TO THE EDITOR:
Recent findings suggest that non-melanoma skin cancer (NMSC) incidence in young adults is rising, particularly among U.S. young women (Christenson et al., 2005). This raises the important question of whether incidence of cutaneous melanoma, the most lethal form of skin cancer, is similarly increasing in young adults. While melanoma incidence among U.S. older adults has been increasing for several decades, there have been indications that incidence may be stabilizing for birth cohorts born after 1945 (Dennis et al., 1993;Hall et al., 1999). However, in an analysis of melanoma trends between 1973 and 1997 in the Surveillance, Epidemiology, and End Results (SEER) Program, Jemal et al. noted evidence of an increase among women born after 1960 (Jemal et al., 2001). Since that analysis, an additional seven years of SEER data have been collected. To better understand recent trends in melanoma incidence among young adults, we report findings from a re-analysis of SEER data, extended through 2004.
Our analysis was restricted to Caucasians from the nine registries that have contributed data to the SEER Program since 1973 (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle, Utah). We calculated annual age-adjusted incidence (SEER Progam, 2007a) and mortality rates (SEER Program, 2007b) of invasive cutaneous melanoma among men and women aged 15–39, standardized to the 2000 U.S. population, using the software SEER*Stat version 6.3.6 (National Cancer Institute: http://seer.cancer.gov/seerstat/). We assessed trends in greater detail using joinpoint regression models, which identify changes in trends over successive segments of time and describe the estimated annual percent change (EAPC) in incidence within each segment (Kim et al., 2000), using the software Joinpoint version 3.0 (National Cancer Institute: http://srab.cancer.gov/joinpoint/). Joinpoint analyses stratifying by melanoma stage (localized vs. regional/distant) and thickness (≤1mm vs. >1mm) were also performed. To describe age-specific trends by year of birth, we calculated incidence by five-year age groups and time periods, and plotted age-specific incidence by calendar year of birth (calculated from the age group midpoint). Additionally, age-period-cohort modeling was used to simultaneously adjust age-specific incidence trends for both calendar period and birth cohort effects (Tarone and Chu, 2000). All p-values are two-sided.
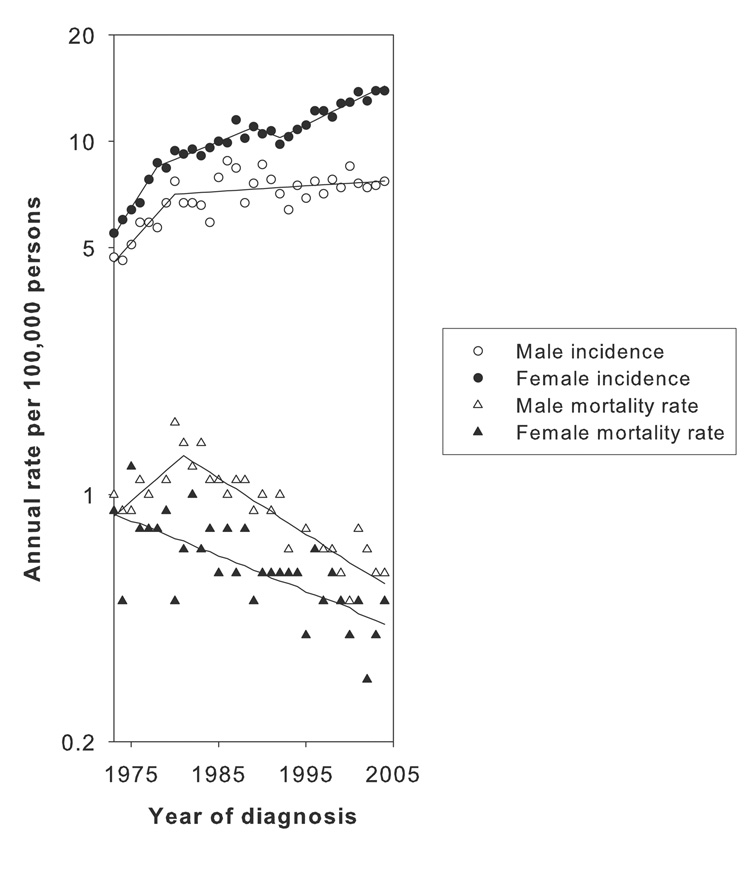
