleosides Analogs.
2. Nucleosides
2.1. Base Modified Nucleosides
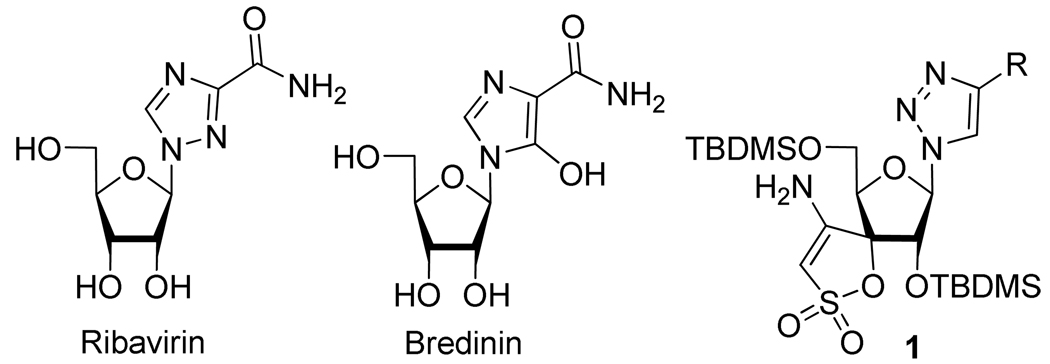
The discovery of clinically useful nucleoside analogs, containing a five-membered heterocyclic base such as Ribavirin,9 Bredinin,10 or compound 111 (Figure 3), provided a catalyst for creative applications of the CuAAC reaction toward medicinally relevant base modified nucleoside analogs.
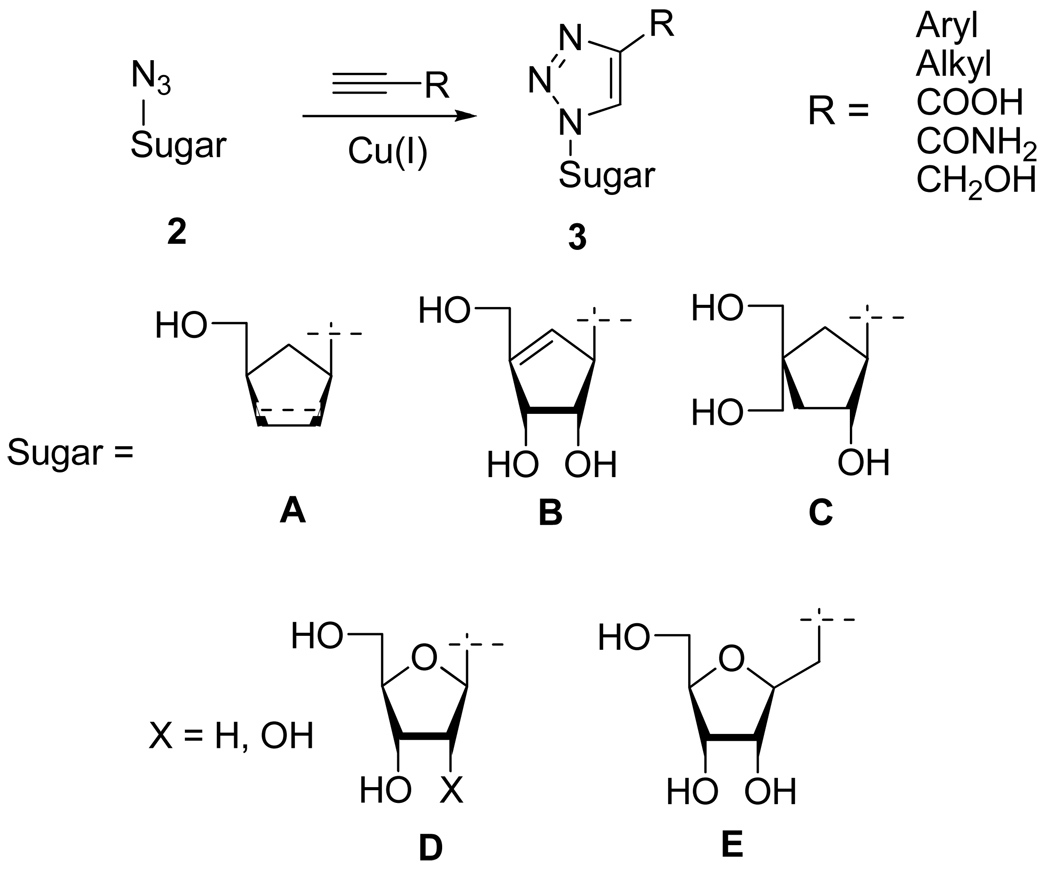
Based on these compounds, the most common application of the Huisgen 1,3-cycloaddition has been the reaction of β-1’-azido sugar moieties 2 with various alkynes in order to form modified nucleosides 3 bearing a substituted 1,2,3-triazole base (Scheme 4). Thus, although some cases required a stoichiometric amount of Cu(I), the cycloaddition has been efficiently applied to different alkyne and sugar azide substrates leading to a variety of new nucleosides analogs.12 Among all the compounds synthesized to date in this series by various laboratories, compound 3B (R = CONH2) exhibits a potent antiviral activity against vaccinia virus with high selectivity index [EC50 = 0.4 µM, selectivity index (SI) > 750].
Scheme 4.
Synthesis of 1,2,3-Triazolo Nucleosides Analogs 3.
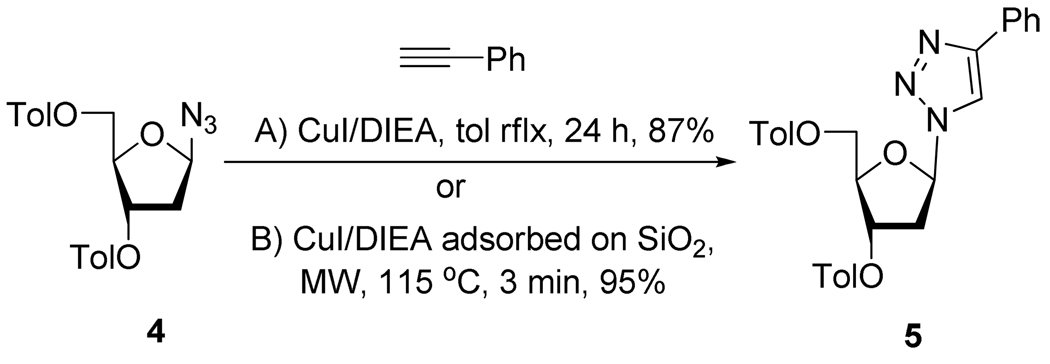
The use of microwave irradiation has been investigated to enhance reaction yields and to accelerate cycloaddition rates. For example, Guezguez et al.13 showed that, starting from compound 4, a stoichiometric amount of CuI and not less that 24h was required to achieve complete reaction (Scheme 5). However, when the reaction was performed under microwave irradiation and in the presence of DIEA and CuI adsorbed on silica gel (1 g/mmol of azide), the dipolar cycloaddition proceeds cleanly in near quantitative yield in 1.5 to 3 min.
Scheme 5.
Synthesis of 1,2,3-Triazolo Nucleosides Analogs 5.
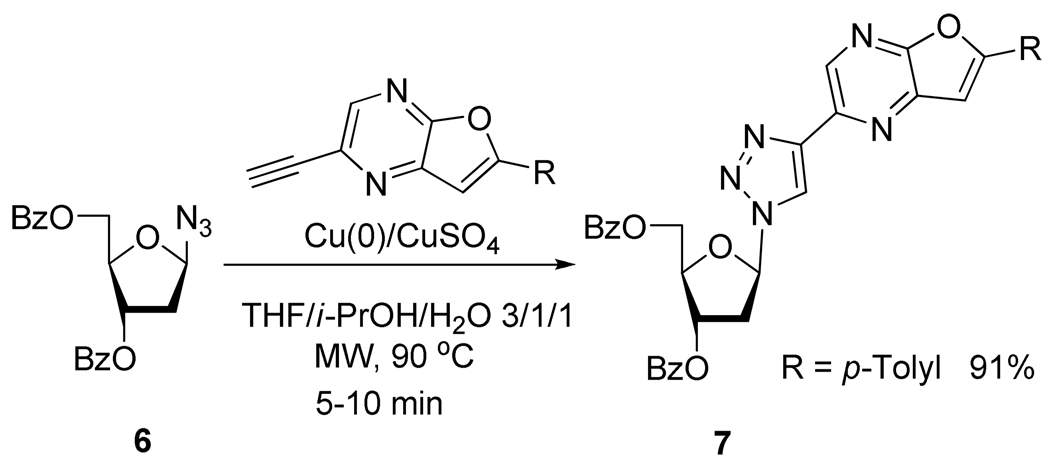
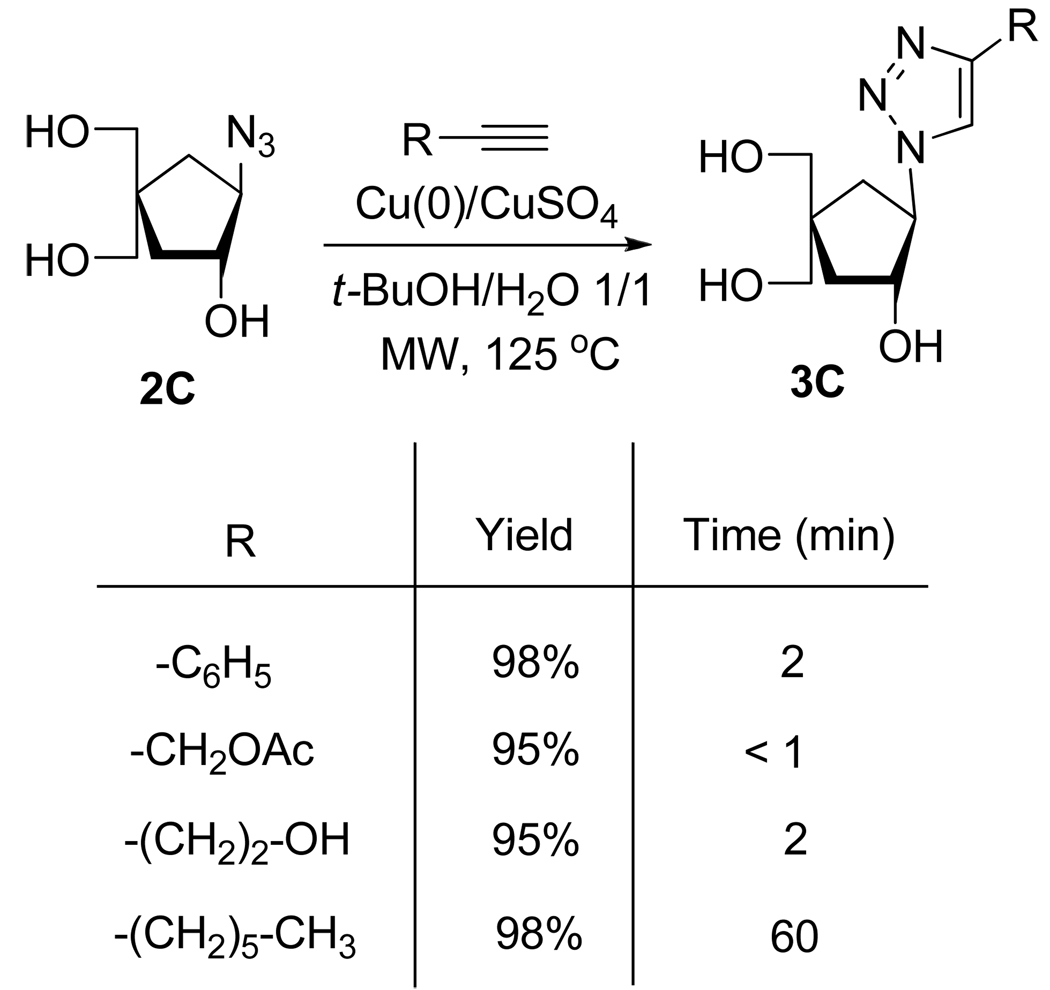
In the same manner, Ermolat’ev et al.14 (Scheme 6) and Broggi et al.15 (Scheme 7) showed that the Huisgen 1,3-cycloaddition can be completed in high yield in only few minutes using microwave irradiation.
Scheme 6.
Synthesis of 1,2,3-Triazolo Nucleosides Analogs 7 Using Microwave Irradiation.
Scheme 7.
Synthesis of 1,2,3-Triazolo Nucleosides Analogs 3C Using Microwave Irradiation.
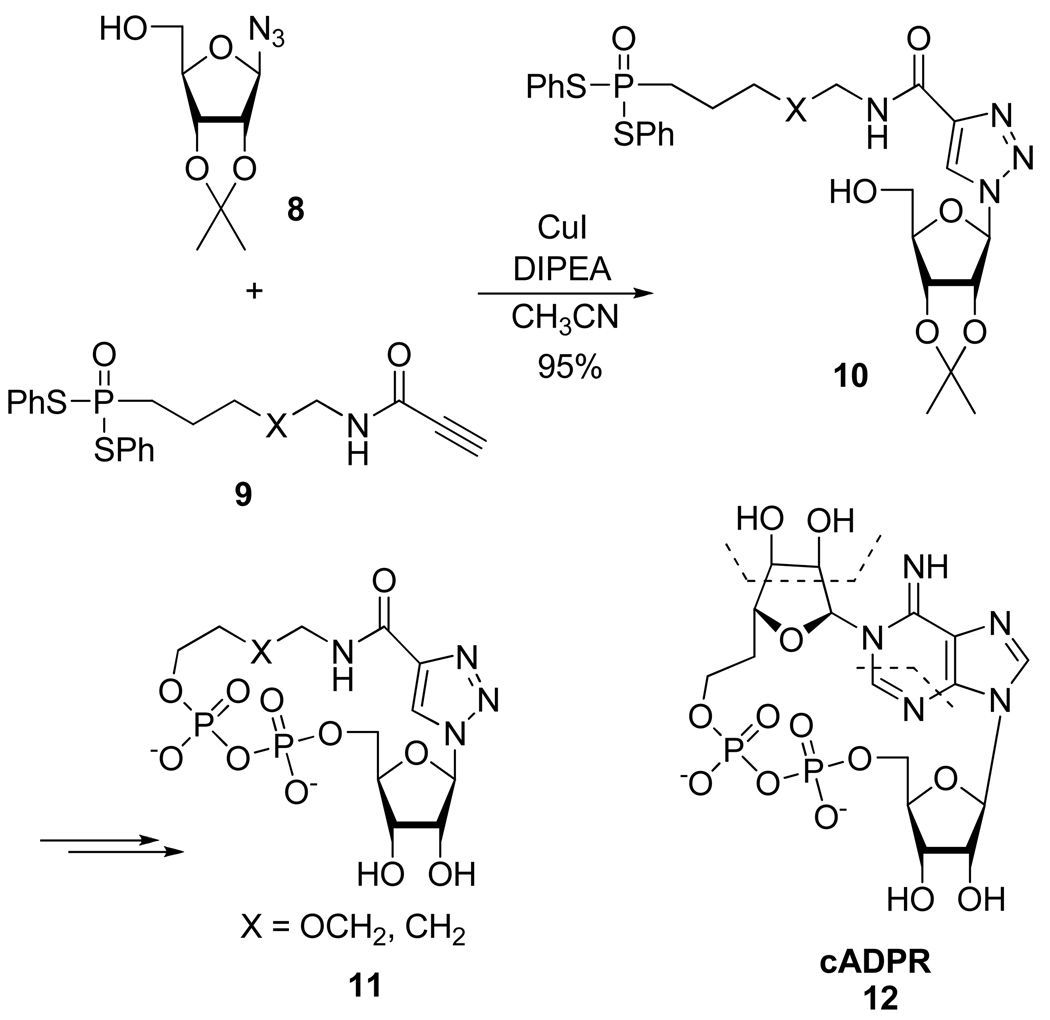
In the search for novel cyclic adenosine diphosphate-ribose (cADPR 12) mimics, Li et al.16 synthesized compounds 11 in 4 steps, through the construction of a 4-amido-1,2,3-triazole nucleobase (Scheme 8). It is noteworthy that like endogenous cADPR, the targeted cyclo-pyrophosphates 11 appeared to induce Ca2+ release in intact human Jurkat T cells.
Scheme 8.
Synthesis of Nucleobase-Simplified cADPR Mimics 11.
