Abstract
Convergent evidence indicates that in later stages of Parkinson's disease raphestriatal serotonin neurons compensate for the loss of nigrostriatal dopamine neurons by converting and releasing dopamine derived from exogenous administration of the pharmacotherapeutic L-3,4-dihydroxyphenyl-L-alanine (L-DOPA). Because the serotonin system is not equipped with dopamine autoregulatory mechanisms, it has been postulated that raphe-mediated striatal dopamine release may fluctuate dramatically. These fluctuations may portend the development of abnormal involuntary movements called L-DOPA-induced dyskinesia (LID). As such, it has been hypothesized that reducing the activity of raphestriatal neurons could dampen supraphysiological stimulation of striatal dopamine receptors thereby alleviating LID. To directly address this, the current study employed the rodent model of LID to investigate the contribution of the rostral raphe nuclei (RRN) in the development, expression and treatment of LID. In the first study, dual serotonin/dopamine selective lesions of the RRN and medial forebrain bundle, respectively, verified that the RRN are essential for the development of LID. In a direct investigation into the neuroanatomical specificity of these effects, microinfusions of ±8-OH-DPAT into the intact dorsal raphe nucleus dose-dependently attenuated the expression of LID without affecting the anti-parkinsonian efficacy of L-DOPA. These current findings reveal the integral contribution of the RRN in the development and expression of LID and implicate a prominent role for dorsal raphe 5-HT1AR in the efficacious properties of 5-HT1AR agonists.
Keywords: Parkinson's disease, serotonin, dopamine, raphe nuclei, L-DOPA, dyskinesia
Introduction
Initial treatment with L-3,4-dihydroxyphenyl-L-alanine (L-DOPA) is exceptionally effective for patients suffering from Parkinson's disease (PD). As PD progresses, higher doses of L-DOPA become necessary and the therapeutic window for dopamine (DA) replacement therapy narrows, increasing the risk for impaired antiparkinsonian efficacy and the development of abnormal involuntary movements called L-DOPA-induced dyskinesia (LID; Jankovic, 2005). Although the mechanism(s) underlying LID are multifaceted, pulsatile supraphysiological release of L-DOPA-derived DA within the striatum leading to abnormal stimulation of supersensitized DA receptors is clearly involved (Cenci & Lundblad, 2006).
Mounting evidence argues that the serotonin (5-HT) system plays a crucial role in this exaggerated release of exogenous DA into the striatum. Serotonergic neurons of the rostral raphe nuclei (RRN), including the dorsal (DRN) and median (MRN) raphe nuclei, hyperinnervate the striatum and are thought to usurp the role of the dopaminergic system upon severe DA denervation (Arai et al., 1998; Maeda et al., 2005; Kannari et al., 2006). Recently, Carta et al. (2007) suggested that DA released from striatal 5-HT terminals acts as a “false neurotransmitter”, unfettered by a lack of DA-sensitive autoreceptors. As a consequence, excessive swings in striatal DA levels following L-DOPA treatment may lead to LID development.
5-HT1A receptor (5-HT1AR) agonists have been shown to possess antidyskinetic properties in both animal and human populations (Bonifati et al., 1994; Bibbiani et al., 2001; Eskow et al., 2007; Goetz et al., 2007). Unfortunately, their utility has been limited by reports of exacerbated parkinsonian symptoms (Iravani et al., 2006) and findings that the 5-HT1AR agonist, sarizotan provided limited antidyskinetic efficacy in phase III clinical trials (Merck KGaA, NCT00105521). Therefore, more effective development and use of these promising compounds requires knowledge of their precise underlying mechanism(s). One prominent theory suggests that 5-HT1AR agonists may be acting at densely expressed somatodendritic 5-HT1A autoreceptors within the RRN, which normally modulate neuronal excitation and consequent release of 5-HT in projection areas (Kreiss & Lucki, 1994; Riad et al., 2000). The RRN send dense serotonergic input to the striatum and previous preclinical studies have indicated that stimulation of RRN 5-HT1ARs may temper pulsatile raphestriatal DA release from 5-HT terminals following DA denervation, curtailing the expression of LID (Kannari et al., 2001; Yamato et al., 2001). However, neither the direct role of the RRN in the development of LID nor the specific neuroanatomical loci for the antidyskinetic effects of 5-HT1AR agonists have been directly investigated. To clarify this critical mechanism, the current study determined the role of the RRN in the development of abnormal involuntary movements and examined the unique contribution of DRN 5-HT1AR in the antidyskinetic effects of 5-HT1AR agonists in hemiparkinsonian rats. The results of the present investigation directly identify that the RRN and intrinsic DRN 5-HT1AR contribute to LID development and the antidyskinetic effects of 5-HT1AR agonists, respectively.
Materials and methods
Animals
Adult male Sprague-Dawley rats were used (n=100; 225-250 g upon arrival; Taconic Farms, Hudson, NY, USA). Animals were housed in plastic cages (22 cm high, 45 cm deep and 23 cm wide) and had free access to standard lab chow (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. The colony room was maintained on a 12/12 h light/dark cycle (lights on at 0700 hrs) at a temperature of 22-23° C. Animals were maintained in strict accordance with the guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academic Press 1996; NIH publication number 85-23, revised 1996).
Experiment 1: Dual DA/5-HT lesions
Surgery
In order to determine how RRN 5-HT lesions impact LID development, rats (250-300 g) received unilateral 6-hydroxydopamine (6-OHDA) lesions of the left medial forebrain bundle (MFB) to destroy DA neurons. Desipramine HCl (25 mg/kg, i.p.; Sigma, St. Louis, MO, USA) was given 30 min prior to the 6-OHDA injection to protect norepinephrine (NE) neurons. Rats were anesthetized with inhalant isoflurane (2-3%; Sigma) in oxygen (2.5 L/min), and then placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The coordinates for 6-OHDA injections were AP: -1.8 mm, ML: +2.0 mm, DV: -8.6 mm relative to bregma, with the incisor bar positioned 5.0 mm below the interaural line (Paxinos & Watson, 1998). Using a 10 μL Hamilton syringe attached to a 26 gauge needle, 6-OHDA (12 μg; Sigma) dissolved in 0.9% NaCl + 0.1% ascorbic acid was infused through a small burr hole in the skull at a rate of 2 μL/min for a total volume of 4 μL. The needle was withdrawn 5 min later. During the same surgery, rats also received bilateral lesions of the serotonergic neurons of the DRN and MRN using 5,7-dihydroxytryptamine creatine sulphate (5,7-DHT; Sigma) at a dose of 12 μg per 2 μL in 1.0% ascorbic acid. A sham 5-HT lesion group was also included, which received 6-OHDA lesions of the MFB followed by injections of vehicle into the DRN and MRN. The coordinates for 5,7-DHT injections were AP: -7.8 mm, ML: 0 mm, DV: -6.8 (DRN) and -8.8 mm (MRN), relative to bregma, with the incisor bar positioned 5.0 mm below the interaural line (Paxinos & Watson, 1998). Using a 10 μL Hamilton syringe attached to a 26 gauge needle, 5,7-DHT was infused through a small burr hole in the skull at a rate of 2 μL/min for a total volume of 2 μL at each DV coordinate. After each infusion and before withdrawal, the needle was held in position for an additional 2 min to allow for diffusion into the tissue. All rats received injections of Buprenex (buprenorphine HCl; 0.03 mg/kg, i.p.; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) as analgesic treatment one h before, one h after and one day after surgery. Post-surgery, rats were pair-housed and soft chow and fruit were provided as needed to facilitate recovery during the first week after surgery.
