Abstract
OBJECTIVE
This study was designed to investigate the long-term effects of simvastatin treatment after traumatic brain injury (TBI) in rats.
METHODS
Adult female Wistar rats (n=24) were injured with controlled cortical impact and divided into three groups. The first two groups were treated with simvastatin 0.5 mg/kg or 1 mg/kg administered orally for 14 days starting one day after TBI. The third group (control) received phosphate-buffered saline (PBS) orally for 14 days. Neurological functional outcome was measured with modified neurological severity scores (mNSS) performed 1 day before TBI and after TBI on Days 1, 4, 7, 14 and biweekly thereafter. All animals were sacrificed 3 months after TBI. Brain tissues of half of the animals were processed for preparation of paraffin-embedded sections for immunohistological studies. The remaining half was frozen for ELISA studies for quantification of brain-derived neurotrophic factor (BDNF) in hippocampus and cortex.
RESULTS
Results showed that both doses of simvastatin significantly improved functional outcome compared to control with no difference between the two doses. Simvastatin treatment of 1 mg/kg increased the number of morphologically intact neurons in hippocampus with 0.5 mg/kg having no significant effect. ELISA studies showed that 0.5 mg/kg of simvastatin significantly increased BDNF levels within hippocampus with 1 mg/kg having no significant effect; neither dose had any effect on BDNF levels within the cortex.
CONCLUSION
Simvastatin treatment provides long-lasting functional improvement after TBI in rats. It also enhances neuronal survival in the hippocampus and increases BDNF levels in the hippocampus secondary to simvastatin treatment.
Keywords: Simvastatin, Traumatic brain injury, Long term, Newly generated cells
INTRODUCTION
TBI remains a major health problem worldwide (28) and despite a number of neuroprotective therapeutic trials, no broadly applicable, safe and efficacious treatment for TBI has been identified (28, 31). Recently, attention has focused on potential therapeutic agents that enhance endogenous neuroplasticity including neurogenesis, angiogenesis and synaptogenesis after brain injury (20–25). One such agent which has shown promise as a therapy for TBI is simvastatin (25). Experimental studies have demonstrated that simvastatin induces neurogenesis and angiogenesis and improves functional outcome after TBI (25). However, until now, all the studies on simvastatin treatment of TBI (25,35, 36) have been of short duration (i.e., following the treatment outcome until one month after injury). Long-term studies are needed to evaluate whether the benefits observed with simvastatin treatment persist over time. Therefore, the present study was conducted to analyze the functional outcome of simvastatin treatment for three months after treatment and to study its effects on neuronal survival in the CA3 region of the hippocampus. We selectively focused on the CA3 region of the hippocampus because of the major role it plays in spatial learning and memory (8). We also studied the long-term effects of simvastatin treatment on the induction of neurotrophic factor, BDNF.
MATERIALS AND METHODS
All procedures have been approved by the Henry Ford Hospital Institutional Animal Care and Use Committee (IACUC).
Animal Model
A controlled cortical impact model in rat was used. Female Wistar rats (300 - 320 g) were anesthetized with chloral hydrate 350 mg/kg intraperitoneally. Rectal temperature was controlled at 37°C with a feedback-regulated water-heating pad. A controlled cortical impact device was used to induce the injury (9). Rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central structure, midway between lambda and bregma. The contralateral craniotomy allowed lateral movement of cortical tissue (30). The dura was kept intact over the cortex. Injury was induced by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 6-mm-diameter tip at the rate of 4 m/s and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer.
Treatment of TBI Rats with Simvastatin
Twenty-four rats were treated with simvastatin or phosphate-buffered saline (PBS) and three groups were studied (see below). Two doses of simvastatin were tested, 0.5 mg/kg or 1.0 mg/kg. Simvastatin was mixed with water and administered directly into the esophagus using a lavage tube daily for 14 days starting 1 day after TBI. A control group received PBS orally for 14 days starting day1 after TBI. Animals were divided into three experimental groups, as follows: Group 1 (8): TBI + 0.5 mg/kg of simvastatin for 14 days; Group 2 (8): TBI + 1.0 mg/kg of simvastatin for 14 days; and Group 3 (8): TBI + PBS. All rats were sacrificed 3 months after TBI. This was not a randomized trial but all studies including functional, histological and ELISA measurements were conducted in a blinded fashion. Four rats died during the study; however, they were replaced and the data of the dead animals was not included.
