The reversible condensation of chromosomes during cell division remains a classic problem in cell biology. Condensation requires the condensin complex 1 in certain experimental systems 2-8, but not in many others 9-15. Anaphase chromosome segregation almost always fails in condensin-depleted cells, leading to the formation of prominent chromatin bridges and cytokinesis failure 4, 9-17. Here, live cell analysis of chicken DT40 cells bearing a conditional knockout of condensin subunit SMC2 reveals that condensin-depleted chromosomes abruptly lose their compact architecture during anaphase and form massive chromatin bridges. The compact chromosome structure can be preserved and anaphase chromosome segregation rescued by preventing the phosphatase targeting subunit Repo-Man from recruiting PP1 to chromatin at anaphase onset. This study identifies an activity critical for mitotic chromosome structure that is inactivated by Repo-Man/PP1 during anaphase. This activity, RCA (regulator of chromosome architecture), cooperates with condensin to preserve the characteristic chromosome architecture during mitosis.
Mitosis is normal in SMC2 conditional knockout (SMC2ON/OFF) chicken DT40 cells grown without doxycycline (SMC2ON) 12. By 30 hours after addition of doxycycline to the culture medium (SMC2OFF) SMC2 mRNA levels drop at least 160-fold (QRT-PCR, Supplementary Figure 1a) and the protein becomes undetectable in immunoblots. The cells begin to die within 24-48 hours as anaphase chromosome segregation fails and massive chromatin bridges block cytokinesis (Figure 1a-d). The loss of SMC2 is accompanied by loss of other condensin subunits (e.g. CAP-H) from mitotic chromosomes (Supplementary Figure 1b-d).
Figure 1. Phenotypes of condensin-depleted cells in anaphase.
(a-d) Stills from movies of SMC2ON/OFF:H2B-RFP /CENPH:GFP cells during anaphase. SMC2ON/OFF:H2B-GFP cells were grown for 30 h in the presence of doxycycline then induced to adhere on concanavalin A-coated coverslips and followed by 2 min interval time-lapse imaging. (a) SMC2ON and (b, c) SMC2OFF cells. The chromosomes condense and align on a metaphase plate (0 min). During early anaphase (6 min) they abruptly lose their compact structure and individual chromosomes can no longer be distinguished. Chromatin bridges persist in cytokinesis which fails, giving rise to a binucleate cell (b, upper panel) or to a cell with a giant nucleus (b, lower pane;). (c, d) higher magnification views of the cell marked in panel (b). The arrows indicate the onset of the loss of chromosome definition. Scale bar, 5 μm.
(e) Measurement of chromosome compaction in anaphase. Values of chromosome compaction were normalised relative to the last metaphase figure in each time-lapse experiment (time 0). Eight independent movies each were analysed for SMC2ON and SMC2OFF cells.
(f-g) Marked chromosomal loci segregate normally in SMC2-depleted cells during anaphase. Anaphases of SMC2OFF cells showing 1:1 segregation of the DHFR:lac O array in two different cell lines. The yellow arrows indicate the position of the integrated sequence visualised by lac I-GFP. The red arrows indicate chromatin bridges. (f-f”) Cells with integration on a micro-chromosome. (g-g”) Cells with integration on a macro-chromosome. Insets in f, g show the localisation of the LacO:DHFR array (green) relative the centromere (INCENP-blue). In both the cell lines the marked loci segregate correctly in the absence of condensin when the chromatin bridging is suppressed by overexpression of cyclin B3 (f”, g”). Scale bar, 5 μm
While this anaphase failure is unlikely to be due to defects in cohesin dynamics (see 18, our unpublished results), it could reflect a loss of DNA topoisomerase II (topo II) function, because topo II localisation is altered in condensin-depleted chromosomes 12, 18, and the activity of extracted Drosophila topo II against an exogenous substrate is decreased following condensin RNAi 18. We therefore examined topo II activity in vivo at a physiological site by quantitating in situ topo II cleavage within the highly characterized 2.1 Mb centromeric α-satellite DXZ1 array of the human X chromosome 19 in four independent SMC2ON/OFF DT40 hybrid cell lines. No significant differences in topo II activity at this site were found in the presence or absence of condensin (Supplementary Figure 2). Therefore, vertebrate topo II does not require condensin for activity in vivo. Functional interactions between topo II and condensin may differ between Drosophila (with a single topo II isoform) and vertebrate cells (with two isoforms).
For quantitative analysis of the segregation of specific chromosomal loci in condensin-depleted cells, we introduced a DNA construct containing 256 copies of the lac operator (lac O) repeat into SMC2ON/OFF cells 20. This array can be detected with lac repressor (lac I)-GFP (Figure 1f,g). Eight independent sub-lines carrying lac O arrays integrated at different chromosomal sites were isolated and analysed. Remarkably, these marked loci segregated normally in 221/222 anaphases in SMC2OFF cells (28±5 anaphases scored for each cell line), even in cells where prominent chromatin bridges were observed (Figure 1f’,g’; Supplementary Figure 1f).