Pharmacological treatments
Three weeks post-surgery rats were primed with L-DOPA methyl ester (L-DOPA; 12 mg/kg, s.c.; Sigma) + DL-serine 2-(2,3,4-trihydroxybenzyl) hydrazine hydrochloride (benserazide; 15 mg/kg, s.c.; Sigma) dissolved in 0.9% NaCl + 0.1% ascorbic acid and administered at a volume of 1.0 mg/kg once daily for 5 days to determine the effects of RRN lesions on the development of AIMs in dual-lesioned rats. Rats were tested for AIMs and rotations (see description below) on days 1, 3, and 5 of L-DOPA priming. While LID would not typically be seen so quickly in PD patients, AIMs develop within even one day of priming following commencement of L-DOPA treatment due to the extensive striatal DA loss (>90%) inflicted by MFB 6-OHDA lesions.
Experiment 2: Intraraphe 5-HT1AR stimulation
Surgery
In order to directly investigate the role of DRN 5-HT1AR in the antidyskinetic effects of 5-HT1AR agonists, rats received unilateral MFB 6-OHDA lesions and chronic cannulae directed at the DRN. The coordinates for intraraphe cannulations were AP: -8.0 mm and ML: -3.15, relative to bregma, and DV: -4.0 mm, relative to the skull surface, with the incisor bar positioned 5.0 mm below the interaural line (Paxinos & Watson, 1998). The stereotaxic arm was set at an angle of 30° to avoid infusion or diffusion of the drug within the cerebral aqueduct. Twenty-two gauge guide cannulae (Plastics One, Roanoke, VA, USA) were unilaterally implanted through a small burr hole in the skull. Twenty-eight gauge dummy cannulae were placed in guide cannulae to ensure patency. After surgery, rats were single-housed and soft chow and fruit were provided as needed to facilitate recovery during the first week after surgery. All rats also received an injection of Buprenex (0.03 mg/kg, i.p.) as analgesic treatment. Two weeks post-surgery, rats were habituated to the microinjection procedure 3 times in the week preceding testing during which they were wrapped loosely in a towel and their dummy cannulae were removed and reinserted.
Pharmacological treatments
Three weeks post-surgery, the effects of direct DRN 5-HT1AR stimulation on LID were determined. Rats (n=61) were primed with L-DOPA for 7 days (12 mg/kg + benserazide, 15 mg/kg, i.p.) and AIMs and rotational data were collected on days 1, 4, and 7 in order to assign rats to equally dyskinetic treatment groups. Only rats displaying ALO AIMs of >30 on day 4 of priming were included in this study, which corresponded with at least 90% DA depletion upon high-performance liquid chromatography (HPLC) analysis of striatal tissue samples (see also Eskow et al., 2007). Thereafter, rats received a treatment regimen of DRN microinjections every 3-4 days which included: Vehicle, ±8-OH-DPAT (0.5, 2.5, 7.5, or 15 μg), the 5-HT1AR antagonist N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (WAY100635; 2.5 μg; Sigma), or ±8-OH-DPAT (2.5 μg) + WAY100635 (2.5 μg). All treatments were dissolved in 0.6 μL of 20% dimethyl sulfoxide (DMSO) + 0.9% sodium chloride (NaCl) in dH2O and injected through an 18.7 mm injector at a rate of 0.4 μL/min for a total of 1.5 min. In order to limit damage and tissue fatigue at the target site, infusions were limited to 3 per animal. Preliminary test infusions with cresyl violet dye (FD Neurotechnologies, Baltimore, MD, USA) in a separate group of rats ensured that the placement and volume of the infusion were appropriate. Five min after each microinjection, rats were treated with L-DOPA (12 mg/kg + Benserazide, 15 mg/kg, i.p.) and tested for AIMs every 10th min for 2 h. Three days after the last microinjection, a final AIMs test ensured the stability of behavioral responses to L-DOPA over the course of testing.
In order to determine the effect of DRN 5-HT1AR stimulation on L-DOPA efficacy, a crossover within-subjects design was employed in an additional group of cannulated rats (n=21). L-DOPA-naïve animals were habituated to the Forepaw Adjusting Steps test 3 times in the week preceding assessment (FAS; described below) beginning 2 weeks after surgery. A baseline measurement of stepping was taken from the last day of habituation which occurred 2 days before initiation of pharmacological testing. Three weeks after surgery, animals received a DRN microinjection of either vehicle or ±8-OH-DPAT (2.5 μg), as described above. Five min after each microinjection, rats were treated with L-DOPA (6 mg/kg + benserazide, 15 mg/kg, i.p.). To allow for peak L-DOPA plasma levels (Sato et al., 1994), FAS testing began 1 h after L-DOPA injection. Each FAS test was separated by 2-3 days.
Behavioral analyses
Abnormal involuntary movements
Rats were monitored for AIMs using a procedure similar to that described in Lundblad et al. (2002) and Bishop et al. (2006). On test days (0900-1400 hrs), rats were individually placed in plastic trays (60 cm × 75 cm) 5 min prior to drug treatments. Following L-DOPA injection, a trained observer blind to treatment condition assessed each rat for exhibition of axial, limb, and orolingual movements (interrater reliability=0.98). In addition, contralateral rotations, defined as complete 360 degree turns away from the lesioned side of the brain, were tallied. Ipsilateral rotations were counted as negative numbers and thus, deducted from total contralateral rotations. Dystonic posturing of the neck and torso, involving positioning of the neck and torso in a twisted manner directed toward the side of the body contralateral to the lesion, were referred to as “axial” AIMs. “Forelimb” AIMs were defined as rapid, purposeless movements of the forelimb located on the side of the body contralateral to the lesion. “Orolingual” AIMs were composed of repetitive openings and closings of the jaw and tongue protrusions. The movements are considered abnormal since they occur at times when the rats are not chewing or gnawing on food or other objects. Every 10th or 20th min for 2 h, rats were observed for 60 s, during which AIMs and rotational behavior were tallied. During the observation periods, a severity score of 0-4 was assigned for each AIMs category: 0 = not present, 1 = present for less than 50% of the observation period (i.e. 1-29 s), 2 = present for more than 50% or more of the observation period (i.e. 30-59 s), 3 = present for the entire observation period (i.e. 60 s) but interrupted by a loud stimulus (a tap on the cage), or 4 = present for the entire observation period and not interrupted by a loud stimulus. For each AIMs category, the scores were summed for the entire 2 h period. Thus, the theoretical maximum score for each type of AIM was 24 (4 × 6 periods with 20 min intervals) or 48 (4 × 12 periods with 10 min intervals) although observed scores were never this severe. For the AIMs subcategories (axial, forelimb, and orolingual; ALO AIMs), scores were summed and broken down into the 1st h (0-60 min) and the 2nd h (70-120 min) of the observation period.
Forepaw adjusting steps
Using a procedure slightly modified from that described in Olsson et al. (1995) and Eskow et al. (2007), the number of adjusting steps taken by the forelimb in order to compensate for lateral movement was counted to determine the effects of lesions and drug treatments on motor performance. Rats were kept L-DOPA-naïve until the initiation of this portion of Experiment 2 and were treated with a lower dose of L-DOPA (6 mg/kg + benserazide, 15 mg/kg, s.c.) in order to avoid the development of severe AIMs that would confound forepaw movement on the FAS test. One h after L-DOPA injection, rats were moved laterally across a table at a steady rate of 90 cm/10 s. The rear part of the torso and the hindlimbs were lifted from the table and one forepaw was held by the experimenter so as to bear weight on the other forepaw. Each stepping test consisted of 6 trials for each forepaw, alternating between directions both forehand (defined as compensating movement toward the body) and backhand (defined as compensating movement away from the body) on the table. Since data derived from the forehand direction has proven to be more sensitive to both lesion and recovery of deficit, only forehand steps were reported. Data were expressed as the sum of forehand steps of the lesioned forelimb divided by the sum of forehand steps of the intact forelimb and multiplying the resultant by 100, yielding a percentage of the intact side which indicates the degree of forehand forepaw disability.
Histology and Neurochemical Analyses
Tissue dissection and cresyl violet staining
One week after the completion of experiments, all rats were killed by decapitation, brains were immediately removed and the striatum was dissected to determine monoamine levels. Tissue was frozen at -80°C and later subjected to monoamine analysis using high performance liquid chromatography with electrochemical detection (HPLC-ED). Rats exhibiting DA levels in the lesioned striatum that were >10% of the intact side were removed from analysis (n=7).