Brain Sample Preparation
Brain tissues from four animals in each group were processed for preparation of paraffin-embedded sections for immunostaining. The remaining four brains in each group were frozen for ELISA studies. For immunostaining, rats were anesthetized intraperitoneally with chloral hydrate and perfused transcardially with saline, followed by 4% paraformaldehyde. Brains were isolated, post fixed in paraformaldehyde at room temperature for 48 hours, and then processed for paraffin sections. For ELISA studies, the ipsilateral, contralateral cortical and hippocampal tissues were separated from the brain, then frozen in liquid nitrogen and stored at − 80°C until used.
Immunohistochemical Staining for Neurons in the Ipsilateral Hippocampus
For identification of neurons, brain sections were deparaffinized and placed in boiling citrate buffer (pH 6) in a microwave oven for 10 minutes. After cooling at room temperature, the sections were incubated overnight in 0.1% saponin-PBS at 4°C. After blocking in normal serum, sections were incubated with mouse anti-MAP-2 antibody (dilution, 1:100; Chemicon International, Temecula, CA) in PBS at 4°C overnight. After sequential incubation with biotin-conjugated anti-mouse immunoglobulin G (dilution, 1:100; Dakopatts, Carpinteria, CA), the sections were treated with an avidin-biotin-peroxidase system (ABC kit, Vector Laboratories, Inc.). DAB was used as a sensitive chromogen for light microscopy. The number of MAP-2 positive cells which were morphologically intact, that is having a well-defined cell boundary surrounding a round nucleus, was counted in the ipsilateral hemisphere using light microscopy (BH-2; Olympus Optical Co, Tokyo, Japan). Neurons demonstrating swelling, scalloping and shrinkage were not included as were neurons showing chromatin condensation. Cell counting was performed using unbiased stereological methods (29).
Neurological Functional Evaluation
Neurological motor measurement was performed using the modified neurological severity scores (mNSS). The test is sensitive to unilateral cortical injury because it reflects multiple asymmetries, including postural, sensory, and forelimb and hindlimb use asymmetries. A detailed description of this functional test has been published previously (33). These tests were performed on all rats 1 day before TBI and after TBI on Days 1, 4, 7, 14 and biweekly thereafter. All measurements were performed by observers blinded to individual treatment.
Enzyme-Linked Immunosorbent Assay
To determine the concentration in each sample, Bradford protein assay was performed (5). Lysis buffer was added to the tissue and sonicated. The protein concentration was obtained by centrifuging the solution at 14000 rpm for 12 minutes at 4°C. Total protein concentration was calculated by reading the plate at 550 nm. Equal amounts of lysates were used for brain-derived neurotrophic factor (BDNF) ELISA. The ELISA kit was purchased from Promega Corp. (Madison, WI). The procedure was performed according to the instructions provided by the company. A 96-well plate was coated with anti-BDNF monoclonal antibody provided by the kit and incubated at 4°C overnight. The plate was blocked the next day for 1 hour and then incubated with BDNF standard and test samples at 4°C overnight. Anti-human polyclonal antibody was added to the plate after washing and incubated for 2 hours at room temperature. After washing, anti-IgY HRP conjugate was added and incubated for 1 hour at room temperature with shaking. Tetramethyl benzidine (TMB) solution was added to the plate and after 10 minutes the reaction was stopped using diluted acid of 1N concentration and the plate was read using a microplate reader at 450 nm. BDNF levels were calculated.
Statistical Analysis
Rats with TBI were enrolled into one of the two different dose treatments with simvastatin and zero dose (PBS) for a total of 3 groups. Rats were evaluated by the mNSS assessment for neurological deficits at 1 day before TBI and after TBI on Days 1, 4, 7 and 14, and then biweekly until sacrificed. mNSS data were evaluated for normality. Ranked data were considered because the data were not normal. A two-way analysis of variance was used. All data are presented as the means ± standard deviation of the original mNSS by groups. A paired t-test was used to test the difference in the cell counts in the ipsilateral hemisphere between the simvastatin-treated groups and the control group. All measurements were analyzed by observers blinded to individual treatments.
