Abstract
Context: Aging, low dehydroepiandrosterone (DHEA), and testosterone are associated with increased adiposity and metabolic risk. Treatment with these hormones may improve these abnormalities.
Objective: The objective of the study was to determine effects of aging, DHEA, or testosterone replacement on adiposity, meal fat partitioning, and postabsorptive lipolysis.
Design: This was a cross-sectional, 2-yr, double-blind, randomized, placebo-controlled trial.
Setting: The study was conducted in the general community.
Patients: Elderly women and men (≥60 yr) with low DHEA sulfate (women and men) and bioavailable testosterone (men) concentrations and young adults.
Interventions: Thirty elderly women each received 50 mg DHEA or placebo daily for 2 yr. Thirty elderly men received 75 mg DHEA, 29 received 5 mg testosterone (patch), and 32 received placebo daily for 2 yr. Thirty young women and 32 young men served as controls.
Main Outcome Measures: In vivo measures of meal fat storage into sc fat, postabsorptive lipolysis, and regional adiposity at baseline and after treatment.
Results: At baseline, the elderly had more body fat, greater systemic lipolysis (women, P = 0.0003; men, P < 0.0001) adjusted for resting energy expenditure, greater meal fat oxidation (women, P = 0.026; men, P = 0.0025), and less meal fat storage in sc fat (women, P = 0.0139; men, P= 0.0006). Although testosterone treatment increased meal fat storage into upper- vs. lower-body fat in elderly men, neither hormone affected regional adiposity, meal fat oxidation, or systemic lipolysis.
Conclusions: Aging, in the context of low DHEA sulfate (women and men) and bioavailable testosterone (men) concentrations, is associated with changes in meal fat partitioning and postabsorptive lipolysis that are not corrected by DHEA and only partly corrected by testosterone replacement.
DHEA or testosterone treatment of hormone-deficient elderly does not normalize adipose tissue lipolysis, but testosterone restores a “youthful” meal fat storage pattern in elderly men.
Aging is characterized by increased fatness and greater accumulation of visceral fat (1,2), which is associated with greater cardiometabolic risk (3). Aging is also associated with hormonal changes such as declining dehydroepiandrosterone sulfate (DHEA-S) concentrations in both men and women, which can fall to about 20% of peak values by the seventh decade of life (2,4,5,6,7): low DHEA-S and/or dehydroepiandrosterone (DHEA) is associated with greater adiposity (2,6,8,9). Testosterone also declines with aging in men (2,10), in whom total serum testosterone concentrations are inversely related to body fatness (2,6,11), and bioavailable testosterone concentration is inversely related to visceral fat mass (12). Based on these well-established associations between aging, changes in DHEA and testosterone, and body fatness, it is reasonable to hypothesize that replacing these hormones in elderly individuals could have beneficial effects on body fat distribution and metabolic risk.
Six months of DHEA replacement in elderly individuals reduced total body (13) and visceral and abdominal sc fat compared with placebo (14). Previous studies reported that 12–36 months of testosterone supplementation in elderly men decreased total body fat (15), reduced fat preferentially in the arms and legs (16), or selectively reduced visceral fat relative to placebo (17). In contrast, we found little change in body fat in response to 2 yr of testosterone replacement in elderly men with low serum concentrations of bioavailable testosterone (18).
Total body and regional fat are determined by the balance of fatty acid storage and release. Dietary fat is the largest source of fatty acids for storage in adipose tissue for most humans and methods have been developed to quantitate this process (19,20). Likewise, adipose tissue lipolysis releases free fatty acids (FFAs) into the circulation to provide energy for lean tissue, and release rates are correlated with resting energy needs in postabsorptive adults (21). There is currently sparse information on the mechanism(s) involved in DHEA and testosterone effects on adipose tissue in humans. Compared with placebo, 5 d of testosterone administration decreased meal fat storage in visceral but not sc abdominal fat (22). This same group reported that 2 months of testosterone reduced meal fat storage in abdominal, but not femoral, sc fat compared with placebo (23).
As part of a 2-yr, double-blinded, placebo-controlled trial to replace DHEA (men and women) or testosterone (men) in elderly with reduced concentrations of these hormones, we measured whether these interventions alter meal fat use and systemic lipolysis as pathways to modifying total body or regional fat distribution. The effects of these interventions on body composition, bone mineral density, physical performance, and quality of life have been presented elsewhere (18).
Subjects and Methods
Participants
This study was approved by the Mayo Institutional Review Board. Informed written consent was obtained from all participants. It was conducted as part of a 2-yr, randomized, placebo-controlled, double-blind trial, which investigated the effects of DHEA and testosterone replacement on body composition, bone mineral density, physical performance and glucose tolerance in elderly people with low androgen levels (18). Briefly, volunteers were 60 yr or older, healthy, with low DHEA-S (women and men) and bioavailable testosterone (men) levels. Women were included if they had a DHEA-S level less than 0.95 μg/ml (2.6 μmol/liter). Men were included if they had a bioavailable testosterone level less than 103 ng/dl (3.6 nmol/liter) and a DHEA-S level less than 1.57 μg/ml (4.3 μmol/liter). The elderly men were also assessed for prostate disease.
We present data from 60 elderly women randomized to 50 mg DHEA (n = 30) or placebo (n = 30) per day, and 91 elderly men randomized to 75 mg DHEA (n = 30) or 5 mg testosterone (patch) (n = 29) or placebo (n = 32) per day. In addition, young women (n = 30) and men (n = 32) were studied concurrently with the elderly at baseline. Detailed information on enrollment and outcomes has previously been presented (18). Briefly, 152 elderly (92 men) and 82 young individuals (39 men) were enrolled in the study. From the initial randomization (18), one elderly male (testosterone group), two elderly women (DHEA group), and 20 young individuals (seven men) did not complete the fat metabolism studies presented here.