Overall, the age-adjusted annual incidence of melanoma among young men increased from 4.7 cases per 100,000 persons (95% confidence limits 3.8, 5.7) in 1973 to 7.7 per 100,000 in 2004 (6.8, 8.7). Among women, age-adjusted annual incidence per 100,000 increased from 5.5 (4.5, 6.6) in 1973 to 13.9 (12.7, 15.2) in 2004. Melanoma incidence increased among young men (EAPC=6.6; 95% CL 2.9, 10.4) and women (9.2; 6.8, 11.7) during the 1970s (Figure 1, Table 1). Starting around 1980, this pattern changed. For men, incidence leveled off between 1980 and 2004 (0.4; −0.2, 0.9). For women, the rate of increase in incidence declined from 1978 to 1987 (2.6; 1.5, 3.8) and stabilized from 1987 to 1992 (−0.6; −3.7, 2.6). After 1992, however, incidence began climbing again (2.7; 2.1, 3.4). Incidence among women from the 1990s onward increased both for thinner and thicker melanomas (≤1mm: 3.1; 2.5, 3.6. >1mm: 2.8; 1.6, 4.0), and was greater for regional and distant tumors (9.2; 3.8, 14.9) compared to localized lesions (1.9; 1.6, 2.3). Melanoma mortality rates for men and women declined from 1981 onward (men: −3.6; −4.5, −2.7. women: −2.3; −3.1, −1.5).
Figure 1.
Age-adjusted (to 2000 U.S. population) annual cutaneous melanoma incidence and mortality rates among Caucasian males and females aged 15–39 in the Surveillance, Epidemiology, and End Results Program areas from 1973 through 2004. The segments of uniform trend from the best-fitting Joinpoint models are also shown.
Table 1.
Estimated annual percent changes (EAPC) in incidence of melanoma and melanoma mortality among Caucasian males and females aged 15–39 in the SEER Program from 1973 through 2004.
| Years1 | Trend 1 EAPC (95% CL) | Years | Trend 2 EAPC (95% CL) | Years | Trend 3 EAPC (95% CL) | Years | Trend 4 EAPC (95% CL) | |
|---|---|---|---|---|---|---|---|---|
| Incidence | ||||||||
| Overall | ||||||||
| Males | 1973–1980 | 6.6 (2.9, 10.4) | 1980–2004 | 0.4 (−0.2, 0.9) | ||||
| Females | 1973–1978 | 9.2 (6.8, 11.7) | 1978–1987 | 2.6 (1.5, 3.8) | 1987–1992 | −0.6 (−3.7, 2.6) | 1992–2004 | 2.7 (2.1, 3.4) |
| By Stage: | ||||||||
| Localized | ||||||||
| Males | 1973–1980 | 9.6 (4.9, 14.5) | 1980–2004 | 0.5 (−0.2, 1.2) | ||||
| Females | 1973–1978 | 15.8 (10.9, 21.0) | 1978–2004 | 1.9 (1.6, 2.3) | ||||
| Regional/Distant | ||||||||
| Males | 1973–2004 | 1.4 (0.6, 2.2) | ||||||
| Females | 1973–1994 | −0.9 (−2.5, 0.8) | 1994–2004 | 9.2 (3.8, 14.9) | ||||
| By Thickness (1988+ only): | ||||||||
| <=1mm | ||||||||
| Males | 1988–2004 | 2.3 (1.2, 3.4) | ||||||
| Females | 1988–2004 | 3.1 (2.5, 3.6) | ||||||
| >1mm | ||||||||
| Males | 1988–2004 | −0.3 (−1.5, 1.0) | ||||||
| Females | 1988–2004 | 2.8 (1.6, 4.0) | ||||||
| Mortality | ||||||||
| Males | 1973–1981 | 5.0 (0.3, 10.1) | 1981–2004 | −3.6 (−4.5, −2.7) | ||||
| Females | 1973–2004 | −2.3 (−3.1, −1.5) |
EAPC = estimated annual percent change in melanoma incidence within jointpoint segment; CL = confidence limits. Statistically significant results in bold face type.
Calendar period within joinpoint segment. Joinpoint modeling was done separately for males and females; hence, sex-specific joinpoint segments may differ.
