Abstract
Objective
FYN is a member of the SRC family of kinases (SFKs), functionally distinct from other SFKs. It interacts with FAK and paxillin (PXN)- regulators of cell morphology and motility. We hypothesized that FYN is upregulated in prostate cancer (CaP).
Patients and Methods
Through datamining in Oncomine; cell line profiling with immunoblotting and quantitative RT-PCR; and immunohistochemical analysis, we describe FYN expression in CaP. This analysis included 32 cases of CaP, 9 prostatic intraepithelial neoplasia (PIN), and 19 normal. Samples were scored for the percentage of stained glands and intensity of staining (from 0-3). Each sample was assigned a composite score generated by multiplying percentage and intensity.
Results
Datamining showed an 8-fold increase in FYN expression in CaP compared to normal tissue. This was specific to FYN and not present for other SFKs. Expression of FYN in CaP cell lines (LNCaP, 22Rv1, PC3, DuPro) was detected using quantitative RT-PCR and immunoblot. Expression of FYN and its signaling partners FAK and PXN was demonstrated in human tissue. Comparing normal to cancer, there was a 2.1-fold increase in median composite score for FYN (p<0.001) 1.7-fold increase in FAK (p<0.001), and a 2-fold increase in PXN (p<0.05). There was a 1.7-fold increase in FYN (p<0.05), a 1.6-fold increase in FAK (p<0.01) in CaP as compared to PIN.
Conclusions
These studies support the hypothesis that the FYN and its related signaling partners are upregulated in CaP and supports further investigation into the role of the FYN as a therapeutic target.
Keywords: FYN, SRC, prostate cancer, paxillin, FAK
Introduction
Prostate cancer is the most common cancer affecting American men accounting for more than 200,000 new cases of cancer diagnosed in 2008 [1]. While a large number of men have disease that is either amenable to local therapy (surgery or radiation), a large number will develop metastatic disease. It is this population who is at risk for morbidity and mortality from both the disease and treatment-related side effects such as osteoporosis or cardiovascular events. Despite advances in therapy, more than 30,000 are expected to die in 2008 from this disease. These figures have driven an aggressive search for promising molecular targets in prostate cancer. Castration is a highly effective and widely used therapy for men with this disease; however, the majority of patients will progress to a castration-resistant state. This progression is associated with increased morbidity and mortality. At this time, only docetaxel-based chemotherapy has been shown to extend survival for this pool of patients. Thus, multiple therapeutic targets have been proposed and explored. Tyrosine kinases are known to be dysregulated in prostate cancer and as clinically usable agents have become available, several of these have been studied in prostate cancer including the epidermal growth factor receptor (EGFR), vascular endothelial growth factor receptor (VEGFR), and B/C raf-kinase (BRAF/CRAF), none of which have yet shown significant clinical efficacy. Gene expression profiling of non-receptor tyrosine kinases in prostate cancer has shown that the SRC family is particularly dysregulated in prostate cancer [2].
The SRC-family of kinases (SFKs) is one of the most studied families of proteins in cancer biology. Since the identification and description of the pp60c-SRC, eight other proteins sharing significant structural homology have been identified. The SFKs have long been recognized as overexpressed in a number of cancers including prostate cancer. Each member is distinguished by a unique region that specifies its respective binding partners and hence functions.
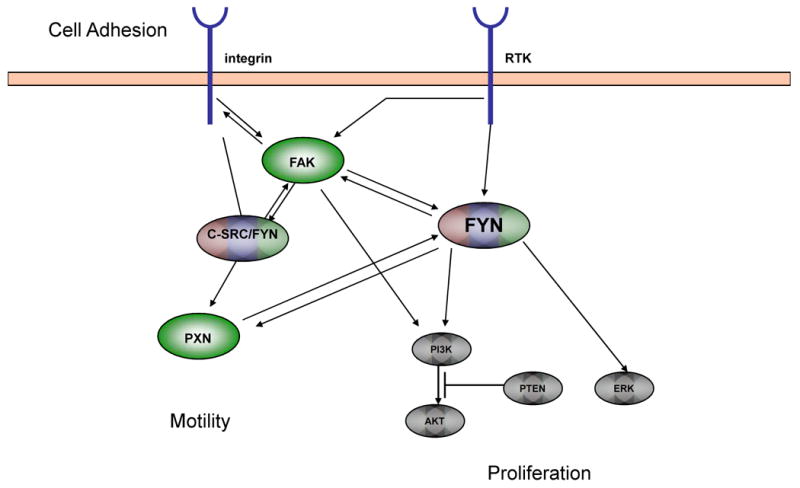
FYN is a 59 kDa member of this family and was one of the first members to be identified. The gene encoding FYN is located on chromosome 6q21. The most abundant transcript encodes a protein composed of 537 amino acids with a structure similar to the other SFKs save the unique region. Like other SFKs, FYN is a non-receptor tyrosine kinase that functions downstream of several cell surface receptors. It is best characterized functions are in neuronal development and T-cell signaling [3], but FYN also induces morphogenic transformation when overexpressed [4]. FYN is recognized as an important mediator of mitogenic signals and as a regulator of cell cycle entry, growth, and proliferation. It is also known to mediate integrin interactions and hence cell-cell adhesion. FYN is known to interact with a number of molecular signals including FAK and Paxillin (PXN) [5, 6] which may account for the described morphogenic transformation and possibly lend insight into its role in cancer.