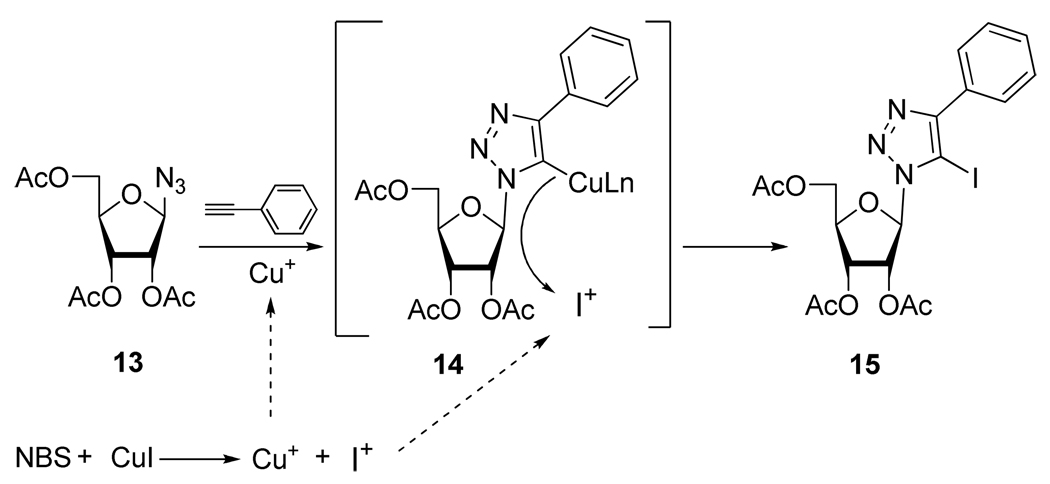
Interestingly, as part of the same project, Li and coworkers17 developed an efficient method for the preparation of 5-iodo-1,4-disubsituted-1,2,3-triazole by a multicomponent one pot reaction of azide 13 and phenyl acetylene in presence of CuI and NBS (Scheme 9), opening new perspectives for further interesting modifications. According to the authors, the catalytic system used for the reaction provides both I+ and Cu+ in situ which allows the one pot trapping of the carbon anion intermediate 14 generated during the cycloaddition process.
Scheme 9.
Plausible Mechanism of Preparation of Compound 15.
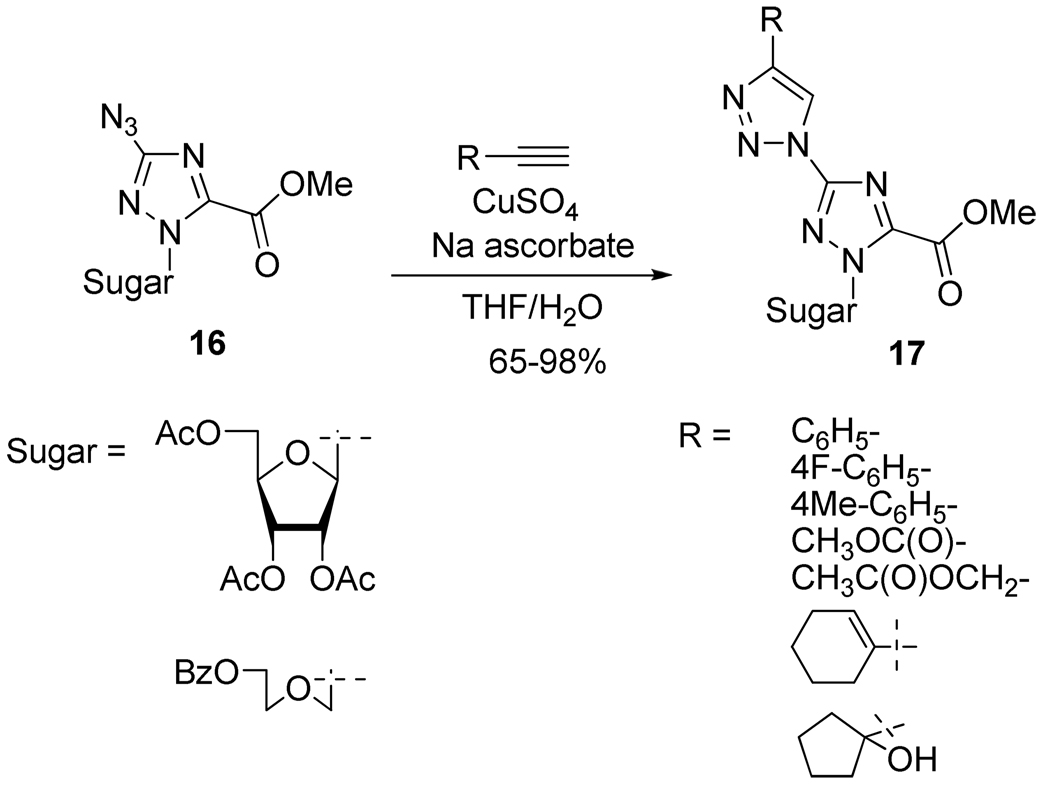
Novel bis-triazolyl nucleosides were synthesized as anti-tobacco mosaic virus (TMV) agents by Xia et al.18 using the azide/alkyne Huisgen reaction. Thus, compound 17 and analogs were obtained in good to excellent yields from 16 in the presence of CuSO4 and sodium ascorbate in a mixture of water and THF, regardless of the nature of the alkyne (Scheme 10).
Scheme 10.
Synthesis of Bis-triazolyl Nucleosides 17.
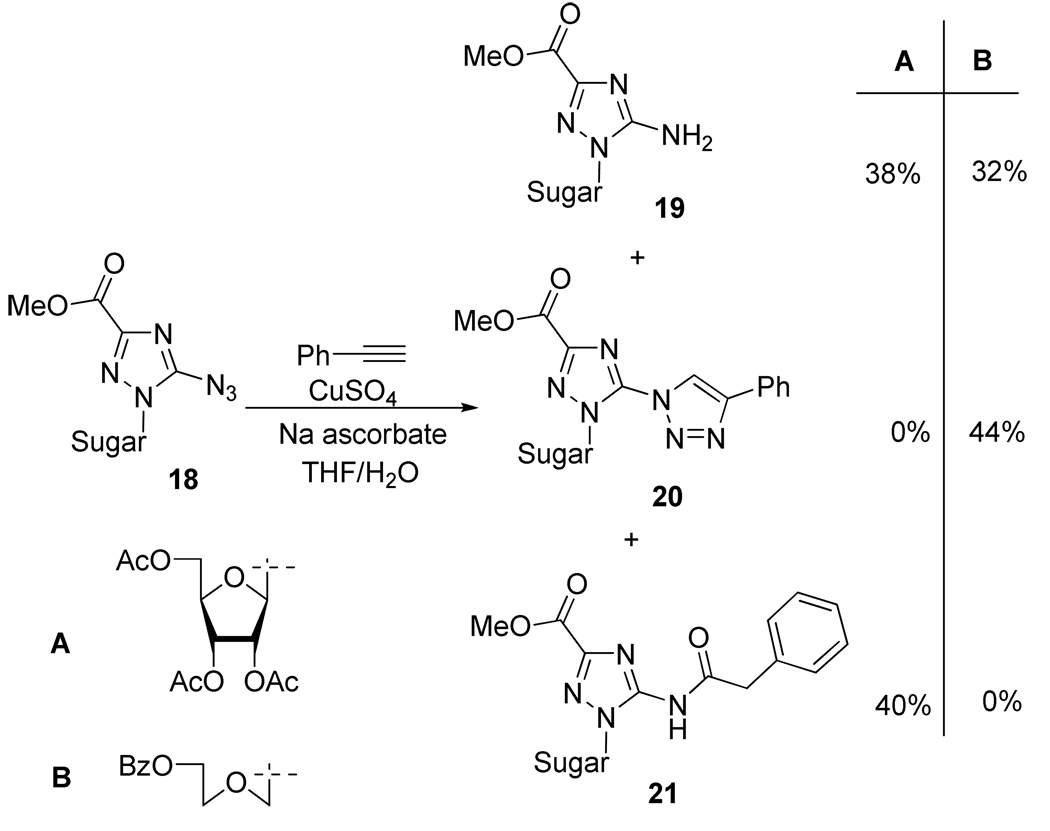
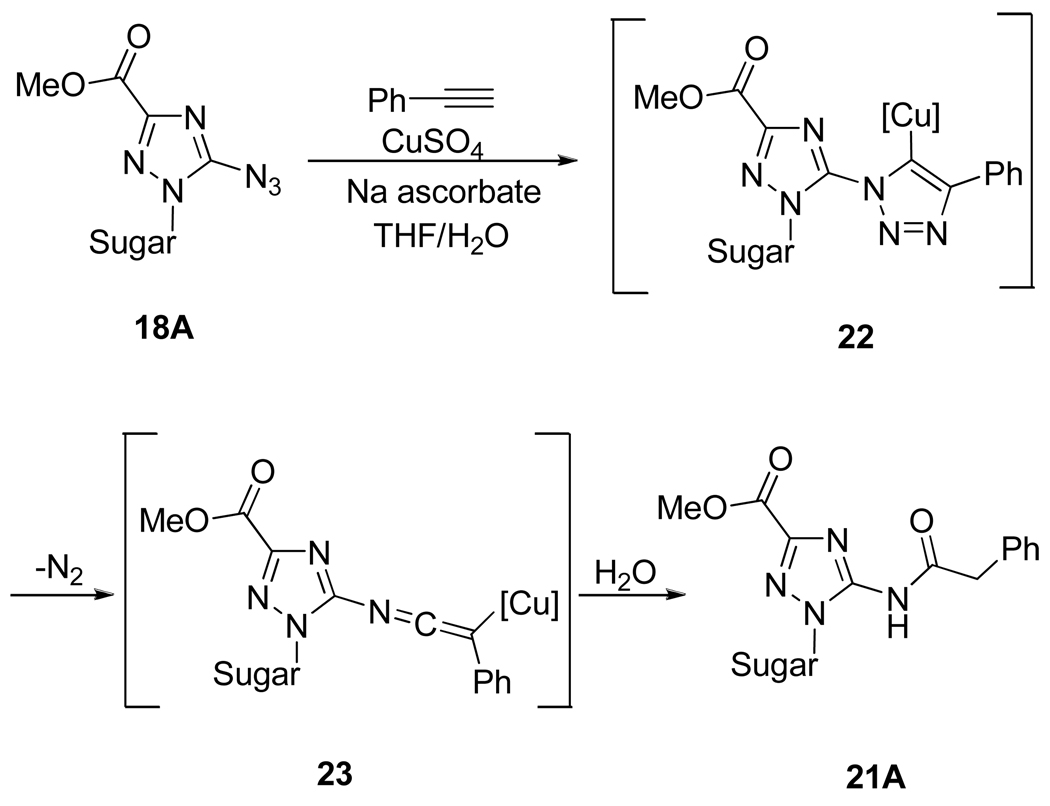
However, in the case of 5-azidotriazoles 18, Cu(I)-promoted 1,2,3-triazole formation was not straightforward (Scheme 11). For instance, when compound 18A was reacted with phenylacetylene, two completely unexpected products were formed, namely, amine 19A and amide derivative 21A. The formation of the anticipated triazole 20A was never observed in presence or absence of copper catalyst under conventional heating or under microwave irradiation. In the case of the acyclo azido derivative 18B, the triazolo compound was formed in moderate yield, but again the reduced product 19B was observed. To explain this unusual reactivity, the authors presumed that the electron-deficient heterocycle that bears the azido group and the steric hindrance induced by the presence of the sugar moiety make 5-azidotriazole compounds 18 rather unsuitable partners for the 1,3-dipolar cycloaddition reaction. Thus, under mild Cu(II)-ascorbate conditions, compound 18A can be reduced to 19B and based on the work of Chang et al.19 on the Cu-catalyzed multiple component reactions, the formation of amide 21A could be explained by the mechanism outlined in Scheme 12.
Scheme 11.
Reactivity of Azido Compound 18 Under CuAAC Conditions.
Scheme 12.
Plausible Mechanism for the Formation of Amide 21A.
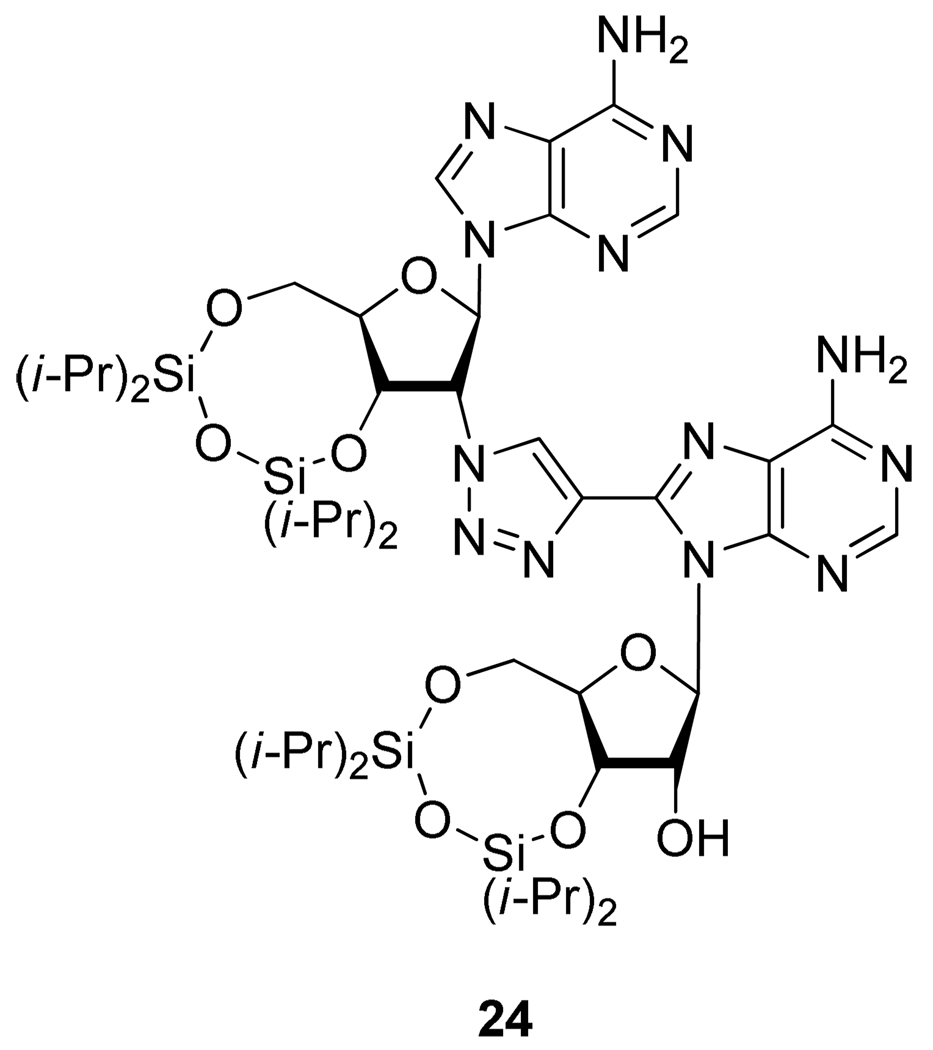
However, despite the previous result, O’Mahony et al.20 demonstrated that this type of reaction was possible with electron deficient rings such as purines by preparing adenosine dimers 24 linked by a 1,2,3-triazole ring (Figure 4).
Figure 4.
Adenosine Dimer 24.