Rats included in Experiment 2 were also examined for verification of injection site within the DRN. To accomplish this, the brain section containing the injection site was rapidly frozen in 3-methylbutane (-30°C) and stored at -20°C. Twenty μm coronal sections with the cannulae placements were taken from each animal using a microtome cryostat (MICROM International GmbH; Model HM505E; Walldorf, Germany). Sections were then post-fixed with 4% paraformaldehyde (Fisher Scientific, Hanover Park, IL, USA) in 0.1 M phosphate-buffered saline (Sigma) and cresyl violet staining was used to determine injection sites and the level of gliosis. Only animals in which the injector tract ended within the DRN and did not exhibit extensive gliosis were included in the final analyses (n=58).
High-performance liquid chromatography
Reverse-phase HPLC-ED was performed on striatal tissue obtained from all rats included in the study, according to the protocol of Kilpatrick et al. (1986), a method for semi-automated catecholamine and indoleamine analysis with coulometric detection. The system included an autoinjector (ESA, Model 542, Chelmsford, MA), a solvent delivery system (ESA, Model 1582), an external pulse dampener (ESA), a Guard-Pak column (ESA), and a MD-150 × 3.2 (150 × 3.2 mm, 3 μm packing) column (ESA). Samples were homogenized in ice-cold perchloric acid (0.1 M), 1% ethanol, and 0.02% EDTA. The homogenates were spun for 30 min at 14,000 g with the temperature maintained at 4°C. Aliquots of supernatant were then analyzed for abundance of DA, 5-HT, NE, 3,4-dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindole-3-acetic acid (5-HIAA). Samples were separated using a mobile phase composed of 90 mM sodium dihydrogen phosphate (monobasic, anhydrous), 0.05 mM EDTA, 1.7 mM octane sulfonic acid, and 9% acetonitrile, adjusted to pH 3.0 with o-phosphoric acid. A coulometric detector configured with 3 electrodes (Coulochem III, ESA) measured content of monoamines and metabolites. An ESA model 5020 guard cell (+350 mV) was positioned prior to the autoinjector. The analytical cell (ESA model 501lA; first electrode at -100 mV, second electrode at +250 mV) was located immediately after the column. The second analytical electrode emitted signals that were recorded and analyzed by EZChrom Elite software via a Scientific Software, Inc. (SS420χ) module. The final oxidation current values were plotted on a standard curve of known concentrations from 10-6 M to 10-9 M, adjusted to striatal tissue weights and expressed as nanogram (ng) of monoamine or metabolite per milligram (mg) tissue (mean ± S.E.).
Data analyses
Striatal monoamine and metabolite levels were analyzed using paired t-tests. Non-parametric Kruskal-Wallis tests determined treatment effects (expressed as means ± S.E.) for summed ALO AIMs. Significant differences between treatments were examined by Mann-Whitney post-hoc comparisons. One or two-way ANOVAs and Fisher's LSD post hoc tests were employed for analyses of rotations and FAS results. Pearson's r correlation coefficients were used to determine correlations between ALO AIMs and the percentage of striatal 5-HT in lesioned animals when compared to control animals (sham 5-HT lesion). In all cases, alpha was set at p<0.05 and statistical analyses were conducted with Statistica Software '98 (Statsoft, Inc., Tulsa, OK, USA).
Results
Experiment 1
The effect of MFB 6-OHDA and RRN 5,7-DHT lesions on striatal monoamine and metabolite levels
Collectively, all rats (n=35) received near-complete, unilateral DA lesions of the nigrostriatal pathway as indicated by significant reductions in ipsilateral striatal DOPAC (>90%) and DA levels (>99%) when compared with the contralateral intact side. A number of rats (n=24) also received 5-HT-selective 5,7-DHT lesions of the RRN to examine the role of 5-HT neurons in the development of LID. At the conclusion of the experiment, striatal 5-HT levels were used to parse animals into 3 statistically-distinct RRN lesion groups: sham 5-HT lesion (control; 100% intact 5-HT), mild 5-HT lesion (68.5 ± 2.6% intact 5-HT), and moderate 5-HT lesion (34.9 ± 4.5% intact 5-HT). Between these groups, 5-HIAA was similarly reduced in mild and moderate 5-HT lesion groups compared to sham-lesioned rats (both p<0.05), while ipsilateral DA and DOPAC levels did not differ. Within each 5-HT lesion group, DA and DOPAC levels within the lesioned striata were significantly less than those in the intact striata and all animals showed at least 99% and 94% depletion, respectively (all p<0.05).
RRN lesions differentially reduce the development of AIMs
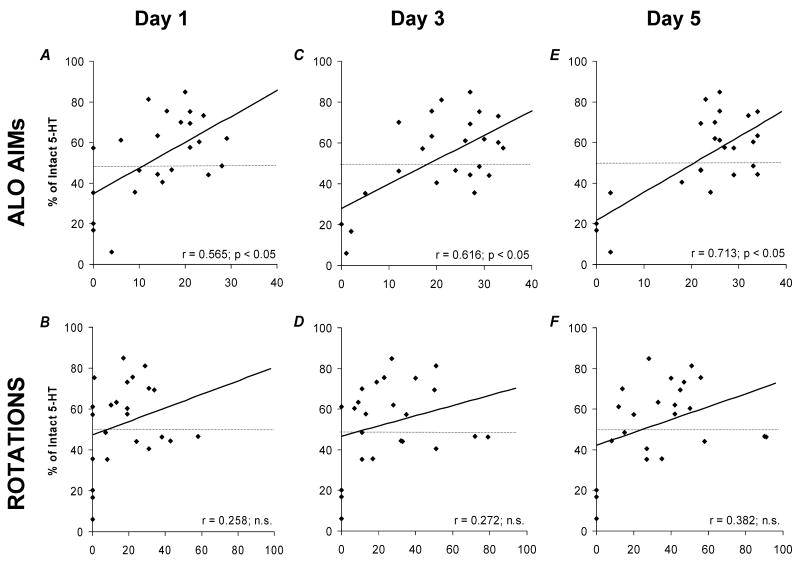
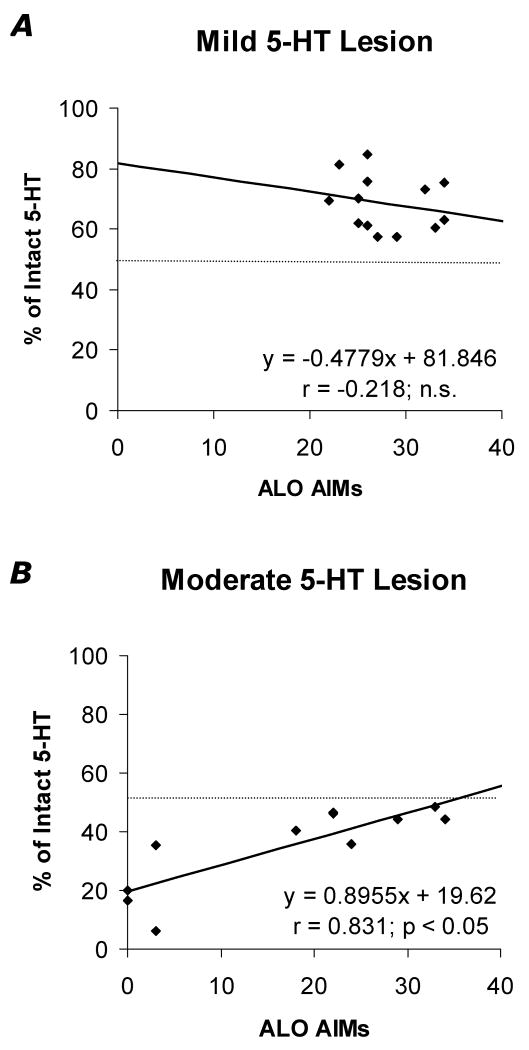
Recent evidence supports the role of 5-HT neurons in LID development (Carlsson et al., 2007; Carta et al., 2007). In order to more directly determine the contribution of the RRN in these effects, the RRN lesion groups described above received 5 consecutive days of L-DOPA treatment (12 mg/kg/day) at a dose known to rapidly induce AIMs (Bishop et al., 2006; Eskow et al., 2007). At study end, L-DOPA-induced ALO AIMs and rotations (X-axes) from days 1, 3, and 5 were plotted against percent intact striatal 5-HT (X-axes; derived from average striatal 5-HT in sham-lesioned rats). As shown in Figure 1(A, C, E), ALO AIMs were positively correlated with striatal 5-HT levels and progressive days of L-DOPA strengthened these effects (all p<0.05). In contrast, striatal 5-HT levels were not significantly correlated with rotational behaviors (Figure 1B, D, F). To better define the degree of 5-HT depletion necessary to modify ALO AIMs, rats within the mild (<50%) and moderate (>50%) 5-HT lesion groups were analyzed separately on day 5 of priming. As shown in Figure 2, only striatal 5-HT depletion of >50% predicted a reduction in ALO AIMS, indicating that mild RRN lesions do not sufficiently disable raphestriatal release of L-DOPA-derived DA.
