Abstract
Context: Children with calcium-deficiency rickets have high 1,25-dihydroxyvitamin D values.
Objective: The objective of the study was to determine whether vitamin D increased calcium absorption.
Design: This was an experimental study.
Setting: The study was conducted at a teaching hospital.
Participants: Participants included 17 children with nutritional rickets.
Intervention: The participants were randomized to 1.25 mg oral vitamin D3 (n = 8) or vitamin D2 (n = 9).
Main Outcome Measure: Fractional calcium absorption 3 da after vitamin D administration was measured.
Results: Mean baseline 25-hydroxyvitamin D concentrations were 20 ng/ml (range 5–31 ng/ml). The increase in 25-hydroxyvitamin D was equivalent after vitamin D3 (29 ± 10 ng/ml) or vitamin D2 (29 ± 17 ng/ml). Mean 1,25-dihydroxyvitamin D values increased from 143 ± 76 pg/ml to 243 ± 102 pg/ml (P = 0.001), and the increase in 1,25-dihydroxyvitamin D did not differ between vitamin D2 and vitamin D3 (107 ± 110 and 91 ± 102 ng/ml, respectively). The increment in 1,25-dihydroxyvitamin D was explained almost entirely by the baseline 25-hydroxyvitamin D concentration (r2 = 0.72; P < 0.001). Mean fractional calcium absorption did not differ before (52.6 ± 21.4%) or after (53.2 ± 23.5%) vitamin D, and effects of vitamin D2 and vitamin D3 on calcium absorption were not significantly different. Fractional calcium absorption was not closely related to concentrations of 25-hydroxyvitamin D (r = 0.01, P = 0.93) or 1,25-dihydroxyvitamin D (r = 0.21, P = 0.24). The effect of vitamin D on calcium absorption did not vary with baseline 25-hydroxyvitamin D values or with the absolute increase in 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D values.
Conclusions: Despite similar increases in 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D with vitamin D2 or vitamin D3, fractional calcium absorption did not increase, indicating that rickets in Nigerian children is not primarily due to vitamin D-deficient calcium malabsorption.
Despite an equivalent increase in 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D after vitamin D2 or vitamin D3, no augmentation of fractional absorption of calcium absorption occurs.
Vitamin D fortification of foods is largely responsible for the near disappearance of nutritional rickets in North America and Europe in the last century. However, nutritional rickets continues to occur in many tropical countries despite abundant sunlight exposure, and a resurgence of the disease has been noted in developed countries, where the disease was thought to have been eradicated.
Nutritional rickets in Nigerian children is associated with low dietary calcium intake, and the disease responds well to treatment with calcium with or without vitamin D (1). Nigerian children with nutritional rickets have similar values for calcium absorption compared with those of control children, with mean fractional absorption values of 60% or greater (2,3,4). The role of vitamin D in mediating the efficiency of calcium absorption in Nigerian children with rickets has not been clarified.
Vitamin D is hydroxylated in the liver to 25-hydroxyvitamin D, and serum concentrations of this metabolite are considered the optimal indicator of vitamin D status. Circulating 25-hydroxyvitamin D is subsequently metabolized in the kidney to 1,25-dihydroxyvitamin D, which acts on vitamin D receptors in the intestine to increase active calcium absorption. Surprisingly, very few human data demonstrate the effects of supplemental vitamin D and its analogs on intestinal calcium absorption, particularly in humans with rickets or osteomalacia.
Children with nutritional rickets due to calcium deficiency have high serum 1,25-dihydroxyvitamin D values, suggesting a compensatory response to maximize calcium absorption. Even so, serum values of 1,25-dihydroxyvitamin D increase nearly 2-fold in response to vitamin D, with peak values occurring 3 d after a single oral dose (5). The increase in 1,25-dihydroxyvitamin D in response to vitamin D implies that there is also an element of coexisting vitamin D deficiency, such that when substrate 25-hydroxyvitamin D is available, the result is a marked increase in 1,25-dihydroxyvitamin D. Dietary calcium deficiency likely increases the requirement for vitamin D, even when 25-hydroxyvitamin D concentrations are considered adequate. Whether the increase in 1,25-dihydroxyvitamin D that can be measured with vitamin D supplementation is associated with a measurable increase in fractional calcium absorption is unknown.
Data from certain studies in adults indicate that calcium absorption increases with increasing 25-hydroxyvitamin D values (6,7,8), but this relationship was not demonstrated in other studies in adolescents (9) and adults (10,11). Instead there were positive correlations between calcium absorption and 1,25-dihydroxyvitamin D values (9,11). In Nigerian children with rickets and healthy control children, fractional calcium absorption was unrelated to either 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D concentrations (2,3,4).
