Abstract
Context: Obesity and type 2 diabetes are associated with elevated intramyocellular lipids (IMCLs) and insulin resistance.
Objective: We tested the hypothesis that skeletal muscle lipases activity could influence IMCL content (including diacylglycerol and ceramides).
Design and Patients: The present study included 48 subjects with a wide range of age (19–68 yr) and body mass index (20–45 kg/m2) who underwent skeletal muscle biopsy, dual-energy x-ray absorptiometry and a hyperinsulinemic euglycemic clamp.
Main Outcome Measures: Insulin sensitivity by hyperinsulinemic clamp, and intramyocellular triacylglycerol (IMTG), diacylglycerol (DAG), and ceramides content, and triacylglycerol and diacylglycerol hydrolase activities were measured in biopsies of vastus lateralis. IMCL was measured by 1H-magnetic resonance spectroscopy in a subgroup of 25 subjects. Multivariate regression analyses were performed to identify the main predictors of IMCL.
Results: Body fat was the main predictor of IMTG independently of the method and the type of muscle; IMTG concentration was higher in females vs. males and obese vs. nonobese subjects. Muscle DAG and ceramides concentrations were elevated in obese and type 2 diabetic subjects and were not related to body fat and fasting free fatty acids, whereas a direct association with the ratio of diacylglycerol hydrolase to triacylglycerol hydrolase activity (an index of incomplete triacylglycerol hydrolysis) was observed, which explained 54 and 38% of the variance in DAG and ceramides (P < 0.001), respectively. DAG content was the main determinant of insulin resistance.
Conclusions: These data suggest that intramyocellular DAG is an independent predictor of insulin resistance in humans and that its levels correlate with lipolytic enzymes activity in skeletal muscle but not with markers of adiposity.
Intramyocellular diacylglycerol content predicts insulin resistance, and is associated with skeletal muscle lipases activity, but not with markers of adiposity in sedentary individuals.
Type 2 diabetes mellitus (T2DM) is commonly associated with disorders in lipid metabolism including elevated plasma free fatty acid (FFA) concentration and ectopic lipid deposition in multiple peripheral tissues such as skeletal muscle (1,2). Ectopic lipids mainly accumulate as triacylglycerol (TAG). An inverse association between intramyocellular triacylglycerol (IMTG) content and peripheral glucose disposal (measured by euglycemic hyperinsulinemic clamp) has been repeatedly reported (3,4,5). It has been proposed that reduced capacity for skeletal muscle fat oxidation, potentially due to mitochondrial dysfunction could contribute to IMTG accumulation, lipotoxicity, and insulin resistance (6,7). However, evidence for a frank mitochondrial dysfunction in skeletal muscle in obesity and T2DM and its role on muscle lipid accumulation is lacking as recently reviewed (8,9). Alternatively, elevated skeletal muscle FFA uptake could also contribute to elevated IMTG in obesity-associated insulin resistant states. It is still unclear at this point whether skeletal muscle FFA transport is increased in obese and type 2 diabetic subjects with studies showing either an increase (10) or no difference (11,12) compared with lean controls. Thus, the cause of IMTG accumulation in sedentary subjects is poorly understood so far.
It is now accepted that it is not IMTG per se that induces insulin resistance but rather some lipotoxic intermediates such as diacylglycerol (DAG) and ceramides, which alter insulin signaling and action (7,13,14,15). We hypothesized first that IMTG accumulate mainly as a consequence of increased adiposity and second that a dysregulation of IMTG turnover and lipolysis in skeletal muscle could contribute to elevated DAG and ceramides content. Lipolysis is the main catabolic reaction leading to the hydrolysis of one molecule of TAG into three fatty acid molecules (16). High rates of lipolysis and FFA release could contribute to de novo ceramides synthesis by generating palmitoyl-CoA as shown in C2C12 myoblasts overexpressing adipose triglyceride lipase (ATGL) (17). In addition, an inability to completely hydrolyze TAG, due to an imbalance of TAG relative to DAG hydrolase activities, could contribute to increased DAG availability (18). To the best of our knowledge, there are currently no data available on the potential anthropometric and biochemical determinants of intramyocellular lipids (including DAG and ceramides) in humans. In the present study, we aimed first to identify the potential determinants of intramyocellular lipids (TAG, DAG, and ceramides) in sedentary humans and second to evaluate the role of skeletal muscle lipolysis as a determinant of markers of intramyocellular lipotoxicity (DAG and ceramides) and insulin sensitivity.