Figure 1.
Effects of 5,7-DHT RRN 5-HT lesions on the development of L-DOPA-induced ALO AIMs and rotations in hemiparkinsonian rats. Correlations between percent striatal 5-HT (Y-axes; as compared to sham 5-HT lesioned rats) and ALO AIMs (X-axes; A, C, E) or rotations (X-axes; B, D, F) on days 1 (A, B), 3 (C, D), and 5 (E, F) of L-DOPA (12 mg/kg + benserazide, 15 mg/kg) priming are shown using Pearson's r correlation coefficients.
Figure 2.
Differential effects of mild (A; <50% 5-HT striatal depletion) versus moderate (B; >50% striatal 5-HT depletion) 5,7-DHT RRN 5-HT lesions on the development of L-DOPA-induced ALO AIMs in hemiparkinsonian rats. Correlations between percent striatal 5-HT (Y-axes) and ALO AIMs (X-axes) on day 5 of priming with L-DOPA (12 mg/kg + benserazide, 15 mg/kg) in rats with mild or moderate 5-HT lesions are reported as Pearson's r correlation coefficients.
Experiment 2
Monoamine & metabolite levels
As in the previous experiment, all rats (n=58) received severe unilateral DA-depleting MFB 6-OHDA lesions. As verified by HPLC-ED analysis, these lesions significantly reduced striatal DOPAC (>90%) and DA levels (>99%), compared with the contralateral intact striatum (both p<0.05). In this group of rats, 5-HIAA levels were slightly elevated on the lesioned side (114%) and there were no differences between contralateral and ipsilateral striatal 5-HT.
Microinjection of ±8-OH-DPAT into the DRN dose-dependently reduces ALO AIMs and rotations
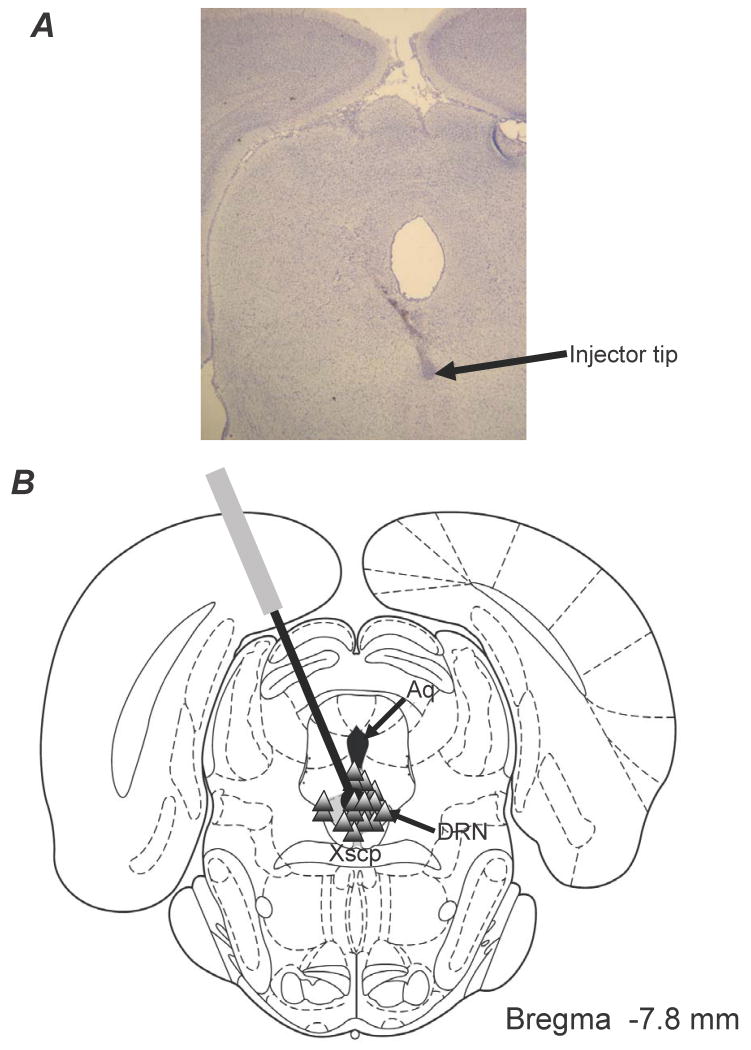
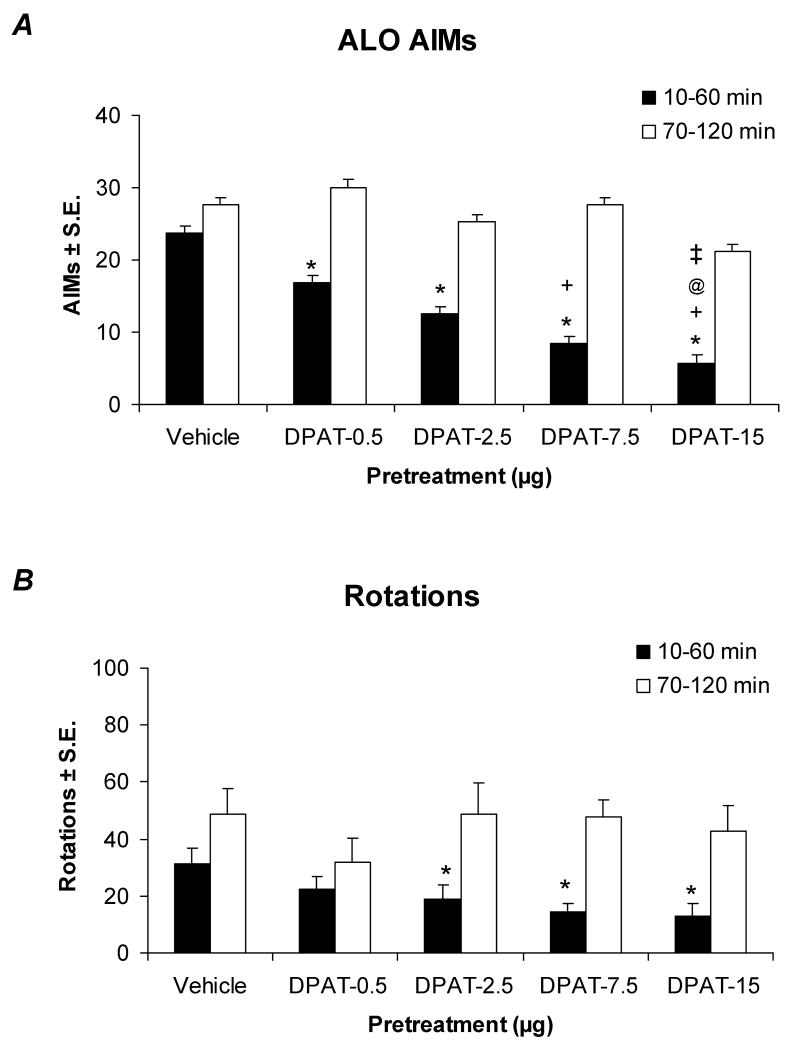
The DRN is known as a major functional contributor of serotonergic innervation to the striatum in both rats and humans (Azmitia & Segal, 1978; McQuade & Sharp, 1997; Hornung, 2003). To date, the precise role of DRN 5-HT1AR in the antidyskinetic effects of 5-HT1AR agonists remains speculative. To clarify this, ±8-OH-DPAT was directly administered via microinfusion into the DRN of hemiparkinsonian, L-DOPA-primed rats through chronic implanted cannulae followed by a systemic injection of L-DOPA (12 mg/kg/day). All rats included in the final analysis had confirmed injector placements within the DRN (Figure 3; n=58, success rate: ∼70%). Direct DRN injections of ±8-OH-DPAT ranging from 0.5 to 15 μg were found to dose-dependently reduce ALO AIMs (Figure 4A), while the attenuation of L-DOPA-induced rotations was limited to the 3 highest doses (Figure 4B). The antidyskinetic effects of ±8-OH-DPAT were most pronounced during the 1st h after administration of L-DOPA and ranged from a 28% reduction with the lowest dose to a 72% reduction with the highest dose, when compared to vehicle-injected animals. There were no significant effects of ±8-OH-DPAT treatment in the 2nd h, corresponding to its 31-46 min half-life within the brainstem of rats (Yu & Lewander, 1997). These results are the first to directly confirm the integral role of the DRN in the antidyskinetic effects of 5-HT1AR agonists.