RESULTS
Modified Neurological Severity Scores
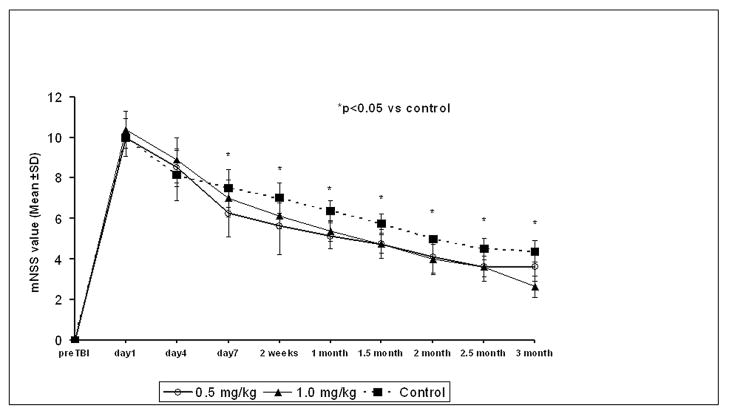
Improvements were seen with simvastatin treatment versus the control group. Simvastatin had a consistent treatment effect on mNSS at early and later time points (up to 3 months) compared with control. There was a trend for the 0.5 mg/kg dose to be better than the 1.0 mg/kg in the first two weeks, but the difference was not significant (Fig. 1).
Figure 1.
Results of modified Neurological Severity Scores (mNSS) before and after TBI. Significant functional improvement was detected in rats treated with 2 different doses of simvastatin (i.e., 0.5 mg/kg and 1 mg/kg) compared with controls. The recovery was evident 7 days after TBI and persisted at all subsequent time points. There was no significant difference among the two treatment groups. * = p < 0.05 vs control
Neuroprotective Effect of Simvastatin on the Hippocampal CA3 Region
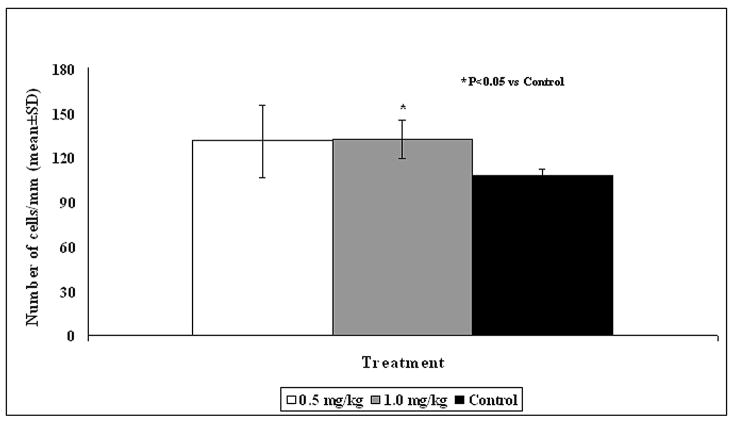
The number of morphologically intact neurons in the CA3 region of the hippocampus (Fig. 2) was significantly higher in animals treated with 1 mg/kg simvastatin versus the control group (P = 0.025, Fig. 3) with 0.5 mg/kg dose having no significant effect.
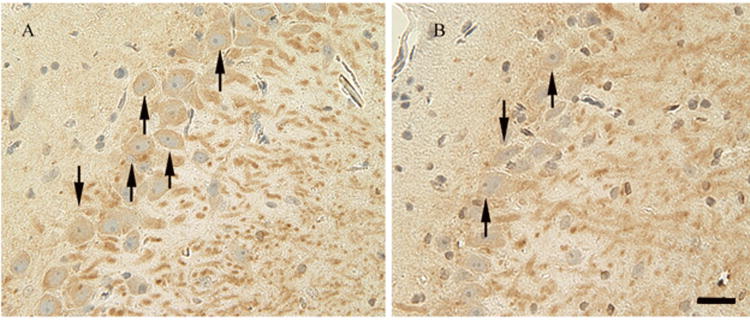
Figure 2.
Fluorescent staining for MAP-2 (brown) counterstained with hematoxylin for labeling of neurons (arrows) in the ipsilateral hippocampal CA3 region in simvastatin-treated (1 mg/kg) (A) and saline-treated (B) animals. Scale bar shown in B = 50μm (Magnification × 200).