Although vitamin D supplementation increases the serum concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, the effect of supplemental vitamin D may depend on the form of vitamin D given. Vitamin D3 is the endogenous form of vitamin D produced by keratinocytes in the skin in response to UVB radiation from sunlight. Vitamin D2 is produced by irradiation of plant ergosterol and is commonly used for supplementation and food fortification. Both forms of vitamin D are hydroxylated in the liver to 25-hydroxyvitamin D. Because this step is not tightly regulated, serum 25-hydroxyvitamin D is considered the primary indicator of vitamin D status. It has been contended that vitamin D3 is superior to vitamin D2 in sustaining adequate 25-hydroxyvitamin D values in adults (12,13) because 25-hydroxyvitamin D2 may bind less avidly to vitamin D binding protein and be cleared more rapidly than 25-hydroxyvitamin D3. However, a recent report indicated that both forms of vitamin D are equally effective in maintaining 25-hydroxyvitamin D levels (14). There are no data comparing the effect of vitamin D2 and vitamin D3 on intestinal calcium absorption.
The relative roles of calcium and vitamin D nutrition in the etiology of rickets in Nigerian children are uncertain. The primary objective of this study was to test the hypothesis that vitamin D supplementation in calcium-deficiency rickets augments calcium absorption. An additional objective was to determine whether vitamin D3 resulted in a greater increase in calcium absorption than vitamin D2. We conducted a randomized controlled trial to determine the acute effects of oral supplementation with vitamin D on intestinal calcium absorption in Nigerian children with rickets and whether the response differed between vitamin D2 and vitamin D3.
Subjects and Methods
Prepubertal children with clinical signs of rickets were recruited from the outpatient department of the Jos University Teaching Hospital (Jos, Nigeria). Subjects required a radiological score of at least 1.5 on a previously validated 10-point scale for assessing the severity of childhood rickets (15). The score is based on the degree of growth plate widening, indicated by cupping and lucency of the long bone metaphyses in radiographs of the wrists and the knees. Children were also required to have achieved bladder control sufficient to allow for a 24-h urine collection.
The Ethical Review Committee of Jos University Teaching Hospital and the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals approved the protocol. The study protocol was explained to potential subjects and their parents in Hausa or English (as they understood), and informed written consent was obtained in all cases.
Using stable isotope methods, fractional calcium absorption was determined at baseline and 3 d after vitamin D administration. We randomly assigned eligible subjects by lottery method to either vitamin D3 (cholecalciferol; Bio-Tech, Fayetteville, AR) or vitamin D2 (ergocalciferol; Pliva, Inc., East Hanover, NJ) as a single oral dose of 1.25 mg (50,000 IU).
For the baseline study, subjects were given a typical Nigerian meal of 150 ml (233 g wet weight) of maize porridge and 50 ml of orange juice to which 120 mg calcium (as calcium glubionate) and 20 μg 46Ca (as calcium chloride) were added. The orange juice containing the isotopes was given after half the porridge had been consumed, and the cup was rinsed with an additional 20 ml of orange juice that the child drank. The remaining porridge was consumed after the orange juice. An iv butterfly needle was inserted to draw a blood sample. After withdrawing blood, 0.5 mg 48Ca was infused slowly, followed by flushing of the line with 5 ml of saline.
A complete urine collection was started immediately before the administration of the isotopes and continued for 24 h. The children remained supervised in the hospital until the urine collection was completed.
Four days after the baseline study, a single oral dose of vitamin D2 or D3 was given under direct observation. Three days after the oral dose of vitamin D (1 wk after the baseline study), calcium absorption was determined again. We chose to measure calcium absorption 3 d after vitamin D administration because this is the time when 1,25-dihydroxyvitamin D is maximal and would be most likely to have an effect on calcium absorption. In the second absorption study, 46Ca (12 μg) was given iv, and 42Ca (1.8 mg) was given orally, following the same procedure and identical diet as in the first study. In previous studies (Abrams, S.A., unpublished data) with the doses used in this study, there is no measurable residual concentration of oral 46Ca 7 d after dosing. Thus, we considered it unnecessary to correct for residual isotope enrichment in the second absorption study.