Figure 3.
(A) Histological example of an injector tip placed within the DRN. (B) Schematic representation of a coronal section of the rat brain depicting the distribution of DRN microinfusion sites. This section was taken from Paxinos & Watson (1998). Triangles denote placements within the DRN (shaded). Relevant anatomical structures are: Aq, aqueduct; Xscp, decussation of the superior cerebellar peduncle.
Figure 4.
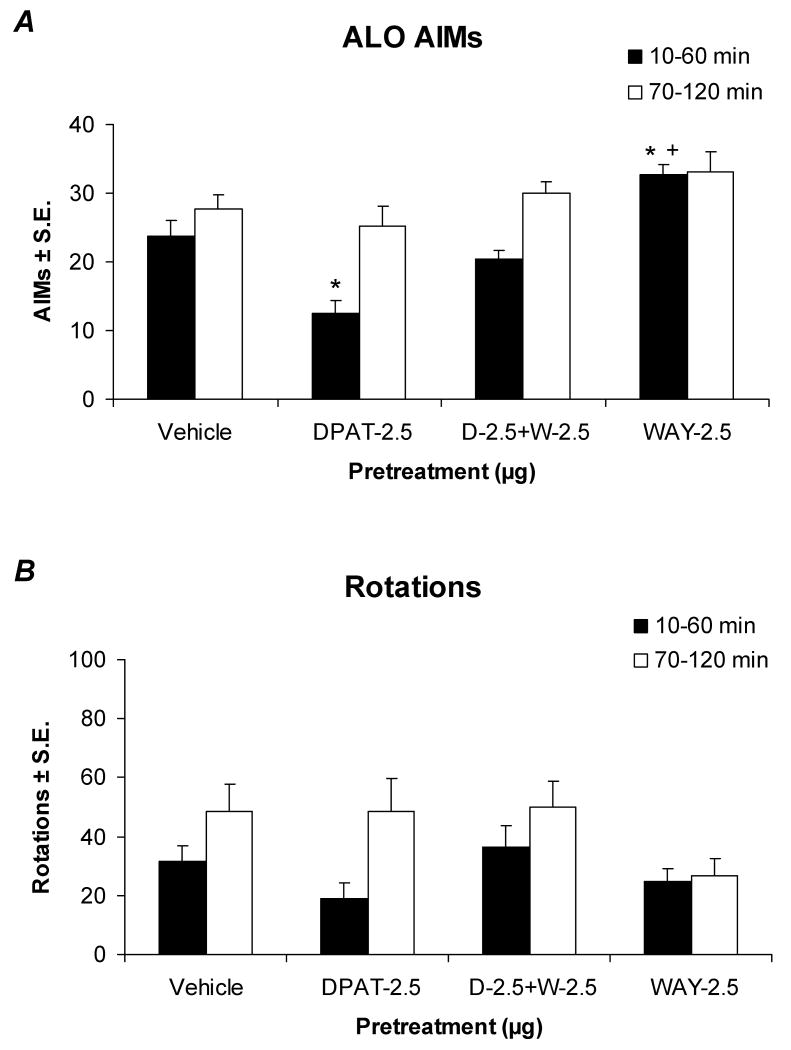
Effects of DRN microinfusion of the 5-HT1AR agonist ±8-OH-DPAT on the expression of ALO AIMs (A) and rotations (B) in L-DOPA-primed rats. Bars denote the effects of Vehicle (20% DMSO, 0.9% NaCl in dH2O; n=12) or ±8-OH-DPAT (DPAT; 0.5, 2.5, 7.5, or 15 μg; n=10-14/group) in the 1st (black bars) or 2nd h (white bars) following L-DOPA (12 mg/kg + benserazide, 15 mg/kg). A, Statistical analyses of ALO AIMs revealed significant effects of treatment in the 1st (χ2=31.30, p<0.05) but not the 2nd h (χ2=7.41, n.s.) after L-DOPA treatment. B, Analyses of rotations demonstrated a similar pattern of significant treatment effects for the 1st h (F4,57=3.07, p<0.05) but not the 2nd h (F4,57=0.74, n.s.). Additional statistical differences from Vehicle (* p<0.05), the 0.5 μg dose (+ p<0.05), the 2.5 μg dose (@ p<0.05), or the 7.5 μg dose (‡ p<0.05) were established with post-hoc comparisons.
The 5-HT1AR antagonist WAY100635 reverses ±8-OH-DPAT's effects and worsens ALO AIMs
In order to ensure the receptor specificity of DRN-infused ±8-OH-DPAT, the selective 5-HT1AR antagonist WAY100635 was utilized. Co-infusion of WAY100635 (2.5 μg) within the DRN reversed the antidyskinetic effects of ±8-OH-DPAT (2.5 μg) on ALO AIMs within the 1st h after L-DOPA treatment (12 mg/kg; Figure 5A), while there were no significant effects upon rotational behaviors (Figure 5B). Surprisingly, administration of WAY100635 alone produced a significant prodyskinetic effect (p<0.05), indicating that it may either block tonic activation of 5-HT1A receptors by endogenous 5-HT or function as an inverse agonist at the level of the DRN. This was further supported by observations that ALO AIMs in WAY100635-treated rats appeared earlier than those in vehicle-pretreated rats (data not shown). In fact, the dyskinetic movements themselves seemed severe enough to impair L-DOPA-induced rotations in the 2nd h of testing (Figure 5B). WAY100635 is purported to be a “silent” 5-HT1AR antagonist when administered systemically (Forster et al., 1995), and therefore the prodyskinetic properties of WAY100635 were unexpected though not unprecedented (Cosi & Koek, 2000). These additional effects promote the assertion that DRN 5-HT1AR regulate the release of raphe-derived striatal DA, as 5-HT1AR blockade would be expected to reverse the auto-inhibition of DA release normally provided by somatodendritic 5-HT1AR stimulation. Collectively, these results support the prominent modulatory actions of DRN somatodendritic 5-HT1A autoreceptors in LID.
Figure 5.
