Abstract
Purpose
The aim of this study was to investigate the prognostic significance of the expression of p53 gene product in operable invasive breast cancer by performing immunohistochemical analysis.
Materials and Methods
Between January 1993 and December 2001, 440 operable invasive breast cancer patients underwent immunohistochemical staining for p53, and we retrospectively analyzed these results together with the clinical outcomes.
Results
The overexpression of p53 was detected in 51.6% of the cases. The overexpression of p53 was inversely correlated with lymph node metastasis (p=0.005). The tumor size, tumor histology, histologic grade, hormonal receptor status and tumor stage were not related to the overexpression of p53. Multivariate Cox regression analysis indicate that lymph node metastasis, tumor size and the p53 expression were the significant prognostic factors for overall survival; lymph node metastasis, the estrogen receptor status and the p53 expression were the significant prognostic factors for relapse free survival. On the subgroup analysis, the p53 non-expressors showed better 7-year overall survival (92.7% vs. 76.7%, respectively, p=0.011) and relapse free survival (74.9% vs. 57.8%, respectively, p=0.032) than did the p53 overexpressors for the patients with lymph node metastasis. However, for the patients without lymph node metastasis, the survival rates were not different for both the p53 non-expressors and the p53 overexpressors.
Conclusion
Immunohistochemical staining of the p53 gene product was an independent prognostic factor for predicting survival of the lymph node positive invasive breast cancer patients.
Keywords: Breast neoplasms, Prognosis, p53, Immunohistochemistry
INTRODUCTION
Breast cancer has become the most common cancer for Korean females since 2002. A population-based cancer registry was established on January 1, 1997 to estimate the incidence of cancer in Daegu, Korea. The age-standardized incidence rates (ASR) of breast cancer were 31.1 per 100,000 females as reported by the Daegu Cancer Registry in 2002. The median age of Korean breast cancer patients (early 40's) is younger than that in the Western developed countries, and the incidence of familiar breast cancer is relatively rare in Korea.
Great insight has recently been gained into the biological properties of tumor cells. The products of tumor suppressor genes are of specific interest as they play important roles in a variety of critical and highly conserved cellular functions, including regulation of the cell cycle and apoptosis, differentiation, surveillance of genomic integrity and repair of DNA errors, signal transduction and cell adhesion. The p53 tumor suppressor gene normally regulates cell proliferation (1) and programmed cell death (2).
Abnormalities of the p53 tumor suppressor gene have been implicated in both tumorigenesis and tumor progression. The importance of the p53 expression has been extensively analyzed by immunohistochemical methods with regard to a plethora of human malignancies, including breast cancer, yet the role of p53 protein in breast cancer is incompletely understood. The prognostic roles of the p53 gene expression with regard to breast cancer remain controversial, and p53 mutation would be not a feasible prognostic marker in the routine diagnostic evaluation of breast cancer.
The purpose of this study was to determine the prognostic significance of immunohistochemical staining the p53 expression in operable breast adenocarcinoma.
MATERIALS AND METHODS
1) Patients and setting
From January 1993 to December 2001, we performed immunohistochemical analyses of p53 gene product in 440 of the 813 operable invasive breast cancers at our hospital. We reviewed the clinicopathologic parameters, the size of the tumor, the pathologic type, histologic grade, nuclear grade, hormone receptor status and the TNM stage. Staging evaluation was done by the guidelines of the American Joint Committee on Cancer, 5th edition.
2) Immunohistochemical staining
Immunohistochemical staining was performed using the avidinbiotin-peroxidase complex with monoclonal antibodies created against p53 (NCL-p53-DO7, Novocastra Laboratories, Newcastle, UK). Representative paraffin blocks containing the tumors isolated from each case were sectioned into 5 µm slices and these were affixed to slides; they were then dried for 1 hour at 60℃. The sections were deparaffinized in xylene and next rehydrated with a descending series of alcohol solutions. The endogenous peroxidase activity was blocked by 3% hydrogen peroxidase for 15 minutes, and this was followed by washing with phosphate buffered saline (PBS), at a pH of 7.2. The sections were then subjected to a heat antigen retrieval process by autoclaving them with 1% zinc sulfate solution for 5 minutes. After cooling for 20 minutes at room temperature, the sections were incubated with 10% normal horse serum (Vectastatin Elite kit) for 30 minutes. After decanting away the excess serum, the sections were incubated with primary antibody for 2 hours at 37.0℃. For the p53 study, DO7 monoclonal antibody was used at a 1 : 100 dilution (Novocastra, Newcastle, UK). The sections were subsequently incubated with prediluted biotinylated anti-mouse immunoglobulin (Vectastain Elite kit) for 30 minutes at 37℃. After washing with PBS, the sections were reacted with peroxidase-conjugated streptoavidin (Dako) at a dilution of 1 : 500 for 30 minutes at 37℃. After washing with PBS, the peroxidase activity was evaluated with 3,3'-deaminobenzidine tetrahydrochloride (DAB), and the sections were counterstained with Meyer's hematoxylin.
