Abstract
Context: Insulin recruits muscle microvasculature, which increases the endothelial exchange surface area to facilitate substrate delivery. Elevated plasma concentrations of free fatty acids (FFAs) cause insulin resistance.
Objectives: The aim of the study was to examine whether FFAs cause insulin resistance in human muscle microvasculature.
Setting: The study was conducted at the General Clinical Research Center at the University of Virginia.
Methods: Twenty-two healthy subjects were studied under two protocols designed to raise plasma insulin concentrations to postprandial levels using either an insulin infusion or a mixed meal challenge. Within each protocol, subjects were studied twice. In random order, they received a 5-h systemic infusion of either saline or Intralipid/heparin. Three hours into the infusion, baseline muscle microvascular blood volume (MBV), microvascular flow velocity, and microvascular blood flow (MBF) were measured. Each subject was then given either the mixed meal or a 1 mU/kg · min insulin clamp for 2 h. Microvascular parameters were again obtained 2 h after the meal or at the end of insulin infusion.
Results: Meal feeding and insulin infusion raised plasma insulin concentrations to approximately 200 pm, and each significantly increased muscle MBV (P = 0.03 and P < 0.01, respectively). MBF trended up after meal feeding (P = 0.08) and increased significantly after insulin infusion (P = 0.02). In the presence of Intralipid, neither the meal nor the insulin infusion increased muscle MBV and MBF.
Conclusions: Compared to saline, lipid infusion raises plasma FFA concentrations and blocks the ability of insulin or meal to recruit muscle microvasculature. High plasma FFA concentrations may contribute to muscle insulin resistance and the microvascular complications of diabetes.
Meal feeding and hyperinsulinemia each increase muscle microvascular perfusion, which is blocked by acute elevation of plasma levels of free fatty acids in healthy humans.
Skeletal muscle capillary perfusion is determined by precapillary terminal arterioles (1). Recent evidence has demonstrated that both insulin and feeding recruit microvasculature within the skeletal muscle (2,3,4,5). This is physiologically important because increased capillary perfusion facilitates the transendothelial transport of oxygen, nutrients, and hormones due to increased delivery of these substances and enlarged endothelial exchange surface area.
Insulin acts on the vascular endothelial cells to activate the phosphatidylinositol 3-kinase (PI3 kinase)/protein kinase B (or Akt)/endothelial nitric oxide synthase (eNOS) pathway (6,7,8), leading to increased nitric oxide (NO) production and the dilatation of peripheral resistance arteries and precapillary terminal arterioles. As a result, insulin increases total blood flow (9,10) and enhances microvascular perfusion in the skeletal muscle (11,12,13,14) and cardiac muscle (15). Insulin’s action on the microvasculature is rapid, within 5–10 min (13), and NO-dependent. Inhibition of eNOS with L-nitro-arginine methyl ester abolishes insulin-mediated increases in microvascular perfusion (12,13).
With insulin resistance, insulin action through the PI3-kinase/Akt/eNOS pathway is blunted (8,16,17). This decreases NO production. Insulin resistance can also involve the muscle microvasculature. In obese Zucker rats, there is a marked impairment in insulin-mediated muscle capillary recruitment (18). Similarly, acute insulin resistance induced experimentally by infusions of α-methylserotonin (19), TNF-α (20), or lipids (14) blocks insulin’s microvascular action in rat skeletal muscle. In humans, the insulin resistance associated with simple obesity blunts insulin-stimulated skeletal muscle microvascular perfusion (2).
Free fatty acids (FFAs) are the major oxidative fuel for resting skeletal muscle. The levels of FFAs are elevated in patients with type 2 diabetes, and high plasma FFAs have been shown repeatedly to induce insulin resistance, inflammation, and endothelial dysfunction (21,22,23). In laboratory animals, high levels of FFAs also cause insulin resistance in muscle microvasculature by blunting insulin-mediated muscle capillary recruitment (14). However, whether FFAs alter muscle microvascular responses to insulin and/or meal feeding (a potent physiological stimulus of insulin secretion) in humans has not been studied. It is of particular interest to examine the effect of FFAs on meal feeding because it represents a more physiological setting.
