Summary
The bioactive lipid sphingosine 1-phosphate (S1P) and its G protein-coupled receptors play critical roles in cardiovascular, immune and neural development and function [1–6]. Despite its importance, many questions remain about S1P signaling, including how S1P, which is synthesized intracellularly, is released from cells. Mutations in the zebrafish gene encoding the S1P receptor Miles Apart (Mil)/S1P2 disrupt the formation of the primitive heart tube [5]. We find that mutations of another zebrafish locus, two of hearts (toh), cause phenotypes that are morphologically indistinguishable from those seen in mil/s1p2 mutants. Positional cloning of toh reveals that it encodes a member of the Spinster-like family of putative transmembrane transporters. The biological functions of these proteins are poorly understood, although phenotypes of the Drosophila spinster and zebrafish not really started mutants suggest that these proteins may play a role in lipid trafficking [7, 8]. Through gain- and loss-of-function analyses, we show that toh is required for signaling by S1P2. Further evidence indicates that Toh is involved in the trafficking or cellular release of S1P.
Keywords: Sphingosine 1-phosphate, G protein-coupled receptor, Spinster, cardiac development
Results and Discussion
The lysophospholipid sphingosine 1-phosphate (S1P) has emerged as a key cellular signaling molecule. Many of the relevant signaling properties of S1P are mediated via its interaction with a family of G protein-coupled receptors (GPCRs). The interaction of these receptors with S1P is known to affect numerous processes including the development and function of the vertebrate cardiovascular system [1, 3–5]. In addition, S1P has been identified as a mediator of immune function [6]. The S1P analog FTY720 has recently been shown to function as a potent immune modulator, promising to improve the treatment of organ transplant recipients [9] as well as those suffering from pathological immune responses following infection [10]. Despite the importance of S1P signaling, little is known about the processes that make this lipid available to bind its receptors.
Previously, we have shown that the miles apart (mil) gene, which is required for the formation of the primitive heart tube in zebrafish, encodes the orthologue of the mammalian receptor S1P2, initially called Edg5 and also known as S1PR2 [5, 11]. Screening mutagenized lines of zebrafish, we have identified another recessive mutation, two of hearts (toh), which causes phenotypes indistinguishable from those caused by mil/s1p2 mutations.
At 36 hours post fertilization (hpf), wild-type embryos have a functioning heart (Figure 1A) while toh (Figure 1D) and mil/s1p2 (Figure 1G) mutants exhibit pericardial edema (arrows) indicating circulatory defects. Circulatory failure and consequential pericardial edema in toh and mil/s1p2 mutants is in fact the result of a defect in early heart tube formation. In zebrafish, as in all vertebrates, the primitive heart tube is formed from bilateral groups of anterior mesodermal cells. These two cell populations migrate to the embryonic midline and fuse to form a single heart tube [12]. In wild-type zebrafish embryos at 19 hpf, the ring shaped primitive heart tube has formed (Figure 1B). At 19 hpf, the myocardial cells of toh (Figure 1E) or mil/s1p2 (Figure 1H) mutants have not migrated to the midline, resulting in the formation of bilateral heart-like structures, a phenotype called cardia bifida. Despite the cardia bifida, differentiation of the myocardial cells in toh mutants appears unaffected, as the bifid heart structures have wild-type-like chamber-specific gene expression and are infiltrated by endocardial cells (data not shown). However, the bifid heart structures in toh and mil/s1p2 mutants are not appropriately connected to the vasculature and thus cannot support circulation.
Figure 1. two of hearts and miles apart mutant phenotypes.
Comparison of wild-type (A–C), toh mutant (D–F), and mil/s1p2 mutant (G–I) embryos. (A, D and G) Lateral brightfield images, anterior to the left, at 36 hpf show pericardial edema (arrow) and epidermal blisters (insets, arrowheads) in the tails of tohsk12 (D) and mil/s1p2m93 (G) mutants. (B, E and H) Examination of cmlc2 expression at 19 hpf shows heart-ring formation in wild-type embryos (B) and a failure in precardiac mesoderm migration in tohs420 (E) and mil/s1p2m93 (H) mutant embryos. Dorsal views, anterior up. (C, F and I) Visualization of anterior endoderm by Tg(−0.7her5:EGFP)ne2067 expression at 18 hpf. In embryos injected with toh (F) and mil/s1p2 (I) MOs, numerous gaps (arrowheads) appear in the endodermal sheet which is also irregularly shaped. The most anterior region of mil/s1p2 morphants lack GFP positive endodermal cells at the midline (asterisk). Dorsal views, anterior up.
In addition to cardiac defects, toh and mil/s1p2 mutants also display blistering in the tip of the tail, first evident around 26 hpf (Figure 1D and G, respectively; arrowheads). This tail blister phenotype is not shared with other cardia bifida mutants in zebrafish [12], suggesting that toh and mil/s1p2 may function in the same pathway. As mil/s1p2 mutant embryos have also been shown to have defects in the morphogenesis of the anterior endoderm [5], we analysed the endoderm in toh mutants. The anterior endoderm of wild-type embryos, visualized at 18 hpf by the expression of the −0.7her5:EGFP transgene [13], forms a contiguous sheet across the embryonic midline (Figure 1C). Embryos lacking toh function display holes in their anterior endodermal sheet (Figure 1F), similar to mil/s1p2 mutants (Figure 1I). These endodermal morphogenesis defects in toh and mil/s1p2 mutants were also observed by examining the expression of the endodermal markers foxa1 and foxa2 (data not shown). Since the endoderm is required for precardiac mesoderm migration [14–17], the cardia bifida phenotype seen in toh and mil/s1p2 mutants is likely due to these endodermal defects.