Figure 3.
Bar Graph shows the density of neurons in the hippocampal CA3 region in different treatment groups. * = P < 0.05 vs control.
Simvastatin Induces the Expression of BDNF after TBI in hippocampus
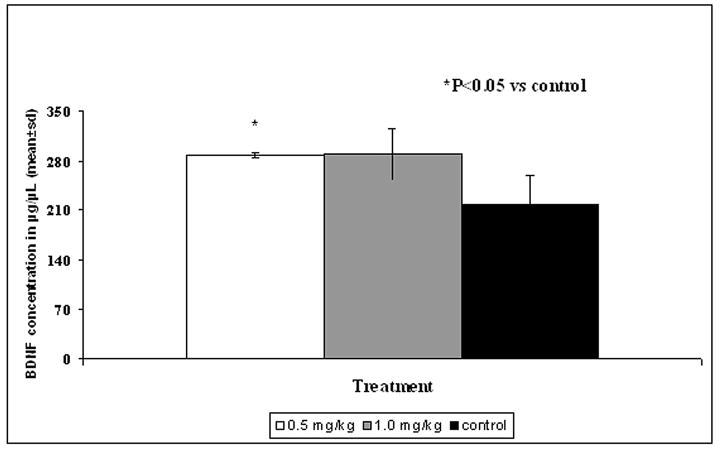
BDNF is a pleiotrophic protein involved in neuronal proliferation, differentiation, synaptic plasticity and survival (2, 13, 15). BDNF levels were evaluated in the left (ipsilateral) cortex and hippocampus (Fig. 4 and 5). BDNF was significantly increased in the ipsilateral hippocampus in the 0.5 mg/kg simvastatin-treated group compared to the control (P = 0.041). There was also a trend for increased BDNF expression in the hippocampus in the 1 mg/kg simvastatin-treated group; however, the increase was not statistically significant (Fig. 4). No difference was seen in the BDNF levels in the cortex with either dose (Fig. 5).
Figure 4.
Bar graph shows the expression of brain-derived neurotrophic factor (BDNF) in the ipsilateral hippocampus of different treatment groups. * = P < 0.05 vs control.
Figure 5.
Bar graph shows the expression of BDNF in the ipsilateral cortex of different treatment groups. No statistical significance was seen in the different treatment groups.
DISCUSSION
Our main findings in this study regarding simvastatin treatment of TBI are as follows: 1) simvastatin administered orally promotes functional recovery until 3 months after treatment; 2) simvastatin treatment reduces neuronal loss in the hippocampus after TBI; and 3) simvastatin treatment induces BDNF expression which persists for 3 months.
Statins (i.e., 3-hydroxy-3 methylglutaryl [HMG]-CoA reductase inhibitors) are potent inhibitors of cholesterol biosynthesis (16, 34). These drugs have generic names like simvastatin, lovastatin, pravastatin, and atorvastatin and are widely prescribed to treat hypercholesteremia. In addition to this cholesterol-lowering effect, many other pleiotropic effects of statins have been identified, including beneficial effects on cerebral vessels, anti-inflammatory and antithrombotic activity in blood and plaques (3, 4, 10, 12, 19, 27, 32), reduction of vascular inflammatory responses (34), modulation of cytokine production (1), promotion of angiogenesis, and decreased oxidative stress (11, 18, 26). These effects extend beyond their lipid-lowering effect.
Simvastatin treatment also had a neuroprotective effect as it reduced neuronal loss in the hippocampus. Our previous studies demonstrated similar neuroprotective effects of simvastatin until 1 month after treatment (25); however, the present study confirms that these effects are long term and persist to at least 3 months. We have focused on the hippocampus since it plays a vital role in spatial memory and learning (8). Also, it is an area especially vulnerable and selectively damaged in TBI (25). Simvastatin treatment increased BDNF expression in the hippocampus 3 months after TBI. BDNF regulates different aspects of neuronal survival, migration, morphological differentiation and synaptic function (2, 13, 15). We understand that many other neurotrophic factors also play an important role in neural recovery after TBI but we selectively focused on BDNF because our previous studies have shown that whereas simvastatin induces many cytokines and neurotrophic factors (36), the induction of all except BDNF is of short duration (36). The present data further validates the long-term effects of simvastatin on BDNF expression. Neural recovery from TBI occurs not only in the acute phase, but persists over a long period and is greatest during the first 6 months post TBI (17). The fact that simvastatin treatment can enhance this neural restoration over a prolonged period significantly augments its potential as a clinical therapy for TBI.
