Abstract
Purposes
Resveratrol is a phenolic compound found in grapes and other food products. In order to assess the availability of resveratrol as an angio-inhibiting drug, we examined whether resveratrol plays an important role in bovine aortic endothelial cells (BAECs) for cell apoptosis and cell migration.
Methods and Materials
Endothelial cell apoptosis was observed as detected by the Hoechst staining and the caspase-3 activity. Additionally, Western blotting was performed for monitoring the activities of various cell signaling molecules.
Results
Resveratrol was shown to act as a pro-apoptotic agent. The pro-apoptotic effect of resveratrol was as great as that of etoposide, a well-known anti-cancer drug. In addition, resveratrol had an inhibitory effect on endothelial cell migration. The demonstrated efficacy of resveratrol suggests that resveratrol may be utilized as an anti-angiogenic drug. To determine the underlying mechanisms, we further investigated which signaling molecules are activated by resveratrol. Extracellular signal-regulated kinase (ERK) was activated by the treatment with resveratrol in BAECs, whereas endothelial nitric oxide synthetase (eNOS), Akt, and Jun N-terminal kinase (JNK) were inhibited. The pretreatment with PD compound, an ERK inhibitor, had no effect on the pro-apoptosis induced by resveratrol.
Conclusion
Resveratrol plays an important role in endothelial cell apoptosis, indicating that resveratrol can be utilized as a potent anti-angiogenic drug.
Keywords: Resveratrol, Endothelial cells, Apoptosis, Caspase-3, Cell signaling
INTRODUCTION
Tumor growth is an angiogenesis-dependent process because a tumor cannot grow over 2 to 3 mm3 in size unless new vessel formation is induced. Accordingly, angioinhibitors are being examined as biological response modifiers (BRMs) that can modulate tumor growth via inhibition of the tumor vasculature (1).
Resveratrol is a natural phytoalexin that is found in grapes, red wines and other food sources. It has been documented that resveratrol acts as an anti-inflammatory and anti-oxidant agent. In addition, resveratrol has also been suggested to have anti-cancer activity (2,3). It is interesting that resveratrol has biphasic effects over its concentration: a low concentration (5 µM) of resveratrol appears to be increase cell proliferation, whereas apoptosis is induced in various cancer cells at 15 µM or higher concentrations (2).
In this study, we focused on the roles of resveratrol in normal endothelial cells to determine whether resveratrol can be utilized as a tumor growth-modulating BRM drug via the blockade of vascular functions. It was recently reported that resveratrol activates extracellular signal-regulated kinase (ERK), a member of the mitogen-activated protein kinase (MAPK) family, and it activates endothelial nitric oxide synthetase (eNOS) at low concentrations in endothelial cells (4). The enhanced ERK and eNOS activities by a low dose of resveratrol promote endothelial cell proliferation. In comparison, little is known about the role of high doses of resveratrol on the endothelium. Therefore, it is essential to determine the role of a high dose of resveratrol on the endothelium for utilizing resveratrol as a tumor growth-modulating BRM drug.
MATERIALS AND METHODS
1) Cell culture and drug treatment
BAECs obtained from descending thoracic aortas were maintained in growth medium (DMEM (1 g/liter glucose, Life technologies, Inc.) that contained 20% fetal bovine serum (FBS, Atlanta Biologicals) without antibiotics) at 37℃ and 5% CO2 (5,6). The cells used in this study were between passages 3 and 10, and they were grown on untreated culture dishes. Before drug treatment, the BAECs were confluently grown in the growth media that contained 50 µg/ml penicillin and 50 µg/ml streptomycin in a 5% CO2 incubator; the cells were then starved for 4~24 h in starvation media (DMEM that contained 0.5% fetal bovine serum with 50 µg/ml penicillin and 50 µg/ml streptomycin). For the apoptotic analysis, the confluent cells were incubated for 36 hours with 0.5% FBS-DMEM that contained 0 or 100 µM etoposide (ETO, Sigma) or 100 µM resveratrol (RES, Sigma) for 36 h.
2) Cell migration
For the in vitro cell migration assay, the cultivated BAECs were wounded with a razor blade after 24 h starvation. The cells were subsequently incubated in media containing 20% fetal bovine serum or various concentrations (1 nM to 100 µM) of resveratrol (RES). The cells were then allowed to migrate for 26 h and we observed the migrated cells under a microscope. To rule out the apoptotic effect of resveratrol, another group of BAECs were incubated with resveratrol for a relatively short time (less than 26 h). For the migration assay, cells that migrated over lines scratched with a blade were considered as migrated cells. Quantification was performed by counting the number of cells that migrated in the same field (7).