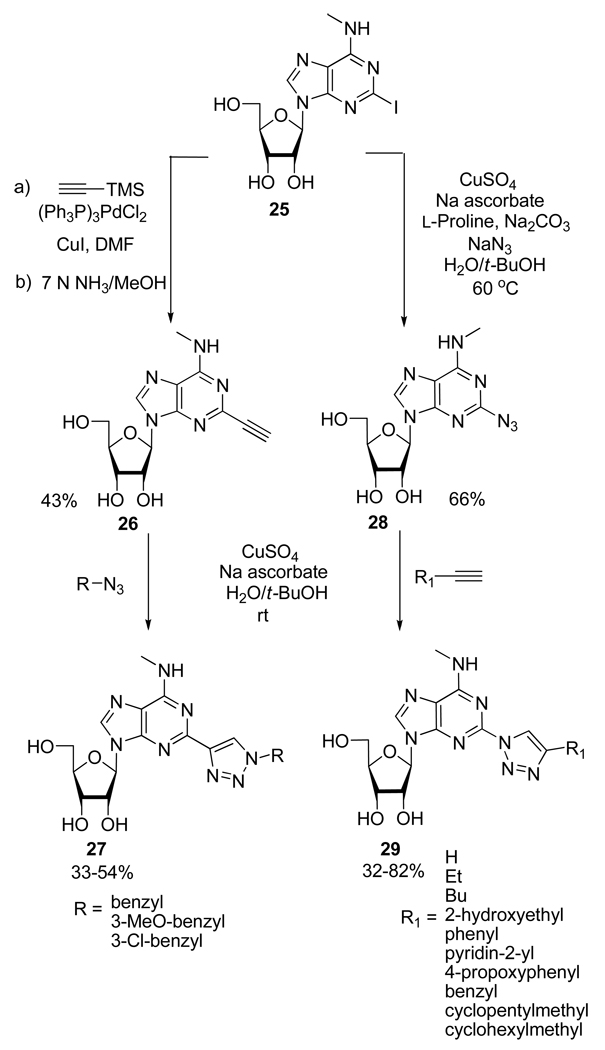
Using the same kind of substrates, Cosyn et al.21 synthesized two series of 2-(1,2,3-triazolyl)adenosine derivatives 27 and 29 using the CuAAC starting from the common intermediate 25 (Scheme 13). Thus, compounds 29 can be prepared in 2 steps first, by introduction of an azido group at the 2-position using a Cu(I)-catalyzed nucleophilic substitution with NaN3 followed by Cu(I)-catalyzed 1,3-dipolar cycloaddition involving various alkynes. Similarly, the 1,2,3-triazol-4-yl analogs 27 were prepared from alkyne derivative 26 by reaction with appropriate azide in presence of CuSO4 and sodium ascorbate at room temperature in a mixture of water and t-BuOH. Among all the compounds prepared, several 2-(1,2,3-triazol-1-yl)-N6-methyl-subsituted adenosine derivatives displayed A3 adenosine receptor affinities in the low nanomolar range with very high A3/A2A and moderate to high A3/A1 selectivity.
Scheme 13.
Synthesis of 2-(1,2,3-Triazolyl)Adenosine Derivatives 27 and 29.
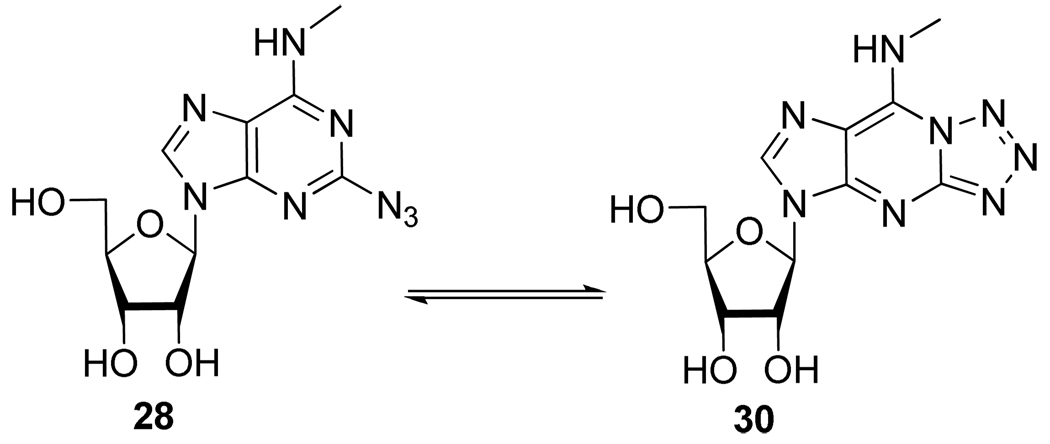
From these results, several observations have been made by the authors. First of all, despite very similar conditions for the Cu(I)-catalyzed nucleophilic substitution of 25 with NaN3 and the conditions necessary for the CuAAC reaction, the one-pot two-step conversion of 25 to 29 was observed in disappointingly low yield. Secondly, during the preparation of 28, the authors observed the formation of the tautomeric fused tetrazole form 30 (17%). Indeed, azide substituted π-deficient nitrogen heterocycles are known to spontaneously cyclizes to the corresponding fused tetrazole (Scheme 14).22 In this case, despite that possible equilibrium, the cycloaddition proceeded smoothly however, some of the lower observed yields for the formation of compounds 29 could possibly be due to a shift of this equilibrium toward compound 30.
Scheme 14.
Azido/Tetrazole Tautomerism of 2-Substituted Adenosine Derivative 28.
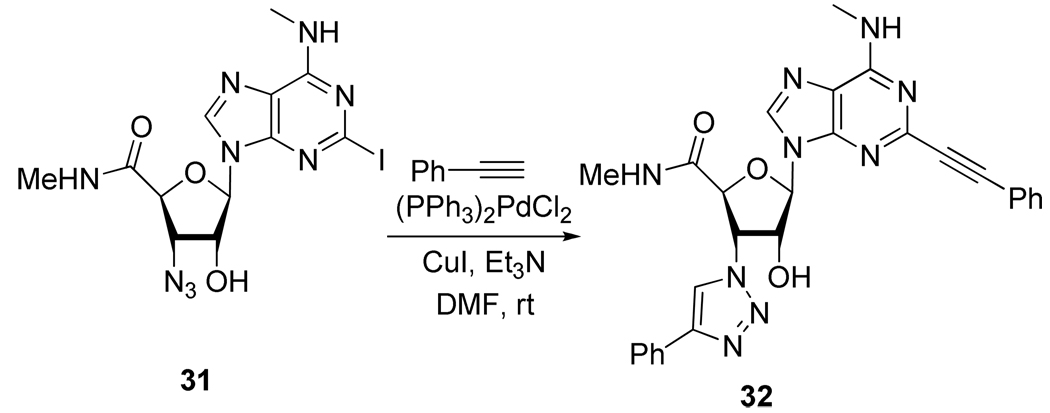
Finally, the same team showed that the copper source used during the Sonogashira coupling could also induce the Huisgen cycloaddition in the presence of an azide group on the molecule (Scheme 15).23
Scheme 15.
Double Effect of the Sonogashira Conditions on Compound 31.
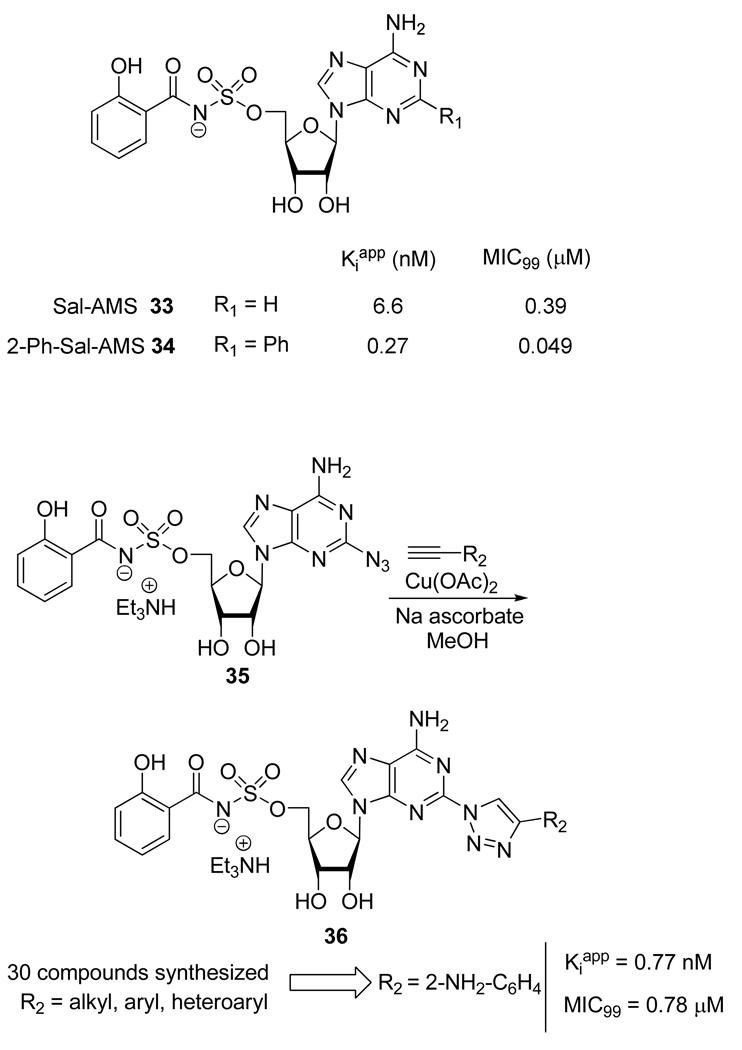
As part of their efforts to find new drugs against tuberculosis (TB), Gupte et al.24 extensively studied the importance of substitutions on their lead compounds, 5’-O-[N-(salicyl)sulfamoyl]adenosine (Sal-AMS, 33) and its analog 2-Ph-Sal-AMS 34 (Scheme 16). Interestingly, modification of the C-2 position of the purine moiety with 4-substituted 1,2,3-triazoles appeared to be well tolerated and a majority of the compounds 36 possessed subnanomolar apparent inhibition constant (Kiapp) against aryl acid adenylating enzymes (AAAE) and submicromolar to micromolar antitubercular activities under iron deficient conditions (minimal inhibitory concentration, MIC99).
Scheme 16.
Synthesis of 2-Triazole Substituted 36, Analogs of Sal-AMS 33.
2.2. Sugar Modified Nucleosides
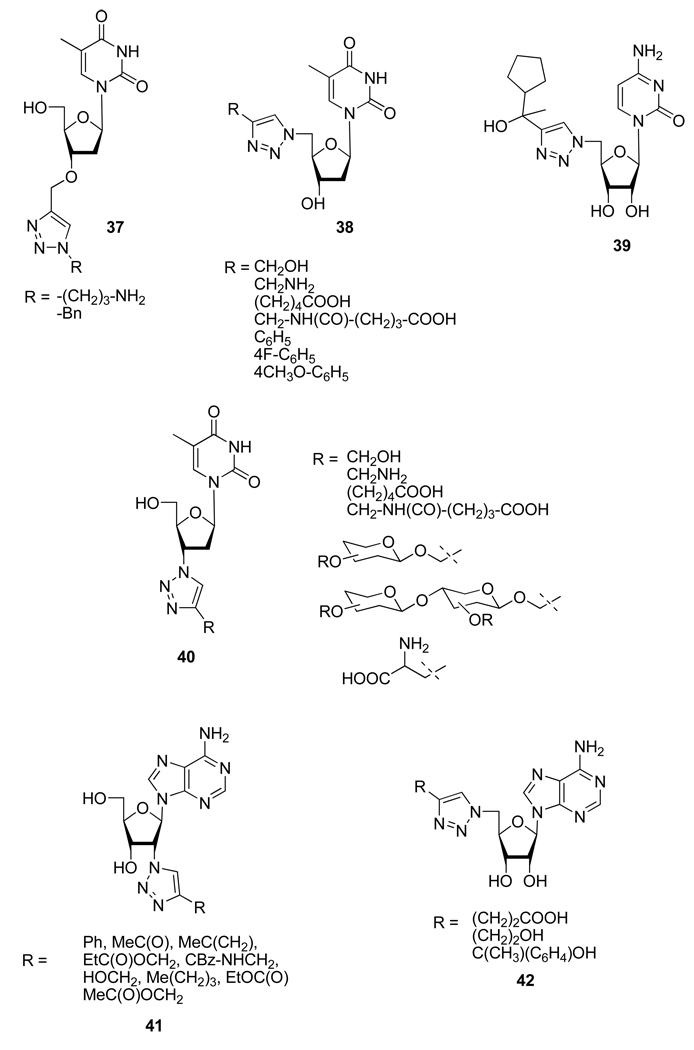
In order to discover new derivatives potentially endowed with biological activity, the copper catalyzed azide/alkyne 1,3-dipolar cycloaddition has also been applied to the functionalization of nucleoside’s sugar moieties. With this in mind, efficient regioselective synthesis of various pyrimidines25 and adenosine26 analogs were achieved by different teams (Figure 5). This strategy allowed Lee et al.22d to identified compound 39 as a new a-2,3-sialyltransferase inhibitor.
Figure 5.
Structures of Compounds 37–42.
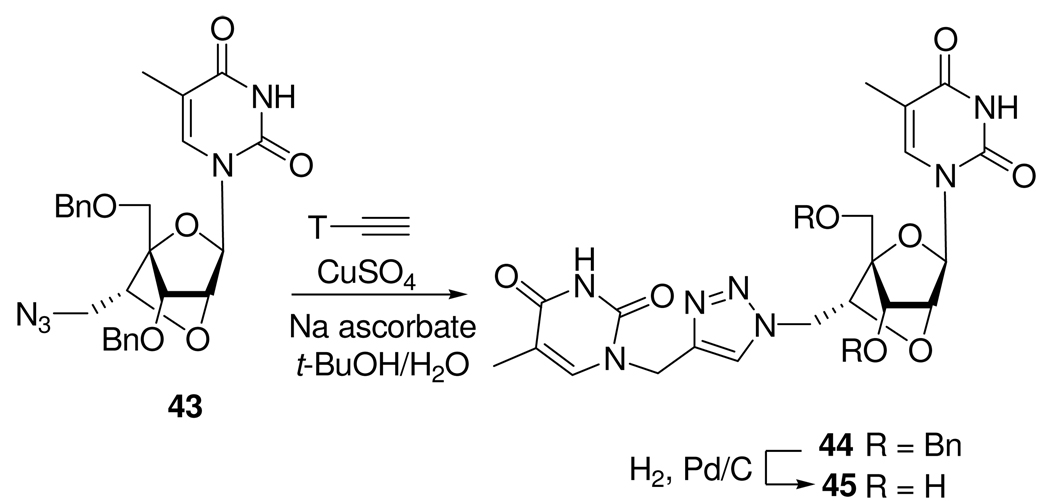
Over the past few years, locked nucleic acid (LNA) has received significant attention as nucleic acid analogs, displaying unprecended recognition of complementary nucleic acids.27 LNA have promising antisense properties and were recognized for their potential in nanoscale engineering and microarray construction. Enderlin et al.28 used the CuAAC for the synthesis of a double-headed nucleoside with a triazole linked to an additional thymine to the 6’-position of a locked nucleic acid -nucleoside monomer (Scheme 17).
Scheme 17.
Synthesis of 6’-Branched Locked Nucleic Acid 45.
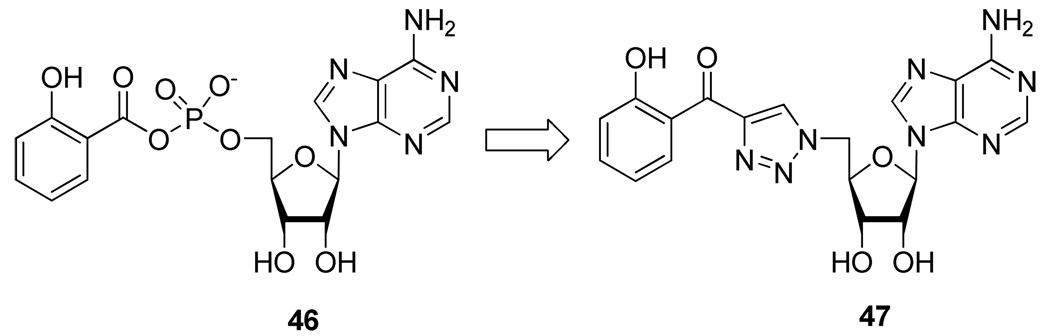
As part of their anti-tuberculosis research program and based on the lead compound 46, Somu et al.29 also studied the potential replacement of the labile acyl phosphate function in compound 47 by a disubstituted triazole (Figure 6).
Figure 6.
Rational for the Design of Compound 47.