To examine more closely the mechanism of anaphase bridge formation in the absence of condensin, we generated a further SMC2ON/OFF subline expressing functional CENP-H:GFP by targeting GFP into the single copy endogenous CENP-H gene 21. The labelled kinetochores enabled us to accurately observe anaphase onset. The chromatin in these cells was labelled with H2B:mRFP so we could also follow the behaviour of the chromosomes.
Before further discussing the role of condensin in mitosis, we must define several terms. We use ‘chromatin compaction’ to refer to changes in the volume occupied by chromosomes that occur in both mitosis and apoptosis, presumably by different mechanisms. Chromatin compaction can be measured as changes in the amount of chromatin per unit volume in chromosomes. We use ‘chromosome condensation’ to refer to the formation of individual compact chromosomes during mitosis. We refer to the distinct, separated chromosomes in mitosis as having a ‘mitotic chromosome architecture’. In chromosome spreads, this is the familiar X-shaped morphology, however in living vertebrate cells chromosomes are often more compact, and separated sister chromatids may not be distinguished prior to anaphase.
Without condensin, chromosome condensation was delayed 10, 12, and kinetochores behaved as though the rigidity of the underlying heterochromatin was reduced 22, 23 (also our unpublished work). In both DT40 (Figure 1 b) and human cells 14, 23 a metaphase alignment was eventually achieved and cells ultimately entered anaphase. Condensin-depleted Drosophila S2 cells enter anaphase without achieving a metaphase alignment 22.
Anaphase in SMC2OFF DT40 cells was highly aberrant: the chromosomes abruptly lost their compact architecture while still moving polewards (Figure 1b-d). When this occurred, individual chromatids were no longer observed and prominent chromatin bridges appeared in >90% of cells within 2-3 minutes of anaphase onset. Importantly, this did not reflect a premature exit of SMC2OFF cells from mitosis. Histone H3-Serine10 dephosphorylation occurred with similar kinetics in SMC2ON and SMC2OFF cells 11 (Supplementary Figure 3a-h) and there was no premature reassembly of the nuclear lamina in SMC2OFF cells (Supplementary Figure 3i-n). Nor did the phenotype simply reflect a premature decondensation of the chromatin.
In SMC2OFF chromosomes at metaphase, chromatin compaction is about 60% that of control SMC2ON chromosomes, as determined by direct measurement on chromosomes in movies (Figure 1e) or independently by measuring the spacing between lac I signals on chromosome arms with integrated lac O arrays (Supplementary Figure 1f). No selective defects in compacting repeated DNA sequences or rDNA were noted, unlike the situation in yeasts (reviewed in 1). Analysis of the movies showed that mitotic chromosome arms normally undergo a slight reduction in chromatin compaction at anaphase onset, followed by re-compaction as they move polewards (Figure 1e). Although the chromatin was less compact in SMC2OFF chromosomes, they nevertheless underwent a similar cycle of relaxation/re-compaction. Therefore, the dramatic change in their morphology during anaphase appears to reflect not a sudden loss of chromatin compaction, but instead a loss of the individual chromosome architecture during anaphase. Together, these observations suggest (in agreement with previous studies 12, 22, 23) that a key role of condensin is the stabilization of chromosome architecture throughout mitosis.
Because chromosomes lacking condensin can condense and function in mitosis until the onset of anaphase, there must be another system that drives chromosome condensation and stabilizes the condensed chromosome architecture when condensin is absent. The availability of the conditional knockout of condensin I and II in DT40 cells has enabled us for the first time to begin characterising this second system, which we provisionally term RCA (regulator of chromosome architecture).
We infer that RCA normally functions only until anaphase onset, since after this point condensin becomes essential for the maintenance of chromosome architecture. This suggests that RCA function may require high levels of CDK activity. We therefore hypothesized that if we could maintain the cytoplasm in a physiological state characteristic of early mitosis, condensin-depleted chromosomes might be able to complete anaphase segregation with RCA maintaining their integrity. One way to do this would be to express in SMC2OFF cells exogenous cyclin B3, which is normally degraded relatively late in mitosis 24, and which is expressed in DT40 cells (Supplementary Figure 1e). Over-expressed or non-degradable forms of cyclin B1 and cyclin B3 can block chromosome decondensation at the end of mitosis if microinjected into Drosophila embryos or mammalian cultured cells 24.
Exogenous expression of GFP-cyclin B3 indeed had a remarkable effect on SMC2OFF cells, efficiently rescuing the anaphase defects in chromosome architecture. Cells accumulated in late anaphase, with separated chromatids remaining fully condensed at the spindle poles (Figure 2b,d). No chromatin bridges were observed, whereas >90% of non-transfected anaphase cells had prominent bridges (see Figures 2c). The condensed chromosomes at the spindle poles correspond to segregated sister chromatids. Lac O/Lac I:GFP-marked loci on the chromosome arms segregated in a 1:1 manner without chromatin bridges in two independent cell lines transfected with GFP-cyclin B3 (Figure 1f”,g”). Further evidence that these cells underwent a bona fide anaphase includes the normal degradation of cyclin B2 (Figure 2a,b) and the transfer of INCENP to the anaphase central spindle and midbody (Figure 2c,d). GFP-cyclin B3 overexpression did not interfere with SMC2 depletion following addition of doxycycline to the transfected cells (data not shown).