Subjects and Methods
Subjects
Forty-eight sedentary male (n = 24) and female (n = 24) subjects were recruited in the study. Ten of 48 subjects had T2DM and were treated mainly with sulfonylurea, metformin, or diets. None of them were taking thiazolidinediones. The subjects had a wide range of body composition, age, insulin sensitivity, and metabolic status (Table 1). The subjects were recruited based on a sedentary lifestyle determined by activity index questionnaire and were not enrolled in any structured sports activities. The protocol was approved by the Institutional Review Board of the Pennington Biomedical Research Center, and all volunteers gave written informed consent. After completing the screening visit, total fat mass was measured on a dual-energy x-ray absorptiometer (QDR 4500A; Hologic Inc., Bedford, MA). The participants were asked to refrain from vigorous physical activity for 48 h before presenting to the Pennington inpatient clinic and ate a weight-maintaining diet consisting of 35% fat, 16% protein, and 49% carbohydrate 2 d before the clamp and the muscle biopsy. The muscle biopsy was performed in the morning after a 10- to 12-h overnight fast and before the clamp. Samples of vastus lateralis weighing 60–100 mg were obtained using the Bergstrom technique, blotted, cleaned, and snap frozen in liquid nitrogen (19).
Table 1.
Anthropometric and clinical characteristics of the subjects
| Lean (n = 16) | Obese (n = 32) | Subjects with 1H-MRS data | |
|---|---|---|---|
| n (female/male) | 6/10 | 18/14 | 9/16 |
| Ethnicity (white/black/Asian) | 8/6/2 | 21/8/3 | 14/10/1 |
| Non-T2DM/T2DM | 16/0 | 22/10 | 15/10 |
| Age (yr) | 24 ± 1 | 41 ± 3a | 35.6 ± 2.4 |
| Body weight (kg) | 72.9 ± 3.4 | 90.8 ± 2.7b | 84.2 ± 2.9 |
| BMI (kg/m2) | 24.1 ± 0.7 | 32.4 ± 0.7b | 28.4 ± 1.0 |
| Body fat (%) | 22.3 ± 2.0 | 35.7 ± 1.3b | 27.7 ± 1.6 |
| GDR (mg/min−1 · kg EMBS−1) | 8.3 ± 0.7 | 6.1 ± 0.4b | 9.9 ± 0.6 |
| Fasting glucose (mg/dl) | 85.6 ± 1.7 | 104.6 ± 4.9a | 101 ± 4 |
| Fasting insulin (mU/liter) | 8.7 ± 0.9 | 12.7 ± 1.4a | 10.5 ± 0.9 |
| Fasting FFAs (mmol/liter) | 0.35 ± 0.04 | 0.52 ± 0.03b | 0.44 ± 0.03 |
| Fasting triglycerides (mg/dl) | 98.7 ± 10.9 | 139.1 ± 14.8a | 110 ± 53 |
EMBS, Estimated metabolic body size.
P < 0.05.
P < 0.01 vs. lean.
Hyperinsulinemic euglycemic clamp
Insulin sensitivity was measured by hyperinsulinemic euglycemic clamp. After an overnight fast, insulin (80 mU/m−2 · min−1) and 20% glucose were administered for 2 h to maintain plasma glucose at 90 mg/dl. At this infusion rate, insulin has been previously shown to suppress more than 95% of hepatic glucose output in subjects with and without T2DM, and the glucose disposal is mainly dependent on skeletal muscle (20,21). Plasma levels of glucose and insulin were measured in triplicate at 5-min intervals at baseline and during steady state from 95 to 120 min. Glucose disposal rate (GDR) expressed in mg/min−1 was adjusted for the estimated metabolic body size (kilograms of fat free mass + 17.7) (22). Fat-free mass was calculated as the difference between body weight and total fat mass.
1H-magnetic resonance spectroscopy (MRS)
Intramyocellular lipids (IMCL) were measured in the soleus and tibialis anterior muscles of the right calf by a 1H-MRS technique on a GE Signa Excite 3T. 12.0-m5 build whole-body imaging and spectroscopy system using the Point Resolved Spectroscopy (PRESS) box technique (23). Measurements were acquired with volunteer lying in the supine position with right leg positioned inside a commercially made radiofrequency 1H knee coil with the knee in the extension and ankle in a neutral position. IMCL contents were determined from the average of the sum of three PRESS boxes in the soleus and one PRESS in the tibialis anterior. Summing the signals increases the signal to noise ratio. Peak positions and areas of interest were determined by time domain fitting using Java-based magnetic resonance user interface and a set of prior knowledge files (24,25). Areas of all peaks were normalized to the corresponding internal water peak as previously described (3).