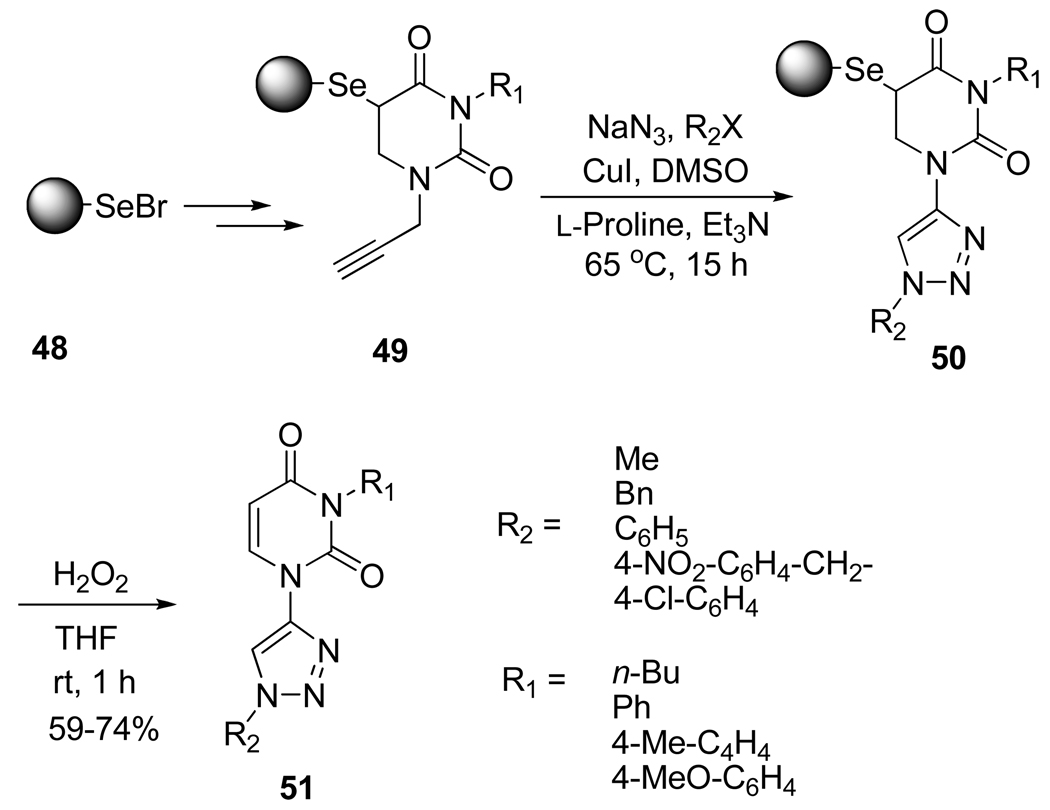
Recently, nucleoside analogues in which the furanose ring has been replaced by heterocyclic moieties have attracted special attention since some of them were reported to show antiviral and anticancer activities. Thus, Cao et al.30 successfully developed an efficient solid phase parallel synthetic route to a bis–heterocycle library of uracil analogs, tethered to triazoles, using a polymer-supported seleno resin and the CuAAC as the key reaction (Scheme 18).
Scheme 18.
Solid-Phase Synthesis of Heterocyclic Nucleosides Analogs 51.
2.3. Nucleosides Bioconjugates
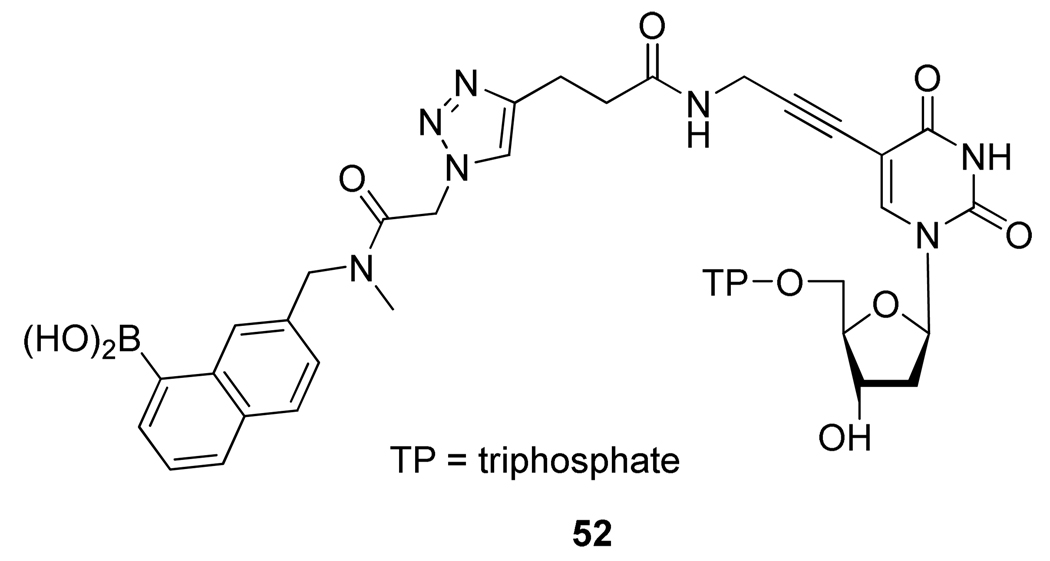
The Cu-catalyzed azide alkyne 1,3-dipolar cycloaddition has also been applied to the synthesis of new nucleoside bioconjugates. Indeed, its efficiency and simplicity rendered this reaction attractive for the covalent linkage of two molecular entities to provide biomolecules with novel properties such as biological activity, altered hydrophobicity, increased bioaffinity, or the ability to carry metal ions. For instance, a boronic acid-labeled thymidine-5’-triphosphate linked through a 14-atom tether using the CuAAC as the key reaction (Figure 7) was synthesized by Lin et al.31 Compound 52 was recognized by a DNA polymerase and has been incorporated into a growing primer strand.
Figure 7.
Chemical Structure of Boronic Acid-Labeled Thymidine-5’-Triphosphate 52.
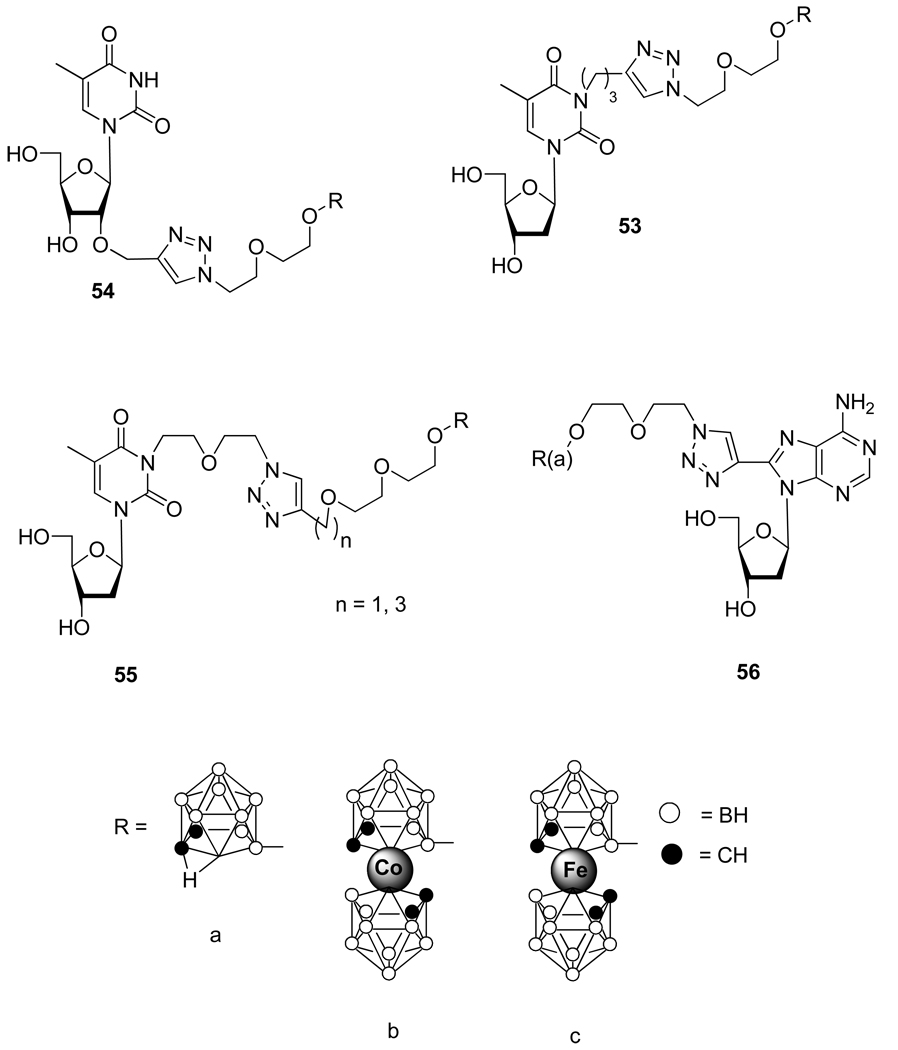
Working also on boron-bearing nucleic acids, Wojtczak et al.32 developed a methodology involving the CuAAC for the synthesis of pyrimidine as well as purines nucleosides conjugates containing carborane and metallocarborane complexes (Figure 8). The behavior of compounds 53–56 designed mainly as potential boron carrier for boron neutron capture therapy (BNCT) of tumors is still under evaluation.
Figure 8.
Structure of Nucleosides Conjugates 53–56.
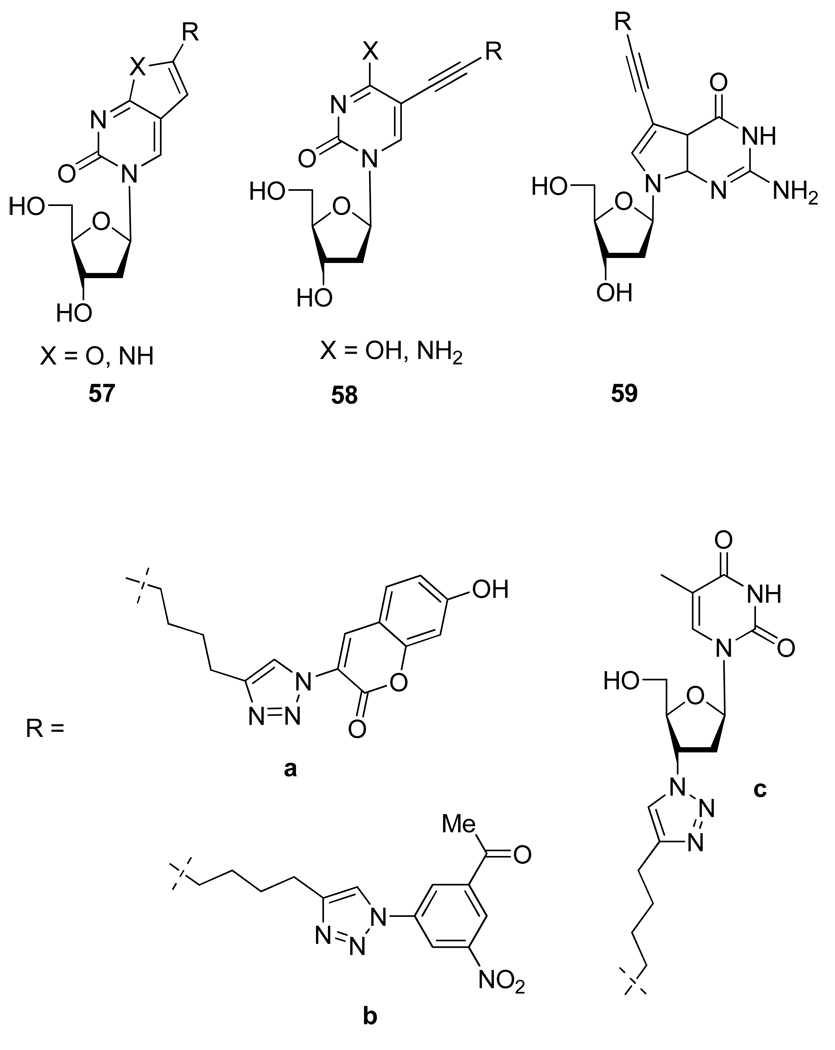
As a part of their study of modified DNA, Seela and coworkers got interested in the use of the CuAAC as an efficient way to label DNA. In order to evaluate the potential of their strategy they first worked at the nucleoside level and have been able to introduce coumarin dyes33 and other azido derivative such as AZT34 on different part of the nucleobase generating notably new fluorescent nucleoside bioconjugates 57a–59a (Figure 9).
Figure 9.
Structure of Nucleosides Conjugates 57–59.
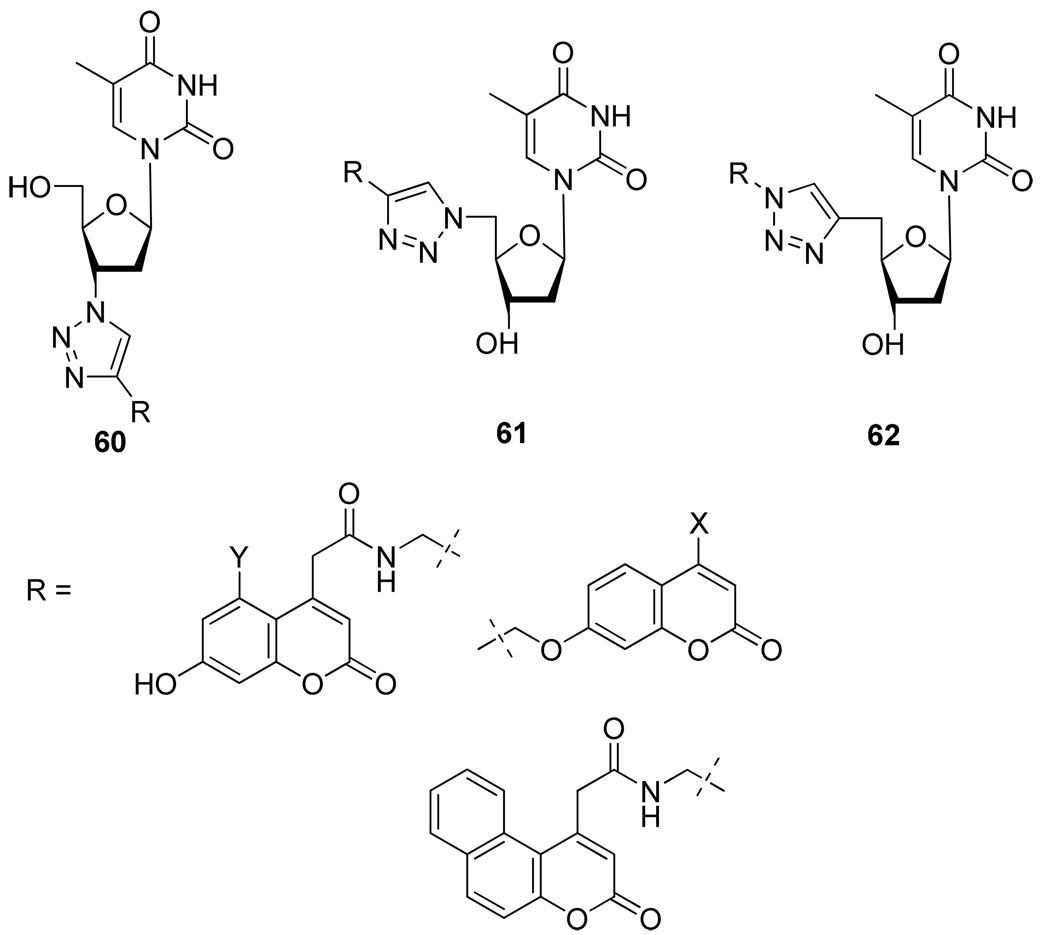
In the same manner, Kosiova et al35. reported the preparation of fluorescent triazole linked coumarin nucleoside conjugates 60–62, the linkage being this time on the sugar moiety (Figure 10).
Figure 10.
Structure of Nucleosides Conjugates 60–62.