Figure 2. Overexpression of GFP-Cyclin B3 rescues the anaphase bridging phenotype in condensin-depleted cells.
(a,b) SMC2OFF cells transiently transfected with GFP-cyclin B3 were fixed and stained for cyclin B2 and tubulin. (a) Transfected cells in early mitosis with high levels of cyclin B2. (b) Transfected cell in cytokinesis showing no chromatin bridging (black arrow) and a low level of cyclin B2. (c,d) SMC2OFF::CENP-H-GFP cells transiently transfected with GFP-cyclin B3 were stained for α-tubulin and INCENP. (c) An untransfected cell with a massive chromatin bridge (red arrow) and (d) a transfected cell with no bridges (black arrow). In both cases INCENP has transferred normally from the chromosomes to the midbody.
(e, f) Inactivation of CDKs with Roscovitine blocks the rescue of chromatin bridging by cyclin B3 in SMC2OFF cells. (e-e’) Untreated cell expressing cyclin B3 showing separated condensed chromosomes without chromatin bridges (black arrow). (f-f’) A transfected cell treated with 50 μM roscovitine for 20′ prior to fixation has a huge chromatin bridge (red arrow). (g-g”) SMC2ON cell arrested in mitosis by colcemid - Repo-Man-GFP is diffusely cytoplasmic. (h-h”) Cell as in (g) treated with 50 μM roscovitine for 15′ prior to fixation. Repo-Man-GFP has relocalized to the chromosomes. Scale bar, 5 μm.
The above experiment is consistent with the hypothesis that RCA is positively regulated by CDK activity. To further test this hypothesis, we treated cells with roscovitine 25, a selective inhibitor that can inactivate Cdk1 even in the presence of high levels of GFP-cyclin B3. Roscovitine treatment of SMC2OFF cells for 10 and 20 minutes before fixation abolished the ability of cyclin B3 to stabilise chromosome architecture in anaphase, and prominent chromatin bridges were again observed (Figure 2e,f).
The mechanism underlying this CDK protection of chromosome architecture in condensin-depleted cells emerged from a study of the targeting of the protein phosphatase PP1 to chromosomes. It was recently reported that a novel targeting subunit, Repo-Man, recruits a pool of PP1γ to chromatid arms at anaphase onset 26. This targeting does not require condensin, as GFP:hPP1γ and GFP:hRepo-Man target normally to anaphase chromosomes in SMC2OFF cells (Supplementary Figure 4). PP1 binding is mediated by a canonical RVXF motif in Repo-Man, and is abolished if this motif is mutated to RAXA 26. Repo-Man-RAXA is a dominant-negative mutant that localises to anaphase chromosomes and can block recruitment of PP1. Its expression in HeLa cells is highly toxic, resulting in apoptotic cell death 26.
Remarkably, GFP:Repo-Man-RAXA overexpression completely restored the ability of condensin-depleted chromosomes to retain their architecture and segregate normally during anaphase in SMC2OFF cells (Figure 3d,e). In controls, when GFP:Repo-Man-wt was overexpressed in SMC2OFF cells, prominent chromatin bridges were observed (Figure 3b,e). This experiment reveals that targeting of PP1 to anaphase chromatin causes the loss of chromosome architecture during anaphase in the condensin-depleted cells.
Figure 3. Expression of GFP-Repo-Man-RAXA mutant suppresses the anaphase bridges in SMC2OFF cells.
SMC2ON and SMC2OFF cells were transiently transfected with GFP-Repo-Man-wt (a,b) or GFP-Repo-Man-RAXA mutant (c,d). SMC2OFF cells transfected with GFP-Repo-Man-wt show anaphase chromatin bridging (b” red arrow) while SMC2OFF cells transfected with GFP-Repo-Man have no bridging (d”, black arrow). Green: GFP-Repo-Man; blue: DAPI. Scale bar, 5 μm. (e) Quantitation of this experiment. U, untransfected cells; T, transfected cells.
The mechanism by which CDK activity protects chromosomal architecture in condensin-depleted cells was revealed by a closer look at the behaviour of Repo-Man. Repo-Man normally has a diffuse cytoplasmic distribution at metaphase, and then binds abruptly to chromosomes upon anaphase onset. This suggests that Repo-Man’s ability to target PP1 to chromatin might be negatively regulated by CDK activity. In support of this hypothesis, Repo-Man has two predicted CDK target sites, the protein is phosphorylated in vitro by Cdk1-cyclin B, and endogenous Repo-Man from cells arrested in prometaphase with the microtubule-disassembling drug colcemid exhibits a mobility shift relative to that in interphase cells (Figure 4g-I - full length gels are shown in Supplementary Figure 4e-g). Furthermore, in colcemid-arrested wild-type cells, transfected GFP-Repo-Man is diffusely cytoplasmic (Figure 2g). Following addition of roscovitine, the GFP-Repo-Man relocated to the chromosomes within 15 minutes (Figure 2h). Importantly, chromosomes with bound Repo-Man rapidly begin to decondense, even though these colcemid-arrested cells contain functional condensin.
