SUMMARY
Little is known about how metalloproteins in the secretory pathway obtain their metal ion cofactors. We used the Pho8 alkaline phosphatase of the yeast Saccharomyces cerevisiae to probe this process in vivo. We found that both Pho8 activity and protein accumulation are zinc dependent and decrease in zinc-limited cells. Low Pho8 accumulation was the result of degradation by vacuolar proteases. Surprisingly, the protective effect of zinc on Pho8 stability was not solely due to Zn2+ binding to the active site indicating that the Pho8 protein is targeted for degradation in zinc-limited cells by another mechanism. Pho8 is a rare example of a metalloprotein whose stability is regulated by its metal cofactor independently of active site binding. We also assessed which zinc transporters are responsible for supplying zinc to Pho8. We found that the Zrc1 and Cot1 vacuolar zinc transporters play the major role while the Msc2/Zrg17 zinc transporter complex active in the endoplasmic reticulum is not involved. These results demonstrate that the vacuolar zinc transporters, previously implicated in metal detoxification, also deliver zinc to certain metalloproteins within intracellular compartments. These data suggest that Pho8 receives its metal cofactor in the vacuole rather than in earlier compartments of the secretory pathway.
INTRODUCTION
Zinc is required as a structural and/or catalytic cofactor by hundreds of different proteins. Many zinc-dependent proteins reside in organelles of the secretory pathway or pass through this pathway on their way to other compartments (e.g. vacuole, lysosomes) within the cell or prior to their secretion into the extracellular environment. Zinc-dependent proteins that are resident in the secretory pathway include chaperone proteins (Scj1, calreticulin) (Guo et al., 2003; Tang and Wang, 2001), protein disulfide isomerases (Solovyov and Gilbert, 2004), and GPI-phosphoethanolamine transferases (GPI-PETs) (Mann and Sevlever, 2001; Sevlever et al., 2001) that are found in the endoplasmic reticulum (ER). Secreted zinc-dependent proteins include angiotensin-converting enzyme (Guy et al., 2005), matrix metalloproteases (Chang and Werb, 2001), and alkaline phosphatases (Coleman, 1992). Both resident and secreted zinc proteins obtain their zinc cofactor within intracellular compartments.
Zinc occurs in biological systems as the Zn2+ ion. Because of its high charge density, transporter proteins are required to move Zn2+ across cellular membranes such as those that make up the organelles of the secretory pathway. One family of metal ion transporters in particular, the CDF or cation diffusion facilitator family, is involved in the transport of zinc into the secretory pathway of eukaryotic cells (Gaither and Eide, 2001; Kambe et al., 2004). CDF proteins in mammals are also known as ZnT or SLC30A proteins (Liuzzi and Cousins, 2004; Palmiter and Huang, 2004). In the yeast Saccharomyces cerevisiae, four different CDF proteins have been implicated in delivering zinc to the secretory pathway (Ellis et al., 2004). Of these, the Msc2 and Zrg17 proteins play particularly important roles. Msc2 and Zrg17 associate as a heteromeric complex to form the functional transporter (Ellis et al., 2005). These two proteins localize to the ER and mutations in either result in ER stress as indicated by the induction of the unfolded protein response (UPR). UPR induction is an indicator of the accumulation of unfolded proteins in the ER. In addition, the Zrc1 and Cot1 zinc transporters of the CDF family also contribute to ER function (Ellis et al., 2004). These proteins primarily localize to the vacuole where they are required for zinc storage and detoxification (Kamizono et al., 1989; MacDiarmid et al., 2000; Miyabe et al., 2001). However, little Zrc1 or Cot1 was detected in the ER. These observations led to the hypothesis that Zrc1 and Cot1 may be active in the ER soon after their synthesis on ER-bound ribosomes and prior to their trafficking to the vacuole. Alternatively, Zrc1 and Cot1 could supply zinc to the early secretory pathway from their primary location in the vacuole via retrograde vesicular trafficking of zinc in the lumen of these vesicles. Zrc1 and Cot1 function independently of each other perhaps as homomeric rather than heteromeric complexes.
While the importance of Msc2/Zrg17, Zrc1, and Cot1 to ER function was established in terms of combating ER stress, the relative roles of these transporters in providing the cofactor to specific zinc-dependent proteins are unclear. To address this issue, we have examined the effects of zinc status and zinc transporter mutations on the accumulation and activity of alkaline phosphatase. In yeast, the major alkaline phosphatase is encoded by the PHO8 gene (Kaneko et al., 1982). Pho8 alkaline phosphatase is a resident vacuolar protein that requires the binding of two Zn2+ ions and one Mg2+ ion in its active site for catalytic activity (Murphy et al., 1995). The structures of several related alkaline phosphatases have been determined allowing the identification of the metal ligand residues in Pho8 (de Backer et al., 2002; Helland et al., 2008; Kim and Wyckoff, 1990; Le Du et al., 2001; Wang et al., 2007). These studies indicate that the two Zn2+ ions are coordinated to aspartate and histidine residues in the protein.
The function of Pho8 is to catalyze the removal of phosphate from phosphopeptides in the vacuole and allow the recycling of the released inorganic phosphate (Donella-Deana et al., 1993). Much is known about the process whereby Pho8 makes its way to the vacuole. Pho8 is initially synthesized in the ER as a type II integral membrane protein (Klionsky and Emr, 1989). A single transmembrane domain located near the amino terminus tethers the protein to the membrane. The short amino-terminal domain extends into the cytosol while the carboxy-terminal catalytic domain protrudes into the vacuole lumen. Pho8 is glycosylated on sites in the catalytic domain as the protein moves through the ER and the Golgi. Alkaline phosphatases also form dimers and where this interaction first occurs in the pathway is unknown (Coleman, 1992). Finally, upon reaching the vacuole, an ~20 amino acid inhibitory propeptide found at the carboxy-terminus of the protein is removed by proteolytic cleavage and the enzyme becomes active. Propeptide cleavage is mediated by the Pep4 aspartyl protease or possibly by Prb1, a serine protease that requires Pep4 processing for its activity (Kaneko et al., 1982; Klionsky and Emr, 1989; Merz and Wickner, 2004). While Pho8 was originally thought to be present only in a membrane-bound form, a soluble active form was recently purified from yeast extracts (Shong, 2006).
While this narrative indicates that we understand much about the many processing events that ultimately lead to Pho8 activity, we still know little about how this zinc metalloprotein, and others like it, receive their metal cofactors during their biogenesis. To explore this issue in vivo, we have assessed the effects of zinc status and zinc transporter mutations on Pho8 accumulation and activity.
RESULTS
Effects of zinc status on Pho8 alkaline phosphatase activity and accumulation
Transcription of the PHO8 gene, encoding the vacuolar alkaline phosphatase of S. cerevisiae, is induced by phosphate deficiency. The zinc-limiting medium used in our many previous studies of zinc homeostasis contains high levels of phosphate causing low PHO8 expression. Therefore, to examine the effects of zinc status on alkaline phosphatase under our standard growth conditions, we expressed PHO8 from a heterologous promoter. The PHO8 open reading frame was inserted adjacent to the GAL1 promoter in a plasmid vector. Expression of the GAL1 promoter was then driven by co-expression of a hybrid transcriptional activator protein (GEV) composed of the Gal4 DNA binding domain, the VP16 activation domain, and the human estrogen receptor steroid response domain. The GEV activator allows for a wide range of expression levels from the GAL1 promoter by adjusting the amount of a steroid inducer, β-estradiol, added to the medium (Gao and Pinkham, 2000). The level of Pho8 protein expression obtained using this system was similar to the levels observed in phosphate-limited cells expressing Pho8 from its own promoter (data not shown).
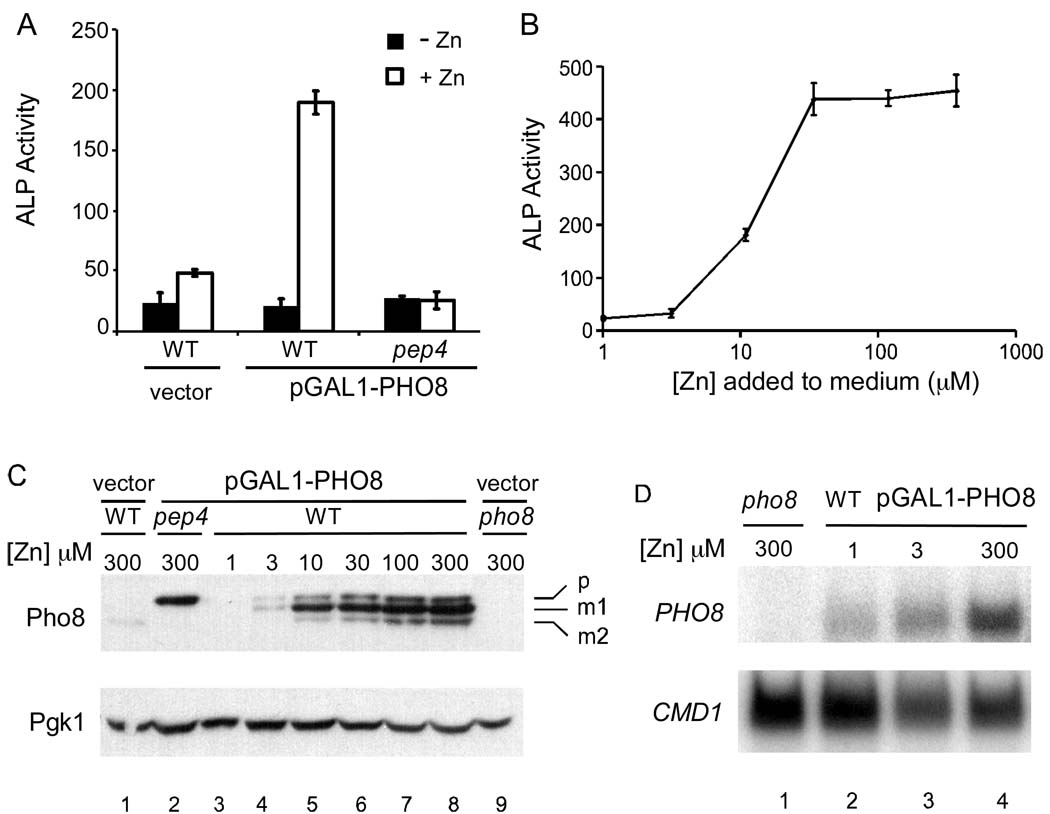
Wild type cells transformed with the GAL1 promoter vector showed little alkaline phosphatase (ALP) activity when grown in either zinc-limiting or replete conditions (Figure 1A). Most of the activity detected in these control cells is likely due to a cytosolic alkaline phosphatase, Pho13, which does not require zinc for its function (Kaneko et al., 1989). Cells bearing the Pho8-expressing plasmid, pGAL1-PHO8, had high activity when grown in a zinc-replete condition but only background levels of activity in zinc-limiting medium. This ALP activity detected in Pho8-expressing cells was dependent on the Pep4 vacuolar protease; activity was low in a pep4 mutant regardless of zinc status. This observation is consistent with the requirement for Pep4 to remove the inhibitory propeptide that blocks activity of the immature Pho8 protein (Klionsky and Emr, 1989). These data demonstrate that Pho8 activity is severely reduced in zinc-deficient cells. The dose response of Pho8 activity to the amount of zinc added to the medium is shown in Figure 1B. Full Pho8 activity was restored when cells were supplemented with 30 µM or higher levels of zinc.