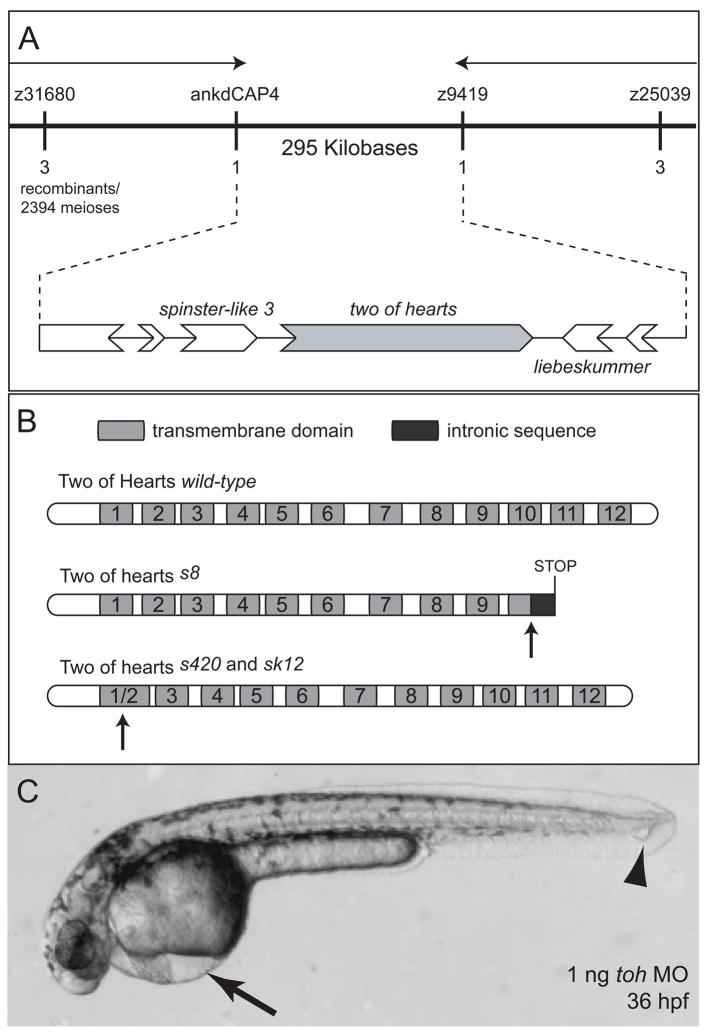
Due to the similarities between the toh and mil/s1p2 mutant phenotypes, we hypothesized that Toh was involved in signaling by Mil/S1P2. In order to test this hypothesis, we isolated the toh gene (Figure 2A). Positional cloning of toh (Figure 2A) is described in detail in the Experimental Procedures. toh encodes a 12 pass transmembrane domain protein of the major facilitator superfamily (MFS) of non-ATP dependent transporters (Figure 2B). The protein is a predicted 504 amino acid member of theSpinster-like family of proteins (Figure S1). This family is named after the Drosophila spinster gene, also called benchwarmer [18, 19]. The lesions found in the spinster-like gene in the toh mutant alleles are described in Figure S2.
Figure 2. Isolation of the two of hearts gene.
(A) Positional cloning of toh. Direction of the chromosomal walk is indicated with black arrows above the marker names. Markers used for mapping are indicated above the line representing the genomic region. The numbers of recombination events out of 2394 meioses found at each marker are indicated below the genomic region. A magnification of the critical region depicts portions of 6 open reading frames (open arrows) in the identified genetic interval, including the previously cloned locus liebeskummer/reptin. The critical region contains two spinster-like genes, one of which was identified as toh (gray filled arrow) and the other we name here spinl3. (B) Schematic diagram of Toh proteins produced from each allele. Arrows point to the site affected in the mutant alleles. Transmembrane domains are indicated in blue and the predicted translated intronic sequence in the s8 allele is indicated in red. Numbers also identify the individual transmembrane domains. (C) Injection of a splice blocking MO against toh into wild-type embryos phenocopies the toh mutations, leading to pericardial edema (arrow) and blistering in the tail (arrowhead).
The identification of the spinster-like gene as the toh locus was further verified by loss- and gain-of-function analyses. First, injection of a morpholino antisense oligonucleotide (MO) blocking toh mRNA splicing at the boundary between exon 4 and intron 4 resulted in the phenotypes seen in toh mutants (Figure 2C). Second, mRNA encoding the putative Toh protein rescued migration of the precardiac mesoderm when injected into maternal-zygotic tohs8 mutants (MZtohs8; generation of these embryos is described in the Experimental Procedures) and zygotic tohs420 mutants (data not shown), leading to functional hearts. Overexpression of the toh mRNA had no effect on wild-type development and did not rescue mil/s1p2 mutants. These loss- and gain-of-function experiments together with the tight genetic linkage and presence of molecular lesions show that we have isolated the toh gene. Interestingly, precardiac mesoderm migration in toh mutants cannot be rescued by overexpressing Drosophila spinster (spin) or either of the two additional spinster-like genes found in the zebrafish genome (Figure S1), not really started (nrs) [20] and a gene we here name spinster-like 3 (spinl3). These data indicate that the Spinster-like proteins have acquired divergent functions.