Figure 4. Repo-Man is the critical factor regulated by CDK-cyclin B3 and required for condensin-depleted chromosomes to retain their compact architecture through anaphase.
(a) Untransfected cells show extensive anaphase bridging, which is eliminated by overexpression of cyclin B3 (b) and unaffected by the expression of GFP:Repo-Man (c). In cells expressing mCherry:Cyclin B3 and moderate levels of GFP:Repo-Man (d), GFP:Repo-Man localisation to chromosomes is much reduced and the chromatids remain condensed with no bridges (black arrow). In contrast, when high levels of GFP:Repo-Man are expressed, the protein can localise to the chromosomes even in the presence of mCherry:Cyclin B3 and chromatin bridges are again observed (e, red arrow). green: GFP:Repo-Man; red, mCherry-Cyclin B3; blue: DAPI. (f) Quantitation of the anaphase bridging phenotype in cells transfected with the constructs indicated in (a-e). (g) Endogenous Repo-Man undergoes a mobility shift in HeLa cells blocked in mitosis with nocodazole. (h) GFP-Repo-Man immunoprecipitated from transfected cells is phosphorylated by Cdk1-Cyclin B. Left panels - Coomassie blue. Right panels - autoradiograph. GFP is not phosphorylated by Cdk1-cyclin B (not shown). (i) Autoradiograph showing recombinant GST-Repo-Man expressed in E. coli is phosphorylated by Cdk1-cyclin B. GST and histone H1 were included as a negative and positive controls, respectively. In (h, i) all lanes had γ32P-ATP. Cdk-cyclin B was added in the lanes indicated with a (+). Scale bar 5 μm.
If Repo-Man were negatively regulated by CDK phosphorylation, then targeting of Repo-Man-wt to chromosomes in cells expressing ectopic cyclin B3 should block the rescue of the condensin depletion phenotype. This is exactly what was seen when cells expressing ectopic cyclin B3 were treated with roscovitine (Figure 2f). Furthermore in SMC2OFF cells simultaneously expressing GFP:Repo-Man-wt plus mCherry:Cyclin B3, if Repo-Man was associated with the anaphase chromosomes (seen in some cells with particularly high levels of Repo-Man overexpression) then loss of chromosome architecture and anaphase bridging occurred even in the presence of Cyclin B3 (Figure 4a,e,f). Thus, Repo-Man is the principal target of CDK1-cyclin B3 that protects the structure of condensin-depleted chromosomes during anaphase.
The rescue of chromosome segregation by transient expression of either cyclin B3 or the Repo-Man-RAXA mutant is not simply due to a lengthening of mitosis. Exogenous expression of Repo-Man-RAXA in HeLa cells does not significantly lengthen mitosis (L.T.-M., unpublished). Furthermore, when mitosis was artificially prolonged by exposing SMC2OFF cells to the microtubule-disassembling drug nocodazole for 4 h (during which time the chromosomes became hypercondensed 12), 91.5% of anaphases had chromatin bridges following release from the block. This compared with 91.9% in SMC2OFF cells undergoing mitosis without drug-induced delay.
Our data reveal that the chromatin bridges observed in condensin-depleted cells do not result from a simple failure to decatenate sister chromatids, but reflect a catastrophic loss of individual chromosome architecture during anaphase. From this, we deduce the existence of an activity, RCA, that can stabilize condensed mitotic chromosomes in the absence of condensin, provided that Repo-Man is prevented from targeting PP1γ to the chromatin. It has gradually become accepted in recent years that chromosomes can condense without condensin, but the present study is the first in which this secondary condensation system has been studied directly.
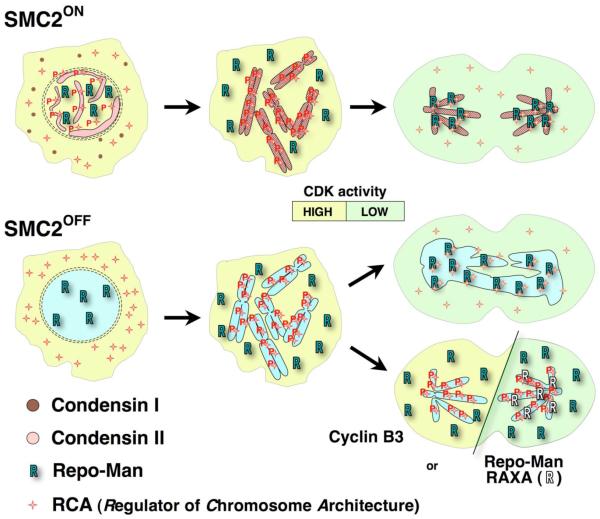
Figure 5 shows a working model for the regulation of mitotic chromosome architecture by condensin and RCA. Prophase chromosome condensation normally occurs as condensin II enters nuclei. Following nuclear envelope breakdown, when CDK activity is maximal, both condensin and RCA (likely activated by phosphorylation) are associated with chromosomes. As cytoplasmic CDK levels drop at anaphase onset, Repo-Man associates with the chromatin, thereby targeting a pool of PP1 to dephosphorylate substrates, including RCA. This dephosphorylation abolishes the ability of RCA to promote chromosome condensation. However, chromosomes remain condensed throughout anaphase because they are stabilised either directly by condensin or by the non-histone proteins whose assembly it promotes (or both) 12.