Lipase activity assays
TAG and DAG hydrolase activities were measured on muscle tissue homogenates as previously described (26). Briefly, triolein and 1(3)-mono-oleyl-2-O-mono-oleylglycerol were emulsified with phospholipids by sonication. Triolein is a triglyceride containing three oleic acid specifically used to determine TAG hydrolase (TAGH) activity. 1(3)-mono-oleyl-2-O-mono-oleylglycerol is a DAG analog used to measure specifically the DAG hydrolase (DAGH) activity because it is not a substrate for monoacylglycerol lipase. Lipase activity data were normalized to total protein content determined in each sample and expressed in nanomoles per minute−1 per milligram−1. We also calculated the ratio of DAGH to TAGH activity as a marker of complete TAG hydrolysis.
TAG and DAG determination by gas chromatography/mass spectrometry
Total lipids were extracted from frozen muscle tissue using the method of Folch et al. (27). The extracts were filtered and lipids recovered in the chloroform phase. TAG and DAG were isolated using thin-layer chromatography on Silica Gel 60 A plates developed in petroleum ether, ethyl ether, and acetic acid (80:20:1) and visualized by rhodamine 6G. The TAG and DAG band was scraped from the plate and methylated using BF3/methanol as described by Morrison and Smith (28). The methylated fatty acids were extracted with hexane and analyzed by gas chromatography using an HP 5890 gas chromatograph equipped with flame ionization detectors, an HP 3365 Chemstation, and a capillary column (SP2380, 0.25 mm × 30 m, 0.25 μm film; Supelco, Bellefonte, PA). Helium was used as a carrier gas. The oven temperature was programmed from 160 C to 230 C at 4 C/min. Fatty acid methyl esters were identified by comparing the retention times to those of known standards. Inclusion of the internal standards, 20:1 (trieicosenoin) and 17:0 (diheptadecanoin), permits quantitation of the amount of TAG and DAG in the sample. The absolute quantity of total and each subspecies of TAG and DAG was calculated and expressed in microgram per milligram wet tissue weight.
Ceramides determination by electrospray ionization tandem mass spectrometry
Ceramides was quantified by electrospray ionization tandem mass spectrometry as previously described (29). Briefly, lipid extracts were prepared by the method of Bligh and Dyer (30) in the presence of non-naturally occurring Cer 14:0, Cer 17:0. Samples were analyzed by direct flow injection on a Quattro Ultima triple-quadrupole mass spectrometer (Micromass, Manchester, UK) using a HTS PAL autosampler (Zwingen, Switzerland) and an Agilent 1100 binary pump (Waldbronn, Germany) with a solvent mixture of methanol containing 10 mm ammonium acetate and chloroform [3:1 (vol/vol)]. A flow gradient was performed starting with a flow of 55 μl/min for 6 sec followed by 30 μl/min for 1 min and an increase to 250 μl/min for another 12 sec. Ceramides was analyzed using a fragment of mass to charge ratio 264 with N-heptadecanoyl-sphingosine as internal standard. Both ions [M+H]+ and [M+H-H2O]+ were used and quantification was achieved by calibration lines generated by addition of Cer 16:0, 18:0, 20:0, 24:1, and 24:0 to tissue samples. Correction of isotopic overlap of ceramides species as well as data analysis was performed by self-programmed Excel macros according to the principles described previously (31). The absolute quantity of total and each subspecies of ceramides was calculated and expressed in nanomoles per milligram wet tissue weight.
Statistical analyses
All statistical analyses were performed using SAS 9.1 Service Pack 4 for Windows (SAS Institute Inc., Cary, NC), and figures were generated using GraphPad Prism 5.0 for Windows (GraphPad Software Inc., San Diego, CA). The relationships between intramyocellular lipid content (IMTG, DAG, and ceramides) and anthropometric and clinical variables were analyzed using Spearman rank correlations. Differences in intramyocellular lipids according to gender (female vs. male), obesity (obese vs. nonobese), and interactions were analyzed using the mixed model. Tukey-Kramer post hoc multiple comparison tests were performed to evaluate specific differences between groups. Significant variables were then included in multivariate stepwise regression analyses after ln transformation to achieve normal distribution to identify the best predictors of IMTG, DAG, and ceramides. All values in figures and tables are presented as mean ± sem. Statistical significance was set at P < 0.05.
Results
Subject characteristics
The anthropometric and clinical characteristics of the population are presented in Table 1. As expected, the obese subjects had higher body weight, percentage of body fat, fasting glucose, FFAs, insulin, and triglycerides compared with the lean. Thus, the obese group had reduced whole-body insulin sensitivity as measured by clamp. The obese group was slightly older and included 10 subjects with T2DM (Table 1).