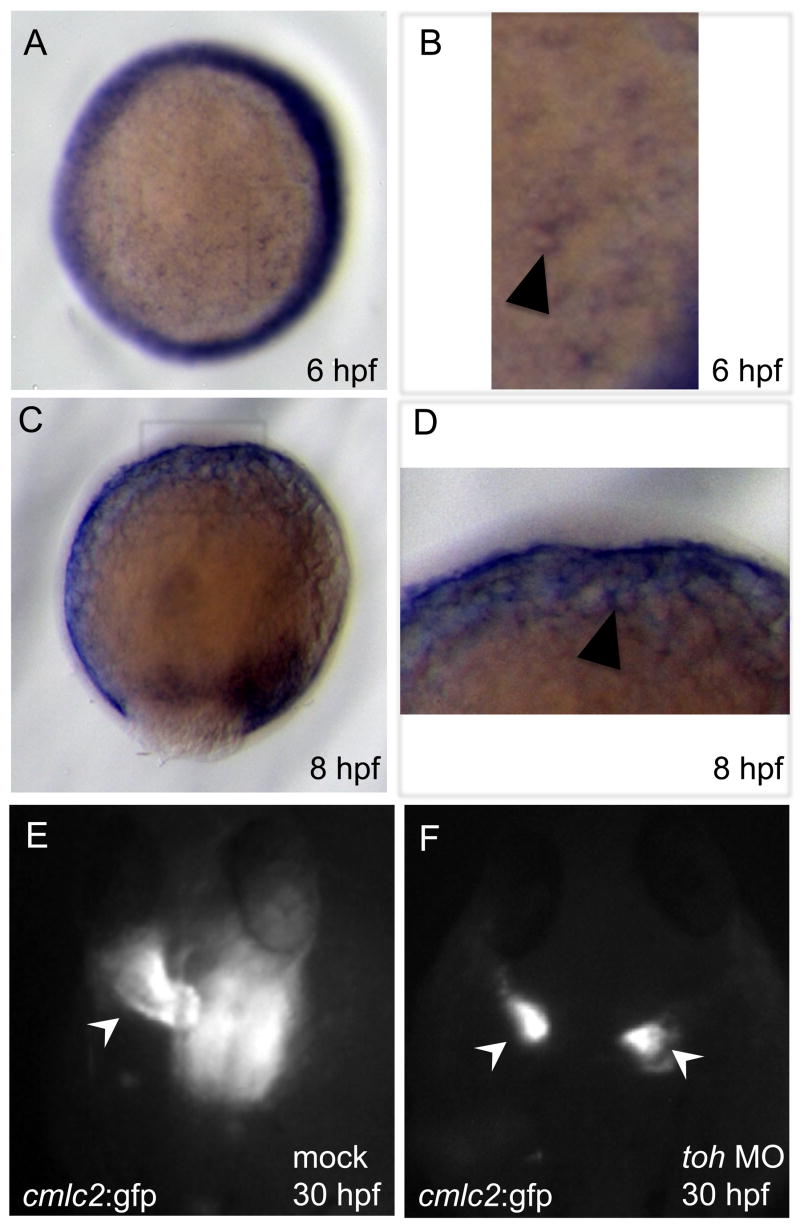
The toh gene is expressed dynamically during early development (Figures 3 and S3). toh mRNA is maternally provided (Figure S3A) and appears to be distributed ubiquitously during cleavage stages (Figure S3B). At the onset of gastrulation, the expression of toh changes. Cells that have undergone involution appear to express heightened levels of toh (Figure S3C, arrowhead). In addition, transcripts can be seen in the yolk syncytial layer, an extraembryonic tissue (Figure 3A and B, arrowhead). At the conclusion of gastrulation, toh is expressed strongly in tissues adjacent to the yolk cell and remains evident in the YSL (Figure 3C and D, arrowhead). This expression continues through early somitogenesis. However, as somitogenesis proceeds, expression domains of toh become evident in the somitic mesoderm and in the endoderm adjacent to the yolk extension. By 24 hpf, toh is strongly expressed in a distinct compartment of the somites (Figure S3D and E; arrowhead), in the endoderm (Figure S3D) and in the heart (Figure S3F and G).
Figure 3. Toh function in the YSL is required for precardiac mesoderm migration.
toh expression at 6 (A and B) and 8 (C and D) hpf, dorsal to the right. (A) Animal pole view, 6 hpf, showing toh expression around the margin and diffusely throughout the YSL. (B) Magnified view of the box in (A) showing toh expression around the YSL nuclei (arrowhead). (C) Lateral view, 8 hpf, showing continued toh expression in cells that have involuted, as well as in the YSL. (D) Magnified view of the box in (C) showing pronounced toh expression in the YSL (arrowhead). (E and F) Dorsal images of Tg(cmlc2:GFP)f1 embryos injected into the YSL with mock solution (E) or 4 ng toh MO (F) and visualized at 30 hpf. Mock injected embryos have a single heart tube (E; arrow), while embryos with loss of Toh function in the YSL very frequently (84%, n = 56) display cardia bifida (F; arrows).