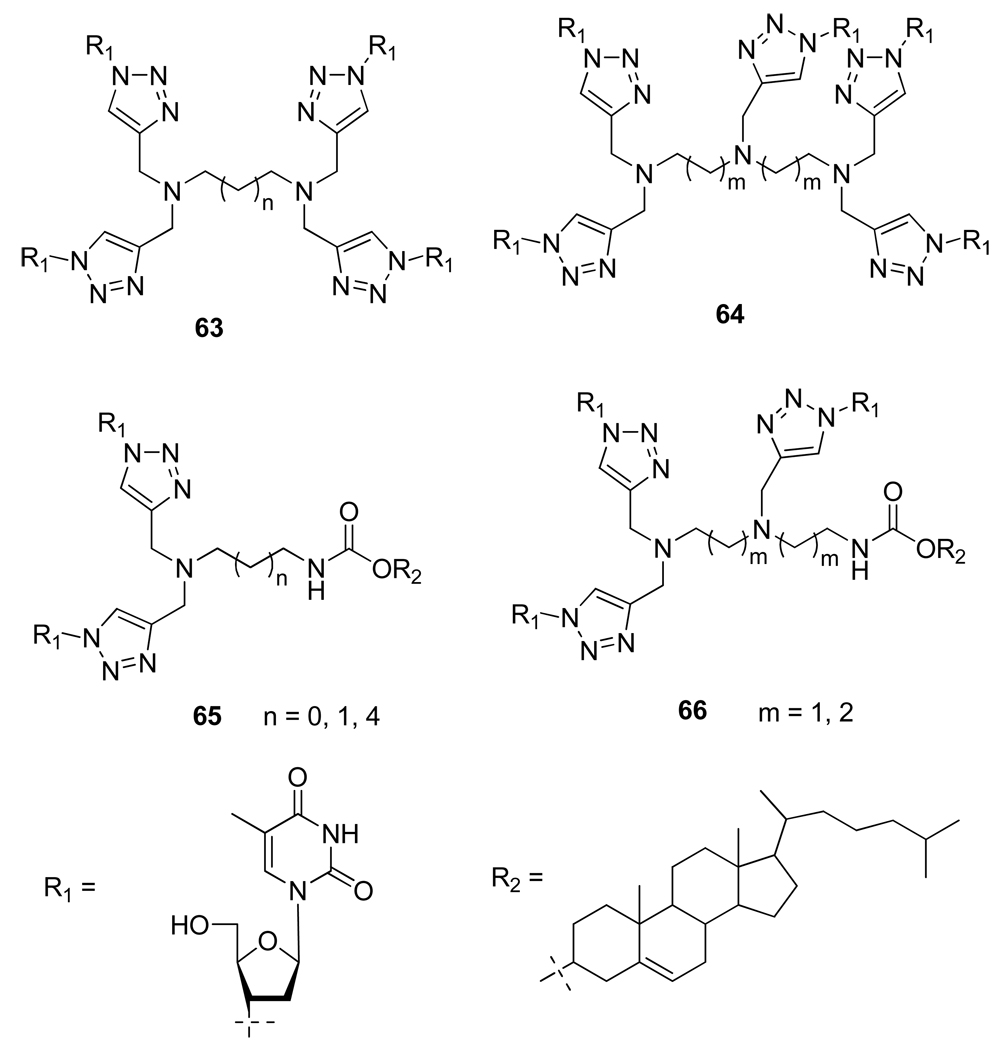
Taking advantage of the versatility of the Cu(I) catalyzed 1,3-cycloaddition, Jin et al.36 prepared a library of novel 1,2,3-triazole-fused oligonucleosides analogs (Figure 11) and interestingly, compound 64 derived from AZT showed a fairly good antibiotic activity against E. coli DH5 α.
Figure 11.
Structure of 1,2,3-Triazole-Fused Oligonucleosides Analogs 63–66.
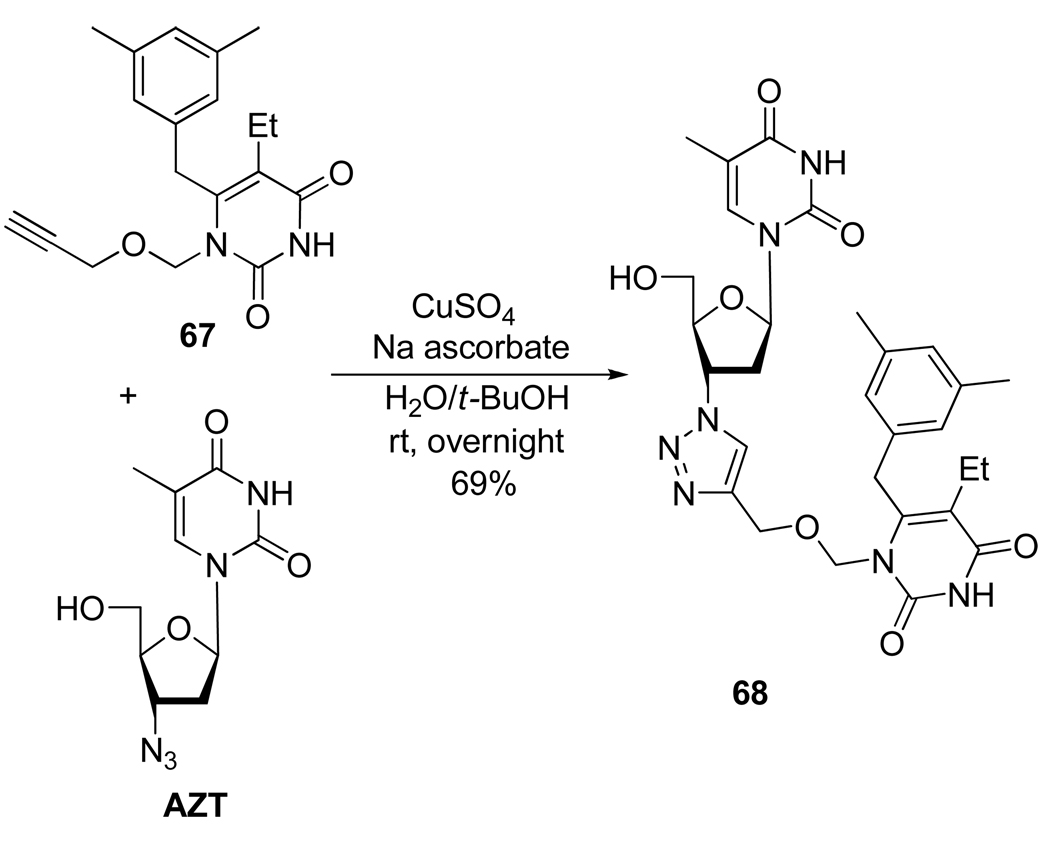
Compound 6837 represents an attempt to synthesize a dual drug by click chemistry combining AZT and HIV-active compound 67 (Scheme 19). Interestingly, this “chimera” showed antiviral activity against wild type HIV-1 and mutant strains comparable to those observed for 67.
Scheme 19.
Synthesis of Compound 68.
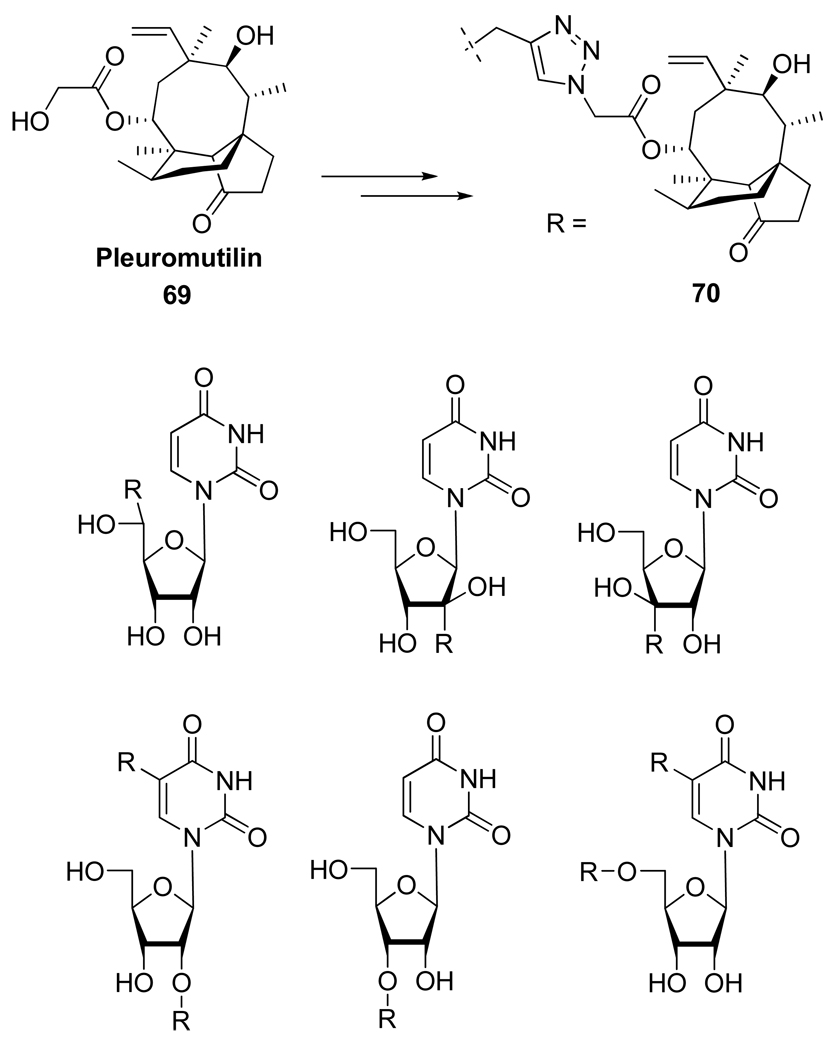
Pleuromutilin 69 is a naturally occurring antibacterial agent (Figure 12) known to bind to bacterial ribosome in the peptidyl transferase center. Due to the presence of a permissible area near this center, numerous modifications of 69 have been investigated including the attachment of nucleoside derivatives in order to induce better binding through their inherent H-bonding properties and stacking abilities. Thus, the CuAAC has been used in a parallel synthetic strategy by Lolk et al.38 to attach a wide range of nucleoside derivatives to Pleuromutilin 69 and it is noteworthy that the bioconjugates 70 kept their antibacterial activity and in some cases showed better affinity to the peptidyl transferase center in the ribosome than the natural Pleuromutilin.
Figure 12.
Structure of Pleuromutilin 69 and Pleuromutilin Bioconjugates 70.
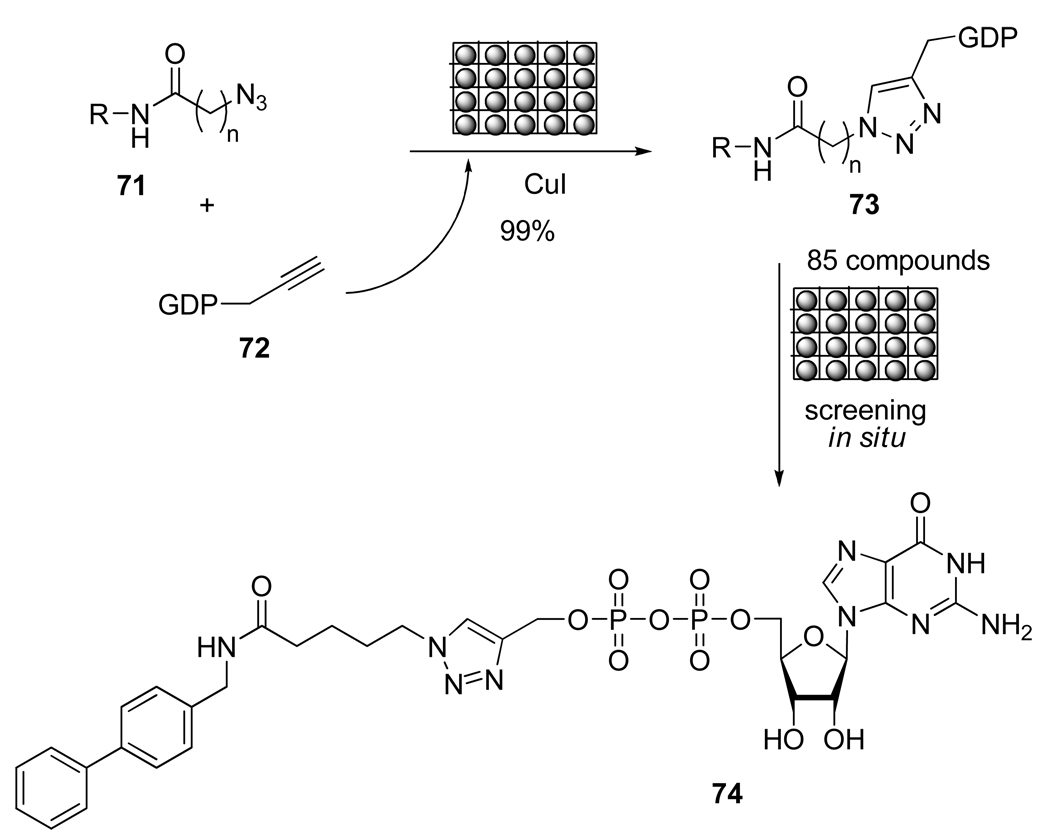
Lee et al.39 investigated the synthesis of potential inhibitors of fucosyl–transferases (Fuc-T). Fuc-T catalyzes the transfer of an L-fucose sugar from a guanosine diphosphate fucose to an acceptor substrate and is involved in several biological processes. Thus, the inhibition of this enzyme may provide a useful therapy for the control of inflammation or for combating tumor growth. Using the CuAAC as the key step of their strategy, the authors prepared a library of guanosine-5’-diphosphate (GDP) triazole. The direct synthesis in a microtiter plate and the absence of protective groups (even for the dianionic phosphate linkage) allowed in situ bio-evaluation (Scheme 20). Among the 85 compounds synthesized, 74 was a nanomolar inhibitor of human α-1,3-fucosyl–transferase.
Scheme 20.
Triazole Synthesis in Microliter Plate for Screening in situ.
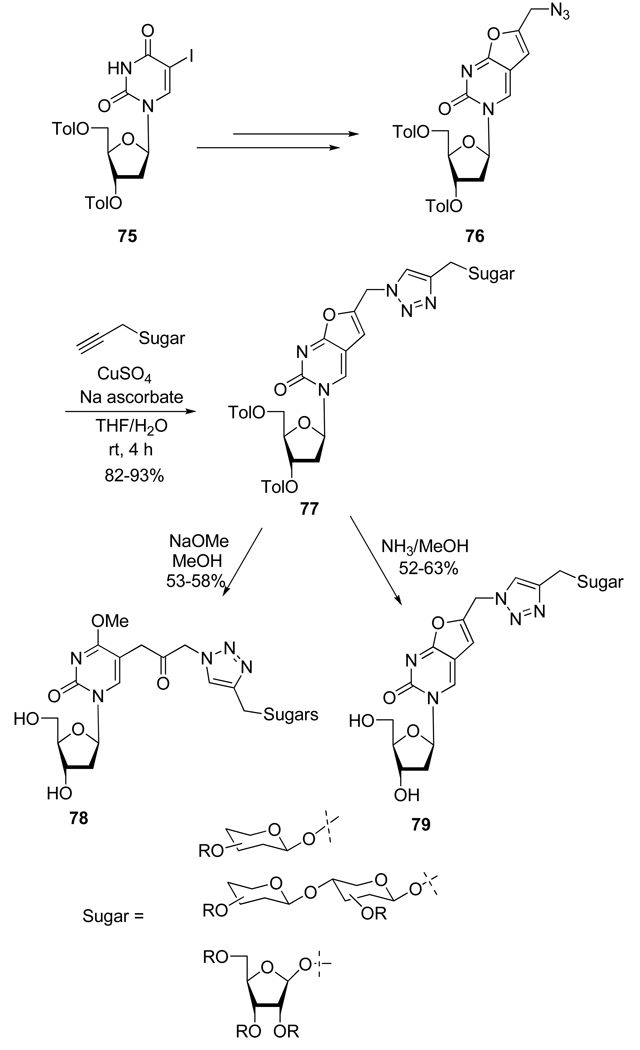
As part of their project to develop anti-varicela-zoster virus (VZV) drugs, Jin et al.40 prepared a set of a new type of carbohydrate conjugated thymidine analogs in order to potentially improve solubility and molecular recognition of active bicyclic furo[2,3-d]pyrimidine nucleosides (Scheme 21). Compound 76, prepared from 75 in 5 steps, was reacted with various propargylic carbohydrates derivatives in presence of CuSO4 and sodium ascorbate to afford the corresponding 1,4-disubstituted 1,2,3-triazoles 77. The subsequent deprotection of 77 with catalytic sodium methoxide in methanol gave the opened ketone-type structure 78 whereas the use of methanolic ammonia only produced the expected compounds 79. The biological study of compounds 78 and 79 is actually underway.
Scheme 21.
Synthesis of Compounds 78 and 79.