In the present study, we used contrast-enhanced ultrasound (CEU) to measure muscle microvascular blood volume (MBV) and microvascular flow velocity (MFV) in response to a meal or to isolated physiological hyperinsulinemia. In the same subjects, we assessed whether acute elevation of plasma concentrations of FFAs by lipid infusion affects these responses in healthy humans compared with saline infusion. Our results indicate that both a mixed meal and an isolated, moderate elevation in plasma insulin potently recruit muscle microvasculature and that elevation of plasma FFA concentrations by lipid infusion to the levels seen in poorly controlled diabetes abolishes this effect.
Subjects and Methods
A total of 22 young and healthy subjects (10 males, 12 females) with no history of obesity, hypertension, diabetes, or hyperlipidemia were studied under two study protocols approved by the human studies Institutional Review Board and the General Clinical Research Center (GCRC) Advisory Committee at the University of Virginia. All subjects gave written informed consent before the study. At the screening visit, blood samples were taken for pregnancy (for women), prothrombin time, partial prothrombin time, complete blood counts with differential analysis, cholesterol, triglycerides, high-density lipoprotein (HDL)-cholesterol, insulin, and glucose measurements. Subjects were excluded from the study if they were pregnant, smokers, anemic, had a bleeding disorder, or were taking any medications or supplements known to affect either endothelial function or glucose metabolism or had a family history of a first-degree relative with diagnosed diabetes. Each subject was studied under either study protocol 1 (mixed meal) or study protocol 2 (insulin clamp), as described below.
Study protocol 1—mixed meal
Ten volunteers (six males and four females; age, 23.9 ± 1.7 yr; body mass index, 22.6 ± 0.7 kg/m2) were studied under this protocol. Each subject was studied twice (saline infusion or Intralipid infusion) in random order with 1–8 wk between the two studies, all after a 12-h overnight fast. Their basal plasma concentrations were 4.9 ± 0.1 mm for glucose and 18.6 ± 2.5 pm for insulin.
Saline (control) infusion
Subjects were admitted to the GCRC the evening before the study. On the morning of the study, catheters were placed in the antecubital veins of both arms. The catheter in the nondominant arm was used for blood sampling of glucose, FFAs, and insulin; the contralateral arm was used for the infusion of normal saline and microbubbles. All subjects received an iv infusion of normal saline at a rate of 45 ml/h, starting at −180 min. After obtaining baseline measurements of muscle MBV and MFV using the CEU technique at time 0 (∼0800 h), each subject drank a liquid meal (Boost, 480 kcal: 72 from fat, 328 from carbohydrate, 80 from protein; Nestlé HealthCare Nutrition, Fremont, MI) within 10 min. Plasma glucose, insulin, and FFAs were measured at 0, 30, 60, 90, and 120 min. At 120 min after the mixed meal, muscle MBV and MFV were again measured using CEU technique.
Intralipid infusion
Subjects were again admitted to the GCRC the evening before the study, and the study was carried out in the exact same fashion as the saline (control) study except that all subjects received an iv infusion of Intralipid (20%) at a rate of 45 ml/h for a total of 5 h. Heparin was given at the same time at a rate of 0.2 U/kg · min to facilitate the conversion of the lipids to FFAs in the body. The mixed meal was ingested 3 h after the initiation of the Intralipid infusion, and the CEU measurements were done before and 120 min after meal ingestion.
Study protocol 2—insulin clamp
Twelve volunteers (four males, eight females; age, 23.3 ± 1.4 yr, body mass index, 21.8 ± 0.7 kg/m2) were studied under this protocol. Each subject was studied twice (saline infusion or Intralipid infusion) in random order with 1–8 wk between the two studies, all after a 12-h overnight fast. Their basal plasma concentrations were 4.5 ± 0.4 mm for glucose and 23 ± 4 pm for insulin.