3) Measurement of apoptosis
Confluent BAECs were starved for more than 4 h and then they were treated with the indicated amounts of resveratrol. The cells were then incubated for an additional period of time (0, 4, 8, 12, 24, and 72 h) to conduct the time-course experiments. After additional incubations, we observed apoptotic cells (round, shrunken cells) under the microscope. For quantification, we counted the apoptotic cells in the same visual field. For Hoechst staining, the confluent cells were incubated with 0.5% FBS-DMEM that contained none (the control: CON), 100 µM etoposide (ETO) or 100 µM resveratrol (RES) for 36h. The BAECs were then fixed with Conroy's fixative for 10 min and washed with phosphate buffered saline (PBS). The cells were next air-dried for 10 min. After air-drying, the cell were stained by Hoechst 33258 (12.5 µg/ml, Sigma) for 30min at room temperature. After incubation, the stained cells were thoroughly washed with PBS. We then observed the nuclei under a fluorescence microscope (Zeiss Autoplan 2).
4) Western blots
The BAECs were confluently grown in 20% FBS-DMEM. They were then washed in ice-cold PBS, scraped in 250 µl RIPA buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS), and solubilized for 15 min to prepare the cell lysate as previously described (6). All the solubilization procedures were performed at 4℃ and the protein content of the soluble cell lysates was measured by using a Bio-Rad DC assay kit (Bio-Rad). The soluble lysates (10 µg each) were resolved by 10% SDS-PAGE; they were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) and probed with antibodies specific to p~ERK (Cell Signaling), p~eNOS (Cell Signaling), p~Akt (Cell Signaling), and caspase-3 (Cell Signaling) for 1 h to overnight. The membrane was then incubated with a secondary antibody at room temperature for 1 h. Goat anti-rabbit IgG conjugated to alkaline phosphatase was used as a secondary antibody. Finally, the membrane was developed by a chemiluminescent detection method (6).
RESULTS
1) A high dose of resveratrol has a pro-apoptotic effect on endothelial cells
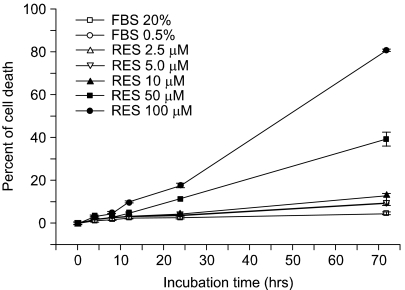
We tested the effect of resveratrol on programmed cell death. The apoptotic activity was determined by counting the number of apoptotic cells that were decreased by a pretreatment with resveratrol. In Fig. 1, the ratio of apoptotic cells was increased as the treated concentration of resveratrol became higher. Whereas about 10% of the cells appeared apoptotic in the cells treated with 10 µM resveratrol for 72 h, 80% of the endothelial cells were apoptotic at 100 µM resveratrol, indicating that resveratrol at a high concentration has a strong pro-apoptotic effect on endothelial cells. In contrast, a low concentration (less than 5 µM) of resveratrol inhibited oxidized LDL-induced cytotoxicity (8), indicating that resveratrol has a biphasic activity for cell viability as a function of its concentration.
Fig. 1.
Resveratrol induces endothelial cell apoptosis. Confluent BAECs were starved for more than 4 h and then the cells were incubated for additional hours (0, 4, 8, 12, 24 or 72 h) with the indicated doses of resveratrol (RES). Consecutively, the cells were incubated for additional hours (0, 4, 8, 12, 24 or 72 h) We then counted the apoptotic cells (round shrunken cells) under the microscope. The line graphs represent the percentages of apoptotic cells (means±S.E.). The experiments were independently performed at least three times.