Because mil/s1p2 and toh mutants have clear defects in the morphogenesis of the anterior endoderm and both genes are expressed in the developing endoderm, we hypothesized that they might function cell-autonomously in the anterior endoderm to regulate precardiac mesoderm migration. To test this hypothesis we performed endoderm transplantation [17] in mil/s1p2 and toh MO injected embryos (morphants). This technique allows one to populate the endoderm of morphants with wild-type cells while leaving other tissues untouched. In four cases, we were able to rescue the mil/s1p2 mutant heart phenotype (data not shown). In these cases, the majority of the anterior endoderm had been replaced with wild-type cells. In cases of partial replacement of the anterior endoderm, or replacement of only posterior endoderm, no rescue was observed. However, in the case of toh morphants, rescue was never observed regardless of the level of endoderm replacement. Altogether, these data indicate that while mil/s1p2 functions cell-autonomously in the endoderm to regulate precardiac mesoderm migration, toh functions in another cell type.
toh is clearly expressed in the YSL (Figure 3A–D), a tissue previously implicated in precardiac mesoderm migration in zebrafish [21]. In order to test whether the YSL expression of toh regulates precardiac mesoderm migration, we injected toh MO into the YSL. Injection of toh MO (Figure 3F), but not mock carrier solution (Figure 3E), into the YSL resulted in cardia bifida at high frequency (84%, n = 56). We also injected 200pg of toh mRNA into the YSL of toh morphants. YSL injection of toh mRNA was able to rescue precardiac mesoderm migration in some toh morphants (38%, n = 32). However, injection of equivalent amounts of nrs mRNA into the YSL never rescued precardiac mesoderm migration. Altogether, these data indicate that Toh function in the YSL is necessary and sufficient for precardiac mesoderm migration.
Little is known about the mechanism by which Spinster-like proteins carry out their function. However, the common phenotypes of toh and mil/s1p2 mutants suggest that toh and mil/s1p2 function in the same pathway. To test this hypothesis, we injected suboptimal amounts of mil/s1p2 and toh MOs alone and in combination. When injected singly, 0.4 ng of mil/s1p2 MO and 0.2 ng of toh MO caused cardia bifida in 2.5% (n=40) and 2.4% (n=85) of the embryos, respectively. However, when embryos were co-injected with 0.4 ng of mil/s1p2 and 0.2 ng of toh MO, 85.7% (n=70) of the embryos exhibited cardia bifida. Therefore, partial loss of function of both Mil/S1P2 and Toh caused cardia bifida much more frequently than the partial loss of either protein alone. We also observed that mil/s1p2; toh double mutants have no additional phenotypes compared to mil or toh single mutants. Together, these data suggest that Mil/S1P2 and Toh may function in a common genetic pathway.
In order to test directly whether toh is required for Mil/S1P2 signaling, we overexpressed mRNA encoding Mil/S1P2 in wild-type and toh mutant embryos. Injection of 100 pg of mil/s1p2 mRNA at the 1-cell stage caused severe morphological defects in wild-type embryos (89%, n = 208; Figure 4A), including cyclopia (asterisk) and disorganized body axis formation. Defects caused by mil/s1p2 overexpression can be traced to problems with cell movements during gastrulation, as embryos overexpressing mil/s1p2 exhibited defects in convergence, extension and epiboly movements (Figure 4D). It is likely that the gastrulation defects seen in mil/s1p2 overexpressing embryos are due to the antagonism between mil/s1p2 and silberblick/wnt11 signaling [22], as mutations in slb/wnt11 cause defects in convergence and extension movements similar in nature to those seen in mil/s1p2 overexpressing embryos [23, 24]. If Toh function is required for signaling by Mil/S1P2, one would expect that loss of Toh function would suppress the phenotypes caused by mil/s1p2 overexpression. Indeed, toh mutants or morphants overexpressing mil/s1p2 only rarely showed morphological defects similar to those seen in control mil/s1p2 overexpressing embryos, as assessed both at 30 hpf (7%, n = 195; Figure 4B) and 8 hpf (Figure 4E). Therefore, Toh function is required for Mil/S1P2 signaling in this overexpression assay. Note that these mil/s1p2 overexpressing embryos still, however, display the phenotypes seen in toh mutants or morphants, including cardia bifida and blister formation in the tail, indicating that mil/s1p2 mRNA is incapable of rescuing loss of toh function.
Figure 4. Exogenous S1P substitutes for Toh function in Mil/S1P2 overexpressing embryos.
