Abstract
A single radial hemolysis assay for parainfluenza-3 virus antibody is described as a simple, sensitive, accurate, and precise alternative to hemagglutination inhibition and serum neutralization tests. A highly significant correlation exists between single radial hemolysis zone areas and hemagglutination inhibition titers of both serum and nasal secretion samples. Antibody conversions equivalent to as little as a twofold rise in hemagglutination inhibition titer are readily and reliably detected in sera and nasal secretions. Single radial hemolysis and hemagglutination inhibition activity was demonstrable in the immunoglobulin G fraction of a hyperimmune parainfluenza-3 virus antiserum.
Full text
PDF
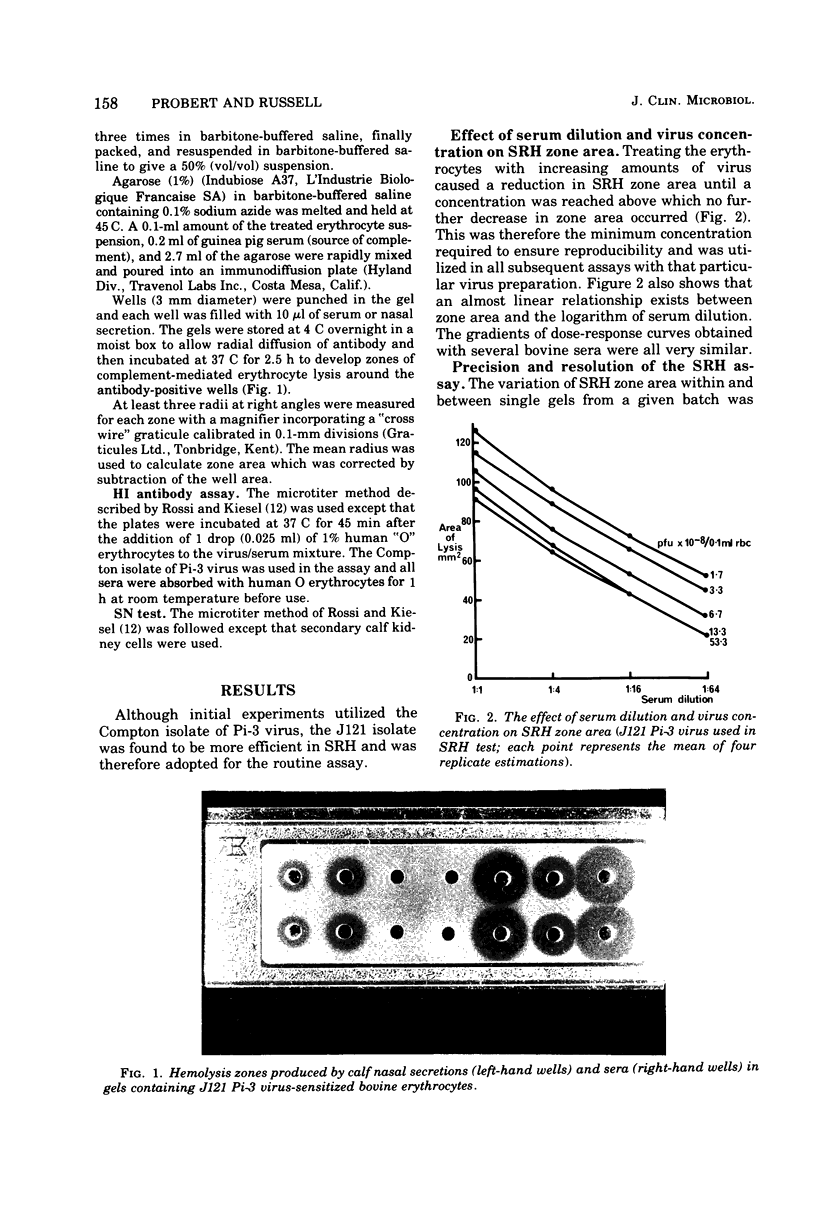
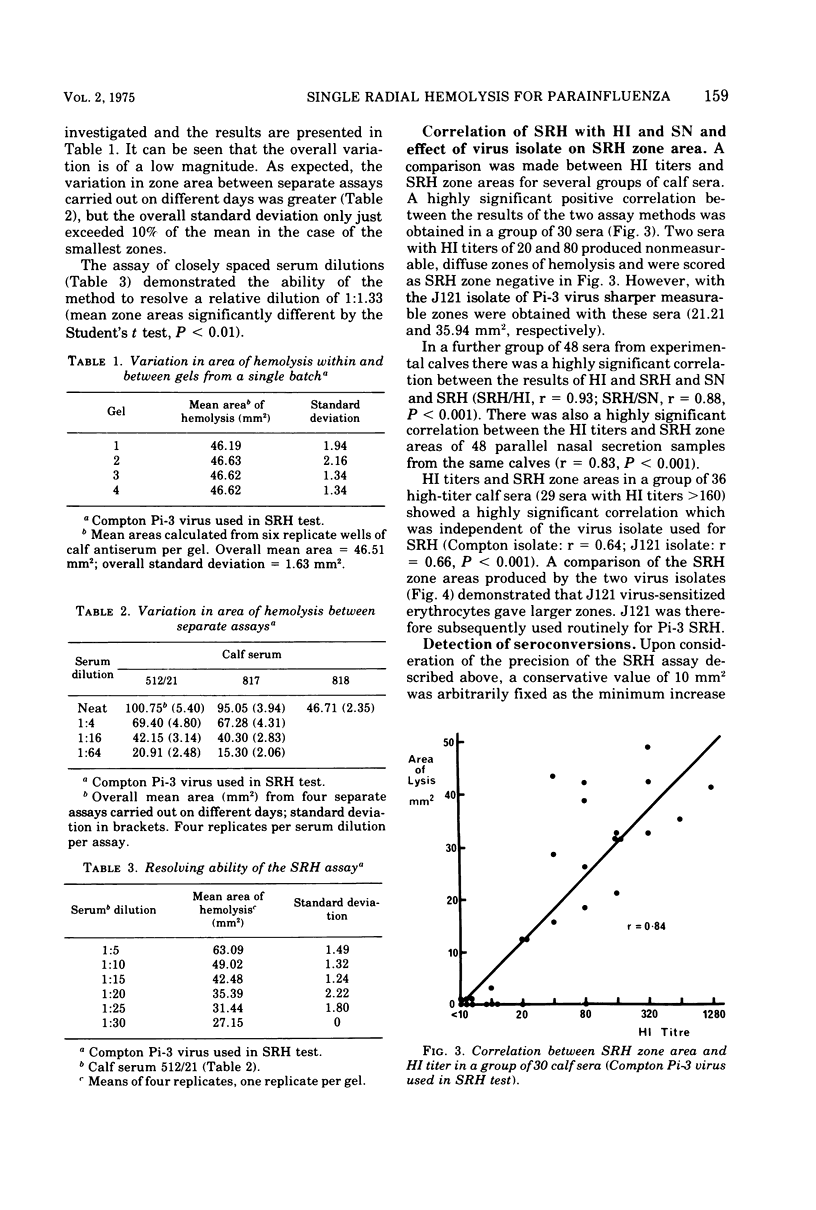


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABINANTI F. R., HOERLEIN A. B., WATSON R. L., HUEBNER R. J. Serologic studies on myxovirus para-influenza 3 in cattle and the prevalence of antibodies in bovines. J Immunol. 1961 May;86:505–511. [PubMed] [Google Scholar]
- HAMPARIAN V. V., WASHKO F. V., KETLER A., HILLEMAN M. R. Laboratory and field investigations of bovine Myxovirus parainfluenza 3 virus and vaccine. III. Evaluation of an SF-4 (shipping fever) virus vaccine in cattle. J Immunol. 1961 Aug;87:139–146. [PubMed] [Google Scholar]
- HOERLEIN A. B., MANSFIELD M. E., ABINANTI F. R., HUEBNER R. J. Studies of shipping fever of cattle. I. Para-influenza 3 virus antibodies in feeder calves. J Am Vet Med Assoc. 1959 Aug 1;135(3):153–160. [PubMed] [Google Scholar]