Effects of DRN microinfusion of the 5-HT1AR agonist ±8-OH-DPAT and the 5-HT1AR antagonist WAY100635 on the expression of ALO AIMs (A) and rotations (B) in L-DOPA-primed rats. The 1st (black bars) and 2nd h (white bars) following pretreatments of Vehicle (20% DMSO, 0.9% NaCl in dH2O; n=12), ±8-OH-DPAT (DPAT; 2.5 μg; n=11), co-administration of ±8-OH-DPAT + WAY100635 (DPAT + WAY; 2.5 μg; n=10), or WAY100635 (WAY; 2.5 μg; n=11) alone and subsequent treatment with L-DOPA (12 mg/kg + benserazide, 15 mg/kg) are displayed. A, Statistical analyses of ALO AIMs found significant effects of treatment during the 1st h (χ2=23.89, p<0.05) but not the 2nd h (χ2=6.77, n.s.) after L-DOPA treatment. B, Analyses of rotations for the 1st h (F3,40=2.40, n.s.) and 2nd h (F3,40=1.73, n.s.) revealed no significant treatment effects. Post hoc comparisons established significant differences from Vehicle (* p<0.05), 2.5 μg of ±8-OH-DPAT (+ p<0.05), or 2.5 μg of WAY100635 (@ p<0.05).
DRN-infused ±8-OH-DPAT does not alter the antiparkinsonian efficacy of L-DOPA
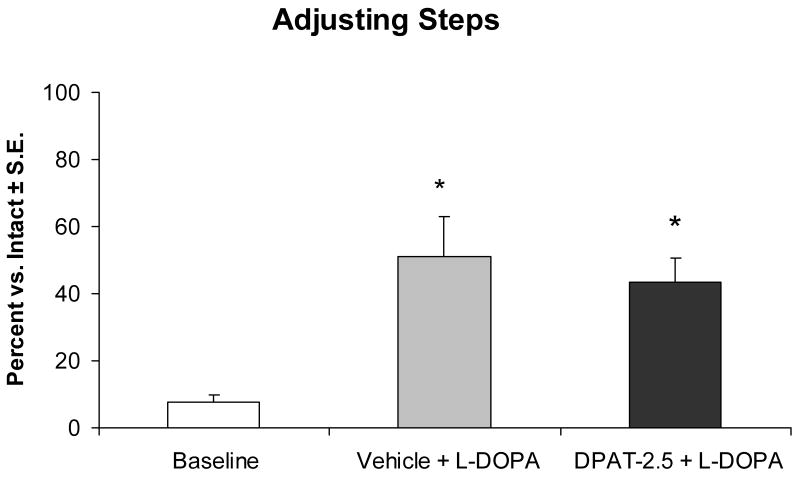
Since systemic administration of 5-HT1AR agonists have been reported to enhance (Bibbiani et al., 2001; Matsubara et al., 2006; Eskow et al., 2007) or reduce (Kannari et al., 2002; Iravani et al., 2006; Fox et al., 2007) the antiparkinsonian efficacy of L-DOPA, a well-established measure of motor performance and akinesia, the FAS test (Olsson et al., 1994; Chang et al., 1999) was employed. As shown in Figure 6, intraraphe administration of ±8-OH-DPAT had no adverse effects on L-DOPA-improved motor performance. Compared to baseline, lesioned forepaw stepping was significantly improved by 43% upon L-DOPA administration (p<0.05). An antidyskinetic dose of DRN ±8-OH-DPAT (2.5 μg) with L-DOPA also significantly improved stepping by 35% (p<0.05), and did not differ from vehicle and L-DOPA. These results corroborate previous systemic work with the 5-HT1AR partial agonist, buspirone (Eskow et al., 2007) and demonstrate that DRN 5-HT1AR stimulation at a dose that provides relief from LID does not exacerbate parkinsonian symptoms in the rodent model.
Figure 6.
Effects of DRN microinfusion of the 5-HT1AR agonist ±8-OH-DPAT and systemic administration of L-DOPA on FAS motor performance in L-DOPA-naïve rats (n=17). Bars denote the lesioned forepaw disability as a percentage of the intact forepaw in response to treatment with Vehicle (20% DMSO, 0.9% NaCl in dH2O) + L-DOPA (6 mg/kg + benserazide, 15 mg/kg) or ±8-OH-DPAT (DPAT; 2.5 μg) + L-DOPA (6 mg/kg + benserazide, 15 mg/kg). Upon finding main effects of treatment (F2,32=9.50; p<0.05), significant improvements from baseline performance in Vehicle + L-DOPA and DPAT-2.5 + L-DOPA were confirmed by post hoc comparisons (* p<0.05).
Discussion
The present results demonstrate that RRN lesions significantly modify the development of L-DOPA-induced AIMs in the hemiparkinsonian rat. Moreover, DRN microinfusions of the 5-HT1AR agonist, ±8-OH-DPAT dose-dependently reduce LID without impairing L-DOPA-improved motor performance. Collectively, these novel findings corroborate and extend previous research supporting an important raphe-mediated mechanism for the striatal release of L-DOPA-derived DA in the hemiparkinsonian, L-DOPA-treated rat and confirm a regulatory role for DRN somatodendritic 5-HT1A autoreceptors in LID.
Role of the RRN in the development of LID
Anatomical studies of rodent and human brains implicate two functionally distinct 5-HT pathways that normally influence striatal function (Azmitia & Segal, 1978; Hornung, 2003). While few of the synaptic contacts within the striatum arise from MRN 5-HT projections, neurons from the DRN deliver the majority of striatal 5-HT via volume transmission (Mamounas et al., 1991; McQuade & Sharp, 1997). While RRN neurons are primarily responsible for 5-HT catabolism, they are also capable of converting L-DOPA to DA via aromatic L-acid decarboxylase (Ng et al., 1971; Gershanik et al., 1979; Arai et al., 1996). Following severe striatal DA depletion, the RRN appears to play a compensatory role, hyperinnervating the striatum and usurping the role of the compromised nigrostriatal dopaminergic system (Zhou et al., 1991; Guerra et al., 1997; Maeda et al., 2003). As such, systemic L-DOPA treatment to DA-denervated rats produces an increase in striatal DA that has been attributed to the release of L-DOPA-derived DA from 5-HT terminals (Tanaka et al., 1999; Kannari et al., 2001; Carta et al., 2007).
Therefore, it has been suggested that raphestriatal neurons are responsible for the development of L-DOPA-induced motor complications in DA-depleted rats. Tanaka and colleagues (1999) have shown an inhibition in the rise of DA concentrations and an 80% decrease in rotational behavior in L-DOPA-treated, hemiparkinsonian rats with extensive 5-HT lesions of the MRN and DRN. Furthermore, non-specific intracerebroventricular 5,7-DHT lesions reduced the development of AIMs in partial DA-lesioned rats and alleviated AIMs expression in both partial and complete DA-lesioned, L-DOPA-primed rats (Carta et al., 2007). However, the effect of direct concurrent RRN 5-HT and MFB DA lesions on the development of LID in L-DOPA-naïve rats has not previously been examined. Our results provide evidence that the RRN are indeed responsible for the development and expression of LID. To the extent that 5-HT levels in the striatum reflect the percentage of intact RRN neurons, ALO AIMs were increasingly associated with the percent intact striatal 5-HT compared to control rats as L-DOPA priming progressed (Figure 1), with the strongest associations observed in animals that had more extensive 5-HT lesions (Figure 2). In fact, animals with mild 5-HT lesions did not show any difference in AIMs development when compared to sham 5-HT-lesioned animals. Thus, it appears that there is a threshold at which 5-HT RRN neurons become less efficient at converting and releasing L-DOPA-derived DA within the striatum. The threshold observed here may also be due to the loss of 5-HT regulatory mechanisms, which include 5-HT1A and 5-HT1B autoreceptors. Serotonin lesions would certainly remove the serotonergic regulation from 5-HT1A and 5-HT1B autoreceptors, both of which have been shown to play an important role in the alleviation of LID (Zhang et al., 2008; Carta et al., 2007; Kannari et al., 2001). Interestingly, similar reductions in AIMs upon L-DOPA treatment are observed regardless of whether 5-HT lesions are given at the same time as the DA-depleting lesion or after the DA-depleting lesion and L-DOPA-priming has taken place (Carta et al., 2007), suggesting both organizational and activational underlying alterations.