(A and B) Lateral views, anterior to the left, of embryos overexpressing mil/s1p2 at 30 hpf. Wild-type embryos injected with 100 pg of mil/s1p2 RNA (A) display a shortened body axis and cyclopia (asterisk). MZtohs8 mutants injected with 100 pg of mil/s1p2 RNA have no body axis defects (B) but develop pericardial edema (arrow) and tail blisters (arrowheads). (C–E) Visualization of axial mesoderm and endoderm by expression of foxa2 at late gastrula stages, dorsal views, anterior up. Wild-type embryos overexpressing mil/s1p2 (D) have broadened axial mesoderm (solid line) compared to uninjected embryos (C). Progression of epiboly (dashed line) is also impaired in embryos overexpressing mil/s1p2 as compared to uninjected siblings. toh MO injected embryos overexpressing mil/s1p2 (E) are indistinguishable from uninjected siblings (C). (F–J) Lateral views of 5.5 hpf embryos injected with mock carrier solution or 10 μM S1P. Control embryos show no response to exogenous S1P (F). Embryos overexpressing Mil/S1P2 do not show more severe phenotypes when injected with mock carrier solution at 5 hpf (G). Mil/S1P2 overexpressing embryos injected with a 10 μM S1P solution at 5 hpf exhibit severe phenotypes (H), including an exaggerated thickened layer of cells at the animal pole (asterisk). Mil/S1P2 overexpressing embryos coinjected with toh MO show no phenotypes when injected with mock solution (I) and resemble controls (F). When injected with a 10 μM S1P solution, Mil/S1P2 overexpressing embryos lacking Toh function show severe defects (J), including a thickened layer of cells at the animal pole (asterisk).
One manner by which Toh might affect the function of the Mil/S1P2 receptor is by regulating the availability of the receptor’s ligand, S1P. To determine whether the phenotypes of Mil/S1P2 overexpression are dependent on receptor-ligand interaction, we generated a mutant form of Mil/S1P2. The E129A mutation in Mil is analogous to mutations that have been shown for other S1P receptors to block the receptor’s ability to interact with S1P without affecting the receptor’s stability or ability to interact with downstream signaling components [25]. Embryos injected with 100 or 200 pg of milE129A mRNA gastrulated normally and displayed none of the phenotypes observed in embryos injected with 100 pg of wild-type mil/s1p2 mRNA. Thus, it appears that the effects seen in Mil/S1P2 overexpressing embryos require an interaction between the exogenous receptor and S1P.
Because the yolk cell contains nutrients as well as some developmental signals necessary for embryonic development [26], we hypothesized that Toh in the YSL was required to make S1P available from the yolk cell to the embryo. One prediction of this hypothesis is that the Mil/S1P2 receptor should be capable of responding to exogenously applied S1P even in the absence of Toh function. To test this prediction, embryos were injected with mil/s1p2 mRNA alone or in combination with toh MO. At the onset of gastrulation (~5 hpf) the embryos were also injected at the animal pole with a mock carrier solution or carrier solution containing 10 μM S1P. Embryos injected with S1P alone showed no effect (Figure 4F). However, embryos injected with mil/s1p2 mRNA showed immediate morphogenetic effects from the application of exogenous S1P regardless of whether or not they had intact Toh expression (Figure 4H, J). These effects were S1P dependent, as mil/s1p2 overexpressing embryos injected with the carrier solution alone did not show this response (Figure 4G and 4I). The presence or absence of Toh function had no effect on the severity of a mil/s1p2 overexpressing embryo’s response to exogenously supplied S1P. In both groups of overexpressing embryos, gastrulation movements paused and the cells of the embryo retreated back to the animal pole, causing a thickening of the embryo (Figure 4H and 4J, asterisks). These data suggest that the reason toh mutants or morphants do not exhibit any defects upon mil/s1p2 overexpression is because of a lack of interaction between overexpressed Mil/S1P2 and endogenous S1P.
The above results and the fact that Toh is a member of a superfamily of transporters raise the possibility that Toh functions as a transporter of S1P. However, Drosophila embryos with mutations in the toh homologue spin display a dramatic expansion of the acidified compartment of the cell, as visualized by Lysotracker staining [7], accompanied by inappropriate accumulations of lipids and sugars in those cellular compartments [19, 27]. Therefore, toh mutations might be affecting S1P release by affecting the storage and trafficking of many lipids, not just S1P or its precursors. We examined the levels of Lysotracker staining in toh morphants and MZtohs8 mutants, and they appeared equivalent to those of wild-type embryos (data not shown), indicating normal lysosomal structure in toh mutants. We also examined the early and recycling endocytic compartments of wild-type and toh MO injected embryos to determine whether the earlier stages of the endocytic pathway might be disrupted in toh mutants. The architecture of the early and recycling endosomal compartments appeared indistinguishable between wild-type and toh MO injected embryos when visualized with a yellow fluorescent protein (YFP) tagged Rab5c protein [28] (data not shown). Therefore, in contrast to Drosophila spin mutants and zebrafish nrs heterozygotes [7, 8], toh mutants do not appear to exhibit gross defects in lipid or carbohydrate trafficking. These findings, together with the failure of Drosophila spin and zebrafish nrs genes to rescue toh mutants, indicate that vertebrate spinster-like genes have diverged in function and that Toh may have a specific role in the trafficking or release of S1P.