Figure 1. Pho8 activity and protein accumulation requires zinc.
(A) Wild type and pep4 mutant cells bearing the vector (pRS316-GAL1-LEU2) or pGAL1-PHO8 were assayed for ALP activity under both low Zn and high Zn conditions. Cells were grown in LZM plus 1 µM ZnCl2 (−Zn, filled bars) or 300 µM ZnCl2 (+Zn, open bars). (B) Wild type cells bearing the pGAL1-PHO8 plasmid were grown overnight in LZM supplemented with the indicated concentration of zinc and then assayed for ALP activity. For panels A and B, each value is the mean of three replicates and the error bars represent ± 1 S.D. (C) Immunoblot analysis of protein samples prepared from cells bearing the vector or pGAL1-PHO8. Wild type cells transformed with pGAL1-PHO8 were grown in LZM supplemented with a range of zinc concentrations. Previous studies showed that cell viability is unaffected by these growth conditions (MacDiarmid et al., 2003). Designations of Pho8 forms are p, precursor; m1, mature form 1; m2, mature form 2. Pgk1 was used as a loading control. (D) S1 nuclease protection assays were performed with pho8 mutant cells or wild type cells bearing pGAL1-PHO8 grown in LZM with three representative zinc concentrations. 32P labeled DNA oligonucleotides that detect PHO8 and CMD1 mRNA were used as probes.
To determine the effects of zinc on Pho8 protein levels, cells were grown over a range of zinc concentrations and Pho8 accumulation was assayed by immunoblotting. The 3-phosphoglycerate kinase (Pgk1) protein served as a loading control for this and subsequent immunoblots. No Pho8 protein was detected in pho8 mutant cells and only a small amount of Pho8 expressed from its chromosomal locus was observed in vector-transformed wild type cells (Figure 1C, lanes 9 and 1, respectively). Surprisingly, three Pho8 bands were detected in zinc replete cells (Figure 1C, e.g. lane 8). One form of Pho8, designated “p” for precursor, was approximately 71 kDa and comigrated with the unprocessed form that accumulated in pep4 mutant cells (Figure 1C, lane 2). The two other forms, designated mature forms “m1” and “m2” were of approximately 69 and 63 kDa molecular mass, respectively. Treatment of lysates with PNGaseF to remove glycosyl groups prior to immunoblotting indicated that all three forms of Pho8 are glycosylated to similar degrees (data not shown). Separation of soluble and membrane proteins by high speed centrifugation, coupled with immunoblotting and activity assays indicated that both m1 and m2 forms are catalytically active (Supplementary Figure 1). However, while m1 was membrane associated, m2 co-fractionated with soluble proteins. Previous studies had detected only the membrane-bound form (Klionsky and Emr, 1989). We suggest that m2 differs from m1 by a proteolytic cleavage event that occurs on the luminal side of the N-terminal transmembrane domain thereby releasing the protein to diffuse into the lumen of the vacuole. A recent study reported the purification of a soluble active form of Pho8 (Shong, 2006). Peptide sequencing of that purified protein indicated that the soluble form lacked the first 62 amino acids including the transmembrane domain. It was not previously clear if the soluble form was produced in vivo or was an in vitro artifact of proteolysis occurring after cell lysis. Our results indicate that m2 is not an in vitro artifact because it was detected in protein samples prepared by lysis in the presence of trichloroacetic acid, a condition in which protease activity is very unlikely. Release of an active phosphatase from the membrane into the vacuole lumen would likely facilitate its interaction with substrates and enhance the recycling of phosphate. We noted that the levels of p, m1, and m2 were variable between experiments. Growth in late log phase appeared to increase the level of m2 relative to the other forms (W. Qiao, unpublished results).
In evaluating the effect of zinc status on Pho8 protein accumulation, we found that replete cells accumulated high levels of protein while very little was detected in zinc-limited cells (Figure 1C, lanes 3–8). The levels of all three forms of Pho8, i.e. p, m1, and m2, were similarly affected by zinc status. Furthermore, the dose response for protein accumulation correlated well with the effects of zinc status on Pho8 activity shown in Figure 1B.
To assess why Pho8 protein fails to accumulate in zinc-limited cells, we first determined by S1 nuclease protection assay whether zinc deficiency affects PHO8 mRNA levels when expressed from the GAL1 promoter. The CMD1 calmodulin mRNA served as a loading control. As expected, no PHO8 mRNA was detected in a pho8 mutant strain (Figure 1D). In cells expressing PHO8 from the GAL1 promoter, approximately 3-fold lower mRNA accumulation was noted in zinc-limited (LZM + 1 µM ZnCl2) cells relative to replete (LZM + 300 µM ZnCl2) cells (Figure 1D). However, the difference in protein level observed in Figure 1C was ~40-fold. These results suggested that the low Pho8 accumulation in zinc-limited cells is not due solely to the small decrease in mRNA levels that we observed. Therefore, we hypothesized that Pho8 is unstable in zinc-limited cells.
Pho8 is unstable in zinc-limited cells
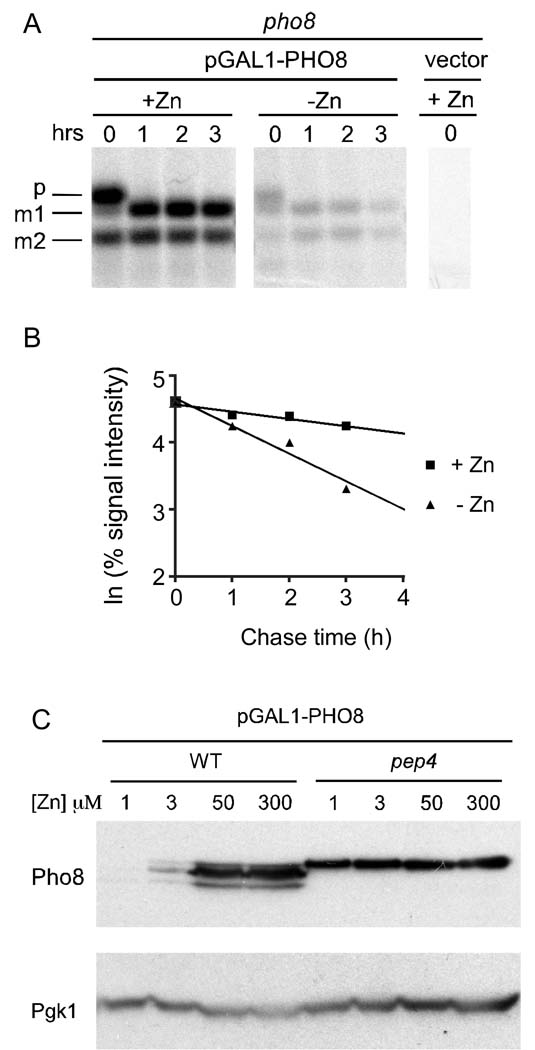
To examine the effects of zinc status on Pho8 protein stability, we used a 35S pulse-chase approach. Zinc-limited and replete cells were pulse-labeled with 35S methionine/cysteine for 12 min and then chased with unlabeled amino acids. Lysates were prepared at various times during the chase period, Pho8 was immunoprecipitated, and the levels of pulse-labeled Pho8 were determined. An example blot is shown in Figure 2A and the signal intensities are plotted in Figure 2B. In both zinc-limited and replete cells, the precursor form was processed to m1 and/or m2 within one hour of pulse labeling. This result is consistent with previous studies that measured the time required for nascent Pho8 to arrive in the vacuole and be processed by Pep4 to be less than 10 min (Klionsky and Emr, 1989). When we assessed the stability of total Pho8 protein, the half-lives in three independent cultures of zinc-replete cells were found to be 6.4, 6.4, and 9.9 hours. The half-lives of total Pho8 in three zinc-limited cultures was measured to be 1.7, 1.7, and 2.2 hours. Thus, Pho8 was degraded at an ~4-fold faster rate in zinc-limited cells than in zinc-replete cells.
Figure 2. Pho8 protein is unstable in zinc-limited cells.
(A) pho8 mutant cells bearing pGAL1-PHO8 were grown in LZM with 1 µM (−Zn) or 300 µM (+Zn) ZnCl2. A 35S pulse-chase experiment was then performed as described in the Materials and Methods. Autoradiography was performed after SDS-PAGE separation of protein samples. The pho8 mutant bearing the vector alone was used as a negative control. (B) Natural log plot of percentage signal intensities (signal intensity at time 0 designated as 100%) over time for the samples in panel A. (C) Immunoblot analysis of lysates from wild type and pep4 mutant cells bearing pGAL1-PHO8 plasmid grown in LZM supplemented with the indicated ZnCl2 concentrations.
Vacuolar proteases are a major system of protein degradation in yeast. To determine whether vacuolar proteases are involved in Pho8 degradation in low zinc, we examined Pho8 accumulation in a pep4 mutant strain that lacks activity of many of these proteases; Pep4 is required for the maturation and activity of several vacuolar proteases. In the pep4 mutant, only the immature form of Pho8 was detected and no decrease in Pho8 protein level was observed in zinc-limited cells (Figure 2C). As discussed previously, Pep4 is known to be required for maturation of Pho8 in zinc-replete cells (Klionsky and Emr, 1989). Our results suggest that degradation of Pho8 in low zinc is also Pep4-dependent. They also suggest that Pho8 degradation in low zinc occurs in the vacuole where Pep4 is active rather than in other compartments of the secretory pathway through which Pho8 passes before arriving in the vacuole. Consistent with this hypothesis, no stabilization of Pho8 in low zinc was observed in mutant strains (hrd2-1, pre1-1 pre2-1) disrupted in proteasome function indicating that the proteasome is not involved in Pho8 turnover (data not shown). Finally, we noted that there was little change in Pho8 accumulation in response to zinc in the pep4 mutant indicating that the small differences in mRNA level observed in Figure 1D likely contribute little to the effect of zinc status on Pho8 accumulation observed in wild type cells.
