Abstract
Purpose
Capecitabine is an oral fluoropyrimidine carbamate and it is known as an effective radiosensitizer. Capecitabine and its metabolite reach their peak concentration in the plasma at 1~2 hours after a single oral administration of capecitabine and the levels fall rapidly thereafter. To verify the radiosensitizing effect of capecitabine that is based on such pharmacokinetic characteristics, we performed a retrospective analysis on the optimal timing of capecitabine administration with performing preoperative chemoradiation for locally advanced rectal cancer.
Materials and Methods
Among 171 patients who were treated with preoperative radiotherapy and concurrent capecitabine administration for rectal cancer, 56 patients were administered capecitabine at 1~2 hours before radiotherapy (group A), and at other time in the other 115 patients (group B). Total mesorectal excision was done at 4 to 6 weeks after the completion of chemoradiation. The radiosensitizing effect of capecitabine was evaluated on the basis of the pathological response.
Results
Complete pathological regression of the primary tumor was observed in 12 patients (21.4%) for group A and in 11 patients (9.6%) for group B (p=0.031). Residual disease less than 0.5 cm (a good response) was observed in 19 patients (33.9%) for group A and in 23 patients (20.0%) for group B (p=0.038). On multivariate analysis, the capecitabine ingestion time showed marginal significance.
Conclusion
When performing preoperative chemoradiation for locally advanced rectal cancer, the radiosensitizing effect of capecitabine was enhanced when it was administered 1 hour before radiotherapy.
Keywords: Rectal neoplasms, Combined modality therapy, Capecitabine
INTRODUCTION
The most important and basic treatment for locally advanced rectal cancer is performing complete radical resection, but the local recurrence rate was reported to range from 17% to 67% in the presence of tumor invasion into the adjacent tissue or when there is lymph node involvement (1~3). Preoperative radiotherapy has been investigated as a neoadjuvant treatment for locally advanced rectal cancer. Many studies have shown that preoperative radiotherapy improved local control and survival for locally advanced rectal cancer (4~7). Some of the chemotherapeutic agents can enhance the effect of radiotherapy, and 5-fluorouracil (5-FU) has been the most popularly used agent to treat rectal cancer (8,9). Yet one study has shown that a combination with leucovorin or levamisole to maximize the effect of 5-FU did not improve the survival rate anymore than using 5-FU alone (10).
Capecitabine is an oral fluoropyrimidine carbamate and it is absorbed from the gastrointestinal tract where it is preferentially converted to 5-FU in tumor cells via the thymidine phophorylase (TP) as compared with normal tissue (11~13). This tumor-preferential activation of capecitabine reduces the systemic exposure to 5-FU and it potentially improves treatment efficacy and safety. Theoretically, the effect of capecitabine was expected to be similar to that of a continuous infusion of 5-FU. In addition, the method of administration of capecitabine is simple and safe (14,15).
However, capecitabine and its metabolite reach their peak level at 0.3~3 hours after single oral administration and they rapidly fall thereafter. Their half-life for the plasma concentration was reported to be between 0.55 and 0.89 hours (16). For that reason, Tepper emphasized that capecitabine should probably be taken daily about one hour prior to radiotherapy to maximize the interaction between the two treatments (17). A few investigators have reported their own results on administering preoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer; however, the time interval between the capecitabine intake and radiotherapy was not mentioned in detail and it was mostly described as "twice a day" or "every 12 hours" (18~20).
We performed this study to verify whether the time interval between administering capecitabine and radiotherapy has an influence on the response to preoperative chemoradiotherapy for locally advanced rectal cancer.
MATERIALS AND METHODS
1) Patients' characteristics
Between January 2002 and April 2004, 171 patients with locally advanced rectal adenocarcinoma who were enrolled in two prospective studies on preoperative chemoradiotherapy at Asan Medical Center were eligible for this retrospective analysis. All of them had clinical stage T3-4 disease or regional lymph node enlargement, but they were without distant metastasis.