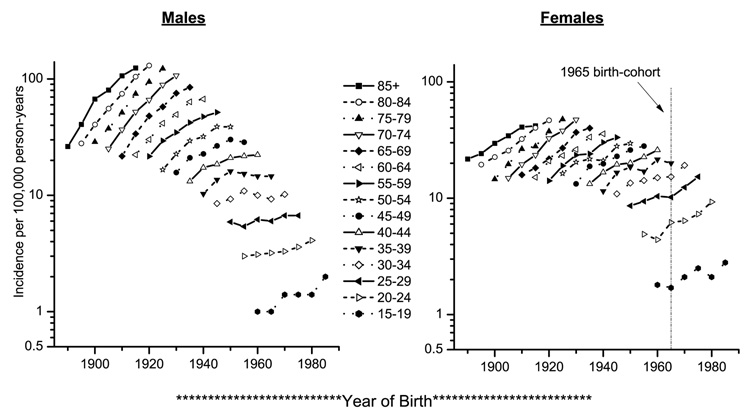
Age-specific incidence patterns by year of birth are presented in Figure 2. Male age-specific incidence rose steadily with each successive birth cohort until 1950, at which time incidence appeared to level off or decrease slightly. Female age-specific incidence by birth cohort increased steadily until around 1950; thereafter, incidence appeared to climb at a slower pace until 1965, at which point incidence appeared to begin accelerating. After adjustment for age and period effects, age-period-cohort modeling confirmed a change in the effect of birth cohort for women born between 1960 and 1965 (Supplementary Figure; slope change parameter = 0.2146; 95% CL 0.0576, 0.3716; p=0.007).
Figure 2.
Age-specific melanoma incidence among Caucasians stratified by sex and birth-cohort year in the SEER program from 1975–1979 through 2000–2004. The points vertically above each cohort year portray the cohort’s age-specific incidence experience. The vertical line at 1965 on the x-axis of the plot for women represents the time point after which melanoma incidence begins increasing in subsequent cohorts.
It is important to consider whether these trends may reflect changes in data quality, diagnosis or surveillance. There is evidence of increased underreporting of melanoma over time within the SEER program, with estimates as high as 17% of all cases (including in situ lesions) in two registries, although such a trend in underreporting cannot explain the observed increase in incidence among women (Seiffert, 1992;Merlino et al., 1997). It is unlikely that a change in melanoma diagnostic criteria would account for our finding, since the effect of such a change would not be expected to be sex-specific. Changes in screening patterns may have led to earlier detection within this time period, with a higher rate of increase seen among superficial localized tumors compared to thicker lesions and regional or metastatic disease overall (Jemal et al., 2001;Welch et al., 2005). Indeed, the observed decrease in melanoma mortality rates after 1981 and previously reported evidence of general improvement in survival by stage over this time period are consistent with a shift towards earlier detection of disease through increased surveillance (Jemal et al., 2001). However, in our analysis, the increasing trend among young women from the early 1990s onward was also observed for thicker and regional/distant tumors, which are less susceptible to misclassification. Moreover, our age-period-cohort analysis suggested that, after adjusting for age and period effects (the latter of which is reflective of changes in disease surveillance), the observed increase in incidence among women born after 1965 is consistent with a birth cohort effect (indicative of changes in disease risk factor prevalence across birth cohorts; (Tarone and Chu, 2000)). Thus, our findings are compatible with a real increase in incidence among young women, although we cannot rule out the effects of changes in surveillance.
The recent increase in incidence among young women parallels reported trends in exposure to ultraviolet radiation (UVR), the primary environmental cause of melanoma (Armstrong and Kricker, 2001). The prevalence of sunburn is increasing among U.S. adult men and women overall, although trends by age group have not been reported (Robinson et al., 1997;Saraiya et al., 2007). Among adolescents aged 16–18, both the prevalence of sunburn and the average number of days spent at the beach increased between sun surveys conducted in 1998 and 2004 (Cokkinides et al., 2006). Tanning bed usage, which has been recently evaluated as a probable cause of melanoma (International Agency for Research on Cancer, 2007), is increasing among U.S. adults and is most prevalent among young women (Robinson et al., 1997;Lazovich and Forster, 2005).
In conclusion, our analysis of SEER data suggests that melanoma incidence is increasing among young women. Additional studies are needed in order to clarify whether the increasing trends for melanoma and NMSC (Christenson et al., 2005) are the result of changes in UVR exposure in this population.