Instability of Pho8 in low zinc is zinc- and Pho8-specific
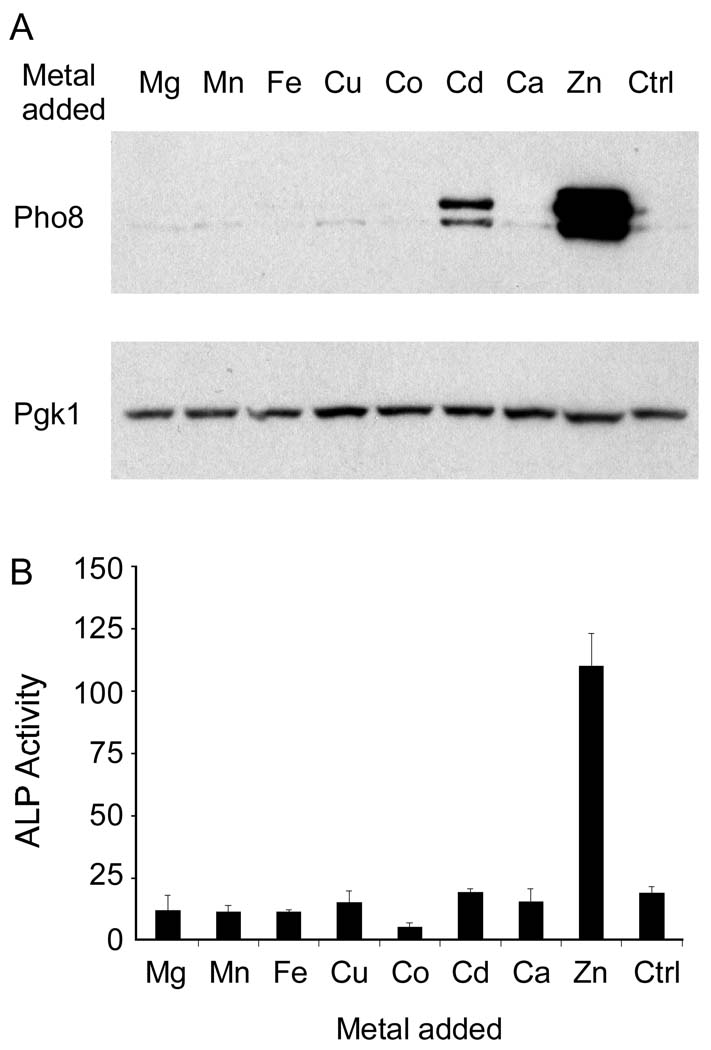
To determine whether the effects of zinc status on Pho8 were zinc specific, we examined the effects of elevated levels of several other metal ions on Pho8 accumulation and activity. Cells were grown in low zinc medium (1 µM Zn) without or with 100 µM concentrations of Mg, Mn, Fe, etc. Only zinc and, to a lesser extent, cadmium increased Pho8 accumulation (Figure 3A). In addition, only Zn increased Pho8 phosphatase activity. Cd had no effect on activity consistent with the specific requirement of alkaline phosphatases for zinc (Figure 3B).
Figure 3. Metal specificity of the effects of zinc on Pho8 accumulation and activity.
(A) Immunoblot analysis of Pho8 in lysates from wild type cells bearing pGAL1-PHO8 plasmid grown in LZM plus 1 µM ZnCl2 supplemented with 100 µM of the indicated metal ions. Metals were provided as MgSO4, MnCl2, FeCl3, CuSO4, CoCl2, CdCl2, CaCl2, and ZnCl2. “Ctrl” denotes the untreated control and Pgk1 was used as a loading control. (B) ALP activity was assayed using cells grown under the same conditions as in panel A.
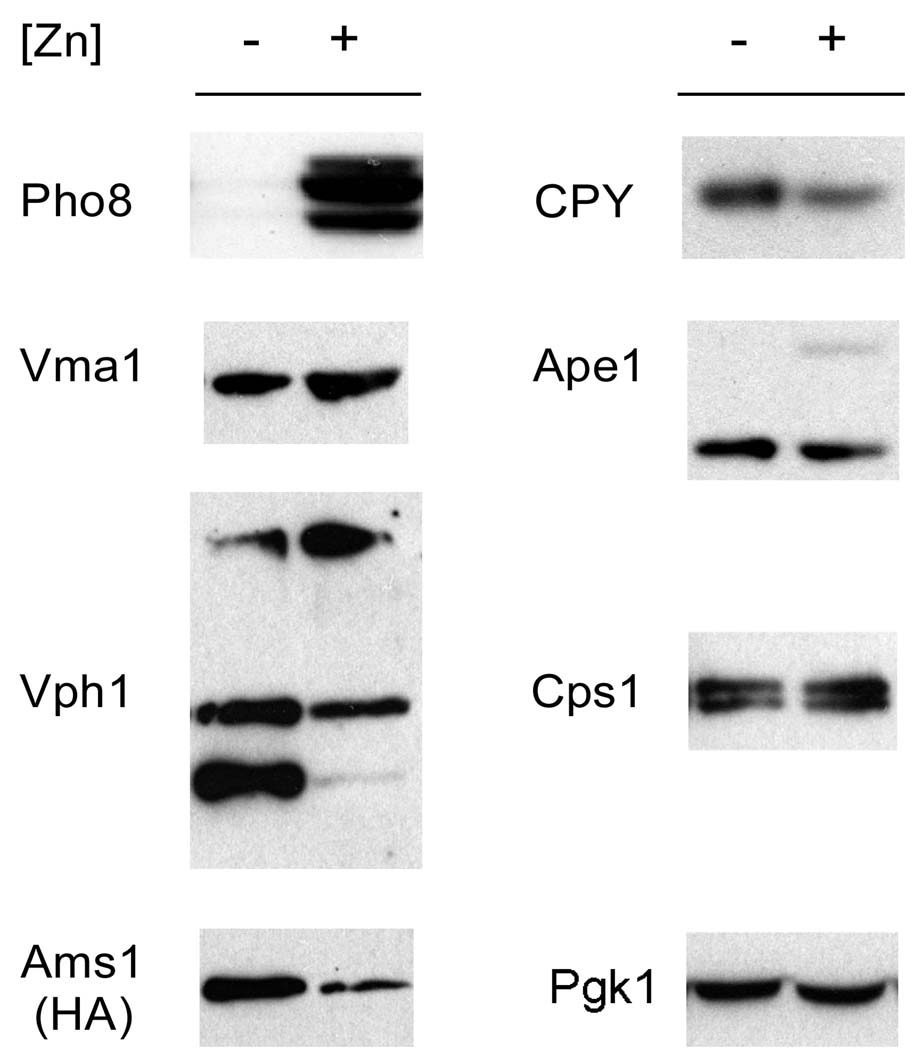
One explanation for the instability of Pho8 is that general remodeling of the vacuole in low zinc results in a decrease in the levels of many vacuolar proteins. Vacuolar autophagic self-degradation may occur under some conditions (Mijaljica et al., 2007). Therefore, to determine whether degradation of Pho8 in low zinc is part of a general phenomenon of vacuolar proteins, we assessed the effects of zinc status on the accumulation of several other vacuolar proteins (Figure 4). This analysis included both membrane (Vma1, Vph1, Ams1) and luminal (CPY, Ape1, Cps1) proteins. Both Ape1 and Cps1 are also zinc-binding proteins. Among these proteins, only Pho8 accumulation was greatly affected by zinc status. These results suggest that Pho8 is selectively targeted for degradation by vacuolar proteases in zinc-limited cells.
Figure 4. Protein instability in zinc-limited cells may be specific to Pho8.
Immunoblot analysis was performed with lysates from wild type cells bearing the pGAL1-PHO8 plasmid grown in LZM supplemented with 1 (−) or 300 (+) µM ZnCl2. Antibodies detecting the indicated vacuolar proteins were used with the exception of Ams1, in which case a plasmid expressing an HA-tagged Ams1 protein from its own promoter was transformed into the strain and an anti-HA antibody was used for the immunoblot. Pgk1 was used as a loading control.
Pho8 degradation in zinc-limited cells is not due to the sensitivity of the apoprotein to proteolysis
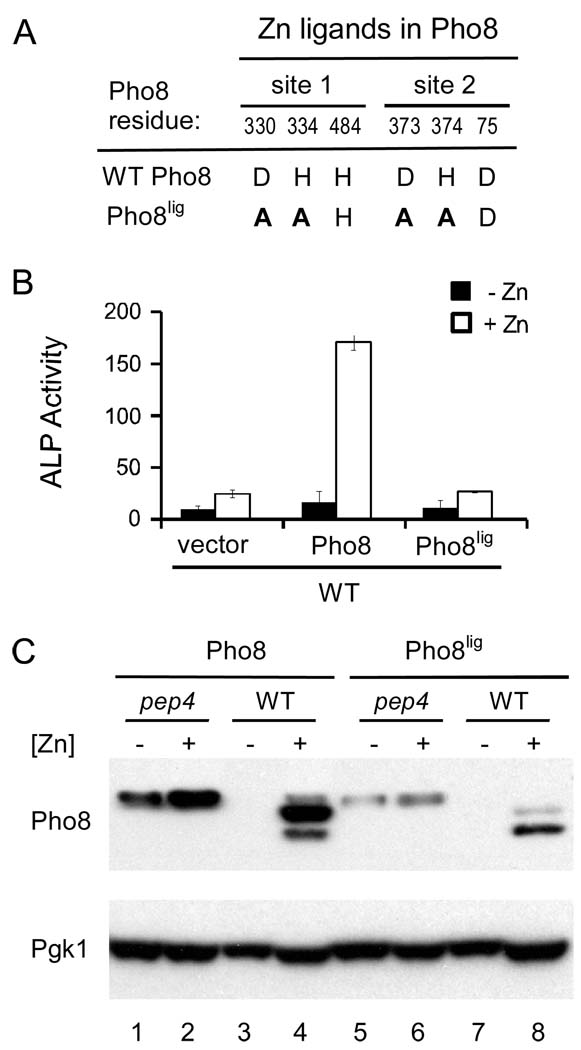
A simple explanation for the degradation of Pho8 in zinc-limited cells is that without zinc bound, the Pho8 apoprotein is more sensitive to proteolysis. To test this hypothesis, we generated a mutant allele of Pho8, designated “Pho8lig”, in which several of the zinc ligand residues were altered such that the protein can no longer bind zinc. Alkaline phosphatases like Pho8 bind two Zn2+ ions. The ligand residues for the two zinc sites are shown in Figure 5A (Murphy et al., 1995) and the changes made in the Pho8lig mutant are also indicated. Specifically, we substituted one histidine ligand and one aspartate ligand with alanines in each of the two zinc sites. As shown in Figure 5B, the Pho8lig zinc ligand mutant was inactive regardless of zinc status indicating the lack of metal cofactor binding. We predicted that if the apoprotein was sensitive to proteolysis, the ligand mutant protein would be unstable under all conditions of zinc status. However, we found that accumulation of the Pho8lig mutant protein was also highly zinc dependent (Figure 5C). Both wild type Pho8 and Pho8lig accumulated in zinc-limited pep4 mutant cells. These results indicate that the Pep4-dependent degradation of Pho8 in low zinc is not simply because the apoprotein is sensitive to degradation. Zinc status can alter Pho8 stability independently of active site zinc binding.