Body composition measurements
Total body and regional fat and lean body masses were assessed with dual-energy x-ray absorptiometry (Lunar Radiation, Madison, WI) (24) and computed tomography (25) (see supplement for details, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.or).
Protocol
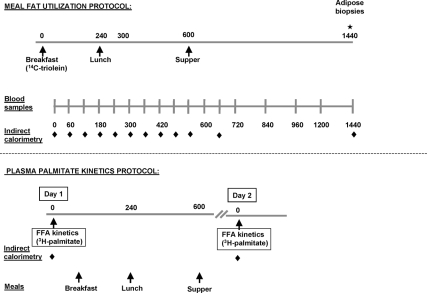
At baseline, each participant was admitted twice, first for assessment of meal fat use and then for assessment of FFA kinetics (adipose tissue lipolysis). The two study visits were performed approximately 7–15 d apart. In the elderly volunteers, the two visits were repeated approximately 2 yr after treatment with placebo, DHEA, or testosterone. A schematic of the study design is presented in Fig. 1.
Figure 1.
Schematic design of the study protocol. At baseline, each participant (elderly and young) was admitted twice, the first time for the assessment of meal fat use and the second time for the assessment of postabsorptive palmitate kinetics (adipose tissue lipolysis). The two study visits were performed approximately 7–15 d apart. In the elderly volunteers, the two visits were repeated at approximately 2 yr after treatment with placebo, DHEA, or testosterone. Additional sc abdominal and femoral fat biopsies (not depicted in the figure) were performed prior to administration of the [l-14C]triolein-labeled meal in the 2-yr visit to account for the amount of 14C remaining in adipose tissue from the baseline study.
Baseline studies
Meal fat use protocol
All participants consumed a weight maintenance diet (55% carbohydrate, 15% protein, and 30% fat) provided by Mayo General Clinical Research Center (GCRC) kitchen for 3 d preceding the study. They were admitted to the GCRC at 1700 h the day before the study and consumed a weight maintenance meal at 1800 h. No additional food was consumed until the next morning. No caffeine was allowed until the end of the study visit.
The following morning at 0700, resting energy expenditure (REE) was determined with indirect calorimetry (DeltaTrac Metabolic Cart, Yorba Linda, CA) for 20 min or longer, and a baseline breath sample was collected for measurement of background 14CO2-specific activity (SA). At 0800 h, volunteers received a liquid test meal (Ensure Plus; Ross Laboratories, Seattle, WA) that provided calories equal to one third of their REE (as assessed the same morning). The meal provided 57% of the energy as carbohydrate, 28% as fat, and 15% as protein. This corresponded to meals of 420 ± 56, 461 ± 68, 551 ± 83, and 617 ± 63 kcal for elderly women, young women, elderly men, and young men, respectively, containing 13 ± 2, 14 ± 2, 17 ± 3, and 19 ± 2 g of dietary fat. Approximately 20 μCi [l-14C] triolein was sonicated into the liquid meal to trace meal fatty acid (FA) metabolism. Solid food meals were provided at 1300 and 1800 h with energy content equal to the previous weight maintenance amounts and with a macronutrient distribution equal to that of the previous 3 d. After consuming the test meal, breath samples were obtained at regular intervals until the next morning at 0800 h for measurement of 14CO2 SA to assess meal FA oxidation. Oxygen consumption and CO2 production rates were also measured with indirect calorimetry until the next morning. Twenty-four hours after the liquid test meal (0800 h), adipose tissue biopsies (∼500 mg) were obtained from the periumbilical sc abdominal and midthigh regions as previously described (26) to measure meal FA storage.
Plasma palmitate kinetics protocol
The weight maintenance diet and GCRC admission protocol were the same as described for the meal fat use protocol. At 0600 h the morning after admission, two iv catheters were placed, one in a forearm vein for tracer infusion and a second in the contralateral hand vein (retrograde) for collection of arterialized blood using the heated box approach (55 C). The volunteers remained in bed after catheter placement. REE was measured at 0800 h for 20 min or longer. After collecting a baseline blood sample for background palmitate SA, a continuous infusion of [9,10-3H]palmitate (∼0.3 μCi/min) commenced. After 30 min for isotopic equilibration, a series of four blood samples were collected at 10-min intervals to measure palmitate kinetics. Participants remained at the GCRC the remainder of the day for measurements of glucose metabolism (27,28). They were provided a research meal at 0900 h (27,28) and a weight maintenance meal at 1630 h. At 2030 h, they were given a standard 10 kcal/kg snack (55% carbohydrate, 15% protein, and 30% fat) and then fasted until the next morning when a second assessment of overnight postabsorptive palmitate kinetics and REE was made using the identical procedures. Thus, each volunteer had four measures of fasting oxygen consumption/CO2 production, two during the palmitate kinetics study and two with the meal fat use study. The average of these measures is presented.
Two-year studies
After about 2 yr of treatment, the elderly participants underwent identical body composition, metabolic, meal fat use, and palmitate kinetics measurement studies a second time. In addition, sc abdominal and femoral fat biopsies were performed prior to the [l-14C]triolein-labeled meal study to correct for any remaining 14C in adipose tissue lipid from the baseline study.
Materials
[l-14C]triolein and [9,10-3H]palmitate were purchased from NEN Life Science Products, PerkinElmer (Boston, MA).