Serum samples were stored at −70 C until they were transported on ice for biochemical analysis to the Mayo Clinic (Rochester, MN). Serum alkaline phosphatase, phosphorus, and calcium were measured on a Roche/Hitachi MODULAR System (Roche Diagnostics, Basel, Switzerland). Serum albumin was measured with a Roche/Hitachi 912 automatic analyzer (Roche Diagnostics). Measurements of serum cholecalciferol (vitamin D3), ergocalciferol (vitamin D2), 25-hydroxyvitamin D3, and 25-hydroxyvitamin D2 were made by isotope-dilution liquid chromatography tandem mass spectrometry on an API 4000 instrument (Applied Biosystems, Forest City, CA), with sample introduction performed by a cohesive four-channel multiplexed system (Thermo-Fisher, Waltham, MA). Total serum 25-hydroxyvitamin D was the sum of measured 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 values. Measurements of serum 1,25-dihydroxyvitamin D were performed using a 125I RIA (DiaSorin, Stillwater, MN). The lower limits of detection were 1 ng/ml (2.5 nmol/liter) for serum vitamin D2 and vitamin D3 and 5 ng/ml (12.5 nmol/liter) for serum 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. Based on our previous studies, plasma PTH was not measured because it does not vary with changes in calcium absorption or with vitamin D administration (2,3,4,5).
Urine samples were transported frozen to the Children’s Nutrition Research Center at the Texas Medical Center. Calcium isotope ratios were initially measured as previously described using magnetic sector thermal ionization mass spectrometry (MAT 261; Thermo Scientific, Bremen, Germany) after purification by oxalate precipitation (4,16). However, because of the extremely low 24-h urine calcium excretion (<1 mg) in these children, calcium isotope ratios in the urine samples were subsequently measured in calcium extracted from larger volumes of urine (20 ml) using magnetic sector thermal ionization mass spectrometry (Thermoquest; Triton TI, Bremen, Germany) at the Division of Nutritional Sciences of Cornell University (17). The methodology used for the collection of isotope ratio data are the same for both mass spectrometers, and the relative sds of the isotope ratios measures are typically 0.1–0.2%. This corresponds to a relative sd of approximately 1% for the actual measured absorption at usual doses and absorption values.
Data entry and statistical analysis were performed with Epi Info 3.2 (Centers for Disease Control and Prevention, Atlanta, GA) and Excel 2003 (Microsoft Corp., Redmond, WA). Mean values of normally distributed continuous variables were compared between the two absorption studies with a paired t test. Medians and ranges are reported for nonnormally distributed variables, which were compared using the Mann-Whitney test. Proportions were compared with the χ2 or Fischer exact test, as appropriate. P <0.05 was considered significant.
Results
A total of 17 Nigerian children with rickets, ages 2–10 yr, were enrolled, and eight were randomly assigned to receive vitamin D3 and nine to receive vitamin D2. The characteristics of the two groups were similar (Table 1). Among all children enrolled, the mean daily dietary calcium intakes were 182 ± 73 mg, and mean baseline serum 25-hydroxyvitamin D concentrations were 20 ng/ml (range 5–31 ng/ml) [50 nmol/liter (range 12–80 nmol/liter)]. Two subjects (12%) had 25-hydroxyvitamin D values in the vitamin D deficient range, less than 12 ng/ml (30 nmol/liter). Baseline alkaline phosphatase concentrations were negatively related to 25-hydroxyvitamin D values (r = −0.66, P = 0.005). Serum 25-hydroxyvitamin D values were unrelated to reported daily sun exposure. Baseline 24-h urinary calcium excretion values were very low, with a median of 0.67 mg (range 0.02–7.6 mg).
Table 1.
Baseline characteristics of study subjectsa
| Characteristic | All subjects (n = 17) | Vitamin D2 group (n = 9) | Vitamin D3 group (n = 8) |
|---|---|---|---|
| Age (months) | 44.5 (28–118) | 44.5 (28–66) | 45.6 (32.5–118) |
| Sex (male/female) | 6/11 | 2/7 | 4/4 |
| Familial rickets | 8 (47%) | 4 (44%) | 4 (50%) |
| Daily sun exposure (h) | 3.25 (0.5–8) | 2.75 (1–8) | 3.5 (0.5–5) |
| Height-for-age z-score | −3.6 (−7.7 to −0.1) | −3.6 (−7.7 to −1.8) | −3.5 (−4.5 to −0.1) |
| Weight-for-height z-score | 0.3 (−1.0 to 1.6) | 0.3 (−1.0 to 1.6) | 0.2 (−0.9 to 0.8) |
| Weight-for-age z-score | −1.9 (−4.6 to −0.3) | −2.0 (−4.6 to −0.3) | −1.6 (−3.1 to −0.3) |
| Daily calcium intake (mg) | 172 (56–309) | 172 (56–261) | 186 (82–309) |
| Radiographic score | 2.75 (1.5–10) | 3.5 (1.5–10) | 2.25 (1.5–8) |
| Serum biochemistriesb | |||
| Calcium (mg/dl), reference range 9.6–10.6 | 8.8 ± 1.0 | 8.6 ± 1.1 | 9.0 ± 1.0 |
| Phosphorus (mg/dl), reference range 3.7–5.4 | 4.0 ± 1.1 | 3.6 ± 0.8 | 4.5 ± 1.3 |
| Albumin (g/dl), reference range 3.5–5.0 | 4.2 ± 0.4 | 4.2 ± 0.3 | 4.1 ± 0.5 |
| Alkaline phosphatase (U/liter), reference range 149–476 | 686 (182–2476) | 751 (182–2476) | 447 (205–1055) |
| 25-Hydroxyvitamin D (ng/ml), optimal range 25–80 | 20 (5–32) | 18 (10–32) | 23 (5–32) |
| 1,25-Dihydroxyvitamin D (pg/ml), reference range24–86 | 143 ± 76 | 156 ± 74 | 130 ± 82 |
Values for nonnormally distributed continuous variables are shown as medians with range in parentheses. Normally distributed variables are shown as means ± sd.