Supplementary Material
Sex-specific maximum likelihood estimates of 5-year birth cohort effects for an age-period-cohort model fit to cutaneous melanoma incidence data for Caucasians in the SEER Program from 1973 through 2004. The vertical line at 1965 on the x-axis of the plot for women represents the point at which there is an apparent change in the slope of cohort effects. Note regarding interpretation of plot: interpretation of individual birth cohort effect estimates is difficult because the parameters are not identifiable (i.e., there is not a unique set of estimates). However, a change in the slope of a birth cohort effects curve is indicative of a change in the magnitude of disease rates. An increase (or decrease) in the slope of the birth cohort effects curve indicates a worsening (or moderation) in the birth-cohort pattern of risk. Changes in birth cohort effects for cancer incidence rates are indicative of changes in disease risk factor prevalence across birth cohorts.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute. The SEER Program is operated by the National Cancer Institute Surveillance Research Program.
Abbreviations
- CI
confidence interval
- EAPC
estimated annual percent change
- NMSC
non-melanoma skin cancer
- SEER
the Surveillance, Epidemiology, and End Results (SEER) Program
- UVR
ultraviolet radiation
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, Roenigk RK. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- Cokkinides V, Weinstock M, Glanz K, Albano J, Ward E, Thun M. Trends in sunburns, sun protection practices, and attitudes toward sun exposure protection and tanning among US adolescents 1998-2004. Pediatrics. 2006;118:853–864. doi: 10.1542/peds.2005-3109. [DOI] [PubMed] [Google Scholar]
- Dennis LK, White E, Lee JA. Recent cohort trends in malignant melanoma by anatomic site in the United States. Cancer Causes Control. 1993;4:93–100. doi: 10.1007/BF00053149. [DOI] [PubMed] [Google Scholar]
- Hall HI, Miller DR, Rogers JD, Bewerse B. Update on the incidence and mortality from melanoma in the United States. J Am Acad Dermatol. 1999;40:35–42. doi: 10.1016/s0190-9622(99)70562-1. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007;120:1116–1122. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–683. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lazovich D, Forster J. Indoor tanning by adolescents: prevalence, practices and policies. Eur J Cancer. 2005;41:20–27. doi: 10.1016/j.ejca.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Merlino LA, Sullivan KJ, Whitaker DC, Lynch CF. The independent pathology laboratory as a reporting source for cutaneous melanoma incidence in Iowa, 1977–1994. J Am Acad Dermatol. 1997;37:578–585. doi: 10.1016/s0190-9622(97)70175-0. [DOI] [PubMed] [Google Scholar]
- Robinson JK, Rigel DS, Amonette RA. Trends in sun exposure knowledge, attitudes, and behaviors: 1986 to 1996. J Am Acad Dermatol. 1997;37:179–186. doi: 10.1016/s0190-9622(97)80122-3. [DOI] [PubMed] [Google Scholar]
- Saraiya M, Balluz L, Wen XJ, Joseph DA. Sunburn prevalence among adults - United States, 1999, 2003 and 2004. MMWR. 2007;56:524–528. [PubMed] [Google Scholar]
- SEER Progam: Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2006 Sub (1973–2004) - Linked To County Attributes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission
- SEER Program: Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State Total U.S. (1969–2004), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007. Underlying mortality data provided by NCHS. ( www.cdc.gov/nchs)
- Seiffert J. Underreporting of melanoma. J Natl Cancer Inst. 1992;84:289. [Google Scholar]
- Tarone RE, Chu KC. Age-period-cohort analyses of breast-, ovarian-, endometrial- and cervical- cancer mortality rates for Caucasian women in the USA. J Epidemiol Biostat. 2000;5:221–231. [PubMed] [Google Scholar]
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sex-specific maximum likelihood estimates of 5-year birth cohort effects for an age-period-cohort model fit to cutaneous melanoma incidence data for Caucasians in the SEER Program from 1973 through 2004. The vertical line at 1965 on the x-axis of the plot for women represents the point at which there is an apparent change in the slope of cohort effects. Note regarding interpretation of plot: interpretation of individual birth cohort effect estimates is difficult because the parameters are not identifiable (i.e., there is not a unique set of estimates). However, a change in the slope of a birth cohort effects curve is indicative of a change in the magnitude of disease rates. An increase (or decrease) in the slope of the birth cohort effects curve indicates a worsening (or moderation) in the birth-cohort pattern of risk. Changes in birth cohort effects for cancer incidence rates are indicative of changes in disease risk factor prevalence across birth cohorts.