Figure 5. Loss of active site Zn binding is not responsible for Pho8 instability in low Zn.
(A) Mutations in the Pho8 zinc ligand mutant, Pho8lig. The number and identity of Zn2+ ligand residues in wild type Pho8 and Pho8lig are shown. Residues that were mutated in Pho8lig are shown in bold. (B) ALP activity is disrupted in Pho8lig. Wild type cells expressing the indicated proteins were assayed for ALP activity when grown in LZM plus 1 µM (− Zn, filled bars) or 300 µM (+ Zn, open bars) ZnCl2. (C) Immunoblot analysis was performed with lysates from wild type and pep4 mutant cells expressing either wild type Pho8 or Pho8lig and grown in LZM supplemented with 1 (−) or 300 (+) µM ZnCl2.
We also noted that accumulation of the Pho8lig mutant protein was reduced relative to wild type Pho8 (Figure 5C). The reason for this lower accumulation is unknown but clearly not the result of the Pep4-dependent, zinc-responsive mechanism of degradation.
Pho8 activity is primarily dependent on the Zrc1 and Cot1 zinc transporters
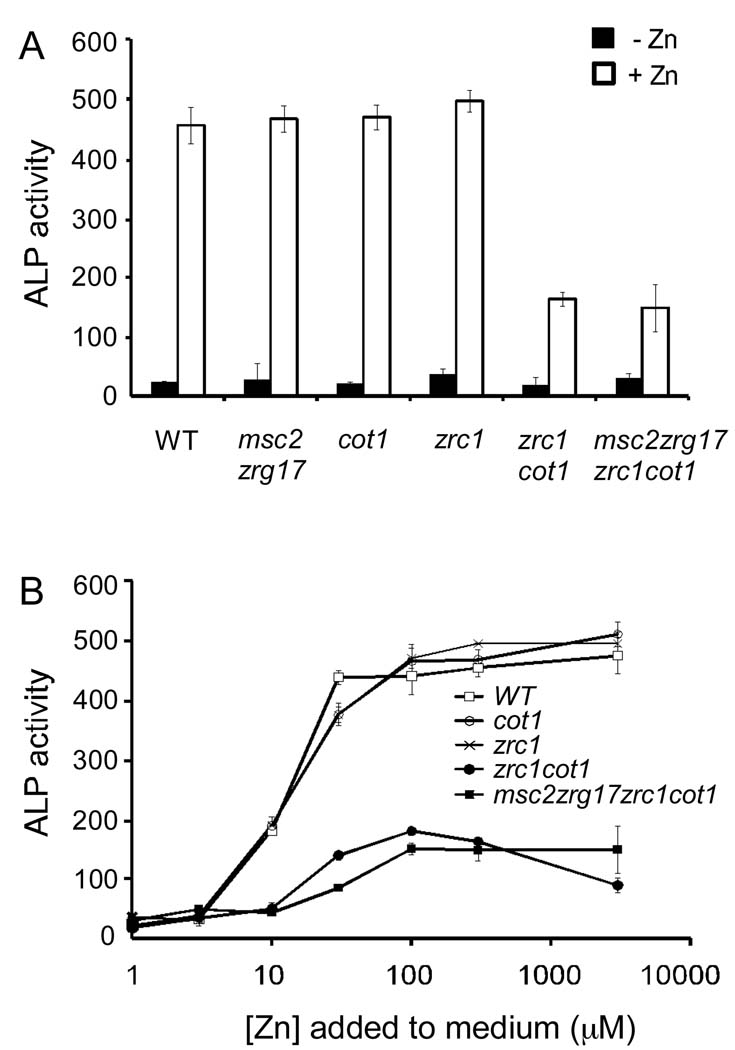
We recently determined that the Msc2/Zrg17 zinc transporter complex, as well as the Zrc1 and Cot1 zinc transporters, deliver zinc to the early compartments of the secretory pathway (Ellis et al., 2004; Ellis et al., 2005). It is in these compartments, i.e. the ER and Golgi, where Pho8 may acquire its zinc for activity. Alternatively, zinc may be delivered to Pho8 after it arrives in the vacuole where Zrc1 and Cot1 play the major roles in zinc transport and the Msc2/Zrg17 complex does not contribute. To determine which zinc transporters are required, we assayed Pho8 activity in wild type cells and various zinc transporter mutants grown in high and low zinc. Mutation of both msc2 and zrg17 did not diminish Pho8 activity in zinc-replete cells indicating that these transporters are not required (Figure 6A). Mutation of zrc1 or cot1 singly did not have a major effect on Pho8 activity either. However, mutation of both zrc1 and cot1 transporters decreased Pho8 activity to ~30% of control values. Inactivation of msc2 and zrg17 in addition to zrc1 and cot1 had no additional impact on Pho8 activity supporting the conclusion that the Msc2/Zrg17 complex is not required. Similar results were obtained when Pho8 activity was assayed over a range of zinc concentrations (Figure 6B). Thus, we conclude that the Zrc1 and Cot1 zinc transporters play redundant roles in providing zinc for Pho8 activity. That some residual Pho8 activity was detected in the msc2 zrg17 zrc1 cot1 mutant indicates that one or more additional pathways for zinc delivery to Pho8 exist in yeast.
Figure 6. Pho8 activity is primarily dependent on Zrc1 and Cot1.
(A) pGAL1-PHO8 was transformed into wild type cells and strains with deletions of genes encoding the indicated transporters. ALP activities were assayed using cells grown in LZM plus 1 µM (−Zn, filled bars) or 300 µM (+Zn, open bars) ZnCl2. (B) Wild type and zinc transporter mutants cells bearing pGAL1-PHO8 were grown overnight in LZM supplemented with the indicated concentrations of zinc and then assayed for ALP activity. Each value is the mean of three replicates. Error bars represent ± 1 S.D.
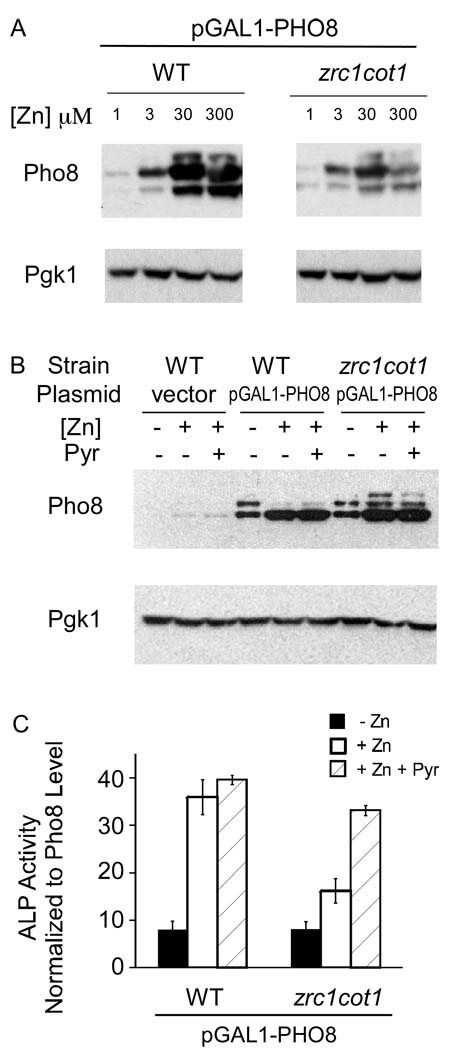
The results shown in Figure 6 suggested that Zrc1 and Cot1 are required for efficient zinc metallation of the Pho8 active site. Alternatively, given our observation that zinc status can alter Pho8 protein levels independently of active site zinc binding, these transporters may play a more indirect role by affecting Pho8 protein stability. To test these hypotheses, we first assessed Pho8 protein accumulation in a zrc1 cot1 mutant grown over a range of zinc (Figure 7A). The maximum level of protein accumulation was lower in the zrc1 cot1 mutant than in wild type cells indicating that Zrc1/Cot1 activity does influence Pho8 protein level. However, the zinc dose response profile was similar to wild type, i.e. Pho8 levels were low in zinc-limited cells and higher in zinc-replete cells. These results indicated that mutation of zrc1 and cot1 does not greatly alter the zinc responsiveness of Pho8 protein accumulation.
Figure 7. Pho8 zinc metallation is impaired in a zrc1 cot1 mutant.
(A) Immunoblot analysis of lysates from wild type and zrc1 cot1 cells bearing pGAL1-PHO8 plasmid and grown in LZM supplemented with the indicated ZnCl2 concentrations. (B) Immunoblot analysis of lysates from wild type or zrc1 cot1 cells bearing either pGAL1-PHO8 plasmid or the vector after overnight induction with different amounts of β-estradiol to equalize expression levels. The low zinc medium for both wild type and zrc1 cot1 strains contained 3 µM zinc and 1 µM β-estradiol. For zinc-replete conditions (300 µM zinc), the wild type cells were induced with 0.01 µM β-estradiol and the zrc1 cot1 cells were induced with 1 µM β-estradiol. (C) Wild type and zrc1 cot1 cells bearing pGAL1-PHO8 were grown as described for panel B and then assayed for ALP activity. The activity values were then normalized to Pho8 (m1 and m2) protein levels quantified from panel B. Zinc-replete cells were also treated with 50 µM pyrithione, a zinc ionophore, for 20 minutes prior to harvesting (+Zn +Pyr, hatched bars). Each value is the mean of three replicates and the error bars represent ± 1 S.D.