The patients were classified into group A or group B according to the time interval between the time of capecitabine ingestion and radiotherapy. As we did not give any specific guidance on the time interval between capecitabine ingestion and radiotherapy to our patients before October 2004, the approximate time of capecitabine intake was identified via phone calls to the individual patient. All of the patients were educated by the medical oncologist to take capecitabine twice a day with a 12 hour interval, and they usually took it in the morning and in the evening after a meal. The time of radiotherapy could be accurately verified from the radiotherapy treatment record. Among the patients, those who had radiotherapy at 0.5~1.5 hour after capecitabine intake were classified into group A and the others were classified into group B. From October 2004, every patient was educated to take capecitabine 1 hour before radiotherapy and so they were classified into group A.
2) Pretreatment evaluation and monitoring during treatment
The pretreatment evaluation included a complete medical history, physical examination, complete blood count, serum biochemical tests, carcinoembryonic antigen (CEA), chest x-ray, colonofiberscopy (CFS), abdominal/pelvic computerized tomography (CT), endorectal ultrasound (EUS), whole body bone scan (in case of CEA > 40 ng/ml) and chest CT (in case of CEA>20 ng/ml). The clinical staging was determined according to abdominal/pelvic CT and EUS findings with using the AJCC TNM cancer staging system.
During chemoradiotherapy, the patients were examined weekly for the safety evaluation and their compliance. A complete blood count, biochemical tests and a documentation of body weight were checked weekly. Safety was evaluated according to the National Cancer Institute Common Toxicity Criteria version 2.0
3) Radiotherapy
All patients received preoperative radiotherapy delivered to the pelvis through three fields (posterior to anterior and two laterals) or four fields (anterior to posterior, posterior to anterior and two laterals) with using an energy level of 6 or 15 MV from a linear accelerator (Varian, Clinac 1800; Varian Medical Systems, Palo Alto, CA) in prone position. The superior border of the radiation field was the bottom of L5, and the inferior border was 3 cm distal to the tumor. The anterior border was located 3 cm anterior to the tumor and the posterior border was 1 cm behind to the posterior margin of the sacrum. The target volume included the primary tumor, the perirectal fat tissue and the internal iliac and presacral lymph nodes. The total dose was 50 Gy with a daily fraction of 2 Gy, 5 days per week. The dose of 46 Gy was delivered to the initial target volume, and this was followed by a 4 Gy boost to the primary tumor.
4) Chemotherapy
A daily dose 1,650 mg/m2 of capecitabine was administered orally twice a day from day 1 to day 25 of radiotherapy without any weekend breaks. Between January 2002 and September 2004, the patients took capecitabine twice a day irrespective of the radiotherapy time; the drug was usually taken 2 times a day, once in the morning and once the evening. After October 2004, the patients were educated to take their medicine 2 times a day, 12 hours apart, and one of the two doses was taken at 1 hour before radiotherapy.
After surgical resection, the adjuvant chemotherapy consisted of four cycles of capecitabine (2,500 mg/m2/day for 14 days, followed by a 1 week break after each cycle); this was started at 4 weeks after surgery. Medicating with capecitabine was checked by a weekly inquiry at the Department of Radiation Oncology and Medical Oncology.
5) Surgery
Surgical resection was performed 4 to 6 weeks after completion of the preoperative chemoradiotherapy; total mesorectal excision was the standard method. All the surgery was done by qualified colorectal surgeons who had performed total mesorectal excision for more than 50 cases per year for the last 5 years.
6) Pathological evaluation and statistical analysis
Pathological examination was generally done by cutting the primary tumor in 5 mm thick sections. When tumor cells were not found in the primary location, an additional thinner slice was taken for conducting a thorough inspection for residual tumor. All the lymphatic tissue and primary tumor were examined by this method. In addition to complete pathological regression, residual disease less than 5 mm was classified as a good response.