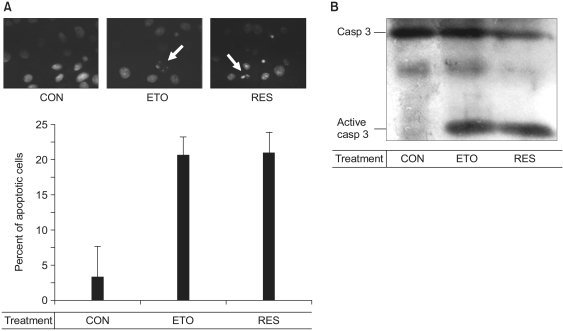
We also tested its pro-apoptotic effect as was detected by Hoechst 33258 staining (Fig. 2A). The apoptotic endothelial cells exhibited the characteristic features of cellular apoptosis, including condensation and fragmentation of the nuclei. As shown in Fig. 2, the fragmentation of nuclei was as great for the cells treated with 100 µM resveratrol as that in the cells treated with 100 µM etoposide, an anti-cancer drug (9). We then determined whether resveratrol activates caspase-3. In Fig. 2B, 100 µM resveratrol induced the cleavage of caspase-3, the same as 100 µM etoposide did, indicating that the resveratrol-induced endothelial apoptosis is mediated by caspase-3. These data confirm that 100 µM resveratrol acts as a pro-apoptotic agent in endothelial cells.
Fig. 2.
The resveratrol-induced apoptosis is mediated by activation of caspase-3. Confluent cells were incubated with 0.5% FBS-DMEM containing none (CON), 100 µM etoposide (ETO) or 100 µM resveratrol (RES) for 36 h. The cell were then stained with Hoechst 33258 and we observed the nuclei under a fluorescence microscope (A). Arrows indicate the fragmented nuclei. In panel B, after the cells were lysed, proteins in the cell lysates were resolved by SDS-PAGE, electrotransferred to PVDF membranes and immunoblotted with caspase-3 antibodies.
2) Resveratrol inhibits the endothelial cell migration
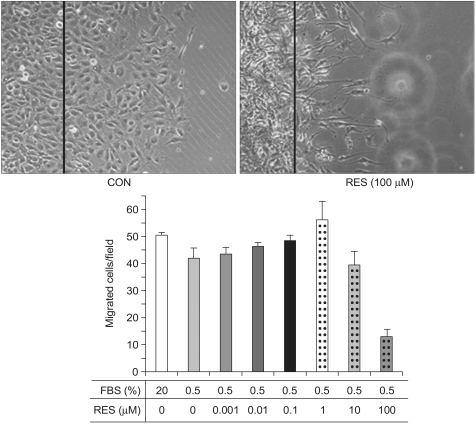
Endothelial cell migration is one of essential processes for a variety of vascular functions such as tumor growth, vascular remodeling and vascular wound healing (10). We investigated whether resveratrol has an effect on endothelial cell migration. Of interest is that resveratrol had an inhibitory effect on cell migration, while fetal bovine serum had the stimulatory effect on the cell migration (Fig. 3). This result indicates that resveratrol can be a potential anti-angiogenic drug, which is consistent with previous reports (11).
Fig. 3.
Resveratrol blocks the endothelial cell migration. Confluent endothelial cells were serum-starved and then scraped with a razor blade after 24 h starvation. The cells were subsequently incubated in media containing 20% fetal bovine serum (CON) or various concentration of resveratrol (RES). After 26 h, we then observed the migrated cells under the microscope. The line on each photograph indicates the boundary lines immediately after scraping. The photographs are representative of at least three different observations. We then counted the cells that migrated over the lines. Quantification was performed by counting the number of cells that migrated in the same field. The bar graphs show the mean number of migrated cells±S.E. (n=3).
3) Does resveratrol activate cell signaling molecules?
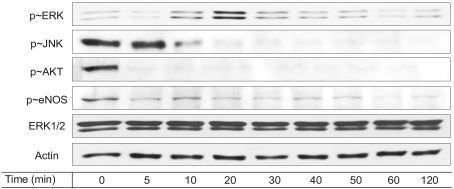
Since ERK and JNK, which are two important MAP kinases (12), are responsible for cell proliferation and apoptosis (13,14), we then determined whether those MAP kinases are modulated by 100 µM resveratrol. As shown in Fig. 4, ERK activation was triggered by the resveratrol treatment, whereas JNK activation was inhibited. Resveratrol-stimulated ERK activation was previously reported in endothelial cells (4). On the other hand, a high concentration of resveratrol stimulates JNK activation in a variety of cancer cells, leading cancer cell apoptosis (15). Accordingly, the stimulatory effect of resveratrol on endothelial ERK was consistent with previous reports, while the inhibitory effect of resveratrol on endothelial JNK was inconsistent with JNK activation in cancer cells. It is likely that these differential responses upon resveratrol stimulation occur in the context of different cells.