To assess whether Zrc1 and Cot1 are required specifically for delivering zinc to the active site of Pho8, we adjusted the levels of the β-estradiol inducer in the medium to generate similar levels of protein accumulation in high and low zinc and in wild type vs. zrc1 cot1 mutants (Figure 7B). Pho8 activity was then measured and normalized to the level of Pho8 (m1 and m2) protein (Figure 7C). Despite relatively high levels of protein accumulation in zinc-limited wild type cells, Pho8 activity remained very low. This result indicated that zinc supplements are required for Pho8 activity even when the protein degradation mechanism is overridden. Pho8 activity was also low in zinc-limited zrc1 cot1 mutant cells again despite relatively high protein accumulation. In zinc-replete cells, Pho8 activity was greatly reduced in the zrc1 cot1 mutant indicating a defect in Pho8 metallation. Consistent with this hypothesis, when zinc-replete zrc1 cot1 cells were treated with zinc plus pyrithione, a zinc ionophore that can move zinc into cells and organelles without the need for transporters, Pho8 activity in the mutant increased to near wild type cells. These results indicate that Zrc1 and Cot1 are required to deliver zinc to Pho8 for active site metallation. Also consistent with this hypothesis, we found that expression of a heterologous zinc transporter, the mouse ZnT-4 protein (Huang and Gitschier, 1997), in zrc1 cot1 mutant cells partially suppressed the defect in Pho8 activity (data not shown).
The data in Figure 7 indicated that zinc metallation of Pho8 specifically requires the activity of Zrc1 and Cot1. What is the mechanism underlying this specific transporter dependence? One possibility is that certain transporters pass zinc directly to Pho8 at some point in the secretory pathway via specific protein-protein interactions reminiscent of metallochaperone-mediated copper trafficking (O'Halloran and Culotta, 2000). The ability of Zn-pyrithione and ZnT-4 expression to restore Pho8 activity in the zrc1 cot1 mutant strongly argues against this model. A second hypothesis is that metallation is, for some reason, restricted to the vacuole where Zrc1 and Cot1 play the major transport role and Msc2/Zrg17 is not active. One way to address this hypothesis is to trap Pho8 in the early secretory pathway and test whether the protein can be metallated in that compartment. Unfortunately, attempts to trap Pho8 in the ER/Golgi using an ER-targeting sequence (HDEL) were unsuccessful because the trapped protein accumulated only to very low levels. In addition, temperature-sensitive mutations blocking ER-to-Golgi trafficking (i.e. sec12ts, sec23ts) or Golgi-to-endosome trafficking (vps41ts) did not sufficiently block delivery of Pho8 to the vacuole to allow us to perform this experiment. Therefore, we used other approaches to address different hypotheses that could explain the specific dependence of Pho8 metallation on Zrc1 and Cot1 activity.
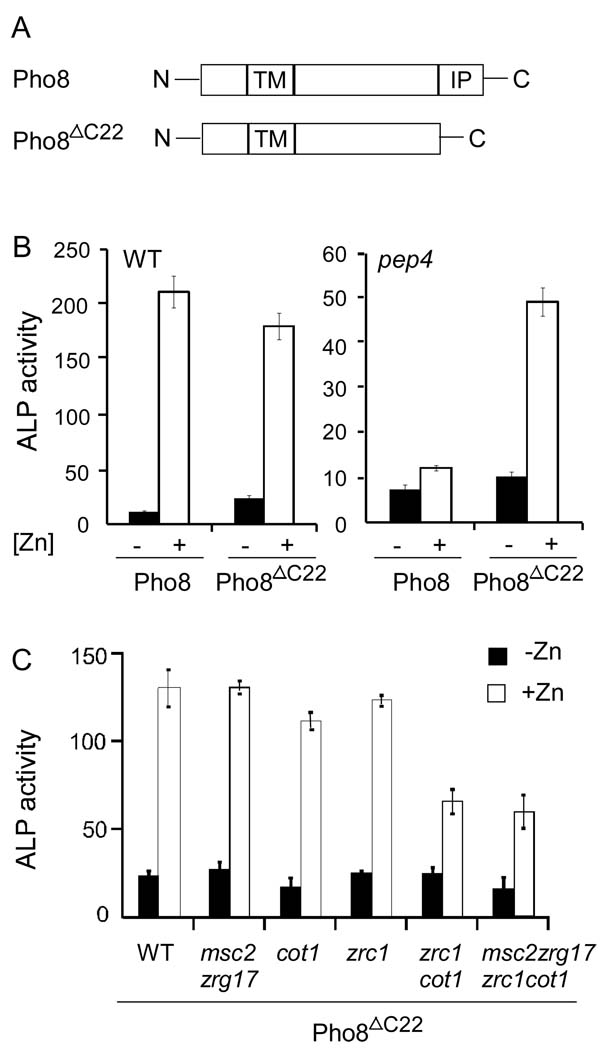
First, we hypothesized that the carboxy-terminal inhibitory propeptide of Pho8 prevented metal binding until the protein’s arrival in the vacuole and the removal of the propeptide by Pep4. If this were the case, we predicted that a truncated form of Pho8 in which the propeptide was removed would now be able to obtain zinc from the Msc2/Zrg17 complex in the ER. We generated a truncated form of Pho8, designated “Pho8ΔC22 “ in which the C-terminal 22 amino acids were removed (Figure 8A). As shown in Figure 8B, the Pho8ΔC22 protein displayed wild-type phosphatase activity that was also similarly zinc dependent. In contrast to wild-type Pho8, however, Pho8ΔC22 was no longer dependent on Pep4 processing for its activity as expected. When assayed for phosphatase activity in response to zinc in the various zinc transporter mutant strains, we found that Pho8ΔC22 showed a similar specific requirement for Zrc1 and Cot1 as the wild type protein (Figure 8C). Therefore, we concluded that the propeptide does not prevent metallation by zinc transported by Msc2/Zrg17 in the ER and dictate Zrc1- and Cot1-dependence.
Figure 8. Pho8 ΔC22 activity remains dependent on Zrc1 and Cot1, and not on Msc2/Zrg17.
(A) Diagram of Pho8 and Pho8ΔC22.TM, transmembrane domain; IP, inhibitory propeptide. (B) Pho8ΔC22 and full-length Pho8 were expressed in the pep4 mutant or wild type cells. ALP activities were measured for these transformants grown in low (1 µM ZnCl2, filled bars) or high (300 µM ZnCl2, open bars) zinc media. (C) Wild type and yeast strains with deletions of genes encoding the indicated transporters were transformed with pPHO8-ΔC22. ALP activities were measured for these transformants grown in low (1 µM ZnCl2, filled bars) or high (300 µM ZnCl2, open bars) zinc media. Each value is the mean of three replicates and the error bars represent ± 1 S.D.
An alternative hypothesis is that the vacuolar environment is better suited for Pho8 metallation than are upstream compartments of the secretory pathway. This model predicted that if we targeted Pho8 to another compartment, it would be poorly metallated when compared to vacuole-localized Pho8. As noted above, trapping of Pho8 in the early secretory pathway was unsuccessful. As an alternative approach, we targeted Pho8 to the cytosol. By removing the amino-terminal transmembrane domain, Pho8 accumulates in the cytosol (Klionsky, 2007; Noda et al., 1995). This approach has been used frequently as a marker for autophagy, i.e. the uptake and degradation in the vacuole of cytosolic constituents and organelles in response to stresses such as nitrogen deficiency (Nair and Klionsky, 2005). We reasoned that we could adapt this system to examine the activity of cytosolic Pho8 as an indicator of zinc metallation. Cytosolic magnesium levels are high (Gunther, 2006) so we did not anticipate that magnesium binding by Pho8 in the cytosol would be disrupted.
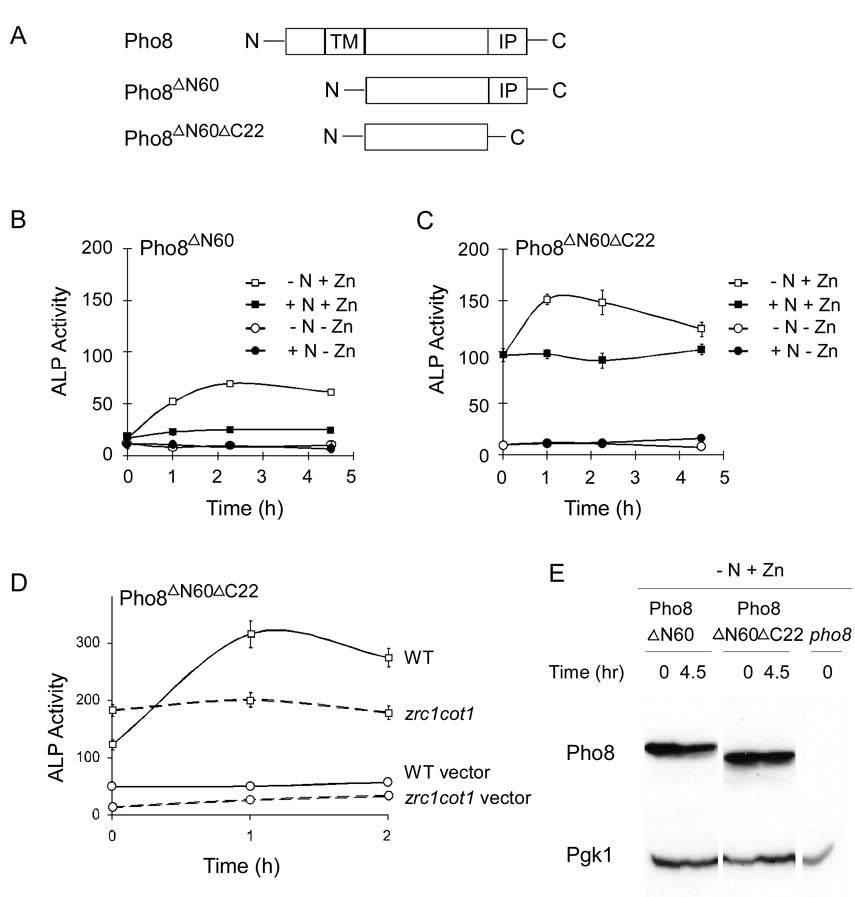
The cytosolic form of Pho8, designated “Pho8ΔN60“, used in previous studies of autophagy has the N-terminal 60 amino acids deleted (Figure 9A). As shown in Figure 9B, cytosolic Pho8ΔN60 showed little activity in zinc-limited cells regardless of nitrogen status. In zinc-replete cells, there was an increase in activity in cells undergoing nitrogen withdrawal relative to nitrogen-replete control cells. This increase in activity is due to the induction of autophagy, uptake of Pho8 ΔN60 in the vacuole, and processing of the protein by Pep4 for activity (Klionsky, 2007; Noda et al., 1995).