Assays
Breath 14CO2 SA was measured by air expired through a solution of benzethonium hydroxide with phenolphthalein, trapping 0.5 mmol CO2. Meal and adipose tissue lipid SA (disintegrations per minute per gram lipid) were assessed by extracting lipids with chloroform-methanol (2:1) and measuring radioactivity to less than 2% counting error (26). The meal was weighed to the nearest milligram and quadruplicate 50-μl aliquots were counted to assess the exact amount of [14C]triolein consumed. Plasma palmitate SA and concentration were measured by HPLC (29). All other metabolic parameters were measured as previously described (18,21).
Calculations
Meal FA storage in adipose tissue was calculated as follows. The adipose lipid SA (disintegrations per minute per gram) was divided by the meal SA (disintegrations per minute per milligram) to predict the meal FA storage (milligram meal fat per gram adipose lipid). To estimate the fraction of tracer stored in each fat depot, we divided the total body adipose fat tissue into upper-body sc (UBSQ), lower-body sc (LBSQ), and visceral fat compartments. The site-specific adipose lipid SA (disintegrations per minute per gram) was multiplied by the depot-specific fat mass (gram) to estimate total meal tracer storage (disintegrations per minute) per depot. The meal tracer storage in an entire depot was divided by the amount of tracer (disintegrations per minute) ingested to calculate the fraction of meal fat that was stored in that depot.
The rate of 14CO2 production (disintegrations per minute per minute) was determined by multiplying the 14CO2 SA (disintegrations per minute per millimole) by the CO2 production rate (millimole per minute; indirect calorimetry) at each time point. A nonlinear model was used to predict nocturnal CO2 production (26) because the nocturnal CO2 production rates were not measured in this study. To calculate the fraction of [14C]triolein oxidized, the area under the 14CO2 production rate vs. time curve was divided by the amount (disintegrations per minute) of [14C]triolein consumed. The recovery of 14CO2 from the oxidation of [1-14C]triolein was corrected for carbon fixation (30).
Palmitate flux was calculated using steady-state formulas.
Statistics
Data comparing elderly (all treatment groups together at baseline) vs. young are represented as means ± sd for near normally distributed data or median with interquartile intervals for nonnormally distributed data. Unpaired t test or Wilcoxon rank-sum test were used to compare the elderly with the young participants within each sex at baseline. A repeated-measures ANOVA was used to compare indirect calorimetry data between elderly and young as well as between treatment and placebo groups at 24 months.
Data comparing the effects of the 24-month treatments in the elderly are presented as the median difference between the changes from baseline in each treatment group vs. the corresponding changes in the placebo group with 95% confidence intervals. Wilcoxon rank-sum test was used to compare the changes in the treatment groups against the changes in the placebo group.
Examining tissue SA values per se alone is of limited value because of tracer dilution in individuals with greater adiposity. For this reason, SA values were used in two ways: 1) the fraction of tracer stored in each fat depot was calculated; this calculation takes into consideration both the SA and degree of adiposity (see Calculations); and 2) SA is presented as a function of fat mass so it is examined per unit of regional fat mass. If the same amount of meal fat is consumed by individuals with varying amounts of body fat mass, the pattern of meal fat storage (milligram meal fat per gram adipose lipid) as a function of body fat mass can provide information on the capacity of adipose tissue to store fat (31,32). Theoretically, storage (milligram meal fat per gram adipose lipid) should decrease in a hyperbolic fashion because, as body fat mass increases, the meal FA are diluted in larger amounts of body fat. If meal fat storage as a function of regional body fat does not follow this pattern, we interpret this as suggesting different relative storage of meal FA in that depot.
Because REE and sex are the best independent predictors of postabsorptive palmitate release rates (micromoles per minute) (21,33) in the present study, we assessed whether age (young vs. elderly) is an additional, independent predictor. Palmitate release rates and REE were not normally distributed and were log transformed to be appropriately tested for inclusion in the multivariate regression analysis.
Results
Elderly vs. young participants at baseline
Subject characteristics
Elderly volunteers had significantly greater body mass index (BMI), whole-body UBSQ, and visceral fat mass than young participants (Table 1). LBSQ fat mass was significantly greater in elderly than young women but did not differ between elderly and young men. Plasma palmitate and glucose concentrations were greater and REE was lower in the elderly than young participants. Elderly volunteers were selected to have low DHEA-S (women and men) and bioavailable testosterone (men). Serum estradiol concentrations were not considered a reliable index of estrogen status for young women because oral contraceptive use was not an exclusion criterion.
Table 1.