To convert values of serum calcium to millimoles per liter, multiply by 0.25; to convert values of serum phosphorus to millimoles per liter, multiply by 0.32; to convert values of albumin to grams per liter, multiply by 10; to convert values of 25-hydroxyvitamin D to nanomoles per liter, multiply by 2.5; to convert values of 1,25-dihydroxyvitamin D to picomoles per liter, multiply by 2.4. Pediatric reference ranges are provided for the age range of children in the study.
At baseline, all subjects had undetectable values of serum vitamin D2, and one had a detectable 25-hydroxyvitamin D2 value of 12 ng/ml (30 nmol/liter), presumably due to recent ingestion of a vitamin D2-fortified food or supplement that was not recalled by the parent. Three had detectable serum vitamin D3 levels [1.6, 1.7, and 15.3 ng/ml (4.2, 4.4, and 40 nmol/liter)] at baseline.
Three days after oral vitamin D, the increase in serum vitamin D3 [52 ± 22 ng/ml (135 ± 57 nmol/liter)] in the vitamin D3 group and the increase in vitamin D2 [48 ± 18 ng/ml (125 ± 47 nmol/liter)] in the vitamin D2 group were equivalent, indicating that the absorption of both drugs was similar. The increase in total serum 25-hydroxyvitamin D was equivalent after administration of vitamin D3 [29 ± 10 ng/ml (72 ± 25 nmol/liter)] or vitamin D2 [29 ± 17 ng/ml (72 ± 42 nmol/liter)]. Mean 1,25-dihydroxyvitamin D values increased from 143 ± 76 pg/ml (343 ± 182 pmol/liter) to 243 ± 102 pg/ml (583 ± 245 pmol/liter) (P = 0.001), and the increase in 1,25-dihydroxyvitamin D did not differ between vitamin D2 and vitamin D3 [107 ± 110 and 91 ± 102 ng/ml (257 ± 264 and 218 ± 245 nmol/liter), respectively].
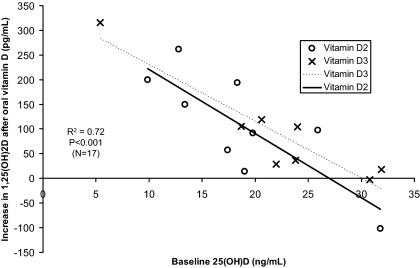
The degree of rise in 1,25-dihydroxyvitamin D values was explained almost entirely by the baseline 25-hydroxyvitamin D concentration (Fig. 1; r2 = 0.72; P < 0.001). The intersection of the regression lines with the x-axis suggests that with 25-hydroxyvitamin D values of 25–30 ng/ml (63–75 nmol/liter), there appears to be little increase in 1,25-dihydroxyvitamin D in response to additional vitamin D. However, there is some variability among subjects with some exhibiting no increase with baseline 25-hydroxyvitamin D values less than 20 ng/ml (50 nmol/liter) and others with a significant rise in 1,25-dihydroxyvitamin D with baseline 25-hydroxyvitamin D values above 25 ng/ml (63 nmol/liter). The increase in serum calcium was similar with vitamin D3 [0.29 ± 0.34 mg/dl (0.072 ± 0.085 mmol/liter)] and vitamin D2 [0.41 ± 0.51 mg/dl (0.10 ± 0.13 mmol/liter); P = 0.57] and significantly greater than baseline values (P = 0.004). The absolute increase in 1,25-dihydroxyvitamin D was greater in those with low baseline 1,25-dihydroxyvitamin D values, but this relationship was not significant (r2 = 0.15, P = 0.13), unlike the relationship with 25-hydroxyvitamin D.
Figure 1.
Change in 1,25-dihydroxyvitamin D in response to supplementation with 50,000 IU oral vitamin D2 or D3. The intersections of the regression lines with the x-axis suggests that with 25-hydroxyvitamin D values of 25–30 ng/ml (63–75 nmol/liter), there appears to be little increase in 1,25-dihydroxyvitamin D in response to additional vitamin D.