Two pathologists, who were kept "blinded" to the clinical outcomes and features of the patients, independently evaluated all the immunohistochemical slides. The tumors with positive nuclear staining in more than 1% of tumor cells were interpreted as having a positive expression.
3) Statistical analysis
Statistical analysis was performed using chi square tests to compare percentages in the cross tabulations, and independent sample T tests were used to compare the means. Survival curves were generated by the Kaplan-Meier method, and they were compared by using the log-rank test. To determine the significant prognostic factors related to overall survival and relapse-free survival (including all of the loco-regional recurrences and distant metastasis), multivariate analysis was performed with the Cox proportional hazards regression model. All the significance levels refer to two-sided tests. p values less than 0.05 were considered significant.
Statistical analyses were performed using SPSS for Windows 12.0.
RESULTS
1) Patients characteristics
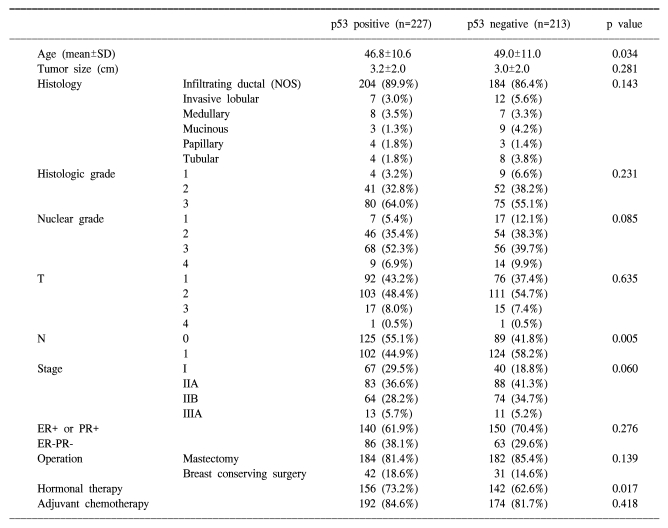
All of the patients were female and the median age of the subjects was 47.8 years. The mean diameter of the tumors was 3.1 cm (range: 0.0~15.0 cm). Histologically, infiltrating ductal cancer was most common (388 patients, 88.2%), and lobular carcinoma was noted in 19 patients (4.3%), medullary carcinoma was noted in 15 patients (3.4%), mucinous carcinoma was noted in 12 patients (2.7%), papillary carcinoma was noted in 7 patients (1.6%), and tubular carcinoma was noted in 12 (2.7%) patients. Mastectomy was performed for 367 patients (83.4%) and breast conserving surgery with radiotherapy was performed for 73 patients (16.6%). Adjuvant hormonal therapy was performed in 298 patients (67.7%) and adjuvamt chemotherapy was performed in 366 patients (83.2%) (Table 1). Adjuvant chemotherapy was performed with the cyclophosphamide +methotrexate+5-FU (CMF) regimen for 239 patients (65.3%), with adriamycin+cyclophosphamide (AC) for 79 patients (21.6%), with 5-FU+epirubicin+cyclophosphamide (FEC) for 40 patients (10.9%), and with other combination chemotherapies for 8 patients.
Table 1.
Clinical characteristics of the operable breast cancer patients according to the p53 expression
2) Correlation with clinicopathologic parameters
The staging was as follows; 168 patients were the T1 stage (40.4%), 214 were T2 (51.4%), 32 were T3 (7.7%), and 2 were T4 (0.5%), 214 patients were N0 (48.6%), 226 patients were N1 (51.4%), and stage I was noted in 107 patients (24.3%), stage IIA was noted in 171 patients (38.9%), stage IIB was noted in 138 patients (31.4%), and stage IIIA was noted in 24 (4.4%) patients. There were 290 patients (66.1%) in the estrogen receptor (ER) positive or progesterone receptor (PR) positive group (66.1%) and 149 patients (33.9%) were in the ER-PR-group.