The pathological response was compared between the two groups using the Chi-Square Test (Fisher's Exact Test). Univariate and multivariate analyses were done via the logistic regression method to determine the prognostic significance of the other factors (age, gender, T-stage, tumor differentiation, CEA level, etc.). But the tumor size was not included in the analysis as this was difficult to measure objectively, based on the CFS and CT scan.
RESULTS
1) Patients' characteristics
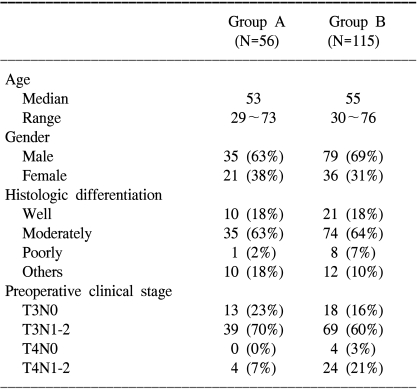
The patients' characteristics are summarized in Table 1. One hundred fifteen patients were classified into group B and 56 patients were classified into group A. The pretreatment variables (age, gender, tumor grade and clinically positive lymph nodes), except the T stage, were well balanced between the two groups. The number of patients clinically classified as T4 stage was 4 (7%) in group A, but there were 28 (24%) such patients in group B.
Table 1.
Patient Characteristics
2) Pathologic response and tumor size change
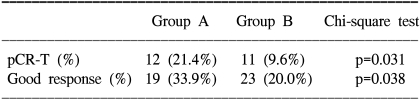
Complete regression of the primary tumor on the pathologic specimen (pCR-T) was noted in 21.4% (12/56) of the group A patients and 9.6% (11/115) of the group B patients (p=0.031 on a Chi-square Test, and p=0.037 on univariate analysis) (Table 2). Other factors did not show statistical significance for pCR-T on univariate analysis; age (<55 vs. ≥55, p=0.096), gender (male vs. female, p=0.555), T-stage (T2 vs. T3 vs. T4, p=0.154), differentiation (well vs. moderate vs. poor, p=0.402), and the CEA level (<20 ng/ml vs. ≥20 ng/ml, p=0.095). On multivariate analysis with factors having a p-value less than 0.1, only the capecitabine ingestion time was marginally significant (p=0.057), but age (p=0.223) and the CEA level (p=0.998) were not significant.
Table 2.
Pathological response to preoperative chemoradiation in all patients
When the analyses were focused on good response, 33.9% (19/56) of the patients in group A and 20.0% (23/115) of the patients in group B (p=0.038 on Chi-square Test) had a good response (Table 2). Yet the p-value was just 0.055 on univariate analysis, and T-stage was the only significant factor (p=0.038). On the multivariate analysis with using the T-stage and capecitabine ingestion time, the T-stage was no longer significant (p=0.940) and the capecitabine ingestion time showed only a trend (p=0.074).
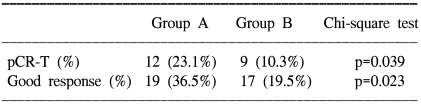
When only the T3 patients were analyzed to exclude the unbalanced distribution of the T-stage between the two groups, the pCR-T was 23.1% (12/52) in group A and 10.3% (9/87) in group B (p=0.039); a good response was noted in 36.5% (19/52) of the group A patients and 19.5% (17/87) of the group B patients (p=0.023) on Chi-square testing (Table 3). On the univariate analysis, the capecitabine ingestion time was the only significant factor for pCR-T (p=0.046) and a good response (p=0.030). The CEA level was marginally significant for a good response on univariate analysis (p=0.065), but not on multivariate analysis.
Table 3.
Pathological response to preoperative chemoradiation in the T3 patients
Downstaging of the T and N classification was noted in 55.4% (31/56) and 76.7% (33/43), respectively, of the group A patients. This was 53.9% (62/115) and 67.7% (63/93), respectively, in the group B patients. There was no significant difference between the two groups.