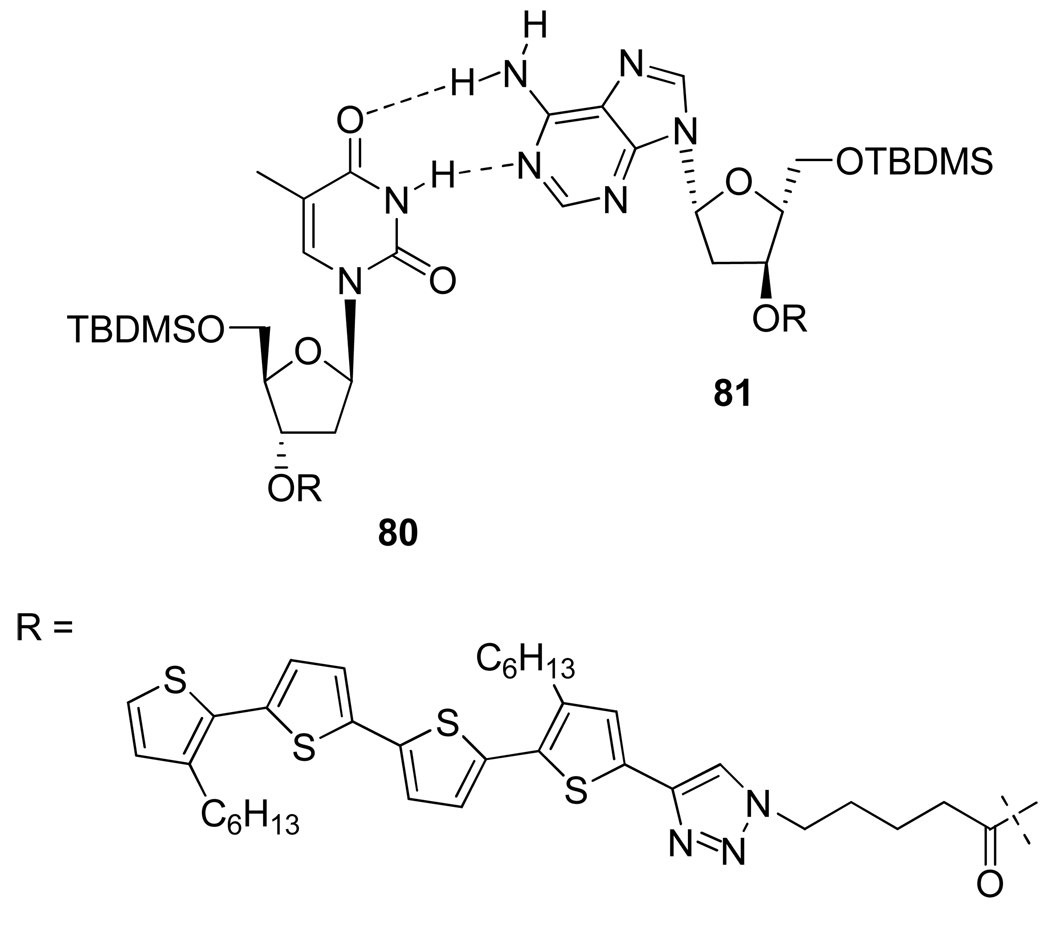
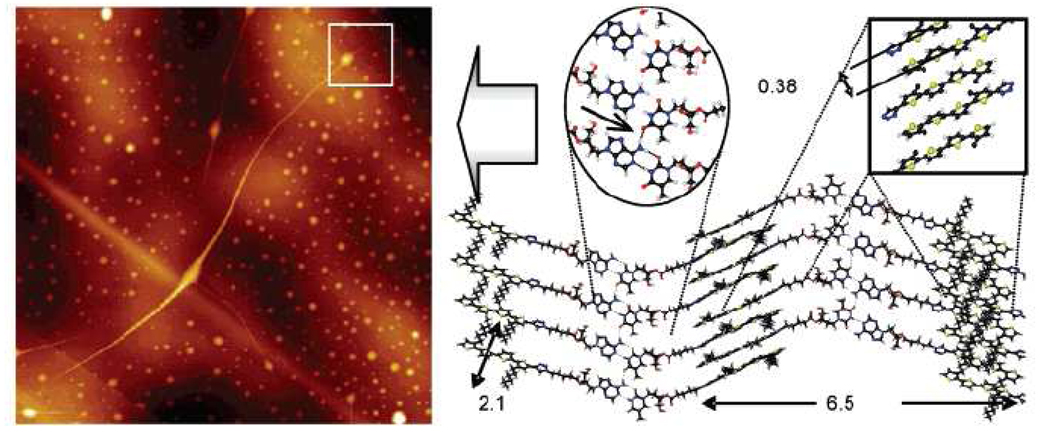
Another application of the Cu(I)-promoted 1,2,3-triazole formation was the synthesis of oligothiophene-nucleoside conjugates 80 and 81 by Jatsh et al.41 (Figure 13). The authors showed that complementary thymidine- and adenosine-functionalized quaterthiophenes form recognition-driven superstructures via hydrogen bonding and other competing intermolecular forces, allowing them to characterize self aggregated fibers up to 30 µm in length (Figure 14).
Figure 13.
Structure of Adenosine-Quaterthiophene 81 and Corresponding Thymidine 80.
Figure 14.
AFM tapping-mode image of a 1:1 mixture of adenosine-quaterthiophene 81 and corresponding thymidine 80 deposited from toluene on HOPG after annealing: topography representation (17 × 17 µm2) (left) and detailed amplitude image (2.5 × 2.5 mm2) (right). In the middle a calculated model for the fiber growth (gray arrow) is shown, including a detail of the molecular interactions involved (oval and square insets) and the molecular dimensions. Reprinted with permission from ref 35. Copyright 2008 American Chemical Society.
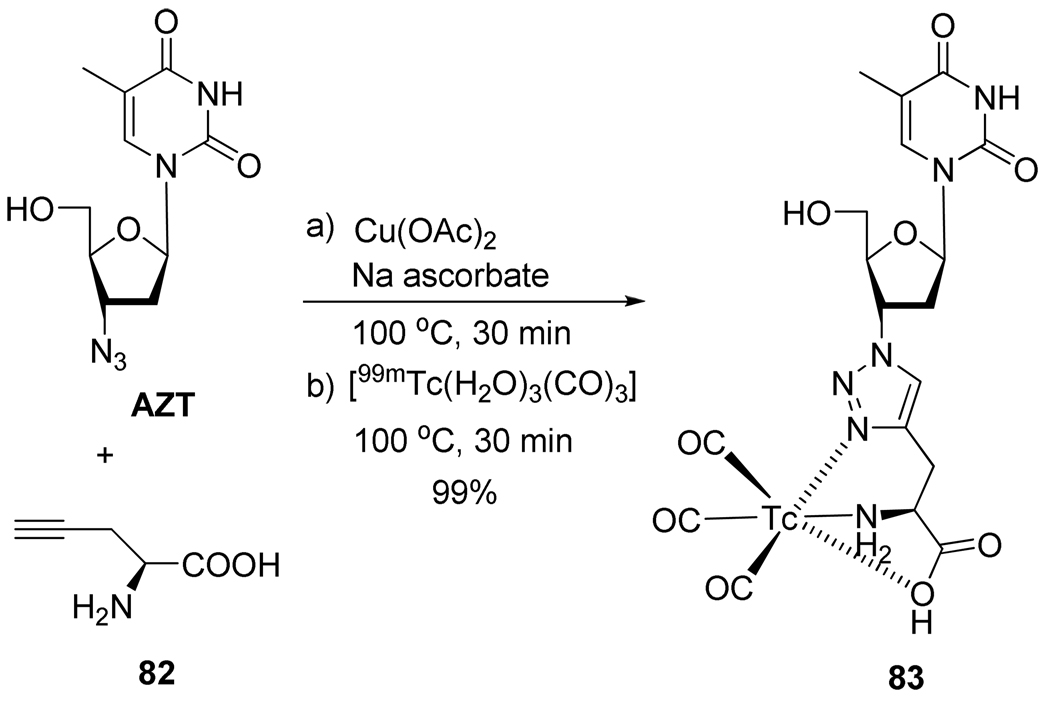
Mindt et al.42 developed the “click to chelate” approach which allowed them to synthesize the metal labeled-nucleoside conjugate 83 in a one pot procedure (Figure 22). They showed that the 1,4-disubstituted triazole forms an integral part of the metal chelating system and facilitates the incorporation of labeled complexes into biomolecules.
3. Oligonucleotides
3.1- 1,2,3-Triazole as Replacement of the Phosphodiester Linkage
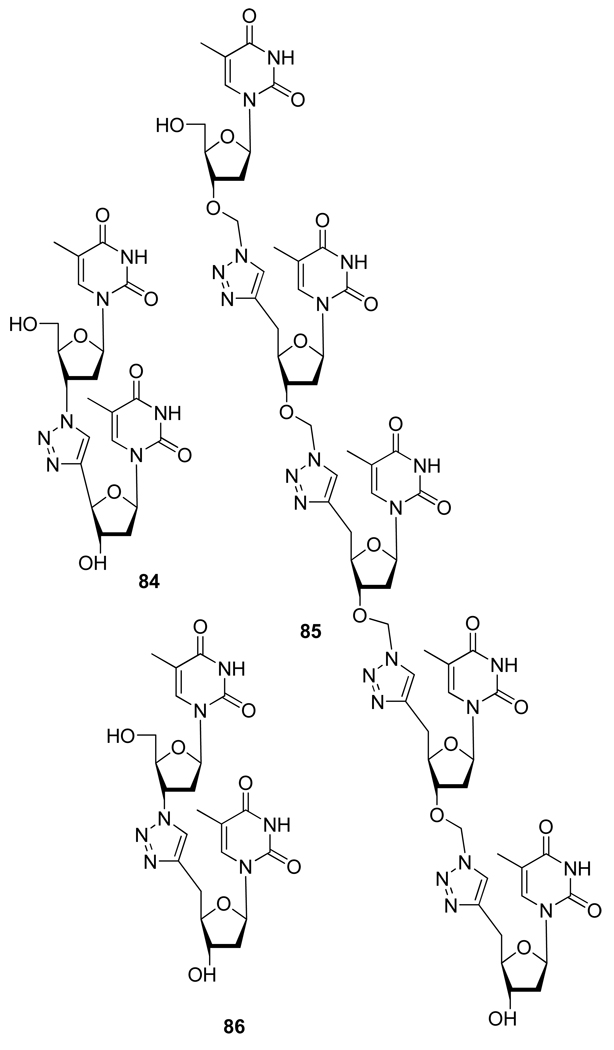
Oligonucleotide chemistry has also benefited from the development of the CuAAC. Thus, given the importance of non-natural oligodeoxyribonucleotide antisense agents acting as post-transcriptional gene silencing agents, several approaches based on repetitions of the Cu(I) catalyzed 1,3-dipolar cycloaddition as a key ligation process have been successfully developed (Figure 15) to replace the phosphodiester linkage in oligonucleotides by a 1,2,3-triazole.43
Figure 15.
Structures of oligonucleotides analogs 84–86
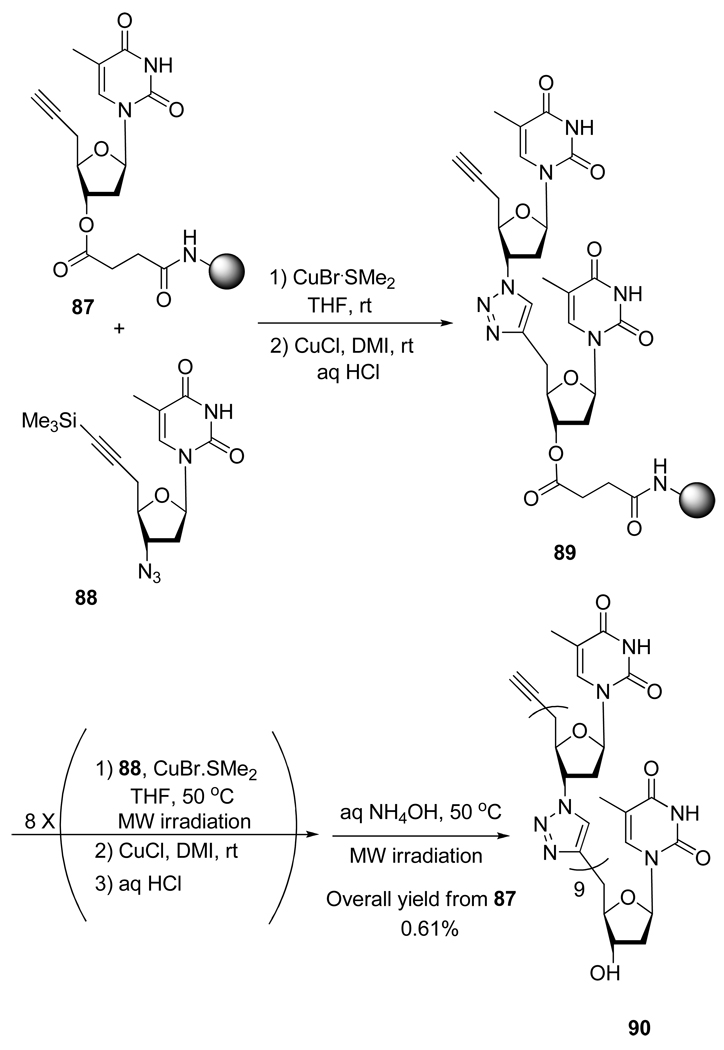
Interestingly, Isobe et al.44 designed and synthesized the new 10-mer triazole-linked analog of DNA (10-mer TLDNA) 90 (Scheme 23). Their approach, optimized on solid phase, used microwave irradiated a CuAAC reaction to realize the chain elongation. Of significance, the artificial 10-mer TLDNA 90 was able to form a stable double strand with the complementary strand of natural DNA.
Scheme 23.
Synthesis of 10-Mer TLDNA 90.
3.2- 1,2,3-Triazole as Linker for Solid Supported Synthesis
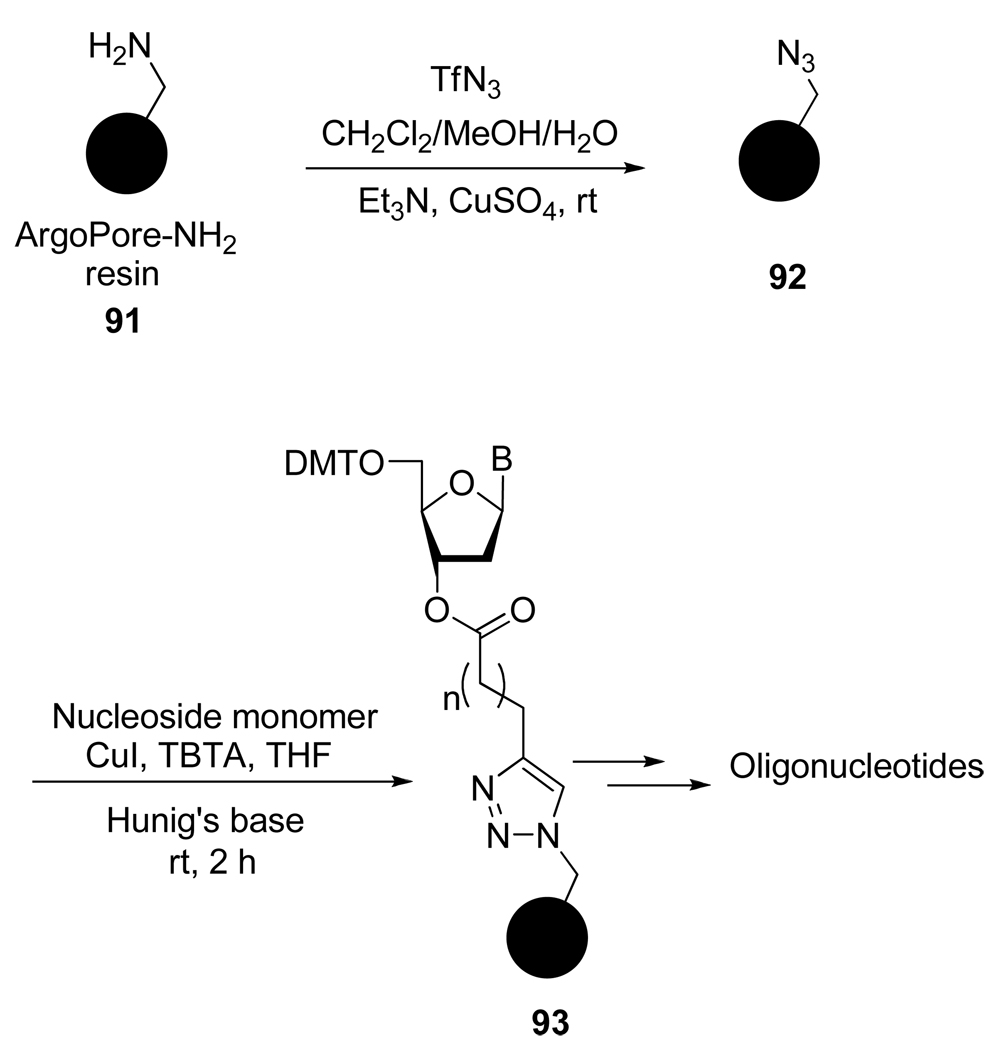
Solid phase synthesis is now the most common method used for the preparation of macromolecules such as peptides or nucleic acids. However the conditions necessary for the covalent attachment of the first monomer can be tricky. For instance, in the case of the use of an aminated solid support the loading can be a slow process and must be accomplished under rigorous exclusion of moisture. Potential partial loading, due these constraints, can result in unwanted side reactions and loss of purity for the final product. To circumvent these problems, Oyelere et al.45 developed the azide-coated support 92 and used the versatility offered by the CuAAC to easily load different alkyne-functionalized nucleosides monomers (Scheme 24). The nucleoside-functionalized support 93 was shown to be suitable for solid phase synthesis of 15-mer and 30-mer oligonucleosides.