Fig. 4.
Resveratrol modulates a variety of cell signaling molecules. Serum-depleted cells (grown in 0.5% FBS-DMEM) were stimulated with 100 µM resveratrol treatment as a function of time. After the cells were lysed, the proteins in cell lysates were resolved by SDS-PAGE, electrotransferred to PVDF membranes and immunoblotted with the appropriate antibodies.
Nitric oxide production has significant effects on the endothelial physiology and pathophysiology (3,4). Therefore, we examined whether eNOS and Akt are modulated by a high dose of resveratrol. In Fig. 4, the basal levels of Akt and eNOS phosphorylation declined with treatment of 100 µM resveratrol. It has been previously documented that NO production is enhanced by resveratrol in MCF breast cancer cells (3). On the contrary, the current results showed that eNOS was inhibited by resveratrol in endothelial cells.
4) The resveratrol-induced ERK activation is not responsible for the resveratrol-dependent apoptosis
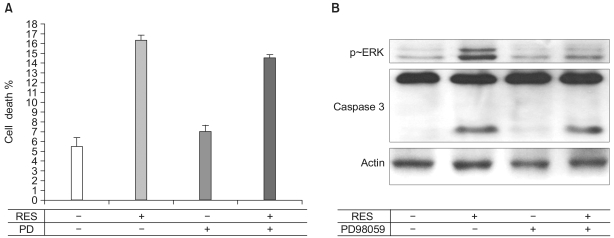
Since ERK was activated by 100 µM resveratrol in endothelial cells, we tested whether the resveratrol-dependent endothelial apoptosis was mediated by the ERK activation. Endothelial cells were pretreated with PD98059, an ERK inhibitor. Subsequently, the caspase-3 activity and apoptosis were assessed. Whereas PD98059 inhibited the ERK activation, the activity of caspase-3 and apoptosis were not altered by the pretreatment of PD98059, indicating that the resveratrol-stimulated ERK activity is not responsible for the resveratrol-stimulated apoptosis (Fig. 5).
Fig. 5.
ERK is not associated with the resveratrol-induced apoptosis. (A) Apoptosis was observed as described in Fig. 1. Interestingly, 10 µM of PD compound had no effect on apoptosis. The bar graphs represent means±S.E. (n=3). (B) Serum-depleted cells (grown in 0.5% FBS-DMEM) were stimulated with 100 µM resveratrol treatment for 24 h. After the cells were lysed, proteins in the cell lysates were resolved by SDS-PAGE, electrotransferred to PVDF membranes and immunoblotted with caspase-3 antibodies.
DISCUSSION
In the last decade, resveratrol has been intensively studied as a potential anti-cancer drug. Resveratrol has biphasic properties according to its concentration. For instance, in androgen-sensitive prostate cancer cells, resveratrol has a proliferative activity at a low dose (5 µM), whereas it has a pro-apoptotic activity at a high dose (15 µM or higher) (3). In endothelial cells, resveratrol activates eNOS at a low dose (~nM) and it inhibits eNOS at a high concentration (100 µM). In addition, the efficacy of resveratrol it still being debated because of its multiplicity of targets and its contradictory functions that seem to depend upon its concentration and the type of target cell. Moreover, less is known for the roles of resveratrol in endothelium. Therefore, this study poses more queries on the uncertain roles of resveratrol in endothelium and (or) it proposes broader roles for the applications of resveratrol.
This study showed resveratrol induces endothelial cell apoptosis and it also inhibits endothelial migration at a high dose. Although it was previously reported that apoptosis of ECV304 endothelial cells is induced by the inhibition of ERK activity (16), we showed here that the resveratrol-stimulated ERK activity was not associated with apoptosis. More clearly, resveratrol inhibits endothelial migration, which is an angiogenic process. Therefore, the high dose (100 µM) of resveratrol may be anti-angiogenic. Since growth, invasion and metastasis of malignant tumors are angiogenesis-dependent processes (17~19), it is possible that high doses of resveratrol can be utilized as an anti-angiogenic drug. Although the underlying mechanisms remain to be solved, the demonstrated effects of high doses of resveratrol provide insight on this drug as a tumor growth-modulating and angio-inhibiting BRM drug.