Positive staining for p53 were found in 227 of the 440 examined tumor samples (51.6%). Lymph node metastasis was more common in the p53 non-expressors (58.2%) than in the p53 overexpressors (44.9%) (p=0.005) (Table 1). No significant correlation were observed with regard to the overexpression of p53 and the tumor size, histologic grade, nuclear grade, vascular invasion, tumor stage or hormonal status.
3) Survival analysis
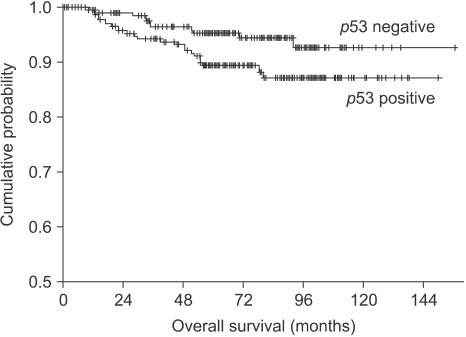
The 7-year overall survival rate and 7-year relapse-free survival rate of the total patient population were 90.7%, and 78.2%, respectively. According to the stage, the 7-year overall survival rate of stage I was 97.9%, it was 94.8% for stage IIA, 82.4% for stage IIB and 75.6% for stage III (p=0.000). The 7-year overall survival rate of the PR positive patients were better than that of the ER negative patients (93.4% vs. 86.7%, respectively, p=0.019), and the 7-year relapse-free survival rate of the ER positive patients was better than that for the ER negative patients (81.3% vs. 74.6%, respectively, p=0.035). The 7-year overall survival rate of patients with p53 overexpression was worse than that of patients with p53 non-expression (87.1% vs. 94.3%, respectively, p=0.041, Fig. 1), but there was no significant differences in terms of the relapse-free survival rate (p=0.072) between the patients with p53 overexpression and the patients with p53 non-expression.
Fig. 1.
Kaplan-Meier overall survival curve in operable breast cancer patients according to p53 expression.
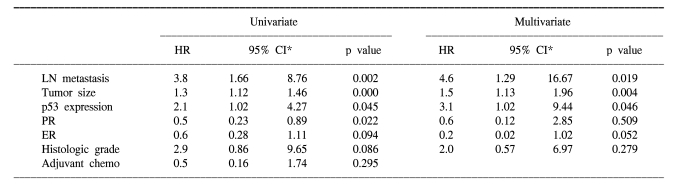
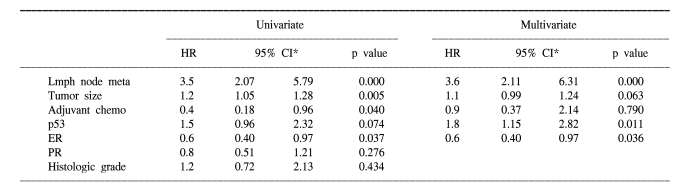
According to multivariate Cox regression analysis, lymph node metastasis, tumor size and p53 expression (hazard ratio=3.1, 95% CI: 1.02-9.44, p=0.046) were the important prognostic factors for the overall survival rates, and lymph node metastasis, ER status and p53 expression (hazard ratio=1.8, 95% CI: 1.15-2.82, p=0.011) were the important prognostic factors for the relapse-free survival rates (Table 2, 3).
Table 2.
Cox regression analysis of overall survival for the operable breast cancer patients
*confidence interval.
Table 3.
Cox regression analysis of relapse free survival for the operable breast cancer patients
*confidence interval.
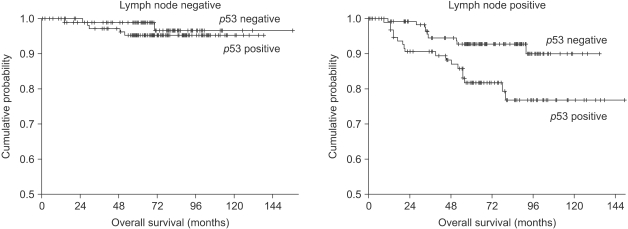
On the subgroup analysis, the p53 non-expressors had better 7-year overall survival and relapse free survival rates than did the p53 overexpressors for the patients with lymph node metastasis (7-year OS: 92.7% vs. 76.7%, respectively, p=0.011, 7-year RFS: 74.9% vs. 57.8%, respectively, p=0.032), but there were no significant differences of the overall survival and relapse free survival rates for the patients without lymph node metastasis (Fig. 2).