Scheme 24.
Azido Resin Derivatization with a Nucleoside Monomer using CuAAC.
3.3- Post- and Presynthetic DNA Modifications
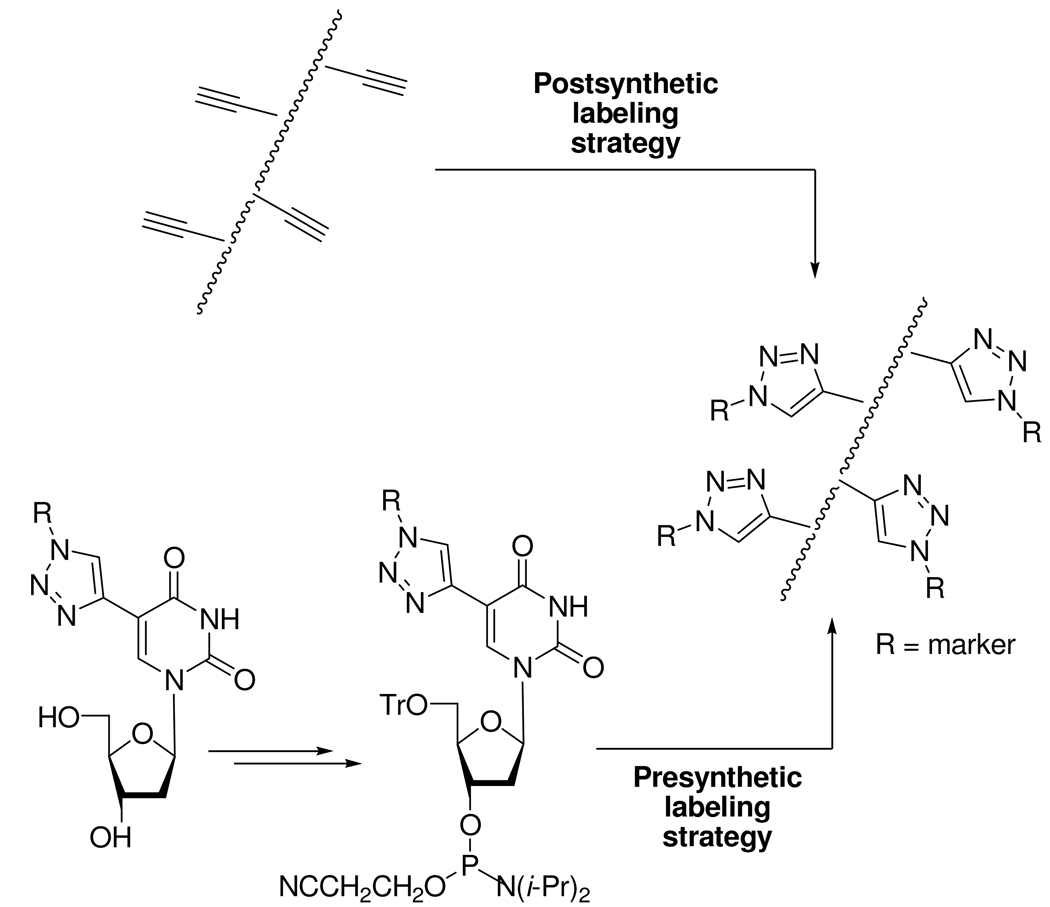
The efficiency and simplicity of azide-alkyne dipolar cycloaddition for coupling organic fragments proved to be an attractive way to “decorate” oligonucleotides strands. In this domain, two main strategies co-exist called pre- and post-synthetic labeling. The post labeling term is employed when the modification occurred on the already formed DNA strand in opposition to the pre-synthetic strategy where the labeling is introduced before the formation of the oligonucleotide (Scheme 25).
Scheme 25.
Postsynthetic and Presynthetic Strategies for DNA Labeling.
An excellent article9 was recently published summarizing the recent applications of the CuAAC for postsynthetic DNA modifications. This application has been successfully used for the preparation of surface immobilized DNA, DNA-protein conjugates, cyclic, and branched DNA structures, but also for analytical purposes, labeling of DNA and for DNA metallization. In light of the recent review, we decided to limit this section to the only pre-synthetic strategy approach.
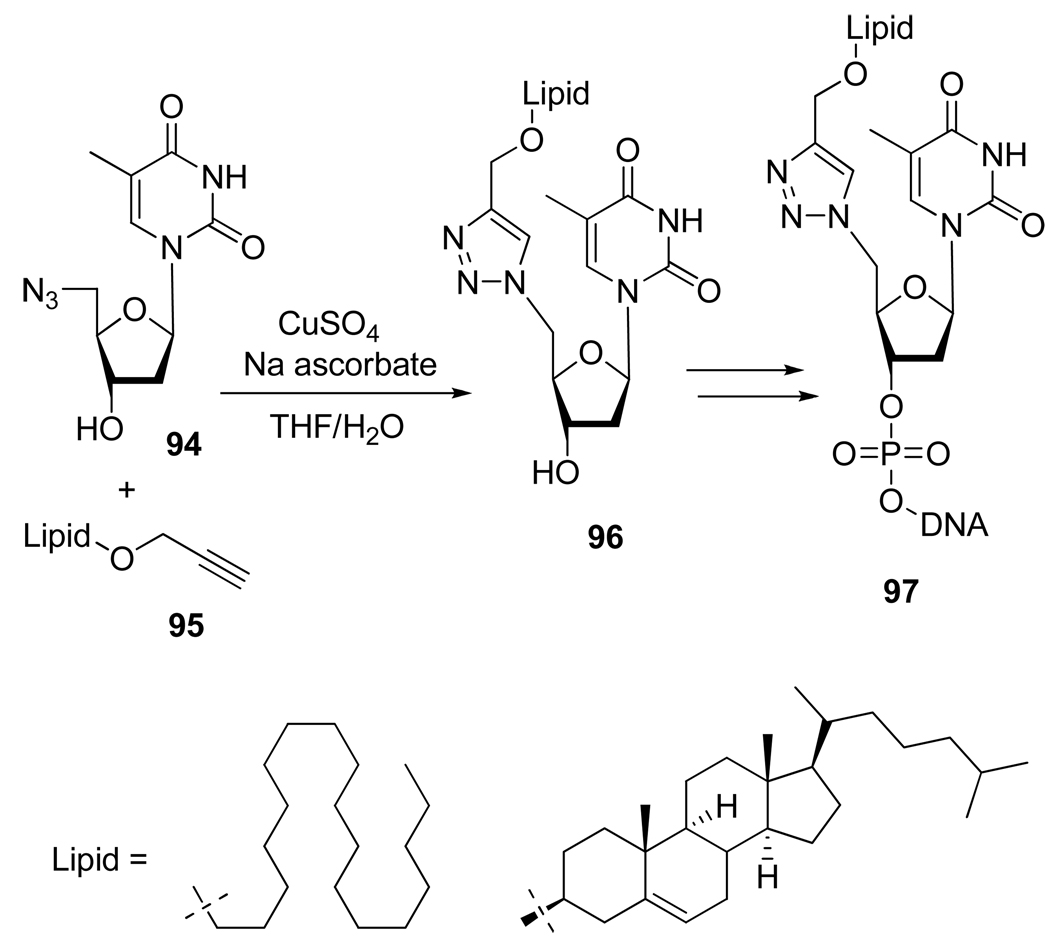
In order to increase the intracellular delivery of nucleotides, Godeau et al.46 used a triazole linker to prepare some lipid-conjugated oligonucleotides 97 (Scheme 25). These compounds appeared to efficiently inhibit HCV internal ribosome entry site (IRES)-mediated translation in human hepatic cells.
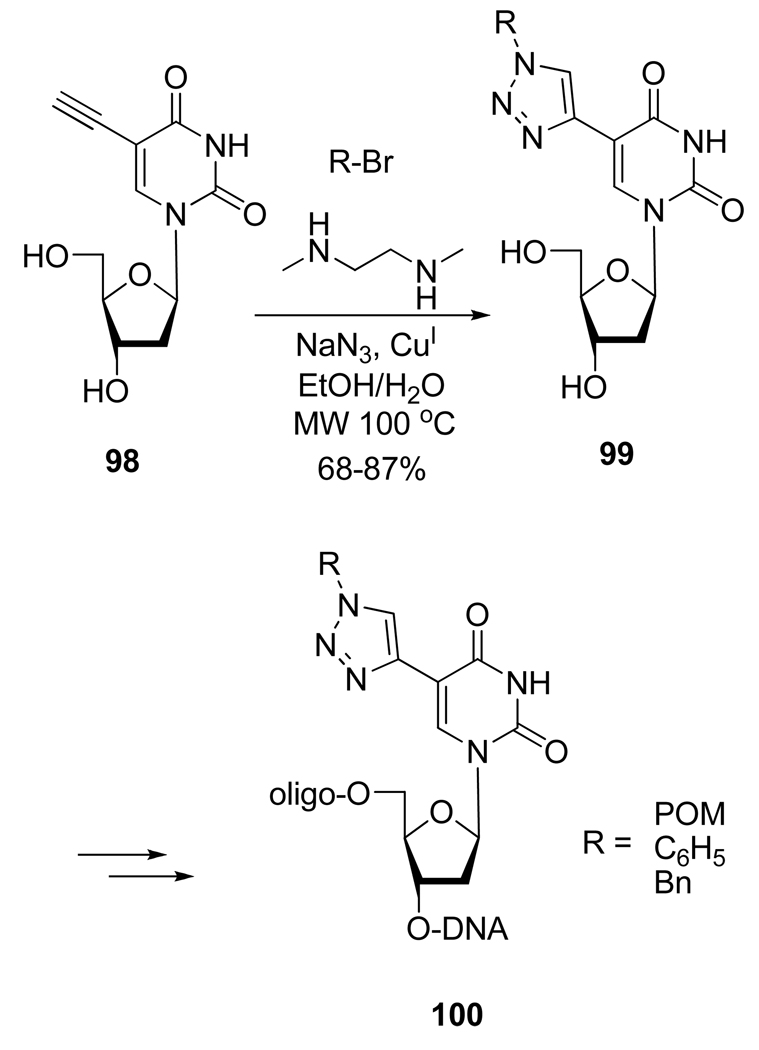
The duplex stability of modified oligonucleotides has also been studied by Kocalka et al.47 who used a one pot azidation procedure under microwave irradiation to form different 2’-deoxyuridines substituted on their 5-position by a 1,2,3-triazole ring. The nucleoside analogs 99 were then introduced into nonamer oligonucleotides by phosphoramidite chemistry (Scheme 27). Interestingly, while single modifications led to decreased duplex stability, the stacking of four consecutive modifications led to enhanced duplex stability, especially for DNA-RNA duplexes.
Scheme 27.
Synthesis of Modified Oligonucleotides 100.
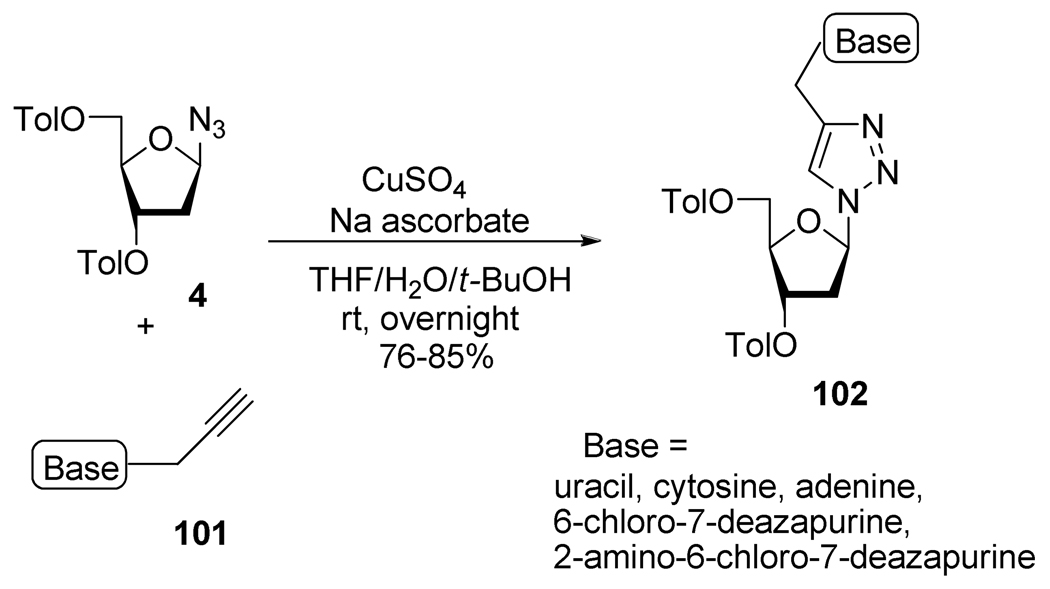
The “fleximers” are a special class of modified nucleosides where the nucleobase is splinted, but still retain the key recognition of DNA bases. Thus, in order to study these unusual nucleoside analogs, Chittepu et al.48 reported the synthesis of new 1,2,3-triazole nucleosides analogs 102 and incorporated their phosphoramidite building blocks into DNA (Scheme 28). In this particular case, these flexible nucleosides appeared to behave as an abasic site with a destabilizing effect on the DNA duplexes.
Scheme 28.
Synthesis of “Fleximers” 102.
4. Conclusion
So far, one might think that the concept of “click chemistry” looks more or less like a big menu proposing a single dish but, as a matter of fact, this unique dish appeared mouth-watering and inviting for a lot of chemists. Because of its modularity, its high yields, and its simple conditions and purification procedures the Cu(I)-catalyzed 1,3-dipolar cycloaddition reaction between alkynes and azides has been suitable for the synthesis of a large number of modified nucleosides and oligonucleotides with a broad range of applications. As we have seen, the possibilities opened by this reaction have not been only attractive for the formation of 1,2,3-triazoles as bioisosteres, or active moieties, but also for the use of 1,2,3-triazoles as a linker to a solid support or to form probes and bioconjugates. In brief, the CuAAC has a huge potential especially if researcher start to really exploit the relative innocuousness of the reactants used during this reaction to bridge the gap between the chemistry and the biology of nucleosides and nucleotides, allowing direct evaluation in specialized bioassays.
Scheme 22.
One-pot Synthesis of Radiolabeled Conjugate 83.
Scheme 26.
Synthesis of Lipid-Oligonucleotides Conjugates 97.
Acknowledgments
This work was supported in part by NIH grant 5P30-AI-50409 (CFAR), 5R37-AI-041980, 5R01-AI-071846 and by the Department of Veterans Affairs. We would like to thank Dr Ethel C. Garnier-Amblard and Dr. Steven J. Coats for helpful discussions and their critical reading of the manuscript.
Biographies

Franck Amblard was born in Châteauroux, France. He studied chemistry at the University of Orléans (France), where he received his Ph.D. in 2004 under the guidance of Professor Luigi A. Agrofoglio working on the synthesis of new nucleosides analogs using metathesis and palladium-catalyzed reactions. In 2005, he moved to the USA to join Professor Raymond F. Schinazi’s research group at Emory University (Atlanta, GA) as a postdoctoral fellow. In 2008 he was appointed Instructor at the Department of Pediatrics, Emory University School of Medicine. His main research interests involve the design, the synthesis and the study of nucleosides analogs as potential antiviral agents.

Jong Hyun Cho was born in Gim-hae, South Korea, in 1967. In 2002, he received his Ph.D. in Organic Chemistry from Seoul National University (South Korea) working on biologically active peptide mimetics under the direction of Professor B. M. Kim. He then joined Professor Chung K. Chu’s group at the Department of Pharmaceutical and Biomedical Sciences, College of Pharmacy, University of Georgia where he studied nucleoside chemistry and medicinal chemistry from 2003 to 2006. He is currently working as a postdoctoral research fellow for Professor Raymond F. Schinazi at Emory University. His research interests include synthetic approaches to nucleosides and peptidomimetics presenting antiviral activities against DNA and RNA viruses.