Baseline characteristics of the elderly and young subjects
| Women
|
Men
|
|||||
|---|---|---|---|---|---|---|
| Elderly (n = 60) | Young (n = 30) | P | Elderly (n = 91) | Young (n = 32) | P | |
| Age, yr | 70.1 ± 5.9 | 22.3 ± 2.9 | a | 68.5 ± 5.6 | 23.5 ± 3.3 | a |
| Body weight, kgb | 70.6 ± 10.5 | 64.7 ± 8.8 | 0.015 | 86.0 ± 11.3 | 81.1 ± 9.1 | 0.0002 |
| BMI, kg/m2 | 27.0 ± 3.5 | 24.1 ± 2.8 | 0.003 | 27.6 ± 3.1 | 24.9 ± 2.8 | 0.002 |
| REE, kcal/d | 1,312 (1,228–1,418) | 1,398 (1,321–1,570) | 0.0014 | 1,652 (1,540–1,790) | 1,808 (1,597–1,949) | 0.009 |
| Lean body mass, kgc | 37.3 ± 3.5 | 39.0 ± 3.5 | 0.040 | 57.4 ± 5.6 | 59.0 ± 4.8 | 0.17 |
| UBSQ fat, kg | 14.5 ± 3.7 | 11.2 ± 3.8 | 0.0002 | 11.4 ± 3.6 | 8.5 ± 3.2 | 0.0002 |
| LBSQ fat, kg | 11.0 ± 3.4 | 7.6 (6.5–11.2) | 0.006 | 6.5 (5.3–8.2) | 6.2 ± 2.6 | 0.20 |
| Visceral fat, kg | 3.8 (2.4–4.8) | 1.3 (0.9–2.1) | <0.0001 | 4.8 (3.4–6.1) | 2.0 (1.3–2.7) | <0.0001 |
| Plasma palmitate, μmol/liter | 104 (93–129) | 90 (87–98) | 0.011 | 90 (76–103) | 70 (52–80) | <0.0001 |
| Glucose, mmol/liter | 5.1 (4.9–5.4) | 4.8 (4.6–5.4) | <0.0001 | 5.2 (5.0–5.4) | 4.9 (4.8–5.0) | <0.0001 |
| Insulin, pmol/liter | 22.0 (17.0–31.0) | 28.0 (19.5–35.5) | 0.09 | 23.6 (18.4–32.0) | 21.0 (17.0–26.5) | 0.28 |
| Epinephrine, pg/ml | 5.1 (4.9–5.4) | 4.8 (4.6–4.9) | 0.14 | 5.2 (5.0–5.4) | 4.9 (4.8–5.0) | 0.24 |
| Sulfated DHEA, μg/ml | 0.34 (0.30–0.53) | 1.40 (1.10–1.90) | <0.0001 | 0.67 (0.42–0.97) | 2.50 (1.90–3.10) | <0.0001 |
| Bioavailable testosterone, ng/dl | N/A | N/A | 57.5 ± 17.7 | 145.2 ± 50.7 | <0.0001 | |
Values are means± sd for normally distributed data or medians (25th to 75th quantiles) for nonnormally distributed data. P values refer to elderly vs. young by unpaired t test for normally distributed data or Wilcoxon rank-sum test for nonnormally distributed data. N/A, Not available.
Not subject to statistical testing.
Obtained with participants dressed but no shoes.
It does not include bone mineral content.
Meal fat use
In both sexes, meal FA storage in sc abdominal and femoral fat (milligram meal fat per gram adipose lipid) was significantly less in elderly than in young individuals (Table 2). This was not merely related to greater fat mass in the elderly because the proportions of meal FA stored in the UBSQ, LBSQ, and whole-body sc (WBSQ) fat depots at 24 h after the test meal were also generally lower in elderly than the young (Table 2). In women, a lesser percentage of meal FA were stored in UBSQ and WBSQ in the elderly, whereas fractional storage in LBSQ fat did not differ significantly between the elderly and young. The percent meal FA storage in sc fat was uniformly, significantly less in elderly than in young men.
Table 2.
Meal FA partitioning and palmitate release rates in the young and elderly subjects at baseline
| Women
|
Men
|
|||||
|---|---|---|---|---|---|---|
| Elderly (n = 60) | Young (n = 30) | P | Elderly (n = 91) | Young (n = 32) | P | |
| Abdominal storage (mg/g lipid) | 0.15 (0.13–0.20) | 0.30 (0.23–0.47) | <0.0001 | 0.15 (0.10–0.20) | 0.32 (0.24–0.57) | <0.0001 |
| Femoral storage (mg/g lipid) | 0.18 (0.14–0.25) | 0.31 (0.23–0.43) | <0.0001 | 0.14 (0.09–0.20) | 0.28 (0.17–0.38) | <0.0001 |
| Abdominal/femoral storage | 0.85 (0.63–1.06) | 0.97 (0.71–1.67) | 0.05 | 1.11 (0.80–1.40) | 1.30 (1.15–1.75) | 0.0053 |
| Meal fat partitioning (%) | ||||||
| Storage in | n = 57 | n = 30 | n = 87 | n = 32 | ||
| UBSQ fat | 16.8 (15.0–20.9) | 23.0 (18.2–28.7) | 0.0005 | 9.7 (6.5–13.7) | 15.2 (10.7–21.6) | 0.0007 |
| LBSQ fat | 16.0 (10.6–21.5) | 18.4 (13.4–25.0) | 0.33 | 5.3 (3.3–7.3) | 8.9 (4.4–12.8) | 0.0027 |
| WBSQ fat | 34.5 (26.0–44.4) | 42.4 (33.0–52.1) | 0.0139 | 15.1 (10.5–23.4) | 26.5 (15.5–34.1) | 0.0006 |
| Oxidation | n = 49 45.1 (39.2–52.9) | n = 30 39.2 (33.3–49.0) | 0.026 | n = 82 54.9 (46.6–62.7) | n = 32 43.1 (35.3–56.9) | 0.0025 |
| Palmitate release (μmol/min) | n = 52 117 (93–144) | n = 30 104 (91–142) | 0.46 | n = 83 122 (101–151) | n = 32 83 (65–108) | <0.0001 |
Values are means± sd for normally distributed data or medians (25th to 75th quantiles) for nonnormally distributed data. P values refer to elderly vs. young by unpaired t test for normally distributed data or Wilcoxon rank-sum test for nonnormally distributed data.