Figure 9. Vacuole localization is required for efficient Pho8 metallation.
(A) Diagram of full-length Pho8, Pho8ΔN60 and Pho8ΔN60ΔC22.TM, transmembrane domain; IP, inhibitory propeptide. (B) Pho8ΔN60 and (C) Pho8ΔN60ΔC22 were expressed in pho8 mutant (BY4743 pho8) cells grown in low or high zinc (1 or 100 µM ZnCl2, respectively) and high nitrogen conditions (+N) or were exposed to nitrogen withdrawal (−N) by transferring cells from high nitrogen to low nitrogen media to induce autophagy. ALP activities were measured at the indicated times. (D) Wild type (solid lines) or zrc1 cot1 mutant (dashed lines) cells expressing either Pho8ΔN60ΔC22 (squares) or the vector (circles) were grown in high zinc (100 µM ZnCl2). Samples were taken at the indicated times after transfer to low nitrogen medium. For panels B-D, each value is the mean of three replicates. Error bars represent ± 1 S.D. (E) Immunoblot of Pho8 protein levels in pho8 cells expressing Pho8 variants as described in panels B and C.
To use this system to compare cytosolic vs. vacuolar activities in response to zinc, we also removed the C-terminal propeptide, generating “Pho8ΔN60ΔC22“, to allow for enzymatic activity in the cytosol (Figure 9A). As expected, little activity was detected in zinc-limited cells (Figure 9C). In zinc- and nitrogen-replete cells, a significant level of activity was detected for Pho8ΔN60ΔC22 indicating that some fraction of the cytosolic protein pool was metallated. Induction of autophagy by nitrogen withdrawal increased activity of Pho8ΔN60ΔC22 by ~50%. This increase in Pho8ΔN60ΔC22 phosphatase activity was likely the result of zinc metallation in the vacuole. This was indicated by the observation that mutation of zrc1 and cot1 prevented the increased activity triggered by nitrogen withdrawal (Figure 9D).
The activity detected for cytosolic Pho8ΔN60ΔC22 protein in zinc- and nitrogen-replete cells (Figure 9C) could be interpreted as efficient metallation of the protein in the cytosol relative to the vacuole. We believe that this is not the case. Triggering autophagy increased activity of the Pho8ΔN60ΔC22 by ~50% yet the fraction of the protein taken up into the vacuole after 4.5 hours of nitrogen withdrawal is less than 10% of the total. This estimate is based on the still undetectable level of processed Pho8ΔN60 (which would co-migrate with Pho8ΔN60ΔC22) observed by immunoblotting (Figure 9E). Thus, the small amount of protein taken up into the vacuole by autophagy appears to be much more effectively metallated than the cytosolic pool of the protein. These results lead us to suggest that the vacuolar environment is optimal for zinc binding by Pho8, at least relative to the cytosol.
DISCUSSION
The goal of this study was to investigate the relationship between zinc status and Pho8 alkaline phosphatase activity in yeast. Many alkaline phosphatases require zinc for their function because two Zn2+ ions are bound in the active site of these enzymes and play co-catalytic roles in the hydrolysis of phosphate from phosphorylated substrates (Coleman, 1992). Here, we have shown that the yeast Pho8 alkaline phosphatase is remarkably zinc dependent in vivo and is inactive in zinc-limited cells. Moreover, we found that Pho8 protein accumulation is also very zinc dependent; Pho8 abundance is very low in zinc-limited cells and rises in zinc-replete cells. We determined that this effect is due, at least in part, to a higher rate of degradation in zinc-limited cells and that this degradation occurs through the action of vacuolar proteases. Intriguingly, our results show that the Pep4 vacuolar protease plays both positive and negative roles in influencing Pho8 activity; Pep4 is required for Pho8 maturation and activity in zinc-replete cells and Pho8 degradation in zinc-limited cells.
The simplest model to explain the instability of yeast Pho8 in low zinc was that when binding of Zn2+ ions in the active site fails to occur, the protein is only partially folded and is therefore susceptible to proteolytic degradation. We were surprised to find, however, that the zinc-binding residues in the active site were not required for the increased stability of Pho8 in high zinc. This result clearly indicated that the simple hypothesis of apoprotein instability was incorrect. There are many examples of metalloproteins that are less stable in vivo in the absence of bound metal cofactor (Grandoni et al., 1989; Li and Merchant, 1995; Mettert and Kiley, 2005). Pho8 is a rare example of a protein whose stability is altered by its cofactor independently of active site metal binding.
Why then is Pho8 stability sensitive to zinc status? An alternative explanation was that some aspect of Pho8 processing en route to the vacuole is disrupted under low zinc conditions thereby rendering the protein more sensitive to proteolysis upon its arrival in that compartment. We showed previously that zinc is required for ER function. Therefore, defects in Pho8 protein folding or modification in the early secretory pathway may occur under low zinc conditions. To test whether Pho8 protein folding was disrupted in low zinc conditions, we expressed Pho8ΔC22, i.e. the form lacking the inhibitory propeptide, in a pep4 mutant allowing the protein to accumulate in zinc-limited cells. Our reasoning was that, if the protein was folded incorrectly in low zinc, supplying the metal to zinc-limited cells expressing Pho8ΔC22 would not increase Pho8 activity. However, when pep4 mutant cells expressing Pho8ΔC22 were transferred from low to high zinc media, Pho8 activity rapidly increased to wild type levels (W. Qiao, unpublished results). These results argued that Pho8 folding is not grossly disrupted in zinc-limited cells. We also considered whether glycosylation of Pho8 was disrupted in zinc-limited cells. Pho8 contains two glycosylation sites in its luminal catalytic domain. However, we note that the degree of glycosylation of Pho8 in a pep4 mutant was unaffected by zinc status (Figure 2C). Finally, Pho8 forms dimers and this association may be inhibited in some way by low zinc conditions. To test whether low zinc disrupted dimerization of Pho8, we used a protein crosslinking/immunoblot assay. Using ethylene glycol bis[succinimidylsuccinate] (EGS) as a crosslinker, we found that the level of crosslinked Pho8 dimers was reduced by 20–30% in zinc-limited cells (W. Qiao, unpublished results). Thus, less efficient dimerization may contribute to the instability of Pho8 in low zinc although we predict that this small effect is likely to be a minor factor.
Another explanation for the sensitivity of Pho8 to degradation in low zinc is that there is a regulatory mechanism that senses zinc and specifically targets Pho8 for degradation. If so, what might be the purpose of this regulation? One possibility is that Pho8 is degraded in zinc-limited cells to make the zinc bound by this protein available for other purposes. Pho8 activity is not essential to cell viability so loss of its activity by protein degradation under low zinc conditions is not detrimental to cell growth. In phosphate-replete cells, the level of Pho8 was determined to be about 3,000 molecules per cell (Ghaemmaghami et al., 2003).
Phosphate limitation induces expression of the PHO8 promoter as much as 10-fold generating perhaps ~30,000 molecules of Pho8 protein per cell (Munsterkotter et al., 2000). With two Zn2+ ions per molecule, 60,000 zinc ions could then be made available following Pho8 degradation for use by other zinc-dependent proteins. This amount represents as much as 5% of the total zinc found in a severely zinc-limited cell (Gitan et al., 1998) and is therefore not an insignificant quantity. If other nonessential zinc proteins are similarly degraded in low zinc, considerable amounts of this nutrient could be redistributed within cells for optimal metabolism under this condition of nutrient stress.
If Pho8 degradation under zinc-limiting conditions is the result of a specific regulatory mechanism, how is the zinc status of the cell sensed and how is that signal translated into altered rates of Pho8 degradation? We have little information regarding these questions at this time. We had previously shown that the genes encoding the Pep4, Prb1, and Prc1 vacuolar proteases are up-regulated in zinc-limited cells and are likely targets of the Zap1 transcription factor (Lyons et al., 2000; Wu et al., 2008). Zap1 induces expression of ~80 genes in zinc-limited cells. Based on these observations, one possible mechanism controlling Pho8 stability is via the expression level of these proteases; increased levels of the vacuolar proteases in zinc-limited cells may cause a higher rate of degradation. This model was found to be unlikely because Pho8 stability was controlled normally in response to zinc status in a zap1 mutant strain (data not shown). Thus, Zap1 is not a component of the zinc-sensing mechanism controlling Pho8 stability. Also, while the specific pool of zinc that controls Pho8 accumulation is unknown, we can exclude the vacuolar zinc pool. Mutation of the ZRC1 and COT1 genes greatly reduce vacuolar zinc levels (MacDiarmid et al., 2000; Simm et al., 2007) yet regulation of Pho8 accumulation in that mutant strain is similar in dose response to that observed in wild type cells (Figure 7A). Thus, the pool of zinc regulating Pho8 accumulation is in the cytosol or some other non-vacuolar compartment.
Finally, we found that metallation of Pho8 was primarily due to zinc transport by the Zrc1 and Cot1 vacuolar zinc transporters. This was an unexpected result because these transporters are well known for their roles in metal detoxification (Eide, 2006). Moreover, the Msc2/Zrg17 transporter complex was previously shown to play the major role in delivering zinc to the early secretory pathway of yeast (Ellis et al., 2004). The Znt-5, ZnT-6, and ZnT-7 transporters, the vertebrate orthologs of Msc2 and Zrg17, are responsible for metallation of alkaline phosphatase in the cells of higher eukaryotes (Ishihara et al., 2006; Suzuki et al., 2005a; Suzuki et al., 2005b). Why is metallation of Pho8 specific to Zrc1 and Cot1 activity? There are several possible mechanisms. First, the specific requirement for Zrc1 and Cot1 may be due to direct transfer of the metal from these transporters to Pho8 by protein-protein interaction. Our results argue strongly against this hypothesis. Second, the Zrc1/Cot1 dependence may be due to the inhibitory propeptide of Pho8 preventing metallation from occurring prior to its removal in the vacuole. Our data indicate that this is not the case either. However, our experiments do not eliminate the possibility that another protein blocks metallation in the early secretory pathway.