In this paper, we present the first series of studies demonstrating the specific importance of FYN in prostate cancer. Our approach used a combination of both datamining and tissue microarray (TMA) immunohistochemical analysis showing overexpression of FYN in human prostate cancer. Our work suggests that FYN and its signaling partners FAK and PXN are upregulated in prostate cancer. Together, these findings suggest that FYN and its related signaling partners should be investigated as potential targets for prostate cancer therapy.
Patients and Methods
Datamining
Expression of FYN in prostate cancer was queried using the Oncomine database (http://www.oncomine.org) in February 2008. This is a publicly available database summarizing gene chip experiments across tissue types [7]. Oncomine provides an infrastructure of datamining tools to query genes and data sets of interest as well as to meta-analyze groups of studies. This database was queried for gene expression data for FYN, SRC, YES, BLK, LCK, FGR, LYN, HCK, and YRK. Studies were included if they compared primary prostate cancers to any of the following: normal or benign epithelium, metastatic prostate cancer, prostatic intraepithelial neoplasia (PIN), benign prostatic hypertrophy (BPH), or hormone refractory prostate cancer (HRPC). The p-values presented are extracted directly from the Oncomine analysis and have not been repeated manually.
Cell lines
All cell lines used were purchased from the American Type Culture Collection (Manassas, VA). Lines used included standard prostate cancer cell lines: LNCaP, CWR22Rv1, PC3, and DuPro. U87 are malignant astrocytes that were used as a positive control for FYN [8]. Cells were grown according to ATCC recommendations: RPMI 1640 with 10% fetal calf serum and penicillin/streptomycin supplement.
Human tissue source
All human tissue samples used in this study were obtained from the University of Michigan through an interSPORE collaboration. Utilization of tissue was performed under an institutional review board approved protocol requiring that all samples were kept anonymous to the primary investigational team.
Tissue was analyzed in the form of a tissue microarray (TMA, TMA100). Microarray fabrication has been described by the University of Michigan group elsewhere [9]. In short, the initial array used contained 120 patient specimens planned to have triplicate representation on the array. Each array element was 0.6 mm in diameter. Tissue samples included primary tumor from prostate cancer patients with Gleason 6 to 9 disease, metastatic tumor sites, PIN, PIA, BPH, prostatic stroma, and normal prostate tissue. The identity of patients was kept blinded to the primary analytic group. Normal glands present on the array were taken either from patients who underwent prostatectomy or cystectomy. A patient's samples was only considered usable if represented at least twice on the array.
Antibodies
Commercially available antibodies were used for all immunoblotting and immunohistochemistry studies. Anti-Fyn was obtained from Millipore (Burlington, MA); Anti-FAK was obtained from Invitrogen (Carlsbad, CA). Anti-Paxillin antibody 5H11 was obtained from Biosource (Invitrogen- Carlsbad, CA).
Protein extraction and Western blotting
Monolayer cells were grown to 80% confluence then washed in ice-cold PBS. Protein lysates were prepared using lysis buffer (10 mmol/L Tris (pH 7.5), 1 mmol/L β-glycerophosphate, 2mmol/L DDT, 1mmol/L EDTA, 150 mmol/L NaCl, 0.5 mmol/L NaF, 2mmol/L NaVO4, 0.1% NP40, 10 μmol/L phenylmethylsulfonyl fluoride (PMSF) 1% Triton X-100 (w/v), 70 units/mL aprotinin, and one Complete Protease Inhibitor Cocktail tablet (Roche, Basel, Switzerland)). Cells were scraped and placed on ice after being passed through a 27-gauge needle and subsequently centrifuged at 14,000 rpm at 4 C for 10 minutes. Protein lysates were quantified using the Pierce Bicinchoninic Acid Protein Assay kit (Rockford, IL); 20 μg of protein were subjected to SDS-PAGE and transferred to a HyBond Enhanced Chemiluminescence nitrocellulose membrane.