We have previously investigated the effects of simvastatin treatment on biochemical and molecular levels (35, 36). Our data have shown that simvastatin activates the pro survival phosphoinositide-3-kinase (P13K)/Akt signaling pathway after TBI (36). The (P13K)/Akt pathway plays a crucial role in cell proliferation and survival. Activated Akt phosphorylates several downstream proteins including Bad, GSK-3β, Forkhead transcription factors and NF-κB to control cell growth, cell survival and protein synthesis (36). Our data have also shown that simvastatin treatment decreases the number of TUNEL-positive apoptotic cells after TBI and suppresses the pro-apoptotic enzyme caspase-3 (35). These effects of simvastatin on growth factors, the P13K/Akt pathway and apoptosis are at least partially responsible for the functional benefit and neuronal survival observed after simvastatin treatment in the present study.
The use of statins as a potential means to restore neurological function has generated great interest in the neuroscience community; however, the majority of research in this field has focused on cerebral ischemia (6, 7, 37). We have shown the benefits of statin treatment after TBI, but our studies until now have been of short duration (21–23, 25). It was therefore imperative to confirm that the benefits of simvastatin treatment persisted over an extended duration, before their laboratory promise could be translated into clinical relevance. Chronic simvastatin treatment has been shown to be neuroprotective in other neurological disorders (14), but the present study is the first one to demonstrate a prolonged efficacy after TBI and adds to our understanding of its biology.
Acknowledgments
This work is supported by NIH grant #5RO1 NS052280-04.
References
- 1.Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- 2.Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 3.Bocan TM. Pleiotropic effects of HMG-CoA reductase inhibitors. Curr Opin Invest Drugs. 2002;3:1312–1317. [PubMed] [Google Scholar]
- 4.Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:556–562. doi: 10.1161/01.atv.20.2.556. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 8.Day LB, Crews D, Wilczynski W. Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim Behav. 1999;57:393–407. doi: 10.1006/anbe.1998.1007. [DOI] [PubMed] [Google Scholar]
- 9.Dixon E, Clifton G, Lighthall JW. A controlled cortical impact model of traumatic brain injury in rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 10.Dujovne CA, Harris WS, Altman R, Overhiser RW, Black DM. Effect of atorvastatin on hemorrheologic hemostatic parameters and serum fibrinogen levels in hyperlipidemic patients. Am J Cardiol. 2000;85:350–353. doi: 10.1016/s0002-9149(99)00745-6. [DOI] [PubMed] [Google Scholar]
- 11.Essig M, Vrtovsnik F, Nguyen G, Sraer JD, Friedlander G. Lovastatin modulates in vivo and in vitro the plasminogen activator/plasmin system of rat proximal tubular cells: role of geranylgeranylation and Rho proteins. J Am Soc Nephrol. 1998;9:1377–1388. doi: 10.1681/ASN.V981377. [DOI] [PubMed] [Google Scholar]
- 12.Eto M, Luscher TF. Modulation of coagulation and fibrinolytic pathways by statins. Endothelium. 2003;10:35–41. doi: 10.1080/10623320303359. [DOI] [PubMed] [Google Scholar]
- 13.Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–80. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- 14.Hoglund K, Thelen KM, Syversen S, Sjogren M, von Bergmann K, Wallin A, Vanmechelen E, Vanderstichele H. The effect of simvastatin treatment on the amyloid precursor protein and brain cholesterol metabolism in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19:256–265. doi: 10.1159/000084550. [DOI] [PubMed] [Google Scholar]
- 15.Huang EJ, Reichardt LFL. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joukhadar C, Klein N, Prinz M, Schrolnberger C, Vukovich T, Wolzt M, Schmetterer L, Dorner GT. Similar effects of atorvastatin, simvastatin and pravastatin on thrombogenic and inflammatory parameters in patients with hypercholesterolemia. Thromb Haemost. 2001;85:47–51. [PubMed] [Google Scholar]