Alleviation of LID via 5-HT1AR stimulation
Given the convergent evidence that the serotonergic RRN play a central role in the development and expression of LID, pharmacotherapies that reduce RRN activity may act to diminish its manifestation. As such, somatodendritic 5-HT1A autoreceptors are a prime target. These receptors are densely expressed in the RRN where they serve an autoregulatory function, tempering the release of native 5-HT and presumably, L-DOPA-derived DA (Lanfurney & Hamon, 2000). In fact, peripheral administration of ±8-OH-DPAT attenuates the supraphysiological rise in striatal DA levels following L-DOPA treatment in hemiparkinsonian rats (Kannari et al., 2001), implicating a mechanism whereby 5-HT1AR agonists alleviate L-DOPA-induced motor complications in rodents (Tomiyama et al., 2005; Dupre et al., 2007), primates (Bibbiani et al., 2001; Iravani et al., 2006), and human PD patients (Bonifati et al., 1994; Olanow et al., 2004; Bara-Jimenez et al., 2005; Goetz et al., 2007).
To date, the role of the DRN in the antidyskinetic effects of 5-HT1AR agonists has been implied by the dense 5-HT1AR pool and striatal afferent projections of this brain region (Azmitia & Segal, 1978; McQuade & Sharp, 1997; Riad et al., 2000). However, the direct role of the DRN 5-HT1AR has not been tested. Through the use of small-volume direct DRN microinfusions of ±8-OH-DPAT, we addressed this issue. As shown in Figure 4, a powerful dose-dependent decrease in dyskinetic behaviors in L-DOPA-primed rats was observed, an effect that proved to be due to selective stimulation of DRN 5-HT1AR (Figure 5). Though not directly studied in the current experiment, DRN 5-HT1AR stimulation is thought to diminish the supraphysiological release of raphestriatal L-DOPA-derived DA, thus alleviating the expression of LID (Tanaka et al., 1999; Kannari et al., 2001; Carta et al., 2007). As additional support, we observed that AIMs were augmented by administration of a 5-HT1AR antagonist alone. Thus, we suggest that 5-HT1AR antagonism may disinhibit the release of striatal DA from DRN 5-HT neurons, resulting in a concomitant rise in AIMs. Collectively, the current results demonstrate that somatodendritic DRN 5-HT1A autoreceptors play a prominent modulatory role and possibly serve to either exacerbate or dampen raphestriatal DA release.
Additionally, it was necessary to ensure that the antidyskinetic effects of DRN 5-HT1AR administration were not due to overall motor impairment by squelching raphestriatal DA release. In agreement with previous studies with systemic 5-HT1AR agonist administration (Eskow et al., 2007), we observed that a low dose of ±8-OH-DPAT that diminished L-DOPA-induced AIMs did not impede L-DOPA-improved motor performance (Figure 6). Other reports of a worsening of parkinsonism upon 5-HT1AR agonist administration (Olanow et al., 2004; Iravani et al., 2006; Carta et al., 2007; Fox et al., 2007) may be resolved by considering the differential populations of receptors (ie. presynaptic autoreceptors and postsynaptic receptors) that are normally stimulated by peripheral adjunct treatment with 5-HT1AR agonists. Presynaptic autoreceptors are known to have a higher sensitivity to pharmacological stimulation than postsynaptic receptors (Carey et al., 2004). Therefore, high doses of potent 5-HT1AR agonists (>0.2 mg/kg) preferentially stimulate extra-raphe postsynaptic receptors, resulting in a spectrum of debilitating motor symptoms including forepaw treading and flat body posture collectively known as “serotonin syndrome” (Goodwin et al., 1987; Blanchard et al., 1993; Bert et al., 2006). Thus, the parkinsonian effects of adjunct +8-OH-DPAT, a more potent 5-HT1AR agonist enantiomer, in MPTP-treated monkeys may have reflected postsynaptic 5-HT1AR stimulation (Iravani et al., 2006). Our results attest that selective presynaptic stimulation of somatodendritic 5-HT1A autoreceptors regulates the supraphysiological swings in raphestriatal L-DOPA-derived DA that lead to LID, and importantly, without adversely affecting L-DOPA-improved motor capacity.
Conclusion
The current study corroborates the integral role of RRN in the development and expression of LID. Though future studies are certainly necessary to determine the mechanisms behind the phenotypic switch that enables the RRN to usurp the role of the nigrostriatal DA system in converting and releasing L-DOPA-derived DA, our novel results directly support the neuroanatomical role of RRN in movement control following DA denervation in the rat 6-OHDA model. The antidyskinetic and motor benefits of DRN-selective 5-HT1AR stimulation observed in the current study attest that this receptor subtype may serve as an effective pharmacological target that could be further explored for the establishment of beneficial adjunct treatments with traditional DA replacement therapies.
Acknowledgments
Financial Support: This work was supported by funds from the American Parkinson Disease Association, the Center for Development and Behavioral Neuroscience at Binghamton University, and NIH NS059600.
Abbreviations
- AIMs
Abnormal Involuntary Movements
- benserazide
DL-Serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride
- DA
Dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- L-DOPA
L-3,4-dihydroxyphenylalanine methyl ester
- HPLC-EC
high performance liquid chromatography coupled to electrochemical detection
- 5-HIAA
5-hydroxyindole-3-acetic acid
- 5-HT
Serotonin
- LID
L-DOPA-induced dyskinesia
- NE
Norepinephrine
- 6-OHDA
6-hydroxydopamine hydrobromide
- PD
Parkinson's disease
- VEH
Vehicle
- WAY100635
N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt
- ±8-OH-DPAT
(±)-8-Hydroxy-2-(di-n-propylamino)tetralin hydrobromide
- FAS
Forelimb Adjusting Steps
- RRN
rostral raphe nuclei
References
- Arai R, Horiike K, Hasegawa Y. Dopamine-degrading activity of monoamine oxidase in locus coeruleus and dorsal raphe nucleus neurons. A histochemical study in the rat. Neurosci Lett. 1998;250:41–44. doi: 10.1016/s0304-3940(98)00429-7. [DOI] [PubMed] [Google Scholar]
- Arai R, Karasawa N, Nagatsu I. Dopamine produced from L-DOPA is degraded by endogenous monoamine oxidase in neurons of the dorsal raphe nucleus of the rat: an immunohistochemical study. Brain Res. 1996;722:181–184. doi: 10.1016/0006-8993(96)00252-1. [DOI] [PubMed] [Google Scholar]
- Aubert I, Guigoni C, Håkansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Bibbiani F, Morris MJ, Dimitrova T, Sherzai A, Mouradian MM, Chase TN. Effects of serotonin 5-HT1A agonist in advanced Parkinson's disease. Mov Disord. 2005;20:932–936. doi: 10.1002/mds.20370. [DOI] [PubMed] [Google Scholar]
- Bert B, Fink H, Hörtnagl H, Veh RW, Davies B, Theuring F, Kusserow H. Mice over-expressing the 5-HT(1A) receptor in cortex and dentate gyrus display exaggerated locomotor and hypothermic response to 8-OH-DPAT. Behav Brain Res. 2006;167:328–341. doi: 10.1016/j.bbr.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Chase TN. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology. 2001;57:1829–1834. doi: 10.1212/wnl.57.10.1829. [DOI] [PubMed] [Google Scholar]
- Bishop C, Taylor JL, Kuhn DM, Eskow KL, Park JY, Walker PD. MDMA and fenfluramine reduce L-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. Eur J Neurosci. 2006;23:2669–2676. doi: 10.1111/j.1460-9568.2006.04790.x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Shepherd JK, Armstrong J, Tsuda SF, Blanchard DC. An ethopharmacological analysis of the behavioral effects of 8-OH-DPAT. Psychopharmacology (Berl) 1993;112:55–63. doi: 10.1007/BF02247363. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G. Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol. 1994;17:73–82. doi: 10.1097/00002826-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Carey R, Depalma G, Damianopoulos E, Muller C, Huston J. The 5-HT1A receptor and behavioral stimulation in the rat: effects of 8-OHDPAT on spontaneous and cocaine-induced behavior. Psychopharmacology (Berl) 2004;177:46–54. doi: 10.1007/s00213-004-1917-4. [DOI] [PubMed] [Google Scholar]