For Western blotting, membranes were blocked at 4° C overnight in TBS-T plus 5% (w/v) Carnation nonfat dry milk. After incubation with each antibody diluted in blocking solution for 1 hour, the membrane was washed for 10 minutes in blocking solution and then washed six times for 5 minutes each in TBS-T. The horseradish peroxidase-conjugated secondary antibody was detected using the Super Signal West Femto Maximum Sensitivity Chemiluminescence Substrate (Pierce) per the manufacturer's directions. Probed membranes were stripped using Pierce Restore Western Blot Stripping Buffer, washed in TBS-T, and blocked overnight before reprobing. The dilutions of antibodies were as follows: anti-FYN 1:1000, anti- FAK 1:1000, anti-PXN 1:500. As a loading control, membranes were probed for actin followed by incubation with a goal anti-mouse IgM-peroxidase-conjugated secondary antibody (Oncogene Research, Uniondale, NY; 1:20,000 and 1:40,000 dilutions of primary and secondary antibodies, respectively).
RNA extraction and quantitative RT-PCR
RNA from cell lines was extracted using an RNAqueous kit (Ambion, Auton, TX) per the manufacturer's recommendations. Samples were stored at -80° C until processed. Customized primers for FYN were prepared by Integrated DNA Technologies (Coralville, IA). Left primer: ATG GAA ACA CAA AAG TAG CCA TAA A; Right primer: TCT GTG AGT AAG ATT CCA AAA GAC C. Data was calibrated to GAPDH expression. Quantitative PCR was performed using SYBR Green dye on an ABI 7700 (Applied Biosystems, Foster City, CA).
Immunohistochemistry
Stained TMA sections were analyzed by a dedicated urological pathologist (HAA) in a blinded fashion. Results were reported semi-quantitatively on a scale of 0-3 for intensity where 0 is negative, 1 is weak, 2 is moderate and 3 is strong. The percentage of tumor staining was reported from 0 to 100% in increments of 10. A composite score was formed using the product of the intensity and percentage of glands staining. Human breast cancer tissue was used as a positive staining control as recommended by the manufacturer [10]. Human leiomyomas were used as a negative control. FYN was stained using an antibody concentration of 1:50; FAK at 1:100; and paxillin at 1:100
Statistical Methods
For the analysis of the TMA data, ANOVA was used to compare expression levels (based on the percent staining or the composite score) across groups. The equal variance assumption was verified using Bartlett's test [11]. Post-hoc pairwise comparisons were performed with a Bonferroni adjustment for multiple comparisons. For comparison of the ordinal, staining intensity score, the Kruskal-Wallis test was utilized. Additionally, a nonparametric trend test [12] was used for further examination of expression levels across the naturally ordered groups. The average of the duplicate or triplicate samples for each subject was used in the analysis. Statistical significance was defined as p < 0.05. Statistical analyses were performed using Stata, Version 9 (StataCorp, College Station, TX).
Results
Expression of FYN in prostate cancer cell lines and tissues
To identify SFKs for analysis, we reviewed available studies in the Oncomine database. In comparing malignant to normal prostate epithelium, the member of this family which arose as the most consistently and strongly overexpressed was FYN which showed an 8-fold increase in cancer (p<0.00005) [13]. There was little to no change in the remainder of the SFKs including LYN, YES, HCK, and FGR. The overexpression of FYN further increased 10-fold in the transition from localized to metastatic cancers while other SFKs were either downregulated (HCK, LCK) or showed no significant changes in expression (LYN, YES, BLK, or SRC) [14].
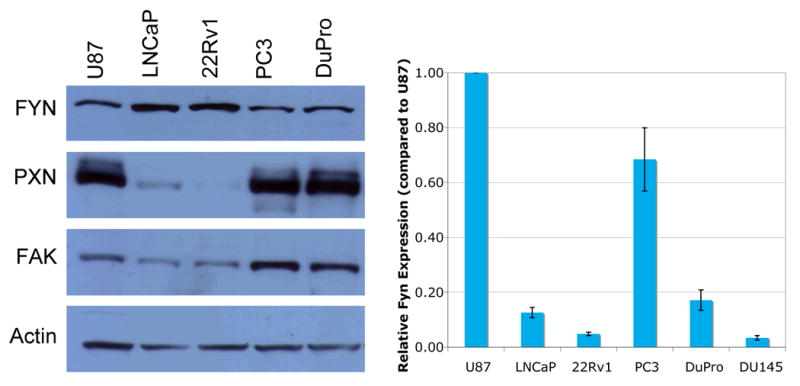
FYN was chosen for further investigation as it was identified as the most upregulated SFK in prostate cancer. Given the homology of the various members of the family, a number of antibodies were tested and eliminated on the basis of sensitivity and specificity (supplemental data- table 1s). Expression of FYN was evaluated in standard prostate cancer cell lines (Figure 1a-top). The U87 cell line was used as a positive control as malignant astrocytes are known to express FYN [8]. Findings were verified by quantitative RT-PCR (Figure 1b). We found expression of FYN RNA and protein in all tested cell lines. Expression of FYN was not seen in human leiomyoma samples (immunoblot verified negative control- data not shown).