Figure 5. Co-operation between condensin and CDKs in establishment and maintenance of mitotic chromosome condensation.
(upper) In normal mitosis, an unknown factor (possibly RCA) promotes chromosome condensation during prophase. Condensin II associates with chromosomes during prophase, and condensin I upon nuclear envelope breakdown. At the onset of anaphase, RCA is inactivated following the targeting of PP1γ to chromosomes by Repo-Man. The chromosomes remain condensed as they migrate to the poles, presumably because they are stabilised by the action of condensin or the non-histone proteins whose assembly it promotes. (lower) In cells lacking condensin, chromosomes condense only after nuclear envelope breakdown. Because condensation can ultimately occur without condensin, at least in some cell types 9, 10, condensin II may normally promote either the nuclear entry, or retention, of a chromosome condensation activity, which for simplicity we provisionally equate with RCA in the diagram. When CDK levels drop during anaphase, RCA is inactivated by Repo-Man/PP1γ. As a result, the chromosomes undergo a catastrophic loss of their compact structure and anaphase fails. Expression of exogenous cyclin B3 maintains the CDK-dependent phosphorylation of RCA preventing Repo-Man from binding to the chromosomes. Alternatively, expression of Repo-Man-RAXA mutant blocks endogenous Repo-Man from targeting PP1γ to the chromosomes. Both treatments allow anaphase chromatids to retain their architecture in the absence of condensin.
In SMC2OFF condensin-depleted cells, we infer that only the RCA system remains functional, as SMC2 protein is undetectable 12. When CDK levels drop during anaphase, Repo-Man binding to chromatin promotes RCA dephosphorylation by PP1. Dephosphorylated RCA can no longer stabilise the condensed chromosomes, which, in the absence of condensin, consequently lose their normal architecture, giving rise to the chromatin bridges that cause anaphase to fail. We note that the bridges could arise through a combination of effects: e.g. when chromosome architecture is altered at anaphase onset, this could also prevent or reduce decatenation of the sister chromatids by topo II. It is possible that when chromosome architecture is preserved by RCA during anaphase following inhibition of Repo-Man function, the polewards tension provided by kinetochore microtubules might provide the directionality that enables topo II to separate the sister chromatids.
The loss of chromosome architecture during anaphase in condensin-depleted cells can be prevented by either maintaining a high level of CDK activity or by expression of Repo-Man-RAXA. Both treatments prevent endogenous Repo-Man from targeting PP1γ to the chromosomes, thereby blocking the dephosphorylation of RCA.
RCA could be a single protein, a multi-protein (or RNA-protein) complex or even a particular form of “histone code” (i.e. a particular spectrum of histone modifications). While this remains to be determined, the present studies allow us to deduce certain of its key attributes. First, at least one essential component of RCA must be a substrate for PP1. Second, PP1 can only act on RCA if targeted by Repo-Man - in cells transfected with Repo-Man-RAXA the high levels of PP1 present throughout the cell are unable to inactivate RCA. Third, RCA is likely either a direct binding partner, or else associated with a binding partner, of the Repo-Man-PP1 complex.
Finally, although this study does not address the mechanism of mitotic chromosome condensation directly, our data raise the possibility that RCA could play a primary role in this process, with condensin serving its essential role in stabilising the resulting condensed state. Further characterization of RCA should provide important new clues concerning the molecular mechanisms regulating the key process of chromosome condensation in mitosis.
MATERIALS AND METHODS
Cell culture, transfections and cell fusions
The chicken DT40 cell culture and repression experiments in the SMC2 conditional knock-out cell line were performed as previously described 12.
SMC2 conditional KO cells expressing H2B-RFP were generated by co-transfection of the H2B-RFP vector with a β-actin-Blasticidin resistant construct at the ratio 10:1. The CENP-H:GFP knock-in construct was provided by T. Fukagawa 21.
For whole cell fusion 1 × 107 cells each of the SMC2 KO line and a human X minichromosome: DT40 hybrid (1aA1/IKNFA3) were washed, mixed and exposed to 1 ml 50% w/v PEG (Roche) for 90 sec at 37°C. After diluting into serum-free medium the cells were washed and plated into basic growth medium overnight. The following day the cells were plated into 96 well dishes and whole cell hybrids selected in conditioned medium in the presence of 10 mM histidinol (Sigma), 2 mg/ml G418SO4 (Gibco), 30 μg/ml blasticidin (ICN) and 2 mg/ml hygromycin (Calbiochem). Clones were characterised by Southern blotting, FISH and indirect IF as described previously 12, 19.