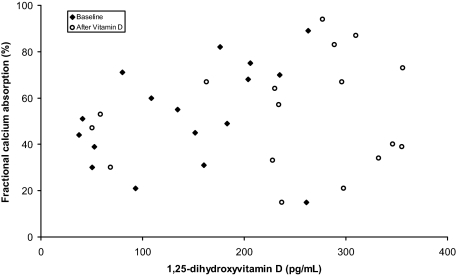
Fractional calcium absorption values were normally distributed but showed a wide variation (ranges 15–89 and 15–94% at baseline and after vitamin D administration, respectively). Mean fractional calcium absorption did not differ before (52.6 ± 21.4%) or after (53.2 ± 23.5%) vitamin D administration (P = 0.93), and there was no significant difference between the effect of vitamin D2 and vitamin D3 on calcium absorption (Table 2). The median 24-h urinary calcium excretion did not differ before (0.67 mg) or after (0.59 mg) vitamin D administration. Fractional absorption of calcium was not closely related to concentrations of 25-hydroxyvitamin D (r = 0.01, P = 0.93) or 1,25-dihydroxyvitamin D (r = 0.21, P = 0.24). Because the baseline relationships may reflect chronic exposure to a given vitamin D status and may differ from those after vitamin D supplementation, we also examined relationship of 1,25-dihydroxyvitamin D with calcium absorption before and after vitamin D administration (Fig. 2). Fractional calcium absorption was not significantly related to 1,25-dihydroxyvitamin D either before (r = 0.34, P = 0.19) or after (r = 0.16, P = 0.54) vitamin D administration. The two children with baseline 25-hydroxyvitamin D values less than 12 ng/ml (30 nmol/liter) had fractional calcium absorption values of 51 and 44%. The effect of vitamin D on calcium absorption did not vary with baseline 25-hydroxyvitamin D values or the absolute increase of either 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D concentrations.
Table 2.
Fractional calcium absorption and urinary calcium excretion in 17 Nigerian children with ricketsa
| Variable | Baseline | After vitamin D |
|---|---|---|
| Fractional calcium absorption (%) | ||
| Combined groups | 52.6 ± 21.4 | 53.2 ± 23.5 |
| Vitamin D2 group | 57.9 ± 23.5 | 51.4 ± 22.8 |
| Vitamin D3 group | 46.8 ± 18.6 | 55.1 ± 25.6 |
| Urinary calcium excretion (mg per 24 h) | ||
| Combined groups | 0.67 (0.02–7.6) | 0.59 (0.05–5.3) |
| Vitamin D2 group | 0.39 (0.02–7.6) | 0.59 (0.07–5.3) |
| Vitamin D3 group | 0.78 (0.35–3.3) | 0.75 (0.05–5.3) |
Values for nonnormally distributed continuous variables are shown as medians with range in parentheses. Normally distributed variables are shown as means ± sd.
Figure 2.
Relationship of calcium absorption to serum 1, 25-dihydroxyvitamin D.
Discussion
This is the first study to examine the effect of both vitamin D2 and vitamin D3 on calcium absorption in children with rickets using validated stable isotope techniques. We were unable to demonstrate a significant effect of pharmacological doses of either vitamin D2 or vitamin D3 on fractional calcium absorption, despite a significant increase in 1,25-dihydroxyvitamin D with both forms of vitamin D. We could not distinguish the effects of vitamin D2 from vitamin D3 on subsequent vitamin D metabolite responses.
An increase in 1,25-dihydroxyvitamin D in response to oral vitamin D has been used to indicate vitamin D inadequacy (5,18,19). Despite the marked rise in 1,25-dihydroxyvitamin D in response to oral vitamin D, consistent with vitamin D inadequacy, no augmentation of fractional absorption of calcium absorption occurred. At 25-hydroxyvitamin D values above 25–30 ng/ml (63–75 nmol/liter), there was a minimal increase in 1,25-dihydroxyvitamin D in response to vitamin D in children with nutritional rickets. There are inadequate data in children to identify the concentration of 25-hydroxyvitamin D that indicates vitamin D sufficiency. Our data suggest that 25-hydroxyvitamin D values above 25–30 ng/ml (63–75 nmol/liter) may be considered adequate in young children, similar to findings in adults (7,20,21). PTH is the major hormonal stimulus of renal 1α-hydroxylase, which converts 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D. Serum PTH levels may have been greater in those with lower 25-hydroxyvitamin D, but we did not demonstrate this in our previous studies (3,5).