- Carlsson T, Carta M, Winkler C, Björklund A, Kirik D. Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesias in a rat model of Parkinson's disease. J Neurosci. 2007;27:8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Cenci M, Lundblad M. Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J Neurochem. 2006;99:381–392. doi: 10.1111/j.1471-4159.2006.04124.x. [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Cosi C, Koek W. The putative “silent” 5-HT1A receptor antagonist, WAY 100635, has inverse agonist properties at cloned human 5-HT1A receptors. Eur J Pharmacol. 2000;401:9–15. doi: 10.1016/s0014-2999(00)00410-6. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernández R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, Ruth TJ, Stoessl AJ. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson's disease: implications for dyskinesias. Brain. 2004;127:2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Negron G, Bishop C. The differential effects of 5-HT(1A) receptor stimulation on dopamine receptor-mediated abnormal involuntary movements and rotations in the primed hemiparkinsonian rat. Brain Res. 2007;1158:135–143. doi: 10.1016/j.brainres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fletcher A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Fox SH, Lang AE, Brotchie JM. Translation of nondopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to Phase IIa clinical studies: Keys to success and roads to failure. Mov Disord. 2007;21:1578–1594. doi: 10.1002/mds.20936. [DOI] [PubMed] [Google Scholar]
- Gershanik O, Heikkila R, Duvoisin R. The role of serotonin neurons in the action of L-DOPA in an animal model of parkinsonism. Neurology. 1979;29:553. [Google Scholar]
- Goetz CG, Damier P, Hicking C, Laska E, Müller T, Olanow CW, Rascol O, Russ H. Sarizotan as a treatment for dyskinesias in Parkinson's disease: a double-blind placebo-controlled trial. Mov Disord. 2007;22:179–186. doi: 10.1002/mds.21226. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR, Heal DJ. The pharmacology of the behavioural and hypothermic responses of rats to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) Psychopharmacology (Berl) 1987;91:506–511. doi: 10.1007/BF00216019. [DOI] [PubMed] [Google Scholar]
- Guerra MJ, Liste I, Labandeira-Garcia JL. Effects of lesions of the nigrostriatal pathway and of nigral grafts on striatal serotonergic innervation in adult rats. Neuroreport. 1997;8:3485–3488. doi: 10.1097/00001756-199711100-00014. [DOI] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Tayarani-Binazir K, Chu WB, Jackson MJ, Jenner P. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist (R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with\ increased motor disability. Pharmacol Exp Ther. 2006;319:1225–1234. doi: 10.1124/jpet.106.110429. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Motor fluctuations and dyskinesias in Parkinson's disease: clinical manifestations. Mov Disord. 2005;20:S11–S16. doi: 10.1002/mds.20458. [DOI] [PubMed] [Google Scholar]
- Kannari K, Yamato H, Shen H, Tomiyama M, Suda T, Matsunaga M. Activation of 5-HT(1A) but not 5-HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered L-DOPA in the striatum with nigrostriatal denervation. J Neurochem. 2001;76:1346–1353. doi: 10.1046/j.1471-4159.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- Kannari K, Kurahashi K, Tomiyama M, Maeda T, Arai A, Baba M, Suda T, Matsunaga M. Tandospirone citrate, a selective 5-HT1A agonist, alleviates L-DOPA-induced dyskinesia in patients with Parkinson's disease. No To Shinkei. 2002;54:133–137. [PubMed] [Google Scholar]
- Kannari K, Shen H, Arai A, Tomiyama M, Baba M. Reuptake of L-DOPA-derived extracellular dopamine in the striatum with dopaminergic denervation via serotonin transporters. Neurosci Lett. 2006;402:62–65. doi: 10.1016/j.neulet.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki L. Differential regulation of serotonin (5-HT) release in the striatum and hippocampus by 5-HT1A autoreceptors of the dorsal and median raphe nuclei. J Pharm Exp Ther. 1994;269:1268–1279. [PubMed] [Google Scholar]
- Lanfurney L, Hamon M. Central 5-HT(1A) receptors: regional distribution and functional characteristics. Nucl Med Biol. 2000;27:429–435. doi: 10.1016/s0969-8051(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kannari K, Shen H, Arai A, Tomiyama M, Matsunaga M, Suda T. Rapid induction of serotonergic hyperinnervation in the adult rat striatum with extensive dopaminergic denervation. Neurosci Lett. 2003;343:17–20. doi: 10.1016/s0304-3940(03)00295-7. [DOI] [PubMed] [Google Scholar]
- Maeda T, Nagata K, Yoshida Y, Kannari K. Serotonergic hyperinnervation into the dopaminergic denervated striatum compensates for dopamine conversion from exogenously administered l-DOPA. Brain Res. 2005;1046:230–233. doi: 10.1016/j.brainres.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Mullen CA, O'Hearn E, Molliver ME. Dual serotoninergic projections to forebrain in the rat: morphologically distinct 5-HT axon terminals exhibit differential vulnerability to neurotoxic amphetamine derivatives. J Comp Neurol. 1991;314:558–586. doi: 10.1002/cne.903140312. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Shimizu K, Suno M, Ogawa K, Awaya T, Yamada T, Noda T, Satomi M, Ohtaki K, Chiba K, Tasaki Y, Shiono H. Tandospirone, a 5-HT1A agonist, ameliorates movement disorder via non-dopaminergic systems in rats with unilateral 6-hydroxydopamine-generated lesions. Brain Res. 2006;1112:126–133. doi: 10.1016/j.brainres.2006.07.003. [DOI] [PubMed] [Google Scholar]
- McQuade R, Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J Neurochem. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- Ng K, Chase T, Colburn R, Kopin I. Dopamine stimulation-induced release from central neurons. Science. 1971;172:487–489. doi: 10.1126/science.172.3982.487. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Damier P, Goetz CG, Mueller T, Nutt J, Rascol O, Serbanescu A, Deckers F, Russ H. Multicenter, open-label, trial of sarizotan in Parkinson disease patients with levodopa-induced dyskinesias (the SPLENDID Study) Clin Neuropharmacol. 2004;27:58–62. doi: 10.1097/00002826-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson W. The rat brain in stereotaxic coordinates. 4th. San Diego: Academic Press; 1998. [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Sato S, Koitabashi T, Koshiro A. Pharmacokinetic and pharmacodynamic studies of L-DOPA in rats. II. Effect of L-DOPA on dopamine and dopamine metabolite concentration in rat striatum. Biol Pharm Bull. 1994;17:1622–1629. doi: 10.1248/bpb.17.1622. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kannari K, Maeda T, Tomiyama M, Suda T, Matsunaga M. Role of serotonergic neurons in L-DOPA-derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. Neuroreport. 1999;10:631–634. doi: 10.1097/00001756-199902250-00034. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Walker PD. Dopamine D1 and D2 receptor contributions to L-DOPA-induced dyskinesia in the dopamine-depleted rat. Pharmacol Biochem Behav. 2005;81:887–893. doi: 10.1016/j.pbb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Yu H, Lewander T. Pharmacokinetic and pharmacodynamic studies of (R)-8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur Neuropsychopharmacol. 1997;7:165–172. doi: 10.1016/s0924-977x(96)00395-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, Andren P, Greengard P, Svenningsson P. Evidence for a role of the 5-HT1B receptor and its adaptor protein, p11, in L-DOPA treatment of an animal model of Parkinsonism. Proc Natl Acad Sci USA. 2008;105:2163–2168. doi: 10.1073/pnas.0711839105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Bledsoe S, Murphy J. Serotonergic sprouting is induced by dopamine-lesion in substantia nigra of adult rat brain. Brain Res. 1991;556:108–116. doi: 10.1016/0006-8993(91)90553-8. [DOI] [PubMed] [Google Scholar]