Figure 1.
Expression of FYN and signaling partners FAK and paxillin (PXN) in prostate cancer cell lines shown by a) immunoblotting and b) quantitative RT-PCR. U87 cells (malignant astrocytes) were used as positive control for FYN expression.
We then verified the Oncomine findings in human tissue samples using immunohistochemical analysis of a tissue microarray obtained from the University of Michigan which contained samples of normal prostate, PIN, and prostate cancer. There were a total of 86 usable patient samples for the FYN analysis (table 1). We stained the array for total FYN (figure 2) and analyzed by generating a composite score from the percentage of tumor cells staining and intensity. A number of candidate antibodies were tested and discarded (supplemental table 1s) if they failed to show sensitivity and specificity to tumor tissues and expected positive control (e.g. lymphocytes) or if the pattern of staining did not correlate with the biology of FYN. For example, an antibody showing predominately nuclear staining in all samples was declared to be erroneous.
Table 1.
Patient Demographics for FYN analysis
| Number | |
|---|---|
| Total usable patients | 86 |
| Tumor | 32 |
| Gleason 3 +3 | 6 |
| Gleason 3 + 4 | 8 |
| Gleason 4 + 3 | 3 |
| Gleason 4 + 4 | 8 |
| Gleason 4 + 5 | 7 |
| Metastases (all sites) | 10 |
| BPH | 8 |
| PIN | 9 |
| Normal Prostate | 19 |
| Median Age (range) | 64 years (43-76) |
| Race | |
| Caucasian | 50 |
| African Descent | 2 |
| Other/Unknown | 34 |
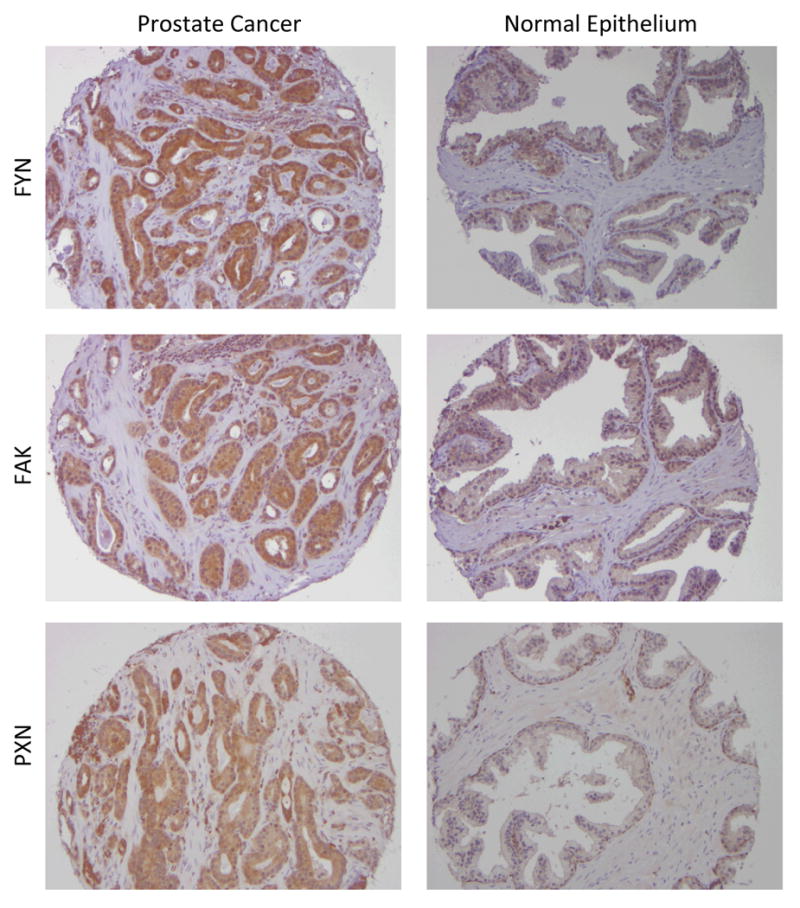
Figure 2.