Based on the apparent poor degree of metallation of Pho8 that occurs in the cytosol, we propose that the vacuole environment is more conducive to zinc binding than early compartments of the secretory pathway. This would explain the Zrc1/Cot1 transporter specificity because the Msc2/Zrg17 complex is not present in the vacuole. One possibility is that the zinc levels are higher in the vacuole than in other compartments and this high zinc is required for Pho8 metallation. Alternatively, some other factor may be influencing zinc binding. For example, the vacuolar pH may play an important role. The pH of the vacuole has been measured to be approximately 5.5 (Nelson et al., 2000). In vitro Pho8 reconstitution experiments indicated that this pH was optimal for metallation and the higher pH’s found in the early secretory pathway resulted in lower activity (W. Qiao, unpublished results). Thus, the acidity of the vacuole may serve as a switch to allow zinc binding when the protein arrives in that compartment. Future studies will be required to address these intriguing hypotheses.
EXPERIMENTAL PROCEDURES
Yeast strains and growth conditions
Yeast strains used were CM100 (MATαcan1-100 his3-11,15 leu2-3,112 trp1-1 ura3-52), WQY11 (CM100 pep4∷KanMX), WQY12 (CM100 pho8∷KanMX), CM102 (CM100 zrc1∷HIS3), CM103 (CM100 cot1∷URA3), CM137 (CM100 zrc1∷HIS3 cot1∷URA3), JSY12 (CM100 27 msc2∷KanMX zrg17∷NatMX), and JSY6 (CM100 msc2∷KanMX zrg17∷NatMX zrc1∷HIS3 cot1∷URA3) (MacDiarmid et al., 2000). WQY11 and WQY12 were constructed by amplifying deletion cassettes from BY4743 pep4∷KanMX and BY4743 pho8∷KanMX strains (Winzeler et al., 1999) by polymerase chain reaction using primers that included 450–750 bp of the promoter and terminator regions of the respective gene. These PCR products were then transformed into CM100 and transformants were selected on media supplemented with G418. Successful gene deletions were confirmed by PCR. Cells were grown in either YPD or in synthetic defined medium with 2% glucose and any necessary auxotrophic supplements. Cells were also cultured in low zinc medium (LZM) prepared as described by Gitan et al. (Gitan et al., 1998). LZM is zinc limiting because it contains 1 mM EDTA and 20 mM citrate as metal buffering agents to control zinc availability. For the nitrogen withdrawal experiments, cells were cultured first in normal, nitrogen-replete LZM. The cells were harvested at an OD600 of ~0.5 (~1 × 107 cells/ml) and then washed twice with low nitrogen LZM (i.e. LZM prepared without ammonium sulfate). The cells were then resuspended in LZM or low nitrogen LZM with either 3 µM or 100 µM ZnCl2. Cells were collected at various times and ALP activities were measured.
Plasmids
Plasmid pSN257 containing PHO8 under the control of the GAL1 promoter was obtained from Dr. Steve Nothwehr (University of Missouri-Columbia). Plasmid pSN257L was constructed by marker swapping the URA3 marker of pSN257 with the LEU2 gene from pUL9 (Cross, 1997). Similarly, pRS316-GAL1 (Liu et al., 1992) was marker swapped with the LEU2 gene to generate pRS316-GAL1-LEU2. Plasmid pPHO8-ΔC22 was constructed by inserting the PHO8 open reading frame lacking the C-terminal 22 residues into pRS316-GAL1-LEU2 by homologous recombination. Plasmids pPHO8-ΔN60 and pPHO8-ΔN60ΔC22 were constructed in a similar fashion. Plasmid pPHO8-lig, in which four zinc-ligand residues are mutated, was constructed by overlap PCR (Ho et al., 1989). All plasmid constructs were confirmed by DNA sequencing. Expression from the GAL1 promoter was obtained using the GEV system (Gao and Pinkham, 2000). For induction using the GEV system, the pGEV-TRP1 plasmid was co-transformed with the GAL1 promoter plasmids. pGEV-TRP1 encodes a hybrid activator protein that contains the Gal4 DNA binding domain, the VP16 activation domain, and the human estrogen receptor steroid response domain. β-estradiol was added to a concentration of 1 µM, unless indicated otherwise, to activate GEV and allow expression of genes under the control of the GAL1 promoter.
Alkaline phosphatase activity assays
Cells were grown overnight and harvested at an OD600 of ~0.5. Harvested cells were chilled on ice and kept on ice throughout processing. The cells were washed in distilled deionized H2O and resuspended in ALP assay buffer (250 mM Tris pH 8.8, 10 mM MgSO4, 50 µM EDTA). The EDTA was included in ALP assay buffer to prevent potential metallation of apo-alkaline phosphatase during cell permeabilization. Control experiments indicated that this level of EDTA was sufficient to prevent metallation of Pho8 in vitro without inhibiting activity of protein that had been metallated in vivo. To 500 µl of each cell suspension, 25 µl 0.1% SDS and 25 µl CHCl3 were added and the cells were permeabilized by vortexing vigorously for 20 seconds. Five µl of 10 mM p-nitrophenol phosphate (PNPP) was added to 50 µl of permeabilized cells to start the assay and the reactions were incubated at room temperature. Reactions were stopped by adding 10 µl 2.4 M NaOH to each reaction. Units are calculated as 100 × ΔA420 / volume of cells assayed (in ml) / reaction time (in minutes) / OD600 of the culture. Cell-free reactions were included as blanks and all assays were performed in triplicate.
Protein extracts and immunoblotting
Protein lysates were prepared by vortexing the cells with glass beads in TCA lysis solution (10% trichloroacetic acid, 10 mM Tris-Cl, 25 mM ammonium acetate, 1 mM EDTA pH 8.0) containing protease inhibitors [1 mM phenylmethylsulfonyl fluoride, 50 µg/ml leupeptin, 10 µg/ml pepstatin, 1 mM EDTA and Complete EDTA-free Protease Inhibitor cocktail (Roche Applied Science)]. The cell suspensions were vortexed 10 × 30 s with 30 s on ice between pulses. Glass beads were removed by carefully transferring the lysate to a new microfuge tube using a micropipette. The lysates were then centrifuged at 13,000 × g for 10 min and the pellets were resuspended for immunoblotting. Antibodies purchased from Molecular Probes were anti-Pgk1, anti-Pho8, anti-Cpy, anti-Vph1, and anti-Vma1. Anti-HA was obtained from Roche and anti-Cps1 and anti-Ape1 were gifts of S. Emr (Cornell University) and D. Klionsky (University of Michigan), respectively.
To fractionate membrane-bound and soluble proteins, cells were collected, washed with distilled deionized H2O, resuspended in MIB buffer (0.6 M mannitol, 20 mM HEPES-KOH, pH 7.4) with protease inhibitors, and the cells were disrupted by vortexing with glass beads. Total protein extracts were collected after low centrifugation (500 × g for 2 min) to remove cell debris. The supernatants were centrifuged at 100,000 × g for 1 hr to separate membrane fractions from soluble fractions.
Metabolic labeling and immunoprecipitation
[35S] methionine- and [35S] cysteine-labeling of yeast cultures, cell lysis, and immunoprecipitation of Pho8 was done as described previously (Nothwehr et al., 1996). In brief, cells were grown in LZM supplemented with 1 µM ZnCl2 (low zinc) or with 300 µM ZnCl2 (high zinc) lacking methionine and cysteine. Cells were grown to mid-log phase (OD600 ~0.5), centrifuged at 1000 × g for 5 min and resuspended in culture medium at an OD600 = 1.0 for zinc-limited cells and 0.5 for zinc-replete cells. [35S] methionine and [35S] cysteine (EXPRE35S35S Protein Labeling Mix, PerkinElmer) were then added to a final isotope concentration of 140 μCi and the cells were incubated for 12 min at 30°C. After this pulse, unlabeled methionine and cysteine were added to final concentrations of 0.5 mg/ml. Samples of 500 µl were removed at the indicated times for immunoprecipitation, SDS-PAGE separation, and autoradiography. Signal intensities were quantified using OPTIQUANT software.
S1 nuclease protection assay
S1 nuclease protection assays were performed with total RNA as described previously (Dohrmann et al., 1992). In each assay sample, 15 µg of total RNA was hybridized to 32P end-labeled oligonucleotide DNA probes specific to PHO8 or CMD1. The samples were then digested with S1 nuclease and separated on a 10% polyacrylamide gel containing 5 M urea. Signal intensities were measured by phosphorimaging analysis (PerkinElmer Life Sciences).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grant GM56285. We thank Steve Nothwehr and Hande Odaman for supplying the Pho8 plasmid and providing helpful advice. We thank Dan Klionsky and Scott Emr for providing antibodies and Liping Huang for supplying plasmids. We also thank Rick Eisenstein, Joel Walker, Avery Frey, and Yi-Hsuan Wu for critical reading of the manuscript.