For transient transfections cells in exponential growth phase were washed and resuspended in OPTIMEM (Gibco) at the concentration of 1.3 × 107/ml and electroporated at 300V, 950μF. For transient transfections with GFP-Cyclin B3 cells were grown in the presence of doxycycline for 8 h, transiently transfected and plated in complete medium + doxycycline for another 21 hr. The experiments were analysed at 29 h after repression of the SMC2 transgene. For the experiments described in Figures 1 (f), 3 and 4 transient transfections were performed using the Nucleofector system (Amaxa). As expected, chicken cyclin B3 (GFP-cyclin B3) caused SMC2ON cells to accumulate in late anaphase (mitotic index 23.6% versus 4.7% for transfected/nontransfected cells).
For the generation of lac O/lac I-GFP cell lines SMC2 conditional KO cells were transfected with pSV2-dhfr8.32 (kindly provided by A. Belmont) and selected in 0.1 μM methotrexate (Sigma). Selected clones were then transfected with a lac I-GFP expression construct, p3’SSdimerCloneEGFP (gift of A. Belmont), and selected in 2.5 mg/ml hygromycin (Roche).
Live cell imaging was performed with a Delta-Vision Microscope in a sealed chamber at 39°C. One single plane was collected for both channels every 2 min.
Quantitative RT-PCR analysis
RNA was reverse-transcribed using Invitrogen Life Technologies Superscript™ First-Strand Synthesis System. cDNA was diluted to 80 μl with sterile H20 and 3 μl of diluted cDNA were used per 20 μl Quantitative PCR (QPCR) reaction. QPCR reaction components included 3 μl cDNA sample, 10 μl 2x Abgene QPCR Master Mix (containing 0.025 units/μl Thermo-Start® Enzyme), 3 μl sterile H20, 2μl SYBR® Green 1 (1:10000 dilution of 10000x concentrate in DMSO, Molecular Probes), 1μl (4 pmol/μl) forward primer and 1μl (4 pmol/μl) reverse primer. Each QPCR reaction was prepared in quadruplicate and amplified using the iCycler iQ™ Real-Time Detection System (BIO-RAD). PCR reactions consisted of an initial 15 minute denaturation step (which was also necessary to activate the Thermo-Start® Enzyme) followed by 40 cycles of melting at 95° C for 15 s, annealing at 58° C for 45 s, and extension at 72° C for 30 s. Primers for detecting SMC2 expression: Forward: 5′-gttttgacctccatagaagac-3′, Reverse: 5′-cagatggagtccaagatg-3′. Primers for cDNA control: Forward: 5′-gggtcttatgaccactgtcca-3′, Reverse: 5′-tggacgctgggatgatgtt-3′.
Generation of the GFP -GgCyclin B3 construct and RT-PCR
The full length GgCyclin B3 cDNA was generated from two overlapping chicken ESTs obtained from MRC geneservice and cloned into pEGFPC1 (ClonTech). mCherry:Cyclin B3 was generated by replacing EGFP with mCherry (gift of Roger Tsien) in the previous vector. Chicken cyclin B3 resembles other canonical cyclins unlike human cyclin B3, which is much larger. Cyclin B3 expression was detected in DT40 cells by RT-PCR using the following primers Fw: gcatccggaggcggcatgccgctggcacgcagc and Rev: gtagtcgaagatctccttggcgtac.
Immunoblotting analysis
Total cell extract or mitotic chromosome preparations 12 were separated in SDS-PAGE and blotted onto nitrocellulose membrane (Amersham). After blotting the membranes were stained with Ponceau S (Sigma). Membranes were blocked with 5% skimmed milk in PBS and processed for ECL by standard methods. Antibodies used were: rabbit anti-ScII M at 1:100027; rabbit anti-CAP-H generated against an amino-terminal portion of GgCAP-H corresponding to aa209-416 of XCAP-H (1:500).
Indirect Immunofluorescence and microscopy
Where not otherwise indicated, cells were fixed with 4% paraformaldeyde for 5′ in cytoskeleton buffer (CB) (137 mM NaCl, 5mM KCl, 1.1 mM Na2PO4, 0.4 mM KH2PO4, 2mM MgCl2, 2 mM EGTA, 5 mM PIPES and 5.5 mM glucose), permeabilized in 0.15% Triton X-100 in CB for 2′ and incubated 30′ with the specific antibodies: mouse anti-α tubulin (SigmaIGMA), anti-INCENP 1186 28 1:750, anti GgCyclin B2 1:100 (gift of E. Nigg), anti phosphoSer10-H3 at 1:200 (Upstate Biotechnology) anti Lamin B1 (Zymed lab) at 1:100. Cells were washed 3 times in PBS, fluorescence-labelled secondary antibodies applied 1:200 (Jackson Immunoresearch) and counter-stained with DAPI. For Supplementary Figure 1 cells were fixed with Methanol: Acetic Acid and processed as previously described 29.
Three-dimensional data sets were collected with a DeltaVision system (Applied Precision) based on an Olympus IX-70 with a Chroma Technology Sedat filter set driven by the SoftWorx software under standard conditions. All image files are archived as raw (r3d), and deconvolved (d3d) files readable by the SoftWorx software. Images were subjected to the standard SoftWorx deconvolution algorithm. Three-dimensional data sets were converted to Quick Projections in SoftWorx, and then converted to TIFF files and imported into Adobe Photoshop for final presentation. Levels were adjusted across each entire image to lower non-specific background haze using the standard Photoshop adjustment of levels.