Expression of FYN, FAK, and PXN in malignant and non-malignant prostate epithelium. Representative photomicrographs of sections of malignant and non-malignant prostate epithelium.
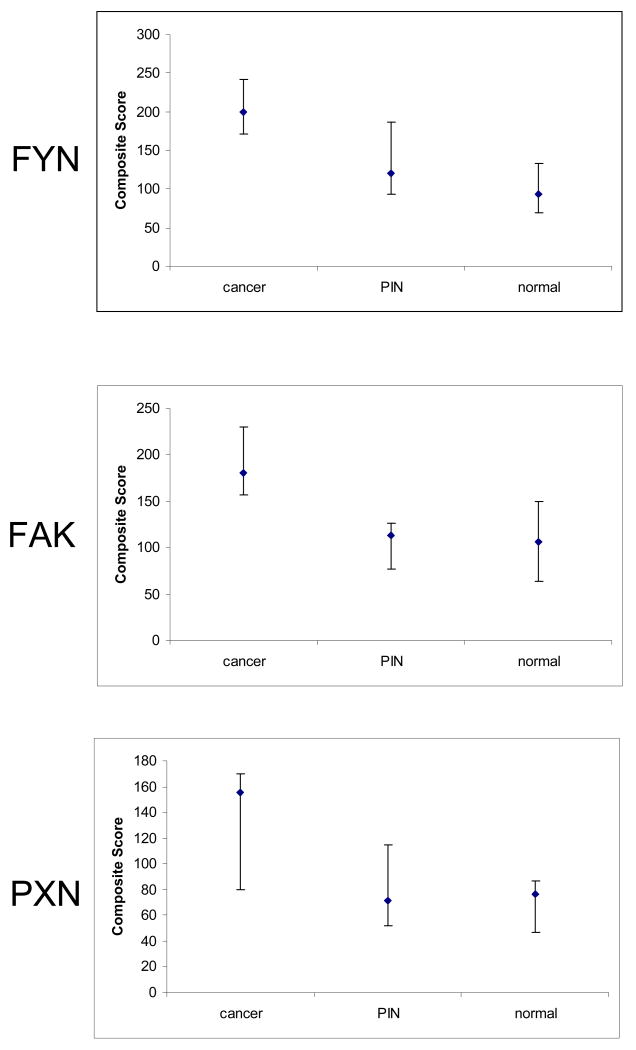
Composite scores for cancer specimens ranged from 23-300 (median 200). Scores did not correlate significantly with Gleason score (data not shown). Nineteen of 32 tumor samples (59%) had scores between 200-300. For normal epithelium, scores ranged from 7-160 (median 93). For PIN, scores ranged from 45-220 (median 120). Figure 3 shows the distribution of composite scores for FYN. Staining of FYN was strong in primary tumor samples in comparison to non-neoplastic tissue (p<0.0001 for overall comparison). Differences in expression between normal and cancer as well as PIN and cancer were both statistically significant based on the composite score. Specifically, there was a 2.1-fold increase in the median composite score comparing normal and cancer (p<0.001). There was a 1.7- fold increase in FYN comparing PIN and cancer (p=0.03). Furthermore, there was evidence for increasing expression levels across these three naturally ordered groups (p<0.001 for trend). Ten metastatic tumors were represented from a variety of sites (lymph node, lung, liver). Scores ranged from 10-290 (median 102). With the limited number of samples, it was not possible to show or deny the absence of a trend in FYN expression which merits further study.
Figure 3.
Plots of A) FYN B) FAK and C) PXN staining in malignant versus non-malignant tissue samples. Composite scores (intensity of staining × percentage of glandular cells staining) are shown on the Y-axis. The median is plotted with the error bars representing the 25th and 75th percentiles.
Signaling partners of FYN (FAK and PXN) are also upregulated in prostate cancer
As FYN interacts with a number of regulators of cellular morphology and attachment, cell lines and human tissue samples were re-examined for FAK and PXN. Immunoblotting showed co-expression of FAK and PXN with FYN (Figure 1a middle, bottom). Both were most highly expressed in the castrate resistant cell lines (PC3 and DuPro) consistent with the datamining presented earlier. Castrate sensitive lines (LNCaP and 22Rv1) did show expression of both FAK and PXN but at a much lower level.