Influence of gender and obesity on IMTG
A significant effect of obesity (P = 0.0094) and gender (P = 0.0004) was found on total IMTG content, with no interaction between gender and obesity (P = 0.43) (Fig. 1). IMTG was higher in females vs. males (8.3 ± 0.9 vs. 3.7 ± 0.4 μg/mg, P < 0.0001) and obese vs. nonobese (6.8 ± 0.7 vs. 2.7 ± 0.4 μg/mg, P = 0.007). Similar findings were observed irrespective to the IMTG subspecies (data not shown). IMCL measured in soleus (P = 0.0001) and tibialis anterior (P = 0.006) by 1H-MRS was also elevated in obese vs. nonobese subjects in a subgroup of 25 subjects (including 15 nonobese and 10 obese).
Figure 1.
Gender difference in total IMTG (A), DAG containing 18:1 fatty acids (B), and saturated ceramide (C) content in lean and obese subjects. Statistical analyses were performed using two-way ANOVA. *, P < 0.05 when compared with lean; #, P < 0.05 when compared with male.
Determinants of IMTG
IMTG was positively correlated with age, body mass index (BMI), body fat, and skeletal muscle TAGH activity (Table 2). Similarly, IMCL both in soleus and tibialis anterior was positively related to age, BMI, body fat, and fasting FFAs (data not shown). Of importance, IMTG was positively correlated with intramyocellular DAG (r = 0.38, P = 0.01) and ceramides (r = 0.51, P = 0.002). We next performed multivariate regression analyses to identify the determinants of IMTG. In a model including age, body fat, gender, obesity, and skeletal muscle TAGH activity as independent variables, body fat was the best predictor of IMTG, explaining 44% of its variance (P < 0.0001) (Table 3). Consistently, using the same regression model, body fat was the main independent determinant of IMCL measured by 1H-MRS in tibialis anterior (r2 = 0.30, P = 0.0054). Body fat remained the best predictor of IMTG in moderately obese subjects with BMI less than 35 kg/m2 (r2 = 0.40, P = 0.0001). The percentage of body fat was a better predictor of IMTG in lean (r2 = 0.70, P = 0.0025) compared with obese subjects (r2 = 0.42, P = 0.02).
Table 2.
Spearman correlations of IMTG, DAG, ceramides, and insulin sensitivity, with various anthropometric and clinical variables
| Independent variables | Dependent variables
|
|||
|---|---|---|---|---|
| IMTG | DAG | Ceramides | GDR | |
| Age (yr) | 0.33a | 0.22 | 0.29 | −0.34 |
| BMI (kg/m2) | 0.32a | 0.12 | 0.22 | −0.48b |
| Body fat (%) | 0.63c | 0.27 | 0.32 | −0.37a |
| Fasting FFAs (mmol/liter) | 0.28 | 0.05 | 0.31 | −0.44a |
| GDR (mg/min−1 · kg EMBS−1) | −0.48b | −0.34a | −0.49b | |
| IMTG (μg/mg) | 0.38a | 0.51b | −0.48b | |
| DAG (μg/mg) | 0.38a | 0.54c | −0.34a | |
| Ceramides (nmol/mg) | 0.51b | 0.54c | −0.49b | |
| Muscle TAGH (nmol/min−1 · mg−1) | 0.42b | 0.34a | 0.44b | −0.04 |
| Muscle DAGH (nmol/min−1 · mg−1) | −0.26 | −0.23 | −0.30 | 0.46b |
| Muscle DAGH/TAGH | −0.57c | −0.50c | −0.44b | 0.36a |
Value are given as the Spearman correlation coefficient.
P < 0.05.
P < 0.01.
P < 0.001.
Table 3.
Determinants of intramyocellular lipids in multivariate stepwise regression analyses
| Dependent variables | Determinants | β | se | Model r2 | Model P |
|---|---|---|---|---|---|
| IMTG | Body fat (%)a | 1.230 | 0.218 | 0.44 | <0.0001 |
| DAG | DAGH/TAGHb | −1.012 | 0.171 | 0.54 | <0.0001 |
| Ceramides | DAGH/TAGHc | −0.428 | 0.099 | 0.38 | 0.0002 |
Independent variables included in the model were age, body fat, obesity, gender, and TAGH activity.
Independent variables included in the model were obesity, gender, ceramides, IMTG, and the ratio of DAGH to TAGH activity.
Independent variables included in the model were obesity, gender, DAG, IMTG, and the ratio of DAGH to TAGH activity.