Chromosome condensation before and after the metaphase:anaphase transition was measured in 3D recordings with a time-lapse of 2 min for 8 SMC2ON and 8 SMC2OFF cells. For each time point, we determined the mean fluorescence intensity in five boxes of 0.46 × 0.73 μm using the Data Inspector tool of the SoftWorx support package. In each cell, this was done for 5 different chromosome arms, and the values obtained were averaged. We have previously used this method to demonstrate that phosphorylation of histone H3 does not correlate with chromosome condensation in Drosophila cultured cells 30. For presentation in Figure 1e, the values obtained were normalised to the average value obtained for the last metaphase prior to anaphase onset for the eight SMC2ON and eight SMC2OFF movies, separately. Normalisation was required because different cells express different levels of H2b-RFP.
Treatment of living cells with the topoisomerase inhibitor etoposide
Etoposide (Sigma) was dissolved in 100% DMSO at 100 mM and stored dark at -20°C. The inhibitor was added to exponentially growing cells to a final concentration of 10 or 50 μM and incubated at 37°C for 15 min. Cells were washed with PBS before embedding in agarose (2 × 107 2n cells/ ml) for PFGE.
For M-phase arrests nocodazole was added to a final concentration of 500 ng/ml to both control and doxycycline-treated cultures at t=48 h. Harvests were made after 24 h (giving a total -/+ doxycycline exposure time of 72 h). As controls for the etoposide assay, DMSO was added at the same volume as for the etoposide-treated samples. The 1.85 and 0.85 Mb DXZ1 cleavage fragments were only detected in the presence of etoposide.
Pulsed field gel electrophoresis (PFGE)
High molecular weight (HMW) DNA without prior restriction enzyme digestion was resolved by PFGE in 0.7 % chromosomal grade agarose (Bio-Rad 162-0136), 0.25x TBE on a Rotaphor Type V apparatus (Biometra), using the following parameters: a 72 h run at 11°C, using a pulse time of 350 - 50 sec. (changing logarithmically), a rotor angle of 110° - 100° (decreasing linearly), a voltage of 120 V - 50 V (decreasing linearly). The DNA was transferred and the Southern blot probed with DXZ1 DNA as described previously 19. Quantitation was carried out on a Packard Instant Imager.
Band shift and In vitro kinase assays
HeLa cells (+/-) treatment with 100 ng/ml nocodazole for 18 h were lysed in ice-cold 50 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 1% (v/v) Nonidet P-40, 1%sodium deochycholate, 0.1% SDS plus complete protease inhibitor cocktail. 40 μg total protein for each sample was separated by electrophoresis on 3-8% Tris-acetate gradient gels (Novex). Proteins were transferred to nitrocellulose for immunoblotting with anti-Repo-Man and anti-rabbit HRP.
EGFP and EGFP-RepoMan were transiently expressed in HeLa cells and affinity purified from lysates using anti-GFP monoclonal antibodies (Roche) covalently coupled to protein G sepharose. Recombinant GST and GST-tagged RepoMan were expressed in E. coli and purified on a glutathione sepharose column.
Immunoprecipitated proteins (on beads), recombinant proteins and histone H1 (Sigma) were incubated with 100 μM 32P-ATP and Cdk1/Cyclin B (4U - Biomol. Intl.) in kinase buffer (50 mM Tris pH 7.5, 1 mM DTT, 5 mM Mg, 0.1 mM EGTA) for 40 min at 30°C on a shaking platform. The reaction was stopped by the addition of SDS sample buffer and the proteins separated by SDS-PAGE on 4-12% Bis-Tris gels, which were dried and exposed to X-ray film.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Laurent Meijer for the gift of a panel of Cdk inhibitors, including roscovitine; Andrew Belmont for the gift of the Lac O/Lac I-GFP plasmids; Tatsuo Fukagawa for the gift of the CENP-H-GFP targeting construct; Erich Nigg for the gift of anti-chicken cyclin B2; Roger Tsien for the gift of mCherry; Fumio Kasai and Malcolm Ferguson-Smith for the chicken Z chromosome paint; Jerome Boudeau and Dario Alessi for help with the CDK1 assays; and Robin Allshire, Margarete Heck and Neville Cobbe for comments on the MS. This work is supported by The Wellcome Trust (P.V., A.L. and W.C.E.), The Caledonian Research Foundation (D.H.) and Cancer Research-UK (J.M.S and C.J.F). WCE and AIL are Principal Research Fellows of The Wellcome Trust.