Raymond F. Schinazi was born in Alexandria, Egypt in 1950. In 1976, he received his Ph.D. in Organic Chemistry from Bath University on ellipticine, a DNA intercallator, under the direction of Professor Malcolm Sainsbury. He then joined Professor William H. Prusoff in the Department of Pharmacology at Yale University. He is currently the Frances Winship Walter Professor of Pediatrics at Emory University and a Senior Research Career Scientist at the Atlanta Department of Veterans Affairs. Professor Schinazi is the recipient of numerous awards, including an Honorary DSc from the University of Bath, the Georgia Biomedical Industry Growth Award, the Bruce Witte Award, and the 2006 Distinguished Scientist Award from the Hepatitis B Foundation. He is coinventor of two of the most widely used anti-HIV and HBV drugs namely, Lamivudine and Emtricitabine. His research interests include the discovery and the development of antiviral and anticancer agents, focusing on nucleoside analogs.
References and notes
- 1.Huisgen R, Szeimies G, Möbius L. Chem.Ber. 1967;100:2494. [Google Scholar]
- 2.For reviews see: Kolb HC, Sharpless KB. Drug Discovery Today. 2003;8:1128. doi: 10.1016/s1359-6446(03)02933-7. Bock VD, Hiemstra H, van Maarseveen JH. Eur. J. Org. Chem. 2006;51 Binder WH, Kluger C. Curr. Org. Chem. 2006;10:1791. Binder WH, Sachsenhofer R. Macromol. Rapid Commun. 2007;28:15. Moorhouse AD, Moses JE. Chem. Med. Chem. 2008;3:715. doi: 10.1002/cmdc.200700334. Binder WH, Sachsenhofer R. Macromol. Rapid Commun. 2008;29:952. Lutz J-F, Zarafshani Z. Adv.Drug. Deliv. Rev. 2008;60:958. doi: 10.1016/j.addr.2008.02.004.
- 3.Kolb HC, Finn MG, Sharpless KB. Angew.Chem. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.a) Tornφe CW, Meldal M. Proceedings of the Second International and the Seventeenth American Peptide Symposium; 2001. p. 263. [Google Scholar]; b) Tornφe CW, Christensen C, Meldal M. J.Org. Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]; c) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2565. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.For Pd-catalyzed Sonogashira reaction on nucleosides see: Agrofoglio LA, Gillaizeau I, Saito Y. Chem. Rev. 2003;103:1875. doi: 10.1021/cr010374q. For the preparation of alkynes from aldehydes: Roth GJ, Liepold B, Mömlller SG, Bestmann HJ. Synthesis. 2004;59 For a review on the preparation of azidonucleosides see: Pathak T. Chem. Rev. 2002;102:1623. doi: 10.1021/cr0104532.
- 6.a) Nucleosides and nucleotides as antitumor and antiviral agents; Chu CK, Baker DC. New York: Plenum Press; 1993. For a general review on antiviral drugs see: De Clercq E. J. Clin. Virol. 2004;30:115. doi: 10.1016/j.jcv.2004.02.009. For a recent review on the anti-HCV compounds in development including nucleosides analogs see: Liu-Young G, Kozal M. J. AIDS Patient Care STDS. 2008;22:449. doi: 10.1089/apc.2007.0199. For a recent review on the current antiviral therapy of chronic hepatitis B see: Ayoub WS, Keefe EB. Aliment. Pharmacol. Ther. 2008;28:167. doi: 10.1111/j.1365-2036.2008.03731.x. For a review on HIV-1 reverse transcriptase inhibitors see: Jochmans D. Virus Res. 2008;134:157. doi: 10.1016/j.virusres.2008.01.003. f) For reviews on nucleosides as antitumor agents see: Blagosklonny MV. Cell Cycle. 2004;3:1035. Robak T, Korycka A, Kasznicki M, Wrzesien-Kus A, Smolewski P. Curr.Cancer Drug Targets. 2005;5:421. doi: 10.2174/1568009054863618. Miura S, Izuta S. Curr.Drug Targets. 2004;5:191. doi: 10.2174/1389450043490578.
- 7.For reviews on therapeutic applications of nucleic acids see: Uhlmann E, Peyman A. A. Chem. Rev. 1990;90:543. Praseuth D, Guieysse AL, Helene C. Biochim. Biophys. Acta. 1999;181 doi: 10.1016/s0167-4781(99)00149-9. Micklefield J. Curr. Med. Chem. 2001;8:1157. doi: 10.2174/0929867013372391.
- 8.Gramlich PME, Wirges CT, Manetto A, Carell T. Angew. Chem. Int. Ed. 2008;47:8350. doi: 10.1002/anie.200802077. [DOI] [PubMed] [Google Scholar]
- 9.Lau JY, Tam RC, Liang TJ, Hong Z. Hepatology. 2002;35:1002. doi: 10.1053/jhep.2002.32672. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 10.Mizushina Y, Matsukage A, Sakaguchi K. Biochim. Biophys. Acta. 1998;1403:5. doi: 10.1016/s0167-4889(98)00027-5. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez R, Valazquez S, San-Felix A, Aquaro S, De Clercq E, Perno C-F, Karlsson A, Balzarini J, Camarasa MJ. J. Med. Chem. 1994;37:4185. doi: 10.1021/jm00050a015. [DOI] [PubMed] [Google Scholar]
- 12.a) Perez-Castro I, Caamano O, Fernandez F, Garcia MD, Lopez C, De Clercq E. Org. Biomol. Chem. 2007;5:3805. doi: 10.1039/b710348d. [DOI] [PubMed] [Google Scholar]; b) Cho JH, Bernard DL, Sidwell RW, Kern ER, Chu CK. J. Med. Chem. 2006;49:1140. doi: 10.1021/jm0509750. [DOI] [PubMed] [Google Scholar]; c) Goeminne A, McNaughton M, Bal G, Surpateanu G, Van der Veken P, De Prol S, Versees W, Steyaert W, Apers S, Haemers A, Augustyns K. Bioorg. Med. Chem. Lett. 2007;17:2523. doi: 10.1016/j.bmcl.2007.02.017. [DOI] [PubMed] [Google Scholar]; d) Broggi J, Diez-Gonzalez S, Petersen JL, Berteina-Raboin S, Nolan SP, Agrofoglio LA. Synthesis. 2008:141. [Google Scholar]; e) Broggi J, Joubert N, Aucagne V, Zevaco T, Berteina-Raboin S, Nolan SP, Agrofoglio LA. Nucleosides Nucleotides. 2007;26:779. doi: 10.1080/15257770701501492. [DOI] [PubMed] [Google Scholar]; f) Pradere U, Roy V, McBrayer TR, Schinazi RF, Agrofoglio LA. Tetrahedron. 2008;64:9044. doi: 10.1016/j.tet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guezguez R, Bougrin K, El Akri K, Benhida R. Tetrahedron Lett. 2006;47:4807. [Google Scholar]
- 14.Ermolat’ev DS, Metha VP, Van der Eycken EV. QSAR Comb. Sci. 2007;26:1266. [Google Scholar]
- 15.a) Broggi J, Joubert N, Aucagne V, Berteina-Raboin S, Diez-Gonzalez S, Nolan SP, Topalis D, Deville-Bonne D, Balzarini J, Neyts J, Andrei G, Snoeck R, Agrofoglio L. Nucleosides Nucleotides. 2007;26:1391. doi: 10.1080/15257770701534139. [DOI] [PubMed] [Google Scholar]; b) Broggi J, Joubert N, Diez-Gonzalez S, Berteina-Raboin S, Zevaco T, Nolan SP, Agrofoglio L. Tetrahedron. 2009;65:1162. [Google Scholar]
- 16.Li L, Lin B, Yang Z, Zhang L, Zhang L. Tetrahedron Lett. 2008;49:4491. [Google Scholar]
- 17.Li L, Zhang G, Zhu A, Zhang L. J. Org. Chem. 2008;73:3630. doi: 10.1021/jo800035v. [DOI] [PubMed] [Google Scholar]
- 18.a) Xia Y, Li W, Qu F, Fan Z, Liu X, Berro C, Rauzy E, Peng L. Org. Biomol. Chem. 2007;5:1695. doi: 10.1039/b703420b. [DOI] [PubMed] [Google Scholar]; b) Li W, Xia Y, Fan Z, Qu F, Wu Q, Peng L. Tetrahedron Lett. 2008;49:2804. [Google Scholar]
- 19.a) Cho SH, Yoo EJ, Bae I, Chang SJ. Am. Chem. Soc. 2005;127:16046. doi: 10.1021/ja056399e. [DOI] [PubMed] [Google Scholar]; b) Yoo EJ, Ahlquist M, Bae I, Sharpless KB, Fokin V, Chang S. J. Org. Chem. 2008;73:5520. doi: 10.1021/jo800733p. [DOI] [PubMed] [Google Scholar]
- 20.O’Mahony G, Ehrman E, Grotli M. Tetrahedron Lett. 2005;46:6745. [Google Scholar]
- 21.Cosyn L, Palaniappan KK, Kim S-K, Duong HT, Gao Z-G, Jacobson KA, Van Calenbergh S. J. Med. Chem. 2006;49:7373. doi: 10.1021/jm0608208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a) Temple C, Kussner CL, Montgommery JA. J. Org. Chem. 1966;31:2210. [Google Scholar]; b) Lioux T, Gosselin G, Mathe C. Eur. J. Org. Chem. 2003;20:3997. [Google Scholar]
- 23.Cosyn L, Gao Z-G, Van Rompaey P, Lu C, Jacobson KA, Van Calenbergh S. Bioorg. Med. Chem. 2006;14:1403. doi: 10.1016/j.bmc.2005.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupte A, Boshoff HI, Wilson DJ, Neres J, Labello N, Somu RV, Xing C, Barry CE, Aldrich CC. J. Med. Chem. 2008;51:7495. doi: 10.1021/jm8008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.a) Zhou L, Amer A, Korn M, Burda R, Balzarini J, De Clercq E, Kern ER, Torrence PF. Antiviral Chem. Chemother. 2005;16:375. doi: 10.1177/095632020501600604. [DOI] [PubMed] [Google Scholar]; b) Ding H, Yang R, Song Y, Xiao Q, Wu J. Nucleosides Nucleotides. 2008;27:368. doi: 10.1080/15257770801944055. [DOI] [PubMed] [Google Scholar]; c) Byun Y, Vogel SR, Phipps AJ, Carnrot C, Eriksson S, Tiwari R, Tjarks W. Nucleosides Nucleotides. 2008;27:244. doi: 10.1080/15257770701845238. [DOI] [PubMed] [Google Scholar]; d) Lee L, Chang K-H, Valiyev F, Liu H-J, Li W-S. J. Chin. Chem. Soc. 2006;53:1547. [Google Scholar]
- 26.O’Mahony G, Svensson S, Sundgreen A, Grφtli M. Nucleosides Nucleotides. 2008;27:449. doi: 10.1080/15257770802086880. [DOI] [PubMed] [Google Scholar]
- 27.Kaur H, Babu BR, Maiti S. Chem. Rev. 2007;107:4672. doi: 10.1021/cr050266u. [DOI] [PubMed] [Google Scholar]
- 28.Enderlin G, Nielsen P. J. Org. Chem. 2008;73:6891. doi: 10.1021/jo801081t. [DOI] [PubMed] [Google Scholar]
- 29.Somu RV, Boshoff HI, Qiao C, Bennet EM, Barry CE, Aldrich CC. J. Med. Chem. 2006;49:31. doi: 10.1021/jm051060o. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, Huang X. J. Comb. Chem. 2008;10:526. doi: 10.1021/cc800034v. [DOI] [PubMed] [Google Scholar]
- 31.a) Lin N, Yan Z, Altier C, Li M, Carrasco N, Suyemoto M, Johnston L, Wang S, Wang Q, Fang H, Caton-Williams J, Wang B. Nucleic Ac. Res. 2007;35:1222. doi: 10.1093/nar/gkl1091. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li M, Lin N, Huang Z, Du L, Altier C, Fang H, Wang B. J. Am. Chem. Soc. 2008;130:12636. doi: 10.1021/ja801510d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.a) Olejniczak A, Wojtczak BA, Lesnikowski Z. Nucl. Nucl. Nucl. Acids. 2007;26:1611. doi: 10.1080/15257770701548733. [DOI] [PubMed] [Google Scholar]; b) Wojtczak BA, Andrysiak A, Gruner B, Lesnikowski Z. J. Chem. Eur. J. 2008;14:10675. doi: 10.1002/chem.200801053. [DOI] [PubMed] [Google Scholar]
- 33.a) Seela F, Sirivolu VR. Org. Biomol. Chem. 2008;6:1674. doi: 10.1039/b719459e. [DOI] [PubMed] [Google Scholar]; b) Seela F, Sirivolu VR, Chittepu P. Bioconjugate Chem. 2008;19:211. doi: 10.1021/bc700300f. [DOI] [PubMed] [Google Scholar]; c) Seela F, Ming X. Helv. Chim. Acta. 2008;91:1181. [Google Scholar]; d) Ming X, Leonard P, Heindl D, Seela F. Nucl. Acid Sym. Ser. 2008;52:471. doi: 10.1093/nass/nrn239. [DOI] [PubMed] [Google Scholar]
- 34.a) Seela F, Sirivolu VR. Chem. Biodiv. 2006;3:509. doi: 10.1002/cbdv.200690054. [DOI] [PubMed] [Google Scholar]; b) Seela F, Sirivolu VR. Helv. Chim. Acta. 2007;90:535. [Google Scholar]; c) Seela F, Sirivolu VR. Nucleosides Nucleotides. 2007;26:597. doi: 10.1080/15257770701490308. [DOI] [PubMed] [Google Scholar]
- 35.Kosiova I, Kovackova S, Kois P. Tetrahedron. 2007;63:312. [Google Scholar]
- 36.Jin P-Y, Jin P, Ruan Y-A, Ju Y, Zhao Y-F. Synlett. 2007:3003. [Google Scholar]
- 37.Danel K, Larsen LM, Pedersen EB, Sanna G, La Colla P, Loddo R. Bioorg. Med. Chem. 2008;16:511. doi: 10.1016/j.bmc.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Lolk L, Pφhlsgaard J, Jepsen AS, Hansen LH, Nielsen H, Steffansen SI, Sparving L, Nielsen AB, Vester B, Nielsen P. J. Med. Chem. 2008;51:4957. doi: 10.1021/jm800261u. [DOI] [PubMed] [Google Scholar]
- 39.Lee LV, Mitchell ML, Huang S-J, Fokin VV, Sharpless B, Wong C-H. J. Am. Chem. Soc. 2003;125:9588. doi: 10.1021/ja0302836. [DOI] [PubMed] [Google Scholar]
- 40.a) Jin X, Yang R, Jin P, Xiao Q, Ju Y. Synthesis. 2007;19:2967. [Google Scholar]; b) Jin X, Ding H, Yang R, Xiao Q, Ju Y. Synthesis. 2008:865. [Google Scholar]
- 41.Jatsch A, Kopyshev A, Mena-Osteritz E, Bäuerle P. Org. Lett. 2008;10:961. doi: 10.1021/ol703090f. [DOI] [PubMed] [Google Scholar]
- 42.Mindt TL, Struthers H, Brans L, Anguelov T, Schweinsberg C, Maes V, Tourwe D, Schibli R. J. Am. Chem. Soc. 2006;128:15096. doi: 10.1021/ja066779f. [DOI] [PubMed] [Google Scholar]
- 43.a) Nuzzi A, Massi A, Dondoni A. QSAR Comb. Sci. 2007;26:1191. [Google Scholar]; b) Lucas R, Neto V, Hadj Bouazza A, Zerrouki R, Granet R, Krausz, Champavier Y. Tetrahedron Lett. 2008;49:1004. [Google Scholar]; c) Lucas R, Zerrouki R, Granet R, Krausz, Champavier Y. Tetrahedron. 2008;64:5467. [Google Scholar]
- 44.Isobe H, Fujino T, Yamazaki N, Guillot-Nieckowski M, Nakamura E. Org. Lett. 2008;10:3729. doi: 10.1021/ol801230k. [DOI] [PubMed] [Google Scholar]
- 45.Oyelere AK, Chen PC, Yao LP, Boguslavsky N. J. Org, Chem. 2006;71:9791. doi: 10.1021/jo0618122. [DOI] [PubMed] [Google Scholar]
- 46.Godeau G, Staedel C, Barthelemy P. J. Med. Chem. 2008;51:4374. doi: 10.1021/jm800518u. [DOI] [PubMed] [Google Scholar]
- 47.Kocalka P, Andersen NK, Jensen F, Nielsen P. Chem. Bio. Chem. 2007;8:2106. doi: 10.1002/cbic.200700410. [DOI] [PubMed] [Google Scholar]
- 48.Chittepu P, Sirivolu VR, Seela F. Bioorg. Med. Chem. 2008;16:8427. doi: 10.1016/j.bmc.2008.08.026. [DOI] [PubMed] [Google Scholar]