DISCUSSION
Capecitabine is highly water-soluble and it is converted to the active metabolite 5-FU by means of a three-step enzymatic pathway (12,13,16). After oral administration, capecitabine is absorbed as an unchanged drug from the gastrointestinal tract, and it is sequentially converted to 5'-deoxy-5-fluorouridine (5'-DFCR) by the carboxylesterase that is primarily located in the liver. 5'-DFCR is then converted to 5'-deoxy-5- fluorouridine (5'-DFUR) by cytidine deaminase, which is also primarily located in the liver and the tumor tissues. Thymidine phosphorylase (TP) is essential for the metabolism of 5'-DFUR to the active FU, and the concentration of TP is 3~10 times higher in many tumor tissues than in normal healthy tissue. This may lead to the preferential activation of capecitabine to active FU in the tumor tissue compared with normal tissue. In fact, capecitabine has demonstrated equivalent activity with lower systemic toxicity than the intravenous administration of 5-FU (20). Schuller et al. have reported that the activities of the TP enzymes are 4 fold higher in colorectal tumors than in the surrounding normal tissues, and the concentration of 5-FU was on average 3.2 times higher in the primary colorectal tumors than in the adjacent healthy tissue (13). Capecitabine can be administered in the outpatient setting as an oral agent and this avoids the complication and pain associated with the intravenous route.
As for its pharmacokinetic aspect, the plasma concentration of capecitabine is extremely variable over the course of time (16,21,22). After taking a single dose of capecitabine, it is rapidly absorbed and metabolized to its active metabolites. It took 0.5~3 hours (median: 2 hours) to reach a peak plasma drug concentration. The time to reach a peak plasma drug concentration for 5'-DFCR, 5'-DFUR and FU was identical to that of capecitabine. The mean eliminated half-life of capecitabine is short, ranging from 0.55 to 0.89 hours, and the mean estimated half-life for 5'-DFCR, 5'-DFUR and FU is 0.7 to 1.3 hours.
On the basis of the pharmacokinetic distinction and Dr. Tepper's advice, we specified the capecitabine ingestion time as "1 hour before radiotherapy" in the protocol that was used from October 2004. Actually, the median value of the peak time for the plasma FU concentration was 2 hours after intake; however, we recommended "1 hour before radiotherapy" with consideration of a little delay for actually performing daily radiotherapy. As a result, we proved that Dr. Tepper's opinion was right by showing that group A with a "1 hour" interval between capecitabine intake and radiotherapy produced a higher pathological response rate than that of group B.
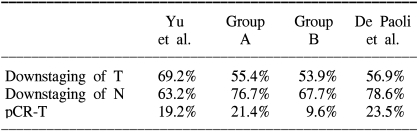
In our previous report on preoperative chemoradiation with 5-FU and leucovorin, a decrease of the T-stage was observed in 69.2% of the patients, a decrease of the N-stage was observed in 63.2% of the patients, and a decrease of the pCR-T was observed in 19.2% of the patients (23). Those results were superior to the outcome of group B (53.9%, 67.7% and 9.6%, respectively), and they were equivalent to those of group A (55.4%, 76.7% and 21.4%, respectively) (Table 4). Although there might be differences in the clinical stage and pretreatment variables among patient populations, we believe the time of capecitabine intake is an important point for achieving an effect of capecitabine that is equivalent or superior to that of the 5-FU/leucovorine combination.
Table 4.
Comparison of the pathological response to the preoperative chemoradiation
De Paoli et al. have recently reported on their results for preoperative chemoradiation with capecitabine in locally advanced rectal cancer (24). Their schedule of capecitabine medication, i.e., 2 hours before radiotherapy, was identical to ours with the exclusion of the time of the first daily dose. Their outcomes were similar to group A (Table 4).
CONCLUSIONS
The radiosensitizing effect of capecitabine was enhanced when it was taken at 1 hour before radiotherapy. However, we need to conduct a study with a larger number of patients and a longer period of follow-up to verify the relationship between the pathologic response and the patients' survival.