- KETLER A., HAMPARIAN V. V., HILLEMAN M. R. Laboratory and field investigations of bovine Myxovirus parainfluenza 3 virus and vaccine. I. Properties of the SF-4 (shipping fever) strain of virus. J Immunol. 1961 Aug;87:126–133. [PubMed] [Google Scholar]
- McKercher D. G., Kaneko J. J., Mills R. J., Wada E. M. A simple method for obtaining undiluted nasal secretions from cattle. Am J Vet Res. 1973 Jun;34(6):837–838. [PubMed] [Google Scholar]
- Morein B. Immunity against parainfluenza-3 virus in cattle. IgA in nasal secretions. Int Arch Allergy Appl Immunol. 1970;39(4):403–414. doi: 10.1159/000230368. [DOI] [PubMed] [Google Scholar]
- Morein B. Immunity against parainfluenza-3 virus in cattle; immunoglobulins in serum and nasal secretions after subcutaneous and nasal vaccination. Z Immunitatsforsch Exp Klin Immunol. 1972 Jul;144(1):63–74. [PubMed] [Google Scholar]
- Rossi C. R., Kiesel G. K. Microtiter tests for detecting antibody in bovine serum to parainfluenza 3 virus, infectious bovine rhinotracheitis virus, and bovine virus diarrhea virus. Appl Microbiol. 1971 Jul;22(1):32–36. doi: 10.1128/am.22.1.32-36.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., McCahon D., Beare A. S. A single radial haemolysis technique for the measurement of influenza antibody. J Gen Virol. 1975 Apr;27(1):1–10. doi: 10.1099/0022-1317-27-1-1. [DOI] [PubMed] [Google Scholar]