CONCLUSIONS
Resveratrol plays an important role in endothelial cell apoptosis and cell migration, indicating that resveratrol can be utilized as a potent anti-angiogenic drug.
ACKNOWLEDGMENT
The authors wish to thank Dr Jaeho Pyee for his constructive advice.
Footnotes
The present work was supported by research funds of Dankook University in 2004.
References
- 1.Gasparini G. The rationale and future potential of angiogenesis inhibitors in neoplasia. Drugs. 1999;58:17–38. doi: 10.2165/00003495-199958010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer Chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 3.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Klinge CM, Blankenship KA, Resinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, et al. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors α and β in endothelial cells. J Biol Chem. 2005;280:7460–7468. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 5.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J Biol Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- 6.Park H, Go YM, St John PL, Maland MC, Lisanti MP, Abrahamson DR, et al. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem. 1998;273:32304–32311. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- 7.Park SG, Kang YS, Ahn YH, Lee SH, Kim KR, Kim KW, et al. Dose-dependent biphasic activity of tRNA synthetase-associating factor, p43, in angiogenesis. J Biol Chem. 2002;277:45243–45248. doi: 10.1074/jbc.M207934200. [DOI] [PubMed] [Google Scholar]
- 8.Ou HC, Chou FP, Sheen HM, Lin TM, Yang CH, Huey-Herng Sheu W. Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells. Clin Chim Acta. 2006;364:196–204. doi: 10.1016/j.cccn.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Lopez AM, Xenaki D, Eden TO, Hickman JA, Chresta CM. MDM2 mediated nuclear exclusion of p53 attenuates ectoposide-induced apoptosis in neuroblastoma cells. Mol Pharmacol. 2001;59:135–143. doi: 10.1124/mol.59.1.135. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 11.Oak MH, Bedoui J, Schini-Kerth VB. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem. 2005;16:1–8. doi: 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos Trans R Soc Lond B Biol Sci. 1996;351:127–134. doi: 10.1098/rstb.1996.0008. [DOI] [PubMed] [Google Scholar]
- 13.Zuckerbraun BS, McCloskey CA, Mahidhara RS, Kim PK, Taylor BS, Tzeng E. Overexpression of mutated IkappaBalpha inhibits vascular smooth muscle cell proliferation and intimal hyperplasia formation. J Vasc Surg. 2003;38:812–819. doi: 10.1016/s0741-5214(03)00427-0. [DOI] [PubMed] [Google Scholar]
- 14.Park H, Park SG, Kim J, Ko YG, Kim S. Signaling pathways for TNF production induced by human aminoacyl-tRNA synthetase-associating factor, p43. Cytokine. 2002;20:148–153. doi: 10.1006/cyto.2002.1992. [DOI] [PubMed] [Google Scholar]
- 15.She QB, Huang C, Zhang Y, Dong Z. Involvment of c-jun NH(2)-terminal kinase in resveratrol-induced activation of p53 and apoptosis. Mol Carcinog. 2002;33:244–250. doi: 10.1002/mc.10041. [DOI] [PubMed] [Google Scholar]
- 16.Sanna B, Debidda M, Pintus G, Tandolini B, Posadino AM, Bennardini F, et al. The anti-metastatic agent imidazolium trans-imidazoledimethylsulfoxide-tetrachlororuthenate induces endothelial cell apoptosis by inhibiting the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway. Arch Biochem Biophys. 2002;403:209–218. doi: 10.1016/s0003-9861(02)00218-7. [DOI] [PubMed] [Google Scholar]
- 17.Ranieri G, Gasparini G. Angiogenesis and angiogenesis inhibitors: a new potential anticancer therapeutic strategy. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:241–253. doi: 10.2174/1568008013341073. [DOI] [PubMed] [Google Scholar]
- 18.Iyer S, Chaplin DJ, Rosenthal DS, Boulares AH, Li Ly, Smulson ME. Induction of apoptosis in proliferating human endothelial cells by the tumor-specific antiangiogenesis agent combretastatin A-4. Cancer Res. 1998;58:4510–4514. [PubMed] [Google Scholar]
- 19.Lenz HJ. Antiangiogenic agents in cancer therapy. Oncology. 2005;19(4) Suppl 3:17–25. [PubMed] [Google Scholar]