References
- 1.Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–1329. doi: 10.1002/1097-0142(19831001)52:7<1317::aid-cncr2820520731>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Million RR, Pfaff WW. Patterns of recurrence in adenocarcinoma of the rectum and rectosigmoid treated with surgery alone: implications in treatment planning with adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:977–985. doi: 10.1016/0360-3016(83)90384-x. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson LL, Sargent DJ, Tepper JE, O'Connell MJ, Allmer C, Smalley SR, et al. Impact of T and N substage on survival and disease relapse in adjuvant rectal cancer; a pooled analysis. Int J Radiat Oncol Biol Phys. 2002;54:386–396. doi: 10.1016/s0360-3016(02)02945-0. [DOI] [PubMed] [Google Scholar]
- 4.Cedermark B, Johansson H, Rutqvist LE, Wilking N Stockholm Colorectal Cancer Study Group. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Cancer. 1995;75:2269–2275. doi: 10.1002/1097-0142(19950501)75:9<2269::aid-cncr2820750913>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 6.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 7.Camma C, Giunta M, Fiorica F, Pagliaro L, Craxi A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 8.Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 9.Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 10.Tepper JE, O'Connell M, Niedzwiecki D, Hollis DR, Benson AB, 3rd, Cummings B, et al. Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control-final report of intergroup 0114. J Clin Oncol. 2002;20:1744–1750. doi: 10.1200/JCO.2002.07.132. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekiguchi F, et al. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998;55:1091–1097. doi: 10.1016/s0006-2952(97)00682-5. [DOI] [PubMed] [Google Scholar]
- 12.Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–1281. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 13.Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45:291–297. doi: 10.1007/s002800050043. [DOI] [PubMed] [Google Scholar]
- 14.Van Cutsem E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097–4106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 15.Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–2292. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 16.Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40:85–104. doi: 10.2165/00003088-200140020-00002. [DOI] [PubMed] [Google Scholar]
- 17.Tepper JE. Cancer of the Colon, Rectum and Anus. ASTRO 2004 refresher course, 305; presented at 46th Annual meeting of the ASTRO (American Society for Therapeutic Radiology and Oncology). [Google Scholar]
- 18.Kim JS, Kim JS, Cho MJ, Song KS, Yoon WH. Preoperative chemoradiation using oral capecitabine in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2002;54:403–408. doi: 10.1016/s0360-3016(02)02856-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim JC, Kim TW, Kim JH, Yu CS, Kim HC, Chang HM, et al. Preoperative concurrent radiotherapy with capecitabine before total mesorectal excision in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63:346–353. doi: 10.1016/j.ijrobp.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 20.Dunst J, Reese T, Sutter T, Zuhlke H, Hinke A, Kolling-Schlebusch K, et al. Phase I trial evaluating the concurrent combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol. 2002;20:3983–3991. doi: 10.1200/JCO.2002.02.049. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy J, Twelves C, Cameron D, Steward W, O'Byrne K, Jodrell D, et al. Bioequivalence of two tablet formulations of capecitabine and exploration of age, gender, body surface, and creatinine clearance as factors influencing systemic exposure in cancer patients. Cancer Chemother Pharmacol. 1999;44:453–460. doi: 10.1007/s002800051118. [DOI] [PubMed] [Google Scholar]
- 22.Reigner B, Verweij J, Dirix L, Cassidy J, Twelves C, Allman D, et al. Effect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patients. Clin Cancer Res. 1998;4:941–948. [PubMed] [Google Scholar]
- 23.Yu CS, Kim JH, Lee JH, Kim TW, Chang HM, Namgung H, et al. Efficacy of preoperative radio-chemotherapy in patients with advanced low rectal cancer. J Korean Soc Coloproctol. 2004;20:46–51. [Google Scholar]
- 24.De Paoli A, Chiara S, Luppi G, Friso ML, Beretta GD, Del Prete S, et al. Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable rectal cancer: a multicentric phase II study. Ann Oncol. 2006;17:246–251. doi: 10.1093/annonc/mdj041. [DOI] [PubMed] [Google Scholar]