In this study, we identify Toh as a novel component of signaling via the zebrafish S1P2 orthologue, Mil. The combination of shared phenotypes between toh and mil/s1p2 mutants, the fact that toh loss-of-function suppresses the deleterious effects of mil/s1p2overexpression, and the synergistic effects of suboptimal mil/s1p2 and toh MO injections suggest that toh is a critical component of the Mil/S1P2 signaling pathway. Furthermore, we have shown that the Mil/S1P2 receptor is capable of signaling in the absence of Toh function when exogenous S1P is applied. Therefore, Toh appears to be a novel contributor to the biosynthesis, trafficking or release of S1P.
Previous analysis has shown that the YSL is critical for precardiac mesoderm migration [21]. This study suggested that the transcription factor gene mtx1, which is expressed exclusively in the YSL, regulates the deposition at the embryonic midline of Fibronectin (FN), an extracellular matrix component critical for precardiac mesoderm migration in mouse [29] and zebrafish [30]. Interestingly, the deposition of FN in mil/s1p2 morphants is also deficient (Figure S4). Furthermore, injection of FN into the midline of mil/s1p2 deficient embryos appears to rescue precardiac mesoderm migration [31]. Thus, mtx1 might regulate toh expression or function in the YSL. The absence of Mtx1 would lead to an absence of Toh function in the YSL and therefore a lack of S1P release from the yolk. Lack of S1P release would, in turn, prevent Mil/S1P2 signalingand the downstream deposition of FN required for endoderm and precardiac mesoderm morphogenesis.
The exact biochemical function of Toh and other Spinster-like proteins remains unclear. The homology of these proteins to small solute transporters suggests the interesting possibility that Toh is involved in the trafficking or actual cellular release of S1P, and that toh mutations affect Mil/S1P2 signaling by limiting available S1P. Our data showing that overexpressed Mil/S1P2 is capable of responding to exogenously supplied S1P strongly supports the idea that the defect observed in animals lacking functional Toh/Spinl2 transporter are due to a reduction in endogenous ligand production or release. Thus, Toh is a strong candidate for a transmembrane transporter that, as previously claimed for ABCC1 [32], may be capable of moving S1P across cellular membranes to make it available for receptor-ligand interactions.
Conclusions
As the relevance of S1P signaling to both basic and clinical sciences becomes more evident, it is critical that the signaling partners of S1P receptors be identified. S1P2 signaling in mammalian systems has been shown to play a vital role in activation of mast cells [33], a cell type thought to contribute to the pathogenesis of asthma [34]. In addition, S1P2 receptor function is known to affect vascular tone [35] and may contribute to the protective effects S1P demonstrates during ischemic challenge to the heart during myocardial infarction [36]. The identification of Toh as a component of S1P2 signaling in zebrafish suggests that the mammalian orthologues of toh may have similar functions in S1P mediated signaling. Therefore, Toh and its orthologues may represent new targets for the manipulation of specific S1P signaling pathways in both normal and pathological states.
Experimental procedures
Zebrafish Strains and Care
Adult and embryonic zebrafish were raised and cared for using standard laboratory procedures [37]. We used the following zebrafish mutant and transgenic strains: tohs8, tohs220, tohsk12, tohs420, milm93, Tg(−0.7her5:egfp)ne2067 [13] and Tg(cmlc2:EGFP)f1 [38]. Maternal-zygotic s8 (MZtohs8) mutant embryos were generated by mating heterozygous tohs8 fish. Escaping homozygous tohs8 embryos were then raised to adulthood.
Immunohistochemistry, Fluorescence microscopy and confocal analysis Embryos were fixed at room temperature for 1 hour in 4% Paraformaldehyde or overnight at 4°C in 2% Paraformaldehyde in PBS. Lysotracker DND-99 (Molecular Probes) was diluted 1:200 in 1/10 Hanks Basic Salt Solution and staining was carried out on live embryos for 1 hour at 28°C, after which embryos were washed 3 times for 5 minutes with 1/10 Hanks Basic Salt Solution, then fixed and processed as above. Images were acquired using a Zeiss LSM5 Pascal confocal microscope. Wholemount fluorescence microscopy was performed with a Zeiss SteREO Lumar.V12 microscope.
Endoderm Transplantation
Endodermal cell transplantation was carried out as described [17], using 100 pg cas/sox32 mRNA to force donor cells into the endodermal lineage. Host embryos were injected with 2 ng mil/s1p2 MO or 1 ng toh MO along with 1 ng cas/sox32 MO to deplete their endoderm.
In situ hybridization
Wholemount in situ hybridization was carried out as described [39] using the following probes: cmlc2 [40], foxa2 [41] and toh. The toh probe was generated using the primers 5′-TTG GAG CCA TCA CAT GTG TGA – 3′ and 5′ – TTA CTT GTT TGG CGG CTT TGT – 3′ to PCR a 516 base pair fragment of toh. The primers were engineered with T3 and T7 promoters, respectively to allow the generation of sense and antisense probes directly from the PCR product.