REFERENCES
- 1.Hirano T. Condensins: organizing and segregating the genome. Curr. Biol. 2005;15:R265–R275. doi: 10.1016/j.cub.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Hirano T, Mitchison TJ. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–458. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 3.Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–521. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- 4.Saka Y, et al. Fission yeast cut3 and cut14, members of the ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. The EMBO J. 1994;13:4938–4952. doi: 10.1002/j.1460-2075.1994.tb06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strunnikov AV, Hogan E, Koshland D. SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation defines a subgroup within the SMC-family. Genes Dev. 1995;9:587–599. doi: 10.1101/gad.9.5.587. [DOI] [PubMed] [Google Scholar]
- 6.Sutani T, et al. Fission yeast condensin complex: essential roles of non-SMC subunits for condensation and Cdc2 phosphorylation of Cut3/SMC4. Genes Dev. 1999;13:2271–2283. doi: 10.1101/gad.13.17.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavoie BD, et al. Mitotic chromosome condensation requires Brn1p, the yeast homologue of Barren. Mol. Biol. Cell. 2000;11:1293–1304. doi: 10.1091/mbc.11.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aono N, et al. Cnd2 has dual roles in mitotic condensation and interphase. Nature. 2002;417:197–202. doi: 10.1038/417197a. [DOI] [PubMed] [Google Scholar]
- 9.Hagstrom KA, Holmes VF, Cozzarelli NR, Meyer BJ. C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis. Genes Dev. 2002;16:729–742. doi: 10.1101/gad.968302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaitna S, et al. The Aurora B Kinase AIR-2 Regulates Kinetochores during Mitosis and Is Required for Separation of Homologous Chromosomes during Meiosis. Curr. Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
- 11.Steffensen S, et al. A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr. Biol. 2001;11:295–307. doi: 10.1016/s0960-9822(01)00096-3. [DOI] [PubMed] [Google Scholar]
- 12.Hudson DF, Vagnarelli P, Gassmann R, Earnshaw WC. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev Cell. 2003;5:323–336. doi: 10.1016/s1534-5807(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 13.Ono T, et al. Differential Contributions of Condensin I and Condensin II to Mitotic Chromosome Architecture in Vertebrate Cells. Cell. 2003;115:109–121. doi: 10.1016/s0092-8674(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 14.Hirota T, et al. Distinct functions of condensin I and II in mitotic chromosome assembly. J. Cell Sci. 2004;117:6435–45. doi: 10.1242/jcs.01604. [DOI] [PubMed] [Google Scholar]
- 15.Savvidou E, et al. Drosophila CAP-D2 is required for condensin complex stability and resolution of sister chromatids. J Cell Sci. 2005;118:2529–2543. doi: 10.1242/jcs.02392. [DOI] [PubMed] [Google Scholar]
- 16.Hirano T, Funahashi S, Uemura T, Yanagida M. Isolation and characterization of Schizosaccharomyces pombe cut mutants that block nuclear division but not cytokinesis. The EMBO J. 1986;5:2973–2979. doi: 10.1002/j.1460-2075.1986.tb04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhalla N, Biggins S, Murray AW. Mutation of YCS4, a budding yeast condensin subunit, affects mitotic and nonmitotic chromosome behavior. Mol. Biol. Cell. 2002;13:632–645. doi: 10.1091/mbc.01-05-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coelho PA, Queiroz-Machado J, Sunkel CE. Condensin-dependent localisation of topoisomerase II to an axial chromosomal structure is required for sister chromatid resolution during mitosis. J. Cell Sci. 2003;116:4763–4776. doi: 10.1242/jcs.00799. [DOI] [PubMed] [Google Scholar]
- 19.Spence JM, et al. Co-localization of centromere activity, proteins and topoisomerase II within a subdomain of the major human X alpha-satellite array. Embo J. 2002;21:5269–5280. doi: 10.1093/emboj/cdf511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belmont AS, Straight AF. In vivo visualization of chromosomes using lac operator-repressor binding. Trends Cell Biol. 1998;8:121–124. doi: 10.1016/s0962-8924(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 21.Fukagawa T, et al. CENP-H, a constitutive centromere component, is required for centromere targeting of CENP-C in vertebrate cells. Embo J. 2001;20:4603–17. doi: 10.1093/emboj/20.16.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira RA, Coelho PA, Sunkel CE. The condensin I subunit Barren/CAP-H is essential for the structural integrity of centromeric heterochromatin during mitosis. Mol. Cell. Biol. 2005;25:8971–8984. doi: 10.1128/MCB.25.20.8971-8984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlich D, et al. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr. Biol. 2006;16:333–344. doi: 10.1016/j.cub.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Parry DH, O’Farrell PH. The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr. Biol. 2001;11:671–83. doi: 10.1016/s0960-9822(01)00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijer L, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–36. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 26.Trinkle-Mulcahy L, et al. Repo-Man recruits PP1gamma to chromatin and is essential for cell viability. J. Cell Biol. 2006;172:679–692. doi: 10.1083/jcb.200508154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitoh N, Goldberg I, Wood E, Earnshaw W,C. ScII: an abundant chromosome scaffold protein is a member of a family of putative ATPases with an unusual predicted tertiary structure. J. Cell Biol. 1994;127:303–318. doi: 10.1083/jcb.127.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckley DM, et al. Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J Cell Biol. 1997;136:1169–83. doi: 10.1083/jcb.136.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earnshaw WC, Ratrie H, Stetten G. Visualization of centromere proteins CENP-B and CENP-C on a stable dicentric chromosome in cytological spreads. Chromosoma (Berl.) 1989;98:1–12. doi: 10.1007/BF00293329. [DOI] [PubMed] [Google Scholar]
- 30.Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and Aurora-B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.