ABBREVIATIONS
- ALP
alkaline phosphatase
- CDF
cation diffusion facilitator
- EDTA
ethylenediaminetetraacetic acid
- ER
endoplasmic reticulum
- GEV
Gal4-estrogen response domain-VP16 activation domain hybrid activator
- LZM
low zinc medium
REFERENCES
- Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE. Structure and mechanism of alkaline phosphatase. Ann Rev Biophys Biomol Struct. 1992;21:441–483. doi: 10.1146/annurev.bb.21.060192.002301. [DOI] [PubMed] [Google Scholar]
- Cross FR. 'Marker swap' plasmids: convenient tools for budding yeast molecular genetics. Yeast. 1997;13:647–653. doi: 10.1002/(SICI)1097-0061(19970615)13:7<647::AID-YEA115>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- de Backer M, McSweeney S, Rasmussen HB, Riise BW, Lindley P, Hough E. The 1.9 A crystal structure of heat-labile shrimp alkaline phosphatase. J Mol Biol. 2002;318:1265–1274. doi: 10.1016/s0022-2836(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 5.Dohrmann PR, Butler G, Tamai K, Dorland S, Greene JR, Thiele DJ, Stillman DJ. Parallel pathways of gene regulation: homologous regulators SWI5 and ACE2 differentially control transcription of HO and chitinase. Genes Dev. 1992;6:93–104. doi: 10.1101/gad.6.1.93. [DOI] [PubMed] [Google Scholar]
- Donella-Deana A, Ostojic S, Pinna LA, Barbaric S. Specific dephosphorylation of phosphopeptides by the yeast alkaline phosphatase encoded by PHO8 gene. Biochim Biophys Acta. 1993;1177:221–228. doi: 10.1016/0167-4889(93)90044-p. [DOI] [PubMed] [Google Scholar]
- Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Ellis CD, Wang F, MacDiarmid CW, Clark S, Lyons T, Eide DJ. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J Cell Biol. 2004;166:325–335. doi: 10.1083/jcb.200401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CD, Macdiarmid CW, Eide DJ. Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J Biol Chem. 2005;280:28811–28818. doi: 10.1074/jbc.M505500200. [DOI] [PubMed] [Google Scholar]
- Gaither LA, Eide DJ. Eukaryotic zinc transporters and their regulation. Biometals. 2001;14:251–270. doi: 10.1023/a:1012988914300. [DOI] [PubMed] [Google Scholar]
- Gao CY, Pinkham JL. Tightly regulated, beta-estradiol dose-dependent expression system for yeast. Biotechniques. 2000;29:1226–1231. doi: 10.2144/00296st02. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Gitan RS, Luo H, Rodgers J, Broderius M, Eide D. Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J. Biol. Chem. 1998;273:28617–28624. doi: 10.1074/jbc.273.44.28617. [DOI] [PubMed] [Google Scholar]
- Grandoni JA, Switzer RL, Makaroff CA, Zalkin H. Evidence that the iron-sulfur cluster of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase determines stability of the enzyme to degradation in vivo. J Biol Chem. 1989;264:6058–6064. [PubMed] [Google Scholar]
- Gunther T. Concentration, compartmentation and metabolic function of intracellular free Mg2+ Magnes Res. 2006;19:225–236. [PubMed] [Google Scholar]
- Guo L, Groenendyk J, Papp S, Dabrowska M, Knoblach B, Kay C, Parker JM, Opas M, Michalak M. Identification of an N-domain histidine essential for chaperone function in calreticulin. J Biol Chem. 2003;278:50645–50653. doi: 10.1074/jbc.M309497200. [DOI] [PubMed] [Google Scholar]
- Guy JL, Lambert DW, Warner FJ, Hooper NM, Turner AJ. Membrane-associated zinc peptidase families: comparing ACE and ACE2. Biochim Biophys Acta. 2005;1751:2–8. doi: 10.1016/j.bbapap.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland R, Larsen RL, Asgeirsson B. The 1.4 A crystal structure of the large and cold-active Vibrio sp. alkaline phosphatase. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbapap.2008.09.020. in press. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat. Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Yamazaki T, Ishida Y, Suzuki T, Oda K, Nagao M, Yamaguchi-Iwai Y, Kambe T. Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J Biol Chem. 2006;281:17743–17750. doi: 10.1074/jbc.M602470200. [DOI] [PubMed] [Google Scholar]
- Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamizono A, Nishizawa M, Teranishi Y, Murata K, Kimura A. Identification of a gene conferring resistance to zinc and cadmium ions in the yeast Saccharomyces cerevisiae. Mol. Gen. Genet. 1989;219:161–167. doi: 10.1007/BF00261172. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Toh-e A, Oshima Y. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol Cell Biol. 1982;2:127–137. doi: 10.1128/mcb.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Toh-e A, Banno I, Oshima Y. Molecular characterization of a specific p-nitrophenylphosphatase gene, PHO13, and its mapping by chromosome fragmentation in Saccharomyces cerevisiae. Mol Gen Genet. 1989;220:133–139. doi: 10.1007/BF00260867. [DOI] [PubMed] [Google Scholar]
- Kim EE, Wyckoff HW. Structure of alkaline phosphatases. Clin Chim Acta. 1990;186:175–187. doi: 10.1016/0009-8981(90)90035-q. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. Embo J. 1989;8:2241–2250. doi: 10.1002/j.1460-2075.1989.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ. Monitoring autophagy in yeast: the Pho8Delta60 assay. Methods Mol Biol. 2007;390:363–371. doi: 10.1007/978-1-59745-466-7_24. [DOI] [PubMed] [Google Scholar]
- Le Du MH, Stigbrand T, Taussig MJ, Menez A, Stura EA. Crystal structure of alkaline phosphatase from human placenta at 1.8 A resolution. Implication for a substrate specificity. J Biol Chem. 2001;276:9158–9165. doi: 10.1074/jbc.M009250200. [DOI] [PubMed] [Google Scholar]
- Li HH, Merchant S. Degradation of plastocyanin in copper-deficient Chlamydomonas reinhardtii. Evidence for a protease-susceptible conformation of the apoprotein and regulated proteolysis. J Biol Chem. 1995;270:23504–23510. doi: 10.1074/jbc.270.40.23504. [DOI] [PubMed] [Google Scholar]
- Liu H, Krizek J, Bretscher A. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics. 1992;132:665–673. doi: 10.1093/genetics/132.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Ann Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Gasch AP, Gaither LA, Botstein D, Brown PO, Eide DJ. Genome-wide characterization of the Zap1p zinc-responsive regulon in yeast. Proc. Natl. Acad. Sci. USA. 2000;97:7957–7962. doi: 10.1073/pnas.97.14.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDiarmid CW, Gaither LA, Eide D. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000;19:2845–2855. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDiarmid CW, Milanick MA, Eide DJ. Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J. Biol. Chem. 2003;278:15065–15072. doi: 10.1074/jbc.M300568200. [DOI] [PubMed] [Google Scholar]
- Mann KJ, Sevlever D. 1,10-Phenanthroline inhibits glycosylphosphatidylinositol anchoring by preventing phosphoethanolamine addition to glycosylphosphatidylinositol anchor precursors. Biochemistry. 2001;40:1205–1213. doi: 10.1021/bi0024512. [DOI] [PubMed] [Google Scholar]
- Merz AJ, Wickner WT. Resolution of organelle docking and fusion kinetics in a cell-free assay. Proc Natl Acad Sci U S A. 2004;101:11548–11553. doi: 10.1073/pnas.0404583101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettert EL, Kiley PJ. ClpXP-dependent proteolysis of FNR upon loss of its O2-sensing [4Fe-4S] cluster. J Mol Biol. 2005;354:220–232. doi: 10.1016/j.jmb.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Mijaljica D, Prescott M, Klionsky DJ, Devenish RJ. Autophagy and vacuole homeostasis: a case for self-degradation? Autophagy. 2007;3:417–421. doi: 10.4161/auto.4441. [DOI] [PubMed] [Google Scholar]
- Miyabe S, Izawa S, Inoue Y. Zrc1 is involved in zinc transport system between vacuole and cytosol in Saccharomyces cerevisiae. Biochem. Biophys. Res. Comm. 2001;282:79–83. doi: 10.1006/bbrc.2001.4522. [DOI] [PubMed] [Google Scholar]
- Munsterkotter M, Barbaric S, Horz W. Transcriptional regulation of the yeast PHO8 promoter in comparison to the coregulated PHO5 promoter. J Biol Chem. 2000;275:22678–22685. doi: 10.1074/jbc.M001409200. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Tibbitts TT, Kantrowitz ER. Mutations at positions 153 and 328 in Escherichia coli alkaline phosphatase provide insight towards the structure and function of mammalian and yeast alkaline phosphatases. J Mol Biol. 1995;253:604–617. doi: 10.1006/jmbi.1995.0576. [DOI] [PubMed] [Google Scholar]
- Nair U, Klionsky DJ. Molecular mechanisms and regulation of specific and nonspecific autophagy pathways in yeast. J Biol Chem. 2005;280:41785–41788. doi: 10.1074/jbc.R500016200. [DOI] [PubMed] [Google Scholar]
- Nelson N, Perzov N, Cohen A, Hagai K, Padler V, Nelson H. The cellular biology of proton-motive force generation by V-ATPases. J. Exp. Biol. 2000;203:89–95. doi: 10.1242/jeb.203.1.89. [DOI] [PubMed] [Google Scholar]
- Noda T, Matsuura A, Wada Y, Ohsumi Y. Novel system for monitoring autophagy in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1995;210:126–132. doi: 10.1006/bbrc.1995.1636. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Bryant NJ, Stevens TH. The newly identified yeast GRD genes are required for retention of late-Golgi membrane proteins. Mol Cell Biol. 1996;16:2700–2707. doi: 10.1128/mcb.16.6.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran TV, Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- Palmiter R, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Archiv. 2004;447:744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- Sevlever D, Mann KJ, Medof ME. Differential effect of 1,10-phenanthroline on mammalian, yeast, and parasite glycosylphosphatidylinositol anchor synthesis. Biochem. Biophys. Res. Comm. 2001;288:1112–1118. doi: 10.1006/bbrc.2001.5900. [DOI] [PubMed] [Google Scholar]
- Shong L. A soluble form of phosphatase in Saccharomyces cerevisiae capable of converting farnesyl diphosphate into E, E-farnesol. Appl. Biochem. Biotechnol. 2006;128:149–157. doi: 10.1385/abab:128:2:149. [DOI] [PubMed] [Google Scholar]
- Simm C, Lahner B, Salt D, LeFurgey A, Ingram P, Yandell B, Eide DJ. Saccharomyces cerevisiae vacuole in zinc storage and intracellular zinc distribution. Eukaryot Cell. 2007;6:1166–1177. doi: 10.1128/EC.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyov A, Gilbert HF. Zinc-dependent dimerization of the folding catalyst, protein disulfide isomerase. Protein Sci. 2004;13:1902–1907. doi: 10.1110/ps.04716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ishihara K, Migaki H, Matsuura W, Kohda A, Okumura K, Nagao M, Yamaguchi-Iwai Y, Kambe T. Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J Biol Chem. 2005a;280:637–643. doi: 10.1074/jbc.M411247200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ishihara K, Migaki H, Nagao M, Yamaguchi-Iwai Y, Kambe T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J Biol Chem. 2005b;280:30956–30962. doi: 10.1074/jbc.M506902200. [DOI] [PubMed] [Google Scholar]
- Tang W, Wang C. Zinc fingers and thiol-disulfide oxidoreductase activities of chaperone DnaJ. Biochemistry. 2001;40:14985–14994. doi: 10.1021/bi0107593. [DOI] [PubMed] [Google Scholar]
- Wang E, Koutsioulis D, Leiros HK, Andersen OA, Bouriotis V, Hough E, Heikinheimo P. Crystal structure of alkaline phosphatase from the Antarctic bacterium TAB5. J Mol Biol. 2007;366:1318–1331. doi: 10.1016/j.jmb.2006.11.079. [DOI] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Davis RW, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Wu CY, Bird AJ, Chung LM, Newton MA, Winge DR, Eide DJ. Differential control of Zap1-regulated genes in response to zinc deficiency in Saccharomyces cerevisiae. BMC Genomics. 2008;9:370. doi: 10.1186/1471-2164-9-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.