As an index of preferential meal FA storage in upper vs. lower body sc fat, we assessed the ratio of the adipose lipid SA in abdominal and femoral fat. The ratio of meal FA storage in sc abdominal to femoral fat was significantly less in the elderly than young participants (Table 2). This indicates that in young adults there was preferential storage of dietary fat in the UBSQ fat region compared with their elderly counterparts.
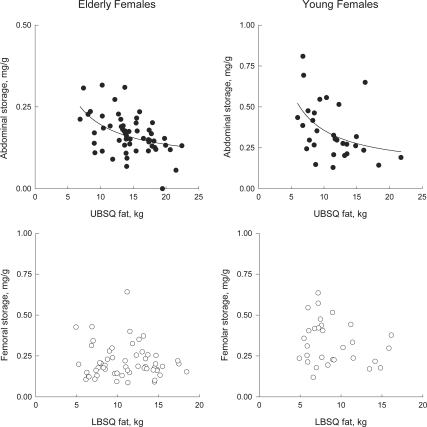
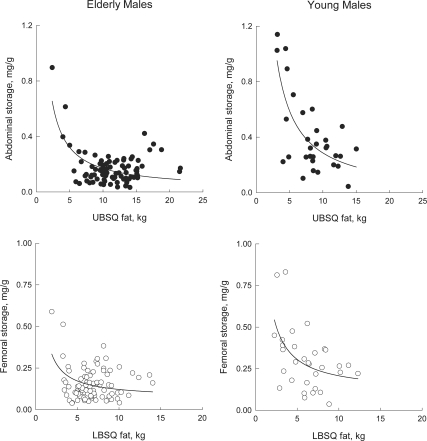
We examined the concentration of meal FA in regional fat as a function of fat mass to understand whether the patterns of storage differed between sites and groups (32). Meal FA storage in abdominal sc fat (milligram meal fat per gram adipose lipid) decreased in a hyperbolic fashion as a function of UBSQ fat mass in elderly (P = 0.0003) and young women (P = 0.011) (Fig. 2, top panels) as well as in elderly (P < 0.0001) and young men (P < 0.0001) (Fig. 3, top panels). Femoral meal fat storage also decreased as a function of LBSQ fat mass in elderly (P = 0.001) and young men (P = 0.004) (Fig. 3, bottom panels). These patterns are consistent with dilution of a relatively fixed amount of meal FA within progressively greater amounts of UBSQ fat (women and men) LBSQ fat (men). In contrast, there was no significant relationship between femoral meal fat storage and LBSQ fat mass in elderly (P = 0.54) or young women (P = 0.40) (Fig. 2, bottom panels). This indicates that as LBSQ fat mass increased in both in elderly and young women, greater amounts of meal FA were stored.
Figure 2.
Relationship between abdominal meal fat storage (milligram meal fat per gram lipid) and UBSQ fat mass (top panels) and between femoral meal fat storage (milligram meal fat per gram lipid) and LBSQ fat mass (bottom panels) in elderly and young women. Subcutaneous adipose tissue biopsies were collected 24 h after the consumption of meal that contained [1-14C]triolein. Note the different scale of the y-axes in the top panels between elderly and young women.
Figure 3.
Relationship between abdominal meal fat storage (milligram meal fat per gram lipid) and UBSQ fat mass (top panels) and between femoral meal fat storage (milligram meal fat per gram lipid) and LBSQ fat mass (bottom panels) in elderly and young men. Subcutaneous adipose tissue biopsies were collected 24 h after the consumption of meal that contained [1-14C]triolein.
The proportion of meal FA oxidized over 24 h was significantly greater in the elderly than the young participants in both sexes, which is consistent with the lesser amounts stored in sc adipose tissue (Table 2).
Plasma palmitate kinetics
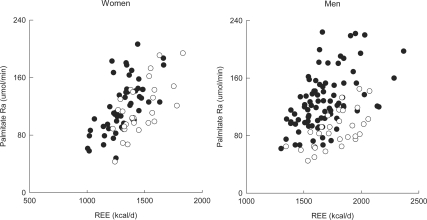
We observed a strong positive association between REE and systemic adipose tissue lipolysis, as measured by palmitate rate of appearance (all P < 0.001) (Fig. 4). Median systemic palmitate release (unadjusted for REE) did not differ between elderly and young women, but it was significantly greater in elderly than young men by about 45% (Table 2). After adjusting for REE, systemic palmitate release was significantly greater in elderly than young women (effect of age: P = 0.0003) as well as in elderly compared with young men (P < 0.0001) (Fig. 4). Therefore, at the same level of REE, systemic FFA release was greater in the elderly than the young.
Figure 4.
The relationship between REE and postabsorptive palmitate rate of appearance (Ra) in elderly (closed circles) and young women (open circles) (left panel) and elderly (closed circles) and young men (open circles) (right panel).
Effects of 2-yr treatment with placebo, DHEA, or testosterone in the elderly
Subject characteristics
Baseline body composition and metabolic parameters did not differ between the treatment groups in either the elderly women or elderly men (18). DHEA treatment increased plasma DHEA-S significantly compared with placebo [difference between placebo and treatment groups in the change from baseline to 24 months in women: 3.78 μg/ml (3.13, 4.10); men: 3.35 μg/ml (2.92, 3.82) (P < 0.0001 for both sexes)]. Compared with placebo, 2 yr of DHEA did not alter REE, BMI, UBSQ, LBSQ, or visceral fat, plasma glucose, palmitate, or epinephrine concentrations in either sex (data not shown).