Fig. 2.
Kaplan-Meier overall survival curve in operable lymph node-negative and lymph node-positive breast cancer patients according to p53 expression.
DISCUSSION
The p53 tumor suppressor gene is located in the short arm of human chromosome 17 (17p13.1), and it encodes a 53-kd multifunctional transcription factor that plays a pivotal role in multiple cellular processes such as cell cycle control, apoptosis, DNA repair and angiogenesis, and it plays an important role for controlling of tumors by regulating the expression of vascular endothelial growth factor. Mutation of the p53 gene is the most common genetic alterations in human cancer. The p53 gene is composed of 11 exons; the region encompassing exons 5 through 9 of the p53 gene contains 80~90% of the known p53 gene mutations (3). Mutation in the p53 gene leads to loss of its usual negative growth regulation and more rapid cell proliferation (4). The levels of wild-type p53 protein are normally extremely low and due to its short half-life, it is undetectable by standard immunohistochemical staining in normal cells and tissues. Conversely, the mutated p53 protein accumulates in the nucleus either by binding with other oncogene proteins or by its half-life being prolonged (5,6). A previous study has shown that the nuclear accumulation of p53 was consistent with the mutation rates, and the nuclear accumulation of p53 exhibited the same subtype specificity; simple immunohistological methods can provide strong evidence of such mutations (7).
The expression of the p53 gene can be easily detected by immunohistochemical methods in a wide variety of human malignancies, including breast cancer, lung cancer (4), colon cancer (8) and ovarian cancer (9). The College of American Pathologists Consensus Statement in 1999 revealed that p53 had a category ll role as a prognostic factor in breast cancer and this remains to be validated via statistically robust studies (10). In previous studies, p53 overexpression was found in 14~58% of breast cancer cases (5,11~15), and it was found in 51.6% (227/440) of our breast cancer cases. The majority of tumors exhibited p53 located in the nucleus. This is consistent with previous studies and it appears to occur via the regulation of transcriptional factors.
Many studies showed a significant relationship between p53 overexpression and high tumor grade (2,10,16), and inverse correlation was noted with the ER status (2,10,16,17). Davidoff et al (5) found significant associations between p53 overexpression and late stage, metastatic spread and a low concentration of progesterone receptors. Lipponen et al (12) found that p53 overexpression was associated with the ductal type tumor, high-grade tumors, dense inflammatory cell infiltrate, a high S-phase fraction, a high mitotic frequency and high values for the nuclear factors. These data suggest that p53 overexpression may be a marker of more aggressive carcinoma. In our study, p53 overexpression was found to be significantly lower in the patients with lymph node matastasis; p53 overexpression was detected in 58.2% (124/213) of the patients without lymph node metastasis, but it was detected in only 44.9% (102/227) of the patients with lymph node metastasis (p=0.005). This is contrast to the results reported by Overgaard et al (18). No significant correlations were detected with regard to the overexpression of p53 and tumor size, vascular invasion, histologic grade, tumor stage, or hormonal status in our study of operable breast cancer patients. Our study data suggest that p53 overexpression may not be a marker of tumor aggressiveness.
Many studies have investigated the association between p53 gene overexpression and the clinical outcome of breast cancer, and most investigators have reported poorer overall and disease-free survival for the breast cancer patients with p53 mutation (11,16,18~20). However, different studies have shown that the prognostic power of p53 overexpression is likely to be weak and it is probably not of clinical value. Barnes et al (11) found that patients who expressed p53 had a worse prognosis for disease-free survival and overall survival, and this was seen for patients with a 10-year median follow-up, and the effect was most apparent for the patients who suffered with infiltrating lobular and grade II infiltrating ductal carcinomas. Meta-analysis of eleven studies revealed that the relative estimated hazard ratio of overall survival was 2.0 (confidence interval: 1.7~2.5) (21). In our study, the seven-year overall survival rate of all the patients was 90.7% and the relapse-free survival rate was 78.2%. The 7-year overall survival rate of the patients without a p53 expression was better than that of the patients with a p53 overexpression (94.3% vs. 87.1%, respectively, p=0.041), but the relapse-free survival rate was not statistically different between the p53 expressors and the p53 non-expressors (82.1% vs. 74.3%, respectively, p=0.072). According to multivariate Cox regression analyses, lymph node metastasis, tumor size and p53 expression were the important prognostic factors for the overall survival rates; lymph node metastasis, the ER status and p53 expression were the important prognostic factors for the relapse-free survival rates in our study.