RNA overexpression and morpholino oligonucleotide generation
All capped mRNA for injection was generated using mMessage mMachine kits (Ambion). mil mRNA was generated as described [5]. pCS2+ mil E129A was generated using the QuikChange II Mutagenesis kit (Stratagene). mRNA encoding toh was generated by linearizing pCS2+ toh with NotI and transcribing with SP6 polymerase. Zebrafish spinl3 mRNA was generated by subcloning the coding region of spinl3 into pCS2+, linearizing this construct with NotI and transcribing with SP6 polymerase. Drosophila spinster mRNA was generated by subcloning spinster-RFP from pUAS spinster-RFP (gift of Sean Sweeny and Graeme Davis) into pCS2+, linearizing with NotI and transcribing with SP6 polymerase. Zebrafish nrs was generated from pCS2+ nrs [20]. rab5c-YFP mRNA was generated from pCS2+ Rab5c-YFP (gift of C–P. Heisenberg) as described [42] and embryos were injected with 100 pg of mRNA. The toh MO (5′-GCA GCT CTT ACC CTC AGT GCC CAG T –3′) was designed by Gene-Tools, Inc. YSL injections of 4ng of toh MO were carried out as described [43].
Exogenous S1P application
S1P (Sigma-Aldrich cat #S9666) was dissolved in 100% methanol at 1 mg/mL. This stock solution was further diluted to 10 μM concentration into a carrier solution of 250 mM potassium chloride containing 0.5% w/v Fatty Acid Free Bovine Serum Albumin (Calbiochem/EMD cat # 126575). Embryos were injected with either 100 pg mil/s1p2 mRNA or mil/s1p2 mRNA plus 1 ng toh MO at the one cell stage. Controls were not injected at this stage. At 5 hpf, the embryos were then injected into the animal pole, amongst the cells of the embryos, with 2.3 nL of 10 μM S1P solution or the carrier solution. They were aged for 30 minutes at 28°C and then imaged.
Cloning of the toh locus
Using bulk segregant analysis, the toh locus was localized to zebrafish chromosome 5. 1197 diploid s220 toh mutant embryos were used to narrow the affected locus to a 295 kilobase region between the CA repeat marker z9419 and a single nucleotide polymorphism (SNP) in a homologue of the mammalian ankhzn gene. This SNP was designated ankdCAP4 and was amplified using primers designed by the dCAPS 2.0 web-based program and detected with HinfI cutting [44]. Zebrafish CA repeat microsatellite primers were obtained from the Massachusetts General Hospital MGH/CVRC Zebrafish Server website (http://zebrafish.mgh.harvard.edu/). The 295 kilobase region is covered entirely by three bacterial artificial chromosomes (BACs): CHORI-211 134D21, CHORI-211 138A6 and DanioKey 7B17. These BACs have been sequenced and assembled by the Sanger Centre Danio rerio Sequencing Project. Full length sequences are available at ftp://ftp.sanger.ac.uk/pub/sequences/zebrafish. Six putative open reading frames (ORFs) were found between z9419 and ankdCAP4 using a combination of the GENSCAN exon prediction software [45] and analysis of conservation of synteny between zebrafish, mouse and human genomic sequence. These ORFs were sequenced from cDNA and genomic DNA in wild-type and mutant samples.
Supplementary Material
Acknowledgments
We thank Holly Field and Jonathan Alexander for assistance with initial studies of toh, Courtney Babbitt for assistance with phylogenetic analysis, Atsuo Kawahara for discussions and sharing unpublished data, Jason Cyster, Ian Scott and Courtney Griffin for helpful comments on the manuscript. This work was supported in part by the NSF (N.O.), the AHA (E.A.O., S-W.J., N.G.H.), the HFSP (H.V.), the NIH (D.Y., D.Y.R.S.) and the Packard foundation (D.Y.R.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun J. Lysophospholipids in the nervous system. Prostaglandins Other Lipid Mediat. 2005;77:46–51. doi: 10.1016/j.prostaglandins.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 4.Wendler CC, Rivkees SA. Sphingosine-1-phosphate inhibits cell migration and endothelial to mesenchymal cell transformation during cardiac development. Dev Biol. 2006;291:264–77. doi: 10.1016/j.ydbio.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- 6.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- 7.Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 8.Kishi S, Bayliss PE, Uchiyama J, Koshimizu E, Qi J, Nanjappa P, Imamura S, Islam A, Neuberg D, Amsterdam A, Roberts TM. The identification of zebrafish mutants showing alterations in senescence-associated biomarkers. PLoS Genet. 2008;4:e1000152. doi: 10.1371/journal.pgen.1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun J, Rosen H. Lysophospholipid receptors as potential drug targets in tissue transplantation and autoimmune diseases. Curr Pharm Des. 2006;12:161–171. doi: 10.2174/138161206775193109. [DOI] [PubMed] [Google Scholar]
- 10.Premenko-Lanier M, Moseley NB, Pruett ST, Romagnoli PA, Altman JD. Transient FTY720 treatment promotes immune-mediated clearance of a chronic viral infection. Nature. 2008;454:894–898. doi: 10.1038/nature07199. [DOI] [PubMed] [Google Scholar]
- 11.Osborne N, Stainier DY. Lipid receptors in cardiovascular development. Annu Rev Physiol. 2003;65:23–43. doi: 10.1146/annurev.physiol.65.092101.142235. [DOI] [PubMed] [Google Scholar]