Influence of gender and obesity on intramyocellular lipases activity
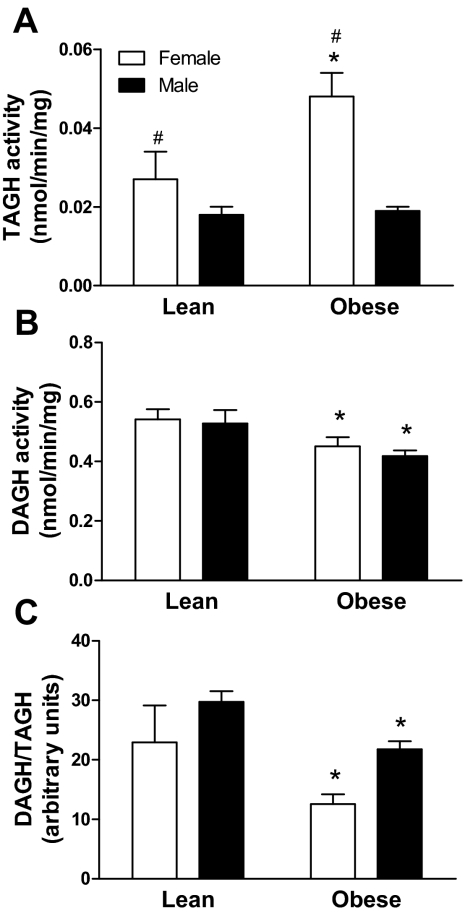
A significant and independent effect of gender (P = 0.015) and obesity (P = 0.037) was found on TAGH activity, with no significant interaction between gender and obesity (P = 0.10). TAGH activity was higher in females vs. males (0.045 ± 0.005 vs. 0.019 ± 0.001 nmol/min−1 · mg−1, P < 0.0001) and obese vs. nonobese (0.035 ± 0.004 vs. 0.021 ± 0.003 nmol/min−1 · mg−1, P < 0.01) (Fig. 2A). DAG hydrolase activity and the ratio of DAGH to TAGH activity were lower in obese vs. nonobese subjects (0.44 ± 0.02 vs. 0.53 ± 0.03 nmol/min−1 · mg−1, P < 0.0001 and 16.4 ± 1.3 vs. 27.7 ± 2.2 arbitrary units, P < 0.0001, respectively). There was no gender difference in DAGH activity and the ratio of DAGH to TAGH activity and no interaction between obesity and gender (Fig. 2, B and C).
Figure 2.
Gender difference in TAGH activity (A), DAGH activity (B), and the ratio of DAGH to TAGH activity (C), in lean and obese subjects. Statistical analyses were performed using two-way ANOVA. *, P < 0.05 when compared with lean; #, P < 0.05 when compared with male.
Influence of gender and obesity on intramyocellular diacylglycerol and ceramides
Intramyocellular DAG and ceramide levels were not different between males and females irrespective of their fatty acid profile. Intramyocellular DAG (0.33 ± 0.05 vs. 0.13 ± 0.01 μg/mg, P < 0.01) and ceramides (0.022 ± 0.002 vs. 0.015 ± 0.002 nmol/mg, P = 0.02) content was elevated in obese vs. nonobese subjects. When we looked specifically into the fatty acid profile, we noticed a significant increase in DAG containing oleic acid (18:1) (P = 0.01) and saturated ceramide concentrations (P < 0.0001) in obese vs. nonobese subjects (Fig. 1, B and C). We found a positive relationship between DAG and ceramides (r = 0.54, P = 0.0009). DAG and ceramides were not correlated with age, BMI, body fat, and fasting FFAs (Table 2). However, these two lipid species were correlated with the skeletal muscle TAGH activity and even stronger with the ratio of DAGH to TAGH activity (Table 2).
Determinants of intramyocellular DAG and ceramides
We next investigated the determinants of intramyocellular DAG concentration. In a model including obesity, gender, IMTG, ceramides, and the ratio of DAGH to TAGH activity as independent variables, the ratio of DAGH to TAGH activity was the strongest determinant of DAG, explaining more than half of the variance (r2 = 0.54, P < 0.0001) (Table 3). Similarly, in a model including obesity, gender, IMTG, DAG, and the ratio of DAGH to TAGH activity as independent variables, ceramide content was best predicted by the ratio of DAGH to TAGH (r2 = 0.38, P = 0.0002) (Table 3). To exclude the confounding effect of T2DM on these relationships, we repeated the analyses in non-T2DM subjects only. The ratio of DAGH to TAGH activity remained the major determinant of skeletal muscle DAG (r2 = 0.65, P < 0.0001) and ceramides (r2 = 0.48, P = 0.0002).