In men, testosterone treatment increased plasma testosterone concentrations significantly compared with placebo [difference between placebo and treatment group in the change from baseline to 24 months: 28.8 pg/dl (10.1, 48.1) (P = 0.002)] achieving low normal concentrations. Testosterone treatment also significantly reduced LH concentrations [difference between placebo and treatment group in the change from baseline to 24 months: −2.70 IU/liter (−3.96, −1.44) (P < 0.0001)]. Testosterone treatment did not alter visceral or sc fat masses compared with placebo but did significantly reduce plasma insulin concentrations [−4.3 pmol/liter (−8.2, −1.3) treatment vs. placebo group) (P = 0.003)].
Meal fat use
Meal FA storage in sc abdominal and femoral fat (milligram meal fat per gram adipose lipid), and the proportion of meal fat tracer oxidized or stored in UBSQ, LBSQ, and WBSQ fat did not differ between placebo and treatment groups at baseline (data not shown). In both sexes, 2 yr of DHEA or testosterone treatment did not alter the proportions of meal FA that were oxidized or stored in UBSQ, LBSQ, and WBSQ compared with placebo (data not shown). The ratio of meal FA storage in sc abdominal to femoral fat significantly increased in the testosterone-treated compared with the placebo group [0.47 (0.05,0.89); P = 0.04 for the change in the testosterone vs. placebo group from baseline to 24 months]. Therefore, testosterone-treated elderly men shifted toward preferential storage of dietary fat in UBSQ adipose tissue, resembling young men. This ratio was not significantly affected by DHEA [0.15 (−0.16,0.52); P = 0.26 for the change in the DHEA vs. placebo group from baseline to 24 months].
Plasma palmitate kinetics
Two years of DHEA or testosterone treatment did not alter postabsorptive palmitate kinetics compared with placebo in either sex (data not shown). For the two sexes combined, the relationships between systemic palmitate release and REE did not change after the 2-yr treatment with placebo (P = 0.97 for the slopes before vs. after treatment), DHEA (P = 0.59), or testosterone (P = 0.34).
Discussion
Body fat is maintained by the balance of storage and release, both on a whole-body basis and within each depot. The elderly participants in this study were selected to have low DHEA-S (women and men) and bioavailable testosterone (men) concentrations. They had more total-body, UBSQ, and visceral fat mass than young adults. Elderly women had more LBSQ fat mass than young women, whereas LBSQ did not differ between elderly and young men. Compared with young adults, the elderly oxidized a greater portion of dietary fat, stored a smaller portion of dietary FA in sc fat, and had greater rates of postabsorptive lipolysis. Treatment with DHEA or testosterone did not change these patterns (except for a regional shift in dietary fat storage in elderly men receiving testosterone), indicating the differences between the young and elderly were not due to the differences in hormone concentrations. These findings indicate major changes in adipose tissue and fatty acid metabolism occur with aging and low androgen levels that are dissociated from the traditional expectations for relating FA release and storage to body composition.
Despite greater regional fat mass, the percent of meal fat stored in both sc adipose depots was less in elderly vs. young men, and less in the UBSQ depot in elderly vs. young women. At the same time, postabsorptive systemic lipolysis, which largely derives from sc fat (34), was substantially greater in the elderly. If FFAs released from adipose tissue were solely destined for oxidation in lean tissue and if storage of meal FA were the major determinant of sc body fat, the elderly should have little sc body fat. Our findings imply that sc adipose tissue in the elderly derives a larger portion of its FA stores from very low-density lipoprotein-triglyceride (35) and/or the direct storage (36), which are normally considered inconsequential. Our results also suggest that the elderly disposed of a larger portion of circulating FFAs through nonoxidative pathways.
The increased oxidation and decreased storage of dietary FAs in adipose tissue in the elderly could be caused by a preferential use of chylomicron triglyceride as a fuel as a result of aging, essentially stealing fatty acids from storage in adipose tissue compared with the young. Alternatively, reduced ability to up-regulate dietary fat storage into sc fat in the postprandial state (37) may result in more FAs being available to lean tissues for oxidation in the elderly, as evident by the lower respiratory exchange ratio in the elderly compared with young (supplemental data). Another possibility is reduced insulin sensitivity in elderly, leading to a preferential oxidation of FAs over glucose. Factors that could limit dietary FA storage in adipose tissue include reduced delivery to adipose tissue (reduced postprandial adipose blood flow), reduced lipoprotein lipase activity, lesser inward FA transport, or esterification. Regardless of the mechanism(s) involved, the greater dietary fat oxidation in the elderly is consistent with the greater glycemic excursion and lower glucose disposal in these elderly participants after a mixed meal that we previously reported (38). We conclude that elderly individuals have an altered ability to regulate fat and carbohydrate metabolism postprandially compared with young adults.
The general pattern of meal FA storage relative to fat mass in elderly women was similar to that of young women in this study and to that we previously observed (32). In LBSQ adipose tissue, the concentration of meal FA did not decrease as fat mass increased (Fig. 2), a pattern substantially different from that seen in men (Fig. 3). Thus, women with greater amounts of LBSQ fat store greater amounts of meal FA in that depot. Our data suggest that the LBSQ depot in women is more active in storing meal FA in women than men, even if the women have chronic sex-steroid deficiency. In contrast, the pattern of meal FA storage in UBSQ was generally similar in young and elderly men and women, The primary factor determining meal FA storage may be different in LBSQ than UBSQ adipose tissue in females, and this mechanism appears to be preserved after menopause.