To extend the studies to clinical material, FAK and PXN expression was evaluated on the TMA. Representative sections stained for FAK and PXN are shown in Figure 2 (middle and bottom). There were a total of 35 usable tumor samples for FAK and 22 samples for PXN analysis. Our findings for the TMA population are represented graphically in Figure 3 (middle and bottom).
FAK scores ranged from 40-300 (median 180) in tumor samples. There was a tendency for higher Gleason tumors to have higher FAK scores, but this association did not reach statistical significance. Twelve of 35 (34%) showed scores in the 200-300 range. Normal epithelium showed FAK scores ranging from 53-253 (median 107). PIN ranged from 35-167 (median 113). In the final analysis, there was a 1.7-fold increase in FAK expression comparing normal and cancer (p<0.001) and a 1.6-fold increase in FAK comparing PIN to cancer (p<0.01). Metastatic lesions showed scores ranging from 57-290 (median 140).
PXN scores for tumor samples ranged from 25-300 (median 155). No clear relationship with Gleason score was seen. Only 2 of 22 (9%) usable specimens showed PXN scores in the 200-300 range (285, 300). Normal prostate samples ranged from 25-160 (median 77). Those for PIN ranged from 40-150 (median 72), but only 4 samples were available for analysis due to poor transfer. There was a 2-fold increase in PXN staining comparing normal to cancer (p<0.05). The limited number of PIN specimens precluded any comparisons between PIN and cancer.
These data point toward an upregulation of FAK and PXN in prostate cancer in comparison to normal epithelium that correlates with FYN overexpression in cancer.
Discussion
Through a combination of datamining, immunoblotting, and immunohistochemistry, we demonstrate the upregulation of FYN, a particular member of the SRC family of kinases, in prostate cancer. The initial Oncomine queries suggested particularly high over expression of FYN when comparing cancer to normal prostate (non-neoplastic, non-hypertrophic) and in situ malignancy (PIN). Expression of FYN was seen both in a panel of prostate cancer cell lines and human tissue samples. This was accompanied by expression of FYN's signaling partners FAK and paxillin (PXN)- factors known to regulate cellular motility and metastasis. There were discrepancies noted between the magnitude of FYN measured by qRT-PCR and immunoblot, however, there are frequent reports of discrepancies between RNA and protein expression in the literature. Specifically, FYN has been shown to undergo posttranscriptional modification which may impact protein expression [15].
Our datamining further suggest that this upregulation of expression is specific to FYN and not the other members of the SRC family. While the SRC kinases share similarities in sequence and structure they do exhibit differences that may be germane to the development of SFK directed therapies. The majority of SFK directed research in cancer has been aimed at the expression of c-SRC. To date, the role of FYN in cancer biology is relatively unexplored. With more than 2300 citations in pubmed referencing the role of SRC and SRC kinases in cancer, there are approximately 200 studies mentioning FYN expression in a variety of cancer models; only a small number of which specifically focus upon FYN biology. FYN has been implicated as a mediator of EGF-driven transformation of JB6 cells [16]. In breast cancer, FYN expression was shown to correlate with poorer survival and also correlated with FAK upregulation [17]. In hematologic malignancies, FYN has been identified as a putative target for treatment of BCR-ABL expressing adult acute lymphoblastic leukemia due to the centrality of its relationship to a number of important molecular signals suspected to drive proliferation of malignant leukemic blasts [18] Compounds active against FYN have demonstrated in vitro anti-proliferative activity in acute lymphoblastic leukemia [19]. In other solid tumors such as melanoma, FYN has been implicated as a mediator of integrin signaling and thus appears to regulate metastatic potential [20].
Interestingly, there is a report of loss of FYN expression in prostate cancer [21]. This group recognized an allelic imbalance at 6q14-22 and sought to identify tumor suppressors associated with this region. They identified FYN as a potential tumor suppressor noting that the highest levels of FYN were seen in benign prostatic hyperplasia (BPH) as compared to malignant tissues which showed little to no FYN expression. While the results appear contradictory, our study does not specifically address the role of FYN in BPH. Members of the SRC family are well known to play a number of different roles in variety of cellular contexts, thus it is entirely possible that in one biochemical context, FYN does serve as a tumor suppressor while in the altered biochemical landscape of neoplastic transformation (i.e. in the change from pre-invasive, to invasive, then again to metastatic) that FYN serves another role altogether. Further studies will be needed to show FYN's biological role in these various settings. This type of dynamic signaling behavior has been seen with other molecular targets (including proposed tumor suppressors) in the setting of prostate cancer [22]. Sørensen et al. performed an immunohistochemical analysis similar to that which we have presented. Our results agree in so far as expression of FYN was seen in normal and hyperplastic epithelium. What requires reconciliation is the absence of FYN staining in tumor tissue reported by Sørensen. In our study, samples from all 32 patients with prostate cancer showed high levels of FYN expression. This may be the result of technical issues such as the choice of antibody the IHC results as we found during our screening. Finally Sørensen's study suggested that by quantitative PCR there was attenuated expression of FYN in tumor samples from patients. The approach taken made use of whole tissue homogenates making epithelial cell content difficult to control. This is especially important given the congruent findings of absent FYN expression in the stromal compartment.