Assessment of the prognostic markers that are independent of the axillary lymph node status for breast cancer is a major concern for the application of adjuvant treatment regimens. In previous studies, the p53 expression was a useful marker for lymph node negative patients (22,23). Rosen et al (19) and Ferrero et al (24) conducted p53 studies on 440 and 297 node-negative breast cancer patients, respectively, with a median follow-up of 10 years. They found that p53 was not a reliable prognostic indicator for the node-negative breast cancer patients. And, Levesque et al (17) and Gohring et al (13) have both identified p53 overexpression as a significant predictor of outcome appear to be limited to the node-positive patients. In our study, for the patients with lymph node metastasis, the p53 non-expressors showed better 7-year overall survival and relapse free survival rates than did the p53 overexpressors (OS: 92.7% vs. 76.7%, respectively, p=0.011; RFS 57.8% vs. 74.9%, respectively, p=0.032), but there were no significant differences of the overall survival and relapse free survival rates for the patients without lymph node metastasis, according to the p53 expression.
CONCLUSIONS
In our retrospective analysis, immunohistochemical staining for p53 protein proved to be a clinically useful prognostic indicator for the cases of operable breast cancer, but this was limited to patients with lymph node metastasis. Further prospective studies are warranted in order to clarify the relationship between tumor suppressor proteins, the breast cancer prognosis and the cell cycle regulation associated with the action of these proteins.
Footnotes
This study was supported by a grant of the Korean Health 21 R&D Project, Ministry of Heath & Welfare, Republic of Korea (0412-CR01-0704-0001).
References
- 1.Adair FE, Berg J, Joubert L, Robbins GF. Long-term follow-up of breast cancer patients: the 30-year report. Cancer. 1974;33:1145–1150. doi: 10.1002/1097-0142(197404)33:4<1145::aid-cncr2820330438>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Friedrichs K, Gluba S, Eidtmann H, Jonat W. Overexpression of p53 and prognosis in breast cancer. Cancer. 1993;72:3641–3647. doi: 10.1002/1097-0142(19931215)72:12<3641::aid-cncr2820721215>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Iggo R, Gatter K, Bartek J, Lane DP, Harris AL. Increased expression of mutant form of p53 oncogene in primary lung cancer. Lancet. 1990;335:675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- 4.Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 5.Davidoff AM, Herndon JE, Glover NS, Kerns BJM, Pence JC, Iglehart JD, et al. Relation between p53 overexpression and established prognostic factors in breast cancer. Surgery. 1991;110:259–264. [PubMed] [Google Scholar]
- 6.Finlay CA, Hinds PW, Tan TH, Eliyahu D, Oren M, Levine AJ. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988;8:531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campo E, de la Calle-Martin O, Miquel R, Palacin A, Romero M, Fabregat V, et al. Loss of heterozygosity of the p53 gene and p53 protein expression in human colorectal carcinomas. Cancer Res. 1991;51:4436–4442. [PubMed] [Google Scholar]
- 8.Marks JR, Davidoff AM, Kerns BJ, Humphrey PA, Pence JC, Dodge RK, et al. Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991;51:2979–2984. [PubMed] [Google Scholar]
- 9.Fitzibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 10.Isola F, Visakorpi T, Holli K, Kallioniemi OP. Association of overexpression of tumor suppressor protein p53 with rapid cell proliferation and poor prognosis in node-negative breast cancer patients. J Natl Cancer Inst. 1992;84:1109–1114. doi: 10.1093/jnci/84.14.1109. [DOI] [PubMed] [Google Scholar]
- 11.Barnes DM, Dublin EA, Fisher CJ, Levison DA, Milles RR. Immunohistochemical detection of p53 protein in mammary carcinoma: an important new independent indicator of prognosis? Hum Pathol. 1993;24:469–476. doi: 10.1016/0046-8177(93)90158-d. [DOI] [PubMed] [Google Scholar]