Body composition and regional fat distribution did not differ between the testosterone and placebo treatment groups. Some investigators who treated with higher doses of testosterone have reported changes in body composition. Marin and colleagues (22,39,40) found significant changes in only visceral fat mass and Allan et al. (17) reported that testosterone treatment prevented increases in visceral fat mass over 1 yr. Our data raise questions about the sustainability of this effect. We were unable to detect differences in whole-body or regional fat composition between placebo and testosterone-treated men with the dose of testosterone we used.
Body composition and fat distribution did not differ between DHEA, and placebo. Although some studies observed changes in fat distribution when DHEA was supplemented for 6 months (14), we found no change in total or regional body fat over a longer period of time.
Testosterone replacement in elderly men did not produce a significant change in absolute regional meal FA storage (milligram per gram) compared with placebo but increased storage in abdominal sc relative to femoral fat. The regional storage pattern after testosterone treatment of the elderly men was nearly identical with that seen in the young men. Despite this, there was no change in regional fat mass, suggesting that meal FA storage is not the only factor determining fat distribution in the elderly. Offsetting change in regional adipocyte lipolysis and/or direct and indirect (very low density lipoprotein) FFA storage may have occurred in the testosterone-treated group to maintain fat distribution. This emphasizes the need to understand the multiple pathways of uptake and the variations in regional release as mechanisms for how fat distribution can be regulated. Previous studies showed that testosterone decreased meal fat storage in the abdominal sc adipose tissue (23). However, those studies included a younger population, conducted over a shorter period of time and the biopsies to measure meal fat storage were performed 4 h after the meal. It is possible that there may have been different findings if the biopsies had performed after meal FA digestion and absorption is complete, which is better accomplished after 24 h (41). Acute testosterone treatment has been shown to reduce meal FA storage into visceral fat but not sc fat 24 h after the ingestion of an experimental meal (22).
To our knowledge, this is the first study to address the issue of the in vivo effects of testosterone or DHEA on systemic lipolysis. Overnight, postabsorptive lipolysis did not differ in the testosterone group vs. placebo after 2 yr of treatment. In vitro studies in human adipocytes looked at the effect of replacement with testosterone, showing decreased lipolysis in response to adrenergic receptor stimulation in adipocytes differentiated from sc adipose tissue preadipocytes (42). In addition, because DHEA has been shown to increase lipolysis in sc adipose tissue in women and in visceral fat in men in vitro (43), consequent changes in body fat distribution were predicted. We found no significant change in whole-body lipolysis, suggesting that in vitro studies may not predict whole-body lipolysis.
Although we excluded a role for physiological levels of DHEA in regulating meal fat partitioning and postabsorptive lipolysis, serum testosterone concentrations were increased only to the low normal range in elderly men. It is possible that if we achieved greater testosterone concentrations, we may have observed a more pronounced effect, although we did detect significant changes in patterns of upper- vs. lower-body meal FA storage. In addition, although our goal was to describe fatty acid metabolism in aging in the face of low DHEA and testosterone and examine the effects of hormone treatment, inclusion of elderly volunteers with normal DHEA and testosterone concentrations would have allowed further comparisons.
In summary, elderly males and females with low DHEA-S (women and men) and bioavailable testosterone (men) concentrations were less efficient than their young counterparts in storing meal fat into UBSQ adipose tissue, even after DHEA or testosterone treatment. Also, the elderly oxidized a greater proportion of meal FA compared with the young. Overnight postabsorptive lipolysis was significantly greater in elderly men and women than the young relative to their energy needs. DHEA or testosterone replacement in elderly androgen-deficient individuals had no effect on body fat composition, meal fat oxidation, total meal fat storage, and plasma FFA kinetics. Although increasing testosterone concentrations in elderly men led to a partial restoration of the pattern of meal fat storage observed in young men, it did not affect regional adiposity. Our findings document that aging in the face of low circulating DHEA-S and testosterone levels is associated with major, unexpected changes in adipose handling of fatty acids that could not be predicted on the basis of body fat and body fat distribution.
Supplementary Material
Acknowledgments
We are indebted to the study participants; Traci Hammer for coordinating the study; Barbara Norby, Jean Feehan, and Laurie Wahlstrom for their nursing support; Peggy Helwig and the Chemistry Core Laboratory of the GCRC for their technical assistance; and members of the GCRC nursing, laboratory, and dietetic staff.
Footnotes
This work was supported by Grants PO1 AG14283 and MO1 RR00585 from the National Institutes of Health; the Department of Medicine, Mayo Clinic; and the Mayo Foundation.
Disclosure Summary: The authors have nothing to declare.
First Published Online June 30, 2009
Abbreviations: BMI, body mass index; DHEA, dehydroepiandrosterone; DHEA-S, dehydroepiandrosterone sulfate; FA, fatty acid; FFA, free fatty acid; GCRC, General Clinical Research Center; LBSQ, lower-body sc; REE, resting energy expenditure; SA, specific activity; UBSQ, upper-body sc; WBSQ, whole-body sc.