FYN is positioned downstream of several important cell surface receptors and upstream of a number of cellular signals important for prostate cancer progression. Like other SRC family members, it is known to mediate a number of cell shape and migration behaviors. As such, its interactions with mediators of cell shape and motility were important to study. Our data also suggests that there is an accompanying upregulation of FAK and PXN, both of which are important regulators of cell shape and interactions with other cells and the extracellular matrix. Both FAK [23-26] and PXN [26, 27] have been recognized as crucial to motility and thus invasion which are cellular processes required for metastatic competence and acquisition of the metastatic phenotype.
Expression of FAK and PXN in prostate cancer have been correlated with disease progression [26, 28]. FAK has been shown to play a role in prostate cancer metastasis by disrupting integrin mediated signaling from the extracellular matrix. The invasive abilities of DU145 cells on fibronectin was inhibited by silencing FAK expression via siRNA [29]. SRC kinases have been implicated as potential means of modulating FAK activity in prostate cancer and SRC inhibitors have been shown to downregulate FAK activation [23]. Overexpression of leupaxin, a member of the PXN family, was shown to cause an increase in cellular motility in PC3 cells [30]. Again, SRC kinase inhibitors have been shown to downregulate activation of PXN which in turn results in decreased cellular motility [5]. Given the overexpression of FYN noted here and the non-specific nature of most SFK inhibitors, it is likely that the bulk of this effect is mediated by FYN.
These findings gain translational relevance with the introduction of SRC-family inhibitors into clinical practice. Dasatinib is commercially available for the treatment of chronic myelogenous leukemia and is currently being evaluated as a treatment for castrate-resistant prostate cancer. Other agents such as AZD0530 and bosutinib are currently in clinical development with a host of others to follow. AZD0530, a potent SRC/ABL inhibitor, has been shown to have a potent effect on cellular motility which is SFK-mediated [31]. While labeled as inhibitors of c-SRC, these drugs are known to have a variety of inhibitor effects upon cellular tyrosine kinases including FYN. Furthermore, several inhibitors of both FAK and PXN are currently in development. This raises the potential for combinatorial approaches with these signal transduction inhibitors in a vertical fashion which may have potent effects on cellular motility and invasion. If relatively non-toxic, such an approach may be an effective treatment after definitive local therapy in concert with or following castration.
In conclusion, our findings show a statistically significant upregulation of FYN and its signaling partners FAK and PXN through datamining, immunoblotting, and immunohistochemistry. It is hoped that further understanding of the role of FYN in prostate cancer development and progression may shed insights into how FYN-inhibitory agents should be used in the clinic. Given our findings, we believe that FYN is a promising molecular target for cancer therapeutics.
Figure 4.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chang YM, Kung HJ, Evans CP. Nonreceptor tyrosine kinases in prostate cancer. Neoplasia. 2007;9:90–100. doi: 10.1593/neo.06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resh MD. Fyn, a Src family tyrosine kinase. Int J Biochem Cell Biol. 1998;30:1159–62. doi: 10.1016/s1357-2725(98)00089-2. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami T, Kawakami Y, Aaronson SA, Robbins KC. Acquisition of transforming properties by FYN, a normal SRC-related human gene. Proc Natl Acad Sci U S A. 1988;85:3870–4. doi: 10.1073/pnas.85.11.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angelucci A, Schenone S, Gravina GL, et al. Pyrazolo[3,4-d]pyrimidines c-Src inhibitors reduce epidermal growth factor-induced migration in prostate cancer cells. Eur J Cancer. 2006;42:2838–45. doi: 10.1016/j.ejca.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Mizutani T, Shiraishi K, Welsh T, Ascoli M. Activation of the lutropin/choriogonadotropin receptor in MA-10 cells leads to the tyrosine phosphorylation of the focal adhesion kinase by a pathway that involves Src family kinases. Mol Endocrinol. 2006;20:619–30. doi: 10.1210/me.2005-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–80. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bare DJ, Lauder JM, Wilkie MB, Maness PF. p59fyn in rat brain is localized in developing axonal tracts and subpopulations of adult neurons and glia. Oncogene. 1993;8:1429–36. [PubMed] [Google Scholar]