Vitamin D-dependent calcium absorption is regarded as critical in situations in which the dietary calcium intakes are very low. Why did vitamin D fail to increase calcium absorption in children with nutritional rickets and low dietary calcium intakes? There are several possible explanations. Because these children had high baseline 1,25-dihydroxyvitamin D values, intestinal vitamin D receptors may have been maximally saturated and stimulated by 1,25-dihydroxyvitamin D at baseline. This is consistent with their high mean baseline fractional calcium absorption of more than 50%, which is greater than the mean fractional absorption of 36% reported among nonrachitic U.S. children (aged 3–5 yr), consuming calcium intakes of approximately 500 mg/d (22). The fractional calcium absorption values observed in the Nigerian children are comparable with those reported among U.S. adolescent girls consuming acute intakes of approximately 300 mg/d (23). It is possible that a positive effect of vitamin D on calcium absorption would be observed in children with very low baseline 25-hydroxyvitamin D values associated with low fractional calcium absorption.
Calcium absorption has been demonstrated to be reduced in adults only when the 25-hydroxyvitamin D concentrations fall to severely deficient values less than 5 ng/ml (12.5 nmol/liter), below which 1,25-dihydroxyvitamin D values also fall (24). Calcium absorption was unrelated to 25-hydroxyvitamin D values above 5 ng/ml (12.5 nmol/liter). Only one child in our study had a 25-hydroxyvitamin D value of 5 ng/ml (12.5 nmol/liter). Nigerian children primarily have nutritional rickets as a result of calcium deficiency, rather than vitamin D deficiency (1). This may explain why we did not observe a reduced calcium absorption in these children with nutritional rickets.
Other evidence is consistent with our findings. In a study of adult men, no change in calcium absorption or calcium excretion was demonstrated with seasonal changes in 25-hydroxyvitamin D values (10). Supplemental vitamin D2 did not augment fractional calcium absorption in elderly women with vitamin D insufficiency who received calcium supplementation (25). However, improvement in fractional calcium absorption with oral 25-hydroxyvitamin D3 administration has been demonstrated in elderly adults with impaired calcium absorption (26). In young adolescents, a significant positive relation to calcium absorption was found for serum 1,25-dihydroxyvitamin but not for 25-hydroxyvitamin D (9). Similar findings have been recently reported in adult women (11). Heaney et al. (6) demonstrated increases in calcium absorption in adult men mediated by both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, with approximately one eighth of vitamin D-like absorptive activity residing in 25-hydroxyvitamin D. In healthy women, dietary fat, dietary fiber, serum 1,25-dihydroxyvitamin D, and alcohol consumption were independent predictors of calcium absorption (27).
The major putative effect of vitamin D metabolites is to increase active intestinal calcium absorption, particularly under conditions of low calcium intakes. However, studies of the effect of vitamin D on calcium absorption in children are lacking. Previous work was limited by the observational cross-sectional design, examining the relationship of calcium absorption with concentrations of 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D (7,9,10,11,24). In Chinese children between the ages of 9 and 17 yr, an unexpected negative relationship between 25-hydroxyvitamin D and fractional calcium absorption was observed (28). In a U.S. study, black adolescent girls had lower 25-hydroxyvitamin D concentrations than white girls, but calcium absorption and retention were unrelated to 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D concentrations (29). In a group of children with arthritis, supplemental vitamin D3, 2000 IU/d for 6 months, had no effect on calcium absorption, irrespective of whether it was given with supplemental calcium (30). One strength of our study is it is the first to compare directly the effect of both vitamin D2 and vitamin D3 administration on calcium absorption in children.
One potential limitation of our study is that 3 d may have been insufficient time to permit maximal up-regulation of duodenal calcium absorption after vitamin D administration. Calcium absorption across the intestinal epithelial cell consists of three components. The first step is the transfer of calcium ions across the brush border membrane through the calcium channel transient receptor potential vanilloid type 6 (TRPV6). This is followed by intracellular diffusion of calcium to the basolateral membrane facilitated by the calcium binding proteins calbindin-D9k and calbindin-D28k. The final step is transfer of calcium across the basolateral membrane by the ATPase PMCA1b. The rate-limiting step of calcium absorption is mediated by the calcium channel TRPV6 localized on the brush border membrane of intestinal epithelium (31). TRPV6 contains a vitamin D response element such that after binding of 1,25-dihydroxyvitamin D with the vitamin D receptor in the intestinal epithelial cell and activation of transcription, intestinal TRPV6 expression increases rapidly over 24 h (32). Thus, it is unlikely that measuring calcium absorption 3 d after administration of vitamin D would not allow sufficient time to detect an effect of vitamin D on calcium absorption. However, more recent data demonstrate that in TRPV6 and calbindin-D9k knockout mice, intestinal calcium transport in response to 1,25-dihydroxyvitamin D can even occur in the absence of TRPV6 and calbindin-D9k, and the molecular mechanisms of 1,25-dihydroxyvitamin D-mediated calcium absorption remain to be fully elucidated (33,34).