- 12.Stainier DY. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- 13.Tallafuss A, Bally-Cuif L. Tracing of her5 progeny in zebrafish transgenics reveals the dynamics of midbrain-hindbrain neurogenesis and maintenance. Development. 2003;130:4307–4323. doi: 10.1242/dev.00662. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi Y, Trinh LA, Reiter JF, Alexander J, Yelon D, Stainier DY. The zebrafish bonnie and clyde gene encodes a Mix family homeodomain protein that regulates the generation of endodermal precursors. Genes Dev. 2000;14:1279–1289. [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter JF, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, Stainier DY. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander J, Rothenberg M, Henry GL, Stainier DY. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–357. doi: 10.1006/dbio.1999.9441. [DOI] [PubMed] [Google Scholar]
- 17.Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;143:949–56. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto D, Nakano Y. Sexual behavior mutants revisited: molecular and cellular basis of Drosophila mating. Cell Mol Life Sci. 1999;56:634–646. doi: 10.1007/s000180050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dermaut B, Norga KK, Kania A, Verstreken P, Pan H, Zhou Y, Callaerts P, Bellen HJ. Aberrant lysosomal carbohydrate storage accompanies endocytic defects and neurodegeneration in Drosophila benchwarmer. J Cell Biol. 2005;170:127–139. doi: 10.1083/jcb.200412001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young RM, Marty S, Nakano Y, Wang H, Yamamoto D, Lin S, Allende ML. Zebrafish yolk-specific not really started (nrs) gene is a vertebrate homolog of the Drosophila spinster gene and is essential for embryogenesis. Dev Dyn. 2002;223:298–305. doi: 10.1002/dvdy.10060. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi T, Kikuchi Y, Kuroiwa A, Takeda H, Stainier DY. The yolk syncytial layer regulates myocardial migration by influencing extracellular matrix assembly in zebrafish. Development. 2006;133:4063–4072. doi: 10.1242/dev.02581. [DOI] [PubMed] [Google Scholar]
- 22.Kai M, Heisenberg CP, Tada M. Sphingosine-1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation. Development. 2008;135:3043–3051. doi: 10.1242/dev.020396. [DOI] [PubMed] [Google Scholar]
- 23.Heisenberg CP, Nusslein-Volhard C. The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev Biol. 1997;184:85–94. doi: 10.1006/dbio.1997.8511. [DOI] [PubMed] [Google Scholar]
- 24.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 25.Parrill AL, Wang D, Bautista DL, Van Brocklyn JR, Lorincz Z, Fischer DJ, Baker DL, Liliom K, Spiegel S, Tigyi G. Identification of Edg1 receptor residues that recognize sphingosine 1-phosphate. J Biol Chem. 2000;275:39379–39384. doi: 10.1074/jbc.M007680200. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Kimelman D. The role of the yolk syncytial layer in germ layer patterning in zebrafish. Development. 2000;127:4681–4689. doi: 10.1242/dev.127.21.4681. [DOI] [PubMed] [Google Scholar]
- 27.Nakano Y, Fujitani K, Kurihara J, Ragan J, Usui-Aoki K, Shimoda L, Lukacsovich T, Suzuki K, Sezaki M, Sano Y, Ueda R, Awano W, Kaneda M, Umeda M, Yamamoto D. Mutations in the novel membrane protein spinster interfere with programmed cell death and cause neural degeneration in Drosophila melanogaster. Mol Cell Biol. 2001;21:3775–3788. doi: 10.1128/MCB.21.11.3775-3788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 29.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 30.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 31.Matsui T, Raya A, Callol-Massot C, Kawakami Y, Oishi I, Rodriguez-Esteban C, Belmonte JC. miles-apart-Mediated regulation of cell-fibronectin interaction and myocardial migration in zebrafish. Nat Clin Pract Cardiovasc Med. 2007;4 Suppl 1:S77–82. doi: 10.1038/ncpcardio0764. [DOI] [PubMed] [Google Scholar]
- 32.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiappara G, Gagliardo R, Siena A, Bonsignore MR, Bousquet J, Bonsignore G, Vignola AM. Airway remodelling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 2001;1:85–93. doi: 10.1097/01.all.0000010990.97765.a1. [DOI] [PubMed] [Google Scholar]
- 35.Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, Ozaki Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res. 2003;58:170–177. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- 36.Karliner JS. Mechanisms of cardioprotection by lysophospholipids. J Cell Biochem. 2004;92:1095–1103. doi: 10.1002/jcb.20129. [DOI] [PubMed] [Google Scholar]
- 37.Westerfield M. A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene, OR: University of Oregon Press; 2000. The Zebrafish Book. [Google Scholar]
- 38.Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- 39.Alexander J, Stainier DY, Yelon D. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev Genet. 1998;22:288–299. doi: 10.1002/(SICI)1520-6408(1998)22:3<288::AID-DVG10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- 41.Odenthal J, Nusslein-Volhard C. fork head domain genes in zebrafish. Dev Genes Evol. 1998;208:245–258. doi: 10.1007/s004270050179. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich F, Krieg M, Schötz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–64. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer’s vesicle in zebrafish. Dev Biol. 2007;310(2):196–210. doi: 10.1016/j.ydbio.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 44.Neff MM, Turk E, Kalishman M. Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 2002;18:613–615. doi: 10.1016/s0168-9525(02)02820-2. [DOI] [PubMed] [Google Scholar]
- 45.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.