Relationship between intramyocellular lipids and insulin sensitivity
Insulin sensitivity was lower in obese vs. nonobese (P = 0.01) subjects, with no significant effect of gender (P = 0.78) and interaction (P = 0.28). As shown in Table 2, insulin sensitivity was negatively correlated with anthropometric indices of fatness such as BMI, body fat, and fasting FFAs as well as to all three intramyocellular lipid species measured (Table 2). This finding was further supported when IMCL measured by 1H-MRS was also inversely related to insulin sensitivity in soleus (r = −0.48, P = 0.02) and tibialis anterior (r = −0.40, P = 0.06). Importantly, the relationship between IMTG and insulin sensitivity was lost after adjustment for intramyocellular DAG content (r = −0.18, P = 0.36). Of interest, insulin sensitivity was positively correlated with the skeletal muscle DAGH activity and the ratio of DAGH to TAGH (Table 2).
Discussion
In the present study, we investigated for the first time in humans the determinants of intramyocellular lipids including the two insulin resistance-inducing lipid species DAG and ceramides and related these to insulin sensitivity. We found that IMTG is primarily related to the percentage of body fat in sedentary populations, whereas intramyocellular DAG and ceramides are mainly determined within the skeletal muscle by the ratio of DAGH to TAGH activity, a marker of lipolysis, and independent of adiposity. An imbalance of TAGH relative to DAGH activity might contribute to intramyocellular lipotoxicity and insulin resistance. These data suggest a previously underappreciated link between skeletal muscle lipolysis, lipotoxicity, and insulin resistance.
The strength of the present study is that we measured simultaneously all three intramyocellular lipids species (TAG, DAG, and ceramides) in biopsy samples obtained from a well clinically phenotyped population. Of importance, intramyocellular DAG and ceramides were measured using gold standard mass spectrometry methods as previously discussed (29). We also investigated for the first time the hypothesis that skeletal muscle lipase activity might determine intramyocellular lipotoxicity and therefore insulin resistance in the fasted condition. Alternatively, increased plasma FFA fractional extraction by the skeletal muscle during the postprandial condition and a mismatch between FFA uptake and oxidation could likely contribute to lipotoxicity and insulin resistance (8,32). One limitation of the present study could be due to the direct measure of IMTG in vastus lateralis biopsies that could be contaminated by infiltrated adipocytes. However, in support of these findings, we observed similar associations between markers of adiposity and IMCL in a subgroup of subjects in which IMCL was measured by 1H-MRS in soleus and tibialis anterior. Another limitation is that the present study may be underpowered to capture significant differences in intramyocellular DAG and ceramides between groups, especially in subjects with T2DM. Therefore, we strictly focused our analysis on the effect of obesity and gender on intramyocellular lipids and lipase activity.
Since the late 90s, an inverse relationship between IMTG and insulin sensitivity has been reported by several independent groups (3,4,5). Later it was shown that this relationship is valid in sedentary populations but modified by the level of aerobic fitness. Indeed, IMTG is adaptatively increased in the muscle of endurance-trained athletes to sustain muscle activity during long-lasting endurance activities and actually positively predicts insulin sensitivity (33). Several groups demonstrated increased IMTG content in obesity and T2DM and in athletes using various methodologies (11,33,34). Here we show that IMTG is mainly determined by the percentage of body fat in sedentary subjects, regardless of the technique used for its determination [i.e. biochemical (ex vivo) or spectroscopic (in vivo)] and the characteristic of the muscle assessed (i.e. vastus lateralis, tibialis anterior, or soleus). This is consistent with a previous report in which IMTG content in tibialis anterior measured by 1H-MRS was positively related to body fat (33) but in contrast with another report showing no association between IMTG measured from vastus lateralis biopsies and indices of adiposity in Pima Indians (4). The discrepancy might be due to the sample size, the methodology used to measure both body fat and IMTG in skeletal muscle, and/or study population characteristics. It has been hypothesized that increased intramyocellular lipid content could result from increased plasma FFA availability and/or reduced fat oxidation, possibly due to mitochondrial dysfunction (7). In the present study, IMCL measured by 1H-MRS was positively related to fasting FFAs. Increased adiposity and plasma FFA concentrations would therefore be sufficient to increase IMCL content unless skeletal muscle FFA uptake is reduced. This is unlikely because leg muscle FFA fractional uptake is either similar (6) or increased (35) in obese compared with lean individuals. It is then highly possible that IMTG mainly accumulate as a consequence of increased adiposity and plasma FFA availability.