Vitamin D2 and vitamin D3 appear to be bioequivalent in their effects on calcium and vitamin D homeostasis in children with nutritional rickets. However, because of the small size of our vitamin D2 and vitamin D3 subgroups, we could detect only a significant difference of at least 24% in fractional calcium absorption with 80% power. Previous studies suggested that after 3 d, 25-hydroxyvitamin D2 may be subject to more rapid degradation and less avid binding to vitamin D binding protein than 25-hydroxyvitamin D3 (13). Because we did not continue to measure vitamin D metabolites beyond 3 d, we are unable to confirm a more rapid decline in 25-hydroxyvitamin D2 compared with 25-hydroxyvitamin D3 after 3 d. Daily intake of vitamin D mitigates these potential differences in vitamin D2 and vitamin D3 metabolism (14).
We conclude that vitamin D does not significantly augment calcium absorption in children with nutritional rickets due to inadequate calcium intake. The short-term effects of vitamin D2 and D3 on calcium and vitamin D homeostasis appear to be bioequivalent in this group of children with nutritional rickets. Our data provide further support that rickets in Nigerian children is not primarily due to vitamin D-deficient calcium malabsorption. These data also challenge the assumption that vitamin D will increase calcium absorption in the setting of a low calcium intake, in the absence of severe vitamin D deficiency. Additional studies to determine the conditions under which vitamin D augments calcium absorption in children and adults are warranted. Evaluation of the functional effects of vitamin D, like fractional calcium absorption, will play a critical role in defining the requirements for vitamin D in children.
Acknowledgments
The authors are grateful for the assistance of Livinia Dangiwa, Emmanuel Silas, Dorothy Aku, Elizabeth Yohanna, Oda Bimma, and Dr. Joseph Dabit in collecting urine and blood samples from the children enrolled in this study and in their care during their hospital admissions.
Footnotes
This work was supported by National Institutes of Health Fogarty Grant R03 TW006428.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 30, 2009
Abbreviations: TRPV6, Transient receptor potential vanilloid type 6.
References
- Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Reading JC, Chan GM 1999 A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N Engl J Med 341:563–568 [DOI] [PubMed] [Google Scholar]
- Graff M, Thacher TD, Fischer PR, Stadler D, Pam SD, Pettifor JM, Isichei CO, Abrams SA 2004 Calcium absorption in Nigerian children with rickets. Am J Clin Nutr 80:1415–1421 [DOI] [PubMed] [Google Scholar]
- Oramasionwu GE, Thacher TD, Pam SD, Pettifor JM, Abrams SA 2008 Adaptation of calcium absorption during treatment of nutritional rickets in Nigerian children. Br J Nutr 100:387–392 [DOI] [PubMed] [Google Scholar]
- Thacher TD, Aliu O, Griffin IJ, Pam SD, O'Brien KO, Imade GE, Abrams SA 2009 Meals and dephytinization affect calcium and zinc absorption in Nigerian children with rickets. J Nutr 139:926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher TD, Fischer PR, Isichei CO, Pettifor JM 2006 Early response to vitamin D2 in children with calcium deficiency rickets. J Pediatr 149:840–844 [DOI] [PubMed] [Google Scholar]
- Heaney RP, Barger-Lux MJ, Dowell MS, Chen TC, Holick MF 1997 Calcium absorptive effects of vitamin D and its major metabolites. J Clin Endocrinol Metab 82:4111–4116 [DOI] [PubMed] [Google Scholar]
- Heaney RP, Dowell MS, Hale CA, Bendich A 2003 Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22:142–146 [DOI] [PubMed] [Google Scholar]
- Devine A, Wilson SG, Dick IM, Prince RL 2002 Effects of vitamin D metabolites on intestinal calcium absorption and bone turnover in elderly women. Am J Clin Nutr 75:283–288 [DOI] [PubMed] [Google Scholar]
- Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO 2005 Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab 90:5576–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger-Lux MJ, Heaney RP 2002 Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab 87:4952–4956 [DOI] [PubMed] [Google Scholar]
- Need AG, Nordin BE 2008 Misconceptions—vitamin D insufficiency causes malabsorption of calcium. Bone 42:1021–1024 [DOI] [PubMed] [Google Scholar]
- Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R 1998 Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 68:854–858 [DOI] [PubMed] [Google Scholar]