- 12.Lipponen P, Ji H, Aaltomaa S, Syrjanen S, Syrjanen K. P53 protein expression in breast cancer as related to histopathological characteristics and prognosis. Int J Cancer. 1993;55:51–56. doi: 10.1002/ijc.2910550110. [DOI] [PubMed] [Google Scholar]
- 13.Gohring UJ, Scharl A, Heckel C, Ahr A, Crombach G. P53 protein in 204 patients with primary breast carcinoma: immunohistochemical detection and clinical value as a prognostic factor. Arch Gynecol Obstet. 1995;256:139–146. doi: 10.1007/BF01314642. [DOI] [PubMed] [Google Scholar]
- 14.Sirotkovic-Skerlev M, Krizanae S, Kapitanovic S, Husnjak K, Unusic J, Pavelic K. Expression of c-myc, erbB-2, p53 and nm23-H1 gene product in benign and malignant breast lesions coexpression and correlation with clinicopathologic parame ters. Exp Mol Pathol. 2005;79:42–50. doi: 10.1016/j.yexmp.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Rahko E, Blanco G, Bloigu R, Soini Y, Talvensaari-Mattila A, Jukkola A. Adverse outcome and resistance to adjuvant antiestrogen therapy in node-positive postmenopausal breast cancer patients-The role of p53. Breast. 2006;15:69–75. doi: 10.1016/j.breast.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Beck T, Weller EE, Weikel W, Brumm C, Wilkens C, Knapstein PG. Usefulness of immunohistochemical staining for p53 in the prognosis of breast carcinomas: correlations with established prognosis parameters and with the proliferation marker, MIB-1. Gynecol Oncol. 1995;57:96–104. doi: 10.1006/gyno.1995.1104. [DOI] [PubMed] [Google Scholar]
- 17.Levesque MA, Katsaros D, Yu H, Giai M, Genta F, Roagna R, et al. Immunofluorometrically determined p53 accumulation as a prognostic indicator in Italian breast cancer patients. Int J Cancer. 1998;79:147–152. doi: 10.1002/(sici)1097-0215(19980417)79:2<147::aid-ijc9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Overgaard J, Yilmaz M, Guldberg P, Hansen LL, Alsner J. TP53 mutation is an independent prognostic marker for poor outcome in both node-negative and node-positive breast cancer. Acta Oncol. 2000;39:327–333. doi: 10.1080/028418600750013096. [DOI] [PubMed] [Google Scholar]
- 19.Rosen PP, Lesser ML, Arroyo CD, Cranor M, Borgen P, Norton L. p53 in node-negative breast carcinoma. an immunohistochemical study of epidemiological risk factors, histologic features, and prognosis. J Clin Oncol. 1995;13:821–830. doi: 10.1200/JCO.1995.13.4.821. [DOI] [PubMed] [Google Scholar]
- 20.Lai H, Ma F, Trapido E, Meng L, Lai S. Spectrum of p53 tumor suppressor gene mutations and breast cancer survival. Breast Cancer Res Treat. 2004;83:57–66. doi: 10.1023/B:BREA.0000010699.53742.60. [DOI] [PubMed] [Google Scholar]
- 21.Pharoah PD, Day NE, Caldas C. Somatic mutations in the p53 gene and prognosis in breast cancer: a meta analysis. Br J Cancer. 1999;80:1968–1973. doi: 10.1038/sj.bjc.6690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansson T, Inganas M, Sjogren S, Norberg T, Lindgren A, Holmberg L, et al. P53 status predicts survival in breast cancer patients treated with or without postoperative radiotherapy: a novel hypothesis based on clinical findings. J Clin Oncol. 1995;13:2745–2751. doi: 10.1200/JCO.1995.13.11.2745. [DOI] [PubMed] [Google Scholar]
- 23.Elledge RM, Fuqua SA, Clark GM, Pujol P, Allred DC, McGuire WL. Prognostic significance of p53 gene alterations in node-negative breast cancer. Breast Cancer Res Treat. 1993;26:225–235. doi: 10.1007/BF00665800. [DOI] [PubMed] [Google Scholar]
- 24.Ferrero JM, Ramaioli A, Formento JL, Francoual M, Etienne MC, Peyrottes I, et al. P53 determination alongside classical prognostic factors in node-negative breast cancer: an evaluation at more than 10-year follow-up. Ann Oncol. 2000;11:393–397. doi: 10.1023/a:1008359722254. [DOI] [PubMed] [Google Scholar]