- 9.Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML. E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum Pathol. 2001;32:690–7. doi: 10.1053/hupa.2001.25902. [DOI] [PubMed] [Google Scholar]
- 10.Garcia S, Dales JP, Charafe-Jauffret E, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum Pathol. 2007;38:830–41. doi: 10.1016/j.humpath.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett M. Properties of sufficiency and statistical tests. Proceedings of the Royal Society, Series A. 1937;160:268–82. [Google Scholar]
- 12.Cuzick J. A Wilcoxon-type test for trend. Statistics in Medicine. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 13.Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 14.Varambally S, Yu J, Laxman B, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Ku L, Chen Y, Feng Y. Developmental abnormalities of myelin basic protein expression in fyn knock-out brain reveal a role of Fyn in posttranscriptional regulation. J Biol Chem. 2005;280:389–95. doi: 10.1074/jbc.M405973200. [DOI] [PubMed] [Google Scholar]
- 16.He Z, Tang F, Ermakova S, et al. Fyn is a novel target of (-)-epigallocatechin gallate in the inhibition of JB6 Cl41 cell transformation. Mol Carcinog. 2008;47:172–83. doi: 10.1002/mc.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia S, Dales JP, Charafe-Jauffret E, et al. Overexpression of c-Met and of the transducers PI3K, FAK and JAK in breast carcinomas correlates with shorter survival and neoangiogenesis. Int J Oncol. 2007;31:49–58. [PubMed] [Google Scholar]
- 18.Juric D, Lacayo NJ, Ramsey MC, et al. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J Clin Oncol. 2007;25:1341–9. doi: 10.1200/JCO.2006.09.3534. [DOI] [PubMed] [Google Scholar]
- 19.Lerma EI, Nguyen VA, Wang T, et al. Novel compounds with antiproliferative activity against imatinib-resistant cell lines. Mol Cancer Ther. 2007;6:655–66. doi: 10.1158/1535-7163.MCT-04-0307. [DOI] [PubMed] [Google Scholar]
- 20.Huang F, Reeves K, Han X, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–38. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen KD, Borre M, Orntoft TF, Dyrskjot L, Torring N. Chromosomal deletion, promoter hypermethylation and downregulation of FYN in prostate cancer. Int J Cancer. 2008;122:509–19. doi: 10.1002/ijc.23136. [DOI] [PubMed] [Google Scholar]
- 22.Lotan TL, Lyon M, Huo D, et al. Up-regulation of MKK4, MKK6 and MKK7 during prostate cancer progression: an important role for SAPK signalling in prostatic neoplasia. J Pathol. 2007;212:386–94. doi: 10.1002/path.2194. [DOI] [PubMed] [Google Scholar]
- 23.Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–63. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- 24.Sumitomo M, Shen R, Walburg M, et al. Neutral endopeptidase inhibits prostate cancer cell migration by blocking focal adhesion kinase signaling. J Clin Invest. 2000;106:1399–407. doi: 10.1172/JCI10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–64. [PubMed] [Google Scholar]
- 26.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68:164–71. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Ye L, Lewis-Russell JM, Kynaston H, Jiang WG. Endogenous bone morphogenetic protein-7 controls the motility of prostate cancer cells through regulation of bone morphogenetic protein antagonists. J Urol. 2007;178:1086–91. doi: 10.1016/j.juro.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Rovin JD, Frierson HF, Jr, Ledinh W, Parsons JT, Adams RB. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate. 2002;53:124–32. doi: 10.1002/pros.10114. [DOI] [PubMed] [Google Scholar]
- 29.Zeng ZZ, Jia Y, Hahn NJ, Markwart SM, Rockwood KF, Livant DL. Role of focal adhesion kinase and phosphatidylinositol 3′-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res. 2006;66:8091–9. doi: 10.1158/0008-5472.CAN-05-4400. [DOI] [PubMed] [Google Scholar]
- 30.Sahu SN, Nunez S, Bai G, Gupta A. Interaction of Pyk2 and PTP-PEST with leupaxin in prostate cancer cells. Am J Physiol Cell Physiol. 2007;292:C2288–96. doi: 10.1152/ajpcell.00503.2006. [DOI] [PubMed] [Google Scholar]
- 31.Chang YM, Bai L, Yang YC, Kung HJ, Evans CP. Proc AACR 2007. Los Angeles, CA: 2007. AZD0530 is a novel Src inhibitor with anti-proliferation and anti-migration properties in prostate cancer (abstr #LB-24) [Google Scholar]