- 17.Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 18.Laufs U. Beyond lipid-lowering: effects of statins on endothelial nitric oxide. Eur J Clin Pharmacol. 2003;58:719–731. doi: 10.1007/s00228-002-0556-0. [DOI] [PubMed] [Google Scholar]
- 19.Laufs U, Liao JK. Rapid effects of statins: from prophylaxis to therapy for ischemic stroke. Arterioscler Thromb Vasc Biol. 2003;23:156–157. doi: 10.1161/01.atv.0000051360.79309.4e. [DOI] [PubMed] [Google Scholar]
- 20.Lie DC, Song Hl, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 21.Lu D, Goussev A, Chen J, Pannu P, Li Y, Mahmood A, Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 22.Lu D, Mahmood A, Goussev A, Qu C, Zhang ZG, Chopp M. Delayed thrombosis after traumatic brain injury in rats. J Neurotrauma. 2004;21:1756–1766. doi: 10.1089/neu.2004.21.1756. [DOI] [PubMed] [Google Scholar]
- 23.Lu D, Mahmood A, Goussev A, Schallert T, Qu C, Zhang ZG, Li Y, Lu M, Chopp M. Atorvastatin reduction of intravascular thrombosis, increase in cerebral microvascular patency and integrity, and enhancement of spatial learning in rats subjected to traumatic brain injury. J Neurosurg. 2004;101:819–827. doi: 10.3171/jns.2004.101.5.0813. [DOI] [PubMed] [Google Scholar]
- 24.Lu D, Mahmood A, Qu C, Goussev A, Zhang ZG, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after TBI. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 25.Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, Mahmood A, Chen J, Li Y, Chopp M. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda T, Kawane T, Horiuchi N. Statins augment vascular endothelial growth factor expression in osteoblastic cells via inhibition of protein prenylation. Endocrinology. 2003;144:681–692. doi: 10.1210/en.2002-220682. [DOI] [PubMed] [Google Scholar]
- 27.Morikawa S, Takabe W, Mataki C, Kanke T, Itoh T, Wada Y, Izumi A, Saito Y, Hamakubo T, Kodama T. The effect of statins on mRNA levels of genes related to inflammation, coagulation and vascular constriction of HUVEC. Human umbilical vein endothelial cells. J Atheroscler Thromb. 2002;9:178–183. doi: 10.5551/jat.9.178. [DOI] [PubMed] [Google Scholar]
- 28.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas Al, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss Kl, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson DA. Quantitative histology using confocal microscopy: implementation of unbiased stereology procedures. Methods. 1999;18:493–507. doi: 10.1006/meth.1999.0818. [DOI] [PubMed] [Google Scholar]
- 30.Postmantur R, Kampel A, Simon R. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:765–787. doi: 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- 31.Royo NC, Shimizu S, Schouten JW, Stover JF, McIntosh TKL. Pharmacology of traumatic brain injury. Review. Curr Opin Pharmacol. 2003;3:27–32. doi: 10.1016/s1471-4892(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 32.Shi J, Wang J, Zheng H, Ling W, Joseph J, Li D, Mehta JL, Ponnappan U, Lin P, Fink LM, Hauer-Jensen M. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagul Fibrinolysis. 2003;14:575–585. doi: 10.1097/00001721-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Shohami E, Novikov M, Bass R. Long term effect of HU-211, a novel non-competitive NMD antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 1995;674:55–62. doi: 10.1016/0006-8993(94)01433-i. [DOI] [PubMed] [Google Scholar]
- 34.White CM. Pharmacological effects of HMG CoA reductase inhibitors other than lipoprotein modulation. J Clin Pharmacol. 1999;39:111–118. doi: 10.1177/00912709922007642. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Increase in phosphorylation of Akt and its downstream signaling targets and suppression of apoptosis by simvastatin after traumatic brain injury. J Neurosurgery. 2008;109:691–698. doi: 10.3171/JNS/2008/109/10/0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu HT, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the P13/Akt pathway and increase of neurogenesis are associated with therapeutic improvement after TBI. J Neurotrauma. 2008;25:130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Zhang ZG, Ding GL, Jiang Q, Liu X, Meng H, Hozeska A, Zhang C, Li L, Morris D, Zhang RL, Lu M, Chopp M. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;112:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]