In the present study, we show that intramyocellular DAG and ceramides were not related to any anthropometric parameters, possibly suggesting that lipotoxicity is mainly determined within the skeletal muscle independently of the degree of adiposity. Here we show that the ratio of DAGH to TAGH activity, a marker of incomplete TAG hydrolysis, is a strong determinant of intramyocellular DAG and ceramide content, explaining 54 and 38% of the variance in both lipid species in our population, respectively. A low ratio of DAGH to TAGH activity is associated with elevated intramyocellular lipotoxic lipid species. Mechanistically, this could be driven by a combination of high rates of TAG hydrolysis and reduced DAG hydrolysis in obese subjects. Increased TAG hydrolase activity may contribute to higher fatty acid release and de novo ceramide synthesis by generating palmitoyl-CoA (17). In addition, reduced DAG hydrolase activity contributes to a lower DAG turnover rate and therefore increased DAG availability. Recent studies indicate the presence of ATGL in human skeletal muscle (36,37). These studies suggest that ATGL might play an important role in the regulation of IMTG besides hormone-sensitive lipase. Thus, a reduced DAGH to TAGH activity ratio in obese subjects is consistent with the observation of Jocken et al. (37) showing a reduced hormone-sensitive lipase protein expression and lower forearm glycerol release in obese insulin-resistant subjects. Our study provides a common mechanism of synthesis of DAG and ceramides through the activity of skeletal muscle lipases. This observation requires further functional investigation.
We next examined the relationship between intramyocellular lipids and insulin sensitivity. All three lipid classes (TAG, DAG, and ceramides) were negatively correlated with insulin sensitivity. IMTG irrespective of the fatty acid profile, DAG (18:1), and total saturated ceramide content were significantly elevated in obese. This is in agreement with other studies that have shown a link between elevated intramyocellular DAG and ceramide content and insulin resistance in humans (15,38). There was an independent effect of obesity on all three lipid species. Interestingly, the association between IMTG and insulin sensitivity was lost after adjustment for intramyocellular DAG, suggesting that this bioactive lipid is more mechanistically associated with insulin resistance. For instance, FFA-induced insulin resistance during lipid infusion studies seems to depend mainly on DAG-mediated Ser phosphorylation of insulin receptor susbtrate-1 and inhibition of insulin signaling (14,15). Insulin resistance, which occurs in response to high saturated fat diets and glucocorticoids, depends more on ceramides (39). Thus, ceramide synthesis is not affected by mono- or polyunsaturated fat (13). The difference between those studies might depend on the nature of the fatty acid cocktail used to prepare lipid infusion or diets. However, it is still largely unknown how these lipotoxic intermediates accumulate and whether they contribute to insulin resistance simultaneously. In the present study, DAG and ceramides were both moderately related to each other and to IMTG. However, we found that only DAG was a significant determinant of insulin sensitivity, suggesting that DAG could be more mechanistically related to skeletal muscle insulin resistance in humans as previously discussed (7).
In conclusion, the data show that IMTG might mainly accumulate as a consequence of higher adiposity, whereas markers of intramyocellular lipotoxicity (DAG and ceramides) are determined within the skeletal muscle. The data show that intramyocellular DAG is a significant determinant of insulin resistance in sedentary humans and that its levels are in large part determined by the activity of lipolytic enzymes within the skeletal muscle independently of adiposity. The next step should focus on mechanistic studies to investigate the functional relationship between skeletal muscle lipolysis, intramyocellular lipotoxicity, and insulin resistance. It will also be important to determine whether primary defects in skeletal muscle lipolysis can cause insulin resistance.
Acknowledgments
We are very grateful to Shantele Thomas, Diana Albarado, and Kori Murray for outstanding technical support. A special thank you also goes to the study participants.
Footnotes
This work was supported by pilot and feasibility Grant 340-40-0123 from the Biomedical Research Center (to C.M.), Grant 2003-34323-14010 from the U.S. Department of Agriculture, Grant P30-DK072476 from the Clinical Nutrition Research Unit, National Institutes of Heath (NIH) (to S.R.S.), NIH Grant T32-DK064584 (to L.L.), and the Commission of the European Communities (Integrated Project HEPADIP, http://www.hepadip.org/), Contract LSHM-CT-2005-018734 (to D.L.). We also thank the Hormone Assay and Analytical Services Core and Vanderbilt Diabetes Research and Training Center supported by NIH Grant DK20593 for TAG and DAG analyses. J.E.G. is supported by a fellowship from the International Nutrition Foundation/Ellison Medical Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 16, 2009
Abbreviations: ATGL, Adipose triglyceride lipase; BMI, body mass index; DAG, diacylglycerol; DAGH, DAG hydrolase activity; FFA, free fatty acid; GDR, glucose disposal rate; IMCL, intramyocellular lipids; IMTG, intramyocellular triacylglycerol; MRS, magnetic resonance spectroscopy; PRESS, Point Resolved Spectroscopy; TAG, triacylglycerol; TAGH, TAG hydrolase activity; T2DM, type 2 diabetes mellitus.