- Armas LA, Hollis BW, Heaney RP 2004 Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89:5387–5391 [DOI] [PubMed] [Google Scholar]
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD 2008 Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Manaster BJ, Reading JC 2000 Radiographic scoring method for the assessment of the severity of nutritional rickets. J Trop Pediatr 46:132–139 [DOI] [PubMed] [Google Scholar]
- Abrams SA 1999 Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr 70:955–964 [DOI] [PubMed] [Google Scholar]
- O'Brien KO, Donangelo CM, Zapata CL, Abrams SA, Spencer EM, King JC 2006 Bone calcium turnover during pregnancy and lactation in women with low calcium diets is associated with calcium intake and circulating insulin-like growth factor 1 concentrations. Am J Clin Nutr 83:317–323 [DOI] [PubMed] [Google Scholar]
- Docio S, Riancho JA, Perez A, Olmos JM, Amado JA, Gonzalez-Macias J 1998 Seasonal deficiency of vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res 13:544–548 [DOI] [PubMed] [Google Scholar]
- Peacock M, Selby PL, Francis RM, Brown WB, Hordon L 1985 Vitamin D deficiency, insufficiency, sufficiency and intoxication. What do they mean? In: Norman AW, Schaefer K, Grigoleit H-G, Herrath D, eds. Vitamin D: a chemical, biochemical and clinical update. Berlin: Walter de Gruyter; 569–570 [Google Scholar]
- Heaney RP 2004 Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr 80:1706S–1709S [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B 2006 Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28 [DOI] [PubMed] [Google Scholar]
- Ames SK, Gorham BM, Abrams SA 1999 Effects of high compared with low calcium intake on calcium absorption and incorporation of iron by red blood cells in small children. Am J Clin Nutr 70:44–48 [DOI] [PubMed] [Google Scholar]
- O'Brien KO, Abrams SA, Liang LK, Ellis KJ, Gagel RF 1996 Increased efficiency of calcium absorption during short periods of inadequate calcium intake in girls. Am J Clin Nutr 63:579–583 [DOI] [PubMed] [Google Scholar]
- Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE 2008 Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 23:1859–1863 [DOI] [PubMed] [Google Scholar]
- Zhu K, Bruce D, Austin N, Devine A, Ebeling PR, Prince RL 2008 Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone-related chemistry in elderly women with vitamin D insufficiency. J Bone Miner Res 23:1343–1348 [DOI] [PubMed] [Google Scholar]
- Francis RM, Peacock M, Storer JH, Davies AE, Brown WB, Nordin BE 1983 Calcium malabsorption in the elderly: the effect of treatment with oral 25-hydroxyvitamin D3. Eur J Clin Invest 13:391–396 [DOI] [PubMed] [Google Scholar]
- Wolf RL, Cauley JA, Baker CE, Ferrell RE, Charron M, Caggiula AW, Salamone LM, Heaney RP, Kuller LH 2000 Factors associated with calcium absorption efficiency in pre- and perimenopausal women. Am J Clin Nutr 72:466–471 [DOI] [PubMed] [Google Scholar]
- Lee WT, Cheng JC, Jiang J, Hu P, Hu X, Roberts DC 2002 Calcium absorption measured by stable calcium isotopes [(42)Ca, (44)Ca] among Northern Chinese adolescents with low vitamin D status. J Orthop Surg (Hong Kong) 10:61–66 [DOI] [PubMed] [Google Scholar]
- Weaver CM, McCabe LD, McCabe GP, Braun M, Martin BR, Dimeglio LA, Peacock M 2008 Vitamin D status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. J Clin Endocrinol Metab 93:3907–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman LS, Cassidy JT, Chanetsa F, Hewett JE, Higgins BJ, Robertson JD 2008 Percent true calcium absorption, mineral metabolism, and bone mass in children with arthritis: effect of supplementation with vitamin D3 and calcium. Arthritis Rheum 58:3255–3263 [DOI] [PubMed] [Google Scholar]
- van de Graaf SF, Boullart I, Hoenderop JG, Bindels RJ 2004 Regulation of the epithelial Ca2+ channels TRPV5 and TRPV6 by 1α,25-dihydroxy Vitamin D3 and dietary Ca2+. J Steroid Biochem Mol Biol 89–90:303–308 [DOI] [PubMed] [Google Scholar]
- Wood RJ, Tchack L, Taparia S 2001 1,25-Dihydroxyvitamin D3 increases the expression of the CaT1 epithelial calcium channel in the Caco-2 human intestinal cell line. BMC Physiol 1:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S 2008 Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 149:3196–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzova GD, Sundersingh F, Vaughan J, Tadi BP, Ansay SE, Christakos S, Deluca HF 2008 TRPV6 is not required for 1α,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc Natl Acad Sci USA 105:19655–19659 [DOI] [PMC free article] [PubMed] [Google Scholar]