References
- Baumgartner RN, Heymsfield SB, Roche AF, Bernardino M 1988 Abdominal composition quantified by computed tomography. Am J Clin Nutr 48:936–945 [DOI] [PubMed] [Google Scholar]
- Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Despres JP, Bouchard C 2000 Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE Family Study. J Clin Endocrinol Metab 85:1026–1031 [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P 2008 Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28:1039–1049 [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH 1984 Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59:551–555 [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H 1992 Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab 75:1002–1004 [DOI] [PubMed] [Google Scholar]
- Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB 1994 The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab 79:1310–1316 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Muller DC, Metter EJ, Maggio M, Harman SM, Blackman MR, Andres R 2007 Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab 92:3568–3572 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Ferrara A 1996 Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: the Rancho Bernardo Study. J Clin Endocrinol Metab 81:59–64 [DOI] [PubMed] [Google Scholar]
- De Pergola G, Zamboni M, Sciaraffia M, Turcato E, Pannacciulli N, Armellini F, Giorgino F, Perrini S, Bosello O, Giorgino R 1996 Body fat accumulation is possibly responsible for lower dehydroepiandrosterone circulating levels in premenopausal obese women. Int J Obes Relat Metab Disord 20:1105–1110 [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR 2001 Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, Seres DS, Rosenfeld RS 1990 Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab 71:929–931 [DOI] [PubMed] [Google Scholar]
- Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, Haug E, Larsen R, Andersen M 2007 Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab 92:2696–2705 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Holloszy JO, Kohrt WM 2000 Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf) 53:561–568 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Holloszy JO 2004 Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA 292:2243–2248 [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG 2001 Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol 56:M266–M272 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL 1999 Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- Allan CA, Strauss BJG, Burger HG, Forbes EA, McLachlan RI 2008 Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab 93:139–146 [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton III LJ, Smith GE, Khosla S, Jensen MD 2006 DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- Marin P, Rebuffe-Scrive M, Bjorntorp P 1990 Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 20:158–165 [DOI] [PubMed] [Google Scholar]
- Romanski SA, Nelson R, Jensen MD 2000 Meal fatty acid uptake in human adipose tissue: Technical and experimental design issues. Am J Physiol Endocrinol Metab 279:E447–E454 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD 2003 Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest 111:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin P, Oden B, Olbe L, Bengtsson B-A, Bjorntorp P 1996 Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 81:1018–1022 [DOI] [PubMed] [Google Scholar]
- Marin P, Oden B, Bjorntorp P 1995 Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab 80:239–243 [DOI] [PubMed] [Google Scholar]
- Jensen MD, Kanaley JA, Roust LR, O'Brien PC, Braun JS, Dunn WL, Wahner HW 1993 Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc 68:867–873 [DOI] [PubMed] [Google Scholar]
- Jensen MD, Kanaley JA, Reed JE, Sheedy PF 1995 Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 61:274–278 [DOI] [PubMed] [Google Scholar]
- Romanski SA, Nelson RM, Jensen MD 2000 Meal fatty acid uptake in adipose tissue: gender effects in non-obese humans. Am J Physiol Endocrinol Metab 279:E455–E462 [DOI] [PubMed] [Google Scholar]
- Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA 2007 Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care 30:1972–1978 [DOI] [PubMed] [Google Scholar]
- Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA 2007 Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes [Erratum (2007) 56:1486] 56:753–766 [DOI] [PubMed] [Google Scholar]
- Miles JM, Ellman MG, McClean KL, Jensen MD 1987 Validation of a new method for determination of free fatty acid turnover. Am J Physiol Endocrinol Metab 252:E431–E438 [DOI] [PubMed] [Google Scholar]
- Votruba SB, Zeddun SM, Schoeller DA 2001 Validation of deuterium labeled fatty acids for the measurement of dietary fat oxidation: a method for measuring fat-oxidation in free-living subjects. Int J Obes Relat Metab Disord 25:1240–1245 [DOI] [PubMed] [Google Scholar]
- Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD 2008 Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes 57:1186–1194 [DOI] [PubMed] [Google Scholar]
- Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD 2007 Meal fatty acid uptake in visceral fat in women. Diabetes 56:2589–2597 [DOI] [PubMed] [Google Scholar]
- Shadid S, Kanaley JA, Sheehan MT, Jensen MD 2007 Basal and insulin-regulated free fatty acid and glucose metabolism in humans. Am J Physiol Endocrinol Metab 292:E1770–E1774 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD 2004 Splanchnic lipolysis in human obesity. J Clin Invest 113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack SW, Fisher RM, Gibbons GF, Humphreys SM, McDonough MJ, Potts JL, Frayn KN 1990 Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci 79:339–348 [DOI] [PubMed] [Google Scholar]
- Shadid S, Koutsari C, Jensen MD 2007 Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 56:1369–1375 [DOI] [PubMed] [Google Scholar]
- Ruge T, Hodson L, Cheeseman J, Dennis AL, Fielding BA, Humphreys SM, Frayn KN, Karpe F 2009 Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab 94:1781–1788 [DOI] [PubMed] [Google Scholar]
- Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA 2006 Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 55:2001–2014 [DOI] [PubMed] [Google Scholar]
- Marin P, Holmäng S, Gustafsson C, Jönsson L, Kvist H, Elander A, Eldh J, Sjöström L, Holm G, Björntorp P 1993 Androgen treatment of abdominally obese men. Obes Res 1:245–251 [DOI] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Marin P, Bjorntorp P 1991 Effect of testosterone on abdominal adipose tissue in men. Int J Obes 15:791–795 [PubMed] [Google Scholar]
- Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA 2003 Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab 285:E1282–E1288 [DOI] [PubMed] [Google Scholar]
- Dicker A, Rydén M, Näslund E, Muehlen IE, Wirén M, Lafontan M, Arner P 2004 Effect of testosterone on lipolysis in human preadipocytes from different fat depots. Diabetologia 47:420–428 [DOI] [PubMed] [Google Scholar]
- Tagliaferro AR, Ronan AM, Payne J, Meeker LD, Tse S 1995 Increased lipolysis to beta-adrenergic stimulation after dehydroepiandrosterone treatment in rats. Am J Physiol Regul Integr Comp Physiol 268:R1374–1380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.