References
- McGarry JD 2002 Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA 2004 Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 88:787–835, ix [DOI] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI 1999 Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42:113–116 [DOI] [PubMed] [Google Scholar]
- Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH 1997 Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46:983–988 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L 1999 Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H–13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48:1600–1606 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA 1999 Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277:E1130–E1141 [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI 2006 Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgani JE, Moro C, Ravussin E 2008 Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 295:E1009–E1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO 2009 Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 89:463S–466S [DOI] [PubMed] [Google Scholar]
- Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ 2004 Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18:1144–1146 [DOI] [PubMed] [Google Scholar]
- Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, Cooney GJ, Hawley JA 2003 Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab 88:5444–5451 [DOI] [PubMed] [Google Scholar]
- Pelsers MM, Tsintzas K, Boon H, Jewell K, Norton L, Luiken JJ, Glatz JF, van Loon LJ 2007 Skeletal muscle fatty acid transporter protein expression in type 2 diabetes patients compared with overweight, sedentary men and age-matched, endurance-trained cyclists. Acta Physiol (Oxf) 190:209–219 [DOI] [PubMed] [Google Scholar]
- Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA 2003 A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 278:10297–10303 [DOI] [PubMed] [Google Scholar]
- Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI 1999 Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G 2002 Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51:2005–2011 [DOI] [PubMed] [Google Scholar]
- Arner P, Langin D 2007 The role of neutral lipases in human adipose tissue lipolysis. Curr Opin Lipidol 18:246–250 [DOI] [PubMed] [Google Scholar]
- Watt MJ, van Denderen BJ, Castelli LA, Bruce CR, Hoy AJ, Kraegen EW, Macaulay L, Kemp BE 2008 Adipose triglyceride lipase regulation of skeletal muscle lipid metabolism and insulin responsiveness. Mol Endocrinol 22:1200–1212 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moro C, Bajpeyi S, Smith SR 2008 Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 294:E203–E213 [DOI] [PubMed] [Google Scholar]
- Bergstrom J 1975 Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35:609–616 [PubMed] [Google Scholar]
- Bonadonna RC, Groop L, Kraemer N, Ferrannini E, Del Prato S, DeFronzo RA 1990 Obesity and insulin resistance in humans: a dose-response study. Metabolism 39:452–459 [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Mandarino LJ, Gerich JE 1988 Quantification of the relative impairment in actions of insulin on hepatic glucose production and peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Metabolism 37:15–21 [DOI] [PubMed] [Google Scholar]
- Lillioja S, Bogardus C 1988 Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev 4:517–540 [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR 2006 Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 14:73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D 2001 Java-based graphical user interface for the MRUI quantitation package. Magma 12:141–152 [DOI] [PubMed] [Google Scholar]
- Rico-Sanz J, Thomas EL, Jenkinson G, Mierisova S, Iles R, Bell JD 1999 Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J Appl Physiol 87:2068–2072 [DOI] [PubMed] [Google Scholar]
- Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Ryden M, Arner E, Sicard A, Jenkins CM, Viguerie N, van Harmelen V, Gross RW, Holm C, Arner P 2005 Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 54:3190–3197 [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH 1957 A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509 [PubMed] [Google Scholar]
- Morrison WR, Smith LM 1964 Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 5:600–608 [PubMed] [Google Scholar]
- Liebisch G, Drobnik W, Reil M, Trumbach B, Arnecke R, Olgemoller B, Roscher A, Schmitz G 1999 Quantitative measurement of different ceramide species from crude cellular extracts by electrospray ionization tandem mass spectrometry (ESI-MS/MS). J Lipid Res 40:1539–1546 [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ 1959 A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917 [DOI] [PubMed] [Google Scholar]
- Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G 2004 High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim Biophys Acta 1686:108–117 [DOI] [PubMed] [Google Scholar]
- Corpeleijn E, Saris WH, Blaak EE 2009 Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev 10:178–193 [DOI] [PubMed] [Google Scholar]
- Thamer C, Machann J, Bachmann O, Haap M, Dahl D, Wietek B, Tschritter O, Niess A, Brechtel K, Fritsche A, Claussen C, Jacob S, Schick F, Haring HU, Stumvoll M 2003 Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 88:1785–1791 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE 2001 Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86:5755–5761 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Simoneau JA 1994 Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsted TJ, Nybo L, Schweiger M, Fledelius C, Jacobsen P,Zimmermann R, Zechner R, Kiens B 2009 Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am J Physiol Endocrinol Metab 296:E445–E453 [DOI] [PubMed] [Google Scholar]
- Jocken JW, Roepstorff C, Goossens GH, van der Baan P, van Baak M, Saris WH, Kiens B, Blaak EE 2008 Hormone-sensitive lipase serine phosphorylation and glycerol exchange across skeletal muscle in lean and obese subjects: effect of β-adrenergic stimulation. Diabetes 57:1834–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams 2nd JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ 2004 Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31 [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA 2007 Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5:167–179 [DOI] [PubMed] [Google Scholar]