Saline (control) infusion
Subjects were admitted to the GCRC the evening before the study. On the morning of the study, catheters were placed in the antecubital veins of both arms. The catheter in the nondominant arm was used for blood sampling of glucose, FFAs, and insulin; the contralateral arm was used for the infusion of normal saline and microbubbles. All subjects received an iv infusion of normal saline at a rate of 45 ml/h, starting at −180 min. After obtaining baseline CEU measurements of muscle MBV and MFV at time 0 (∼0800 h), each subject received a 2-h iv infusion of regular insulin (2 mU/min · kg × 10 min and 1 mU/min · kg × 110 min). Blood samples were taken for plasma glucose measurements every 5 min throughout the 120-min insulin infusion, and 20% dextrose was infused at variable rates to maintain euglycemia. We avoided heating the hand to arterialize sampled blood because this alters blood flow both in the heated and contralateral arm (15,24). At end of the clamp, MBV and MFV were again measured. Plasma insulin and FFAs were measured at 0, 30, 60, 90, and 120 min.
Intralipid infusion
Subjects were again admitted to the GCRC the evening before the study, and the study was carried out in the exact same fashion as the saline (control) study except that all subjects received an iv infusion of Intralipid (20%) and heparin for a total of 5 h as described under the mixed meal protocol. The insulin clamp started 3 h after the initiation of the Intralipid infusion, and the CEU measurements were done before and after 120 min of insulin infusion.
CEU
CEU was performed using either a SONOS 7500 or 5500 ultrasound system and an S3 phased array transducer (Philips Medical Systems, Andover, MA) while the subject was in the supine position. The contrast agent (Definity; Bristol-Myers Squibb, Princeton, NJ) used with CEU is commercially produced microbubbles composed of a lipid shell filled with a perfluorocarbon gas. Definity microbubbles (1.5 ml diluted in normal saline to a total volume of 30 ml) were infused iv at a rate of 1.5 ml/min for 8 min. Once the systemic microbubble concentration reached steady state (∼3 min), intermittent imaging of the forearm was then performed in a transaxial plane 5 cm distal to the antecubital fossa using either ultraharmonic or power Doppler imaging, with pulsing intervals (PIs) ranging from 1 to 20 sec. The PI is the time between successive ultrasound pulses, and the progressively longer PI enables progressively greater replenishment of the ultrasound beam elevation between destructive pulses. Three images were captured at each PI. A mechanical index of 1.3–1.5, which is capable of destroying all microbubbles within the ultrasound beam, was used. Depth, focus, and gains (overall gain, time-gain compensation, and lateral-gain compensation) were optimized at the beginning of each study and held constant throughout each study. Intermittent imaging was performed using an internal timer.
CEU imaging analyses
All muscle CEU images were blindly analyzed by one of the authors (J.L.), who had no knowledge of the study design, using either the QLAB software (Philips Medical Systems) or proprietary CEU software (25,26). The video intensity in the region of interest placed around the deep forearm flexor muscles was determined at each PI. After background subtraction, the PI (time) vs. video-intensity curve was generated and fitted to an exponential function: y = A (1 − e−βt) (25,26), where y is the video intensity at a PI t, A is the plateau video intensity representing microvascular blood volume (MBV), and β is the rate constant reflecting the rate of rise of video intensity (i.e. MFV). Microvascular blood flow (MBF) is derived from the product of MBV and MFV (i.e. MBF = MBV × MFV).
Biochemical analysis
Plasma cholesterol, HDL-cholesterol, and triglycerides were measured by the University of Virginia clinical chemistry laboratories. Plasma glucose was measured using a YSI glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured using the Immulite 2000 Automated Immunoassay Analyzer (Siemens Healthcare Diagnostics Inc., Deerfield, IL). Plasma FFAs were quantified using an in vitro enzymatic colorimetric assay with a Wako HR Series NEFA-HR kit (Wako Diagnostics, Richmond, VA).
Statistical analysis
Data are presented as mean ± sem. Statistical analyses were performed using SigmaStat 3.1 software (Systat Software, Inc., Chicago, IL). Each subject served as his/her own control. Comparisons between basal and mixed meal or basal and insulin were made using either a two-tailed Student’s paired t test or repeated-measures ANOVA where appropriate. A P value of less than 0.05 was considered statistically significant.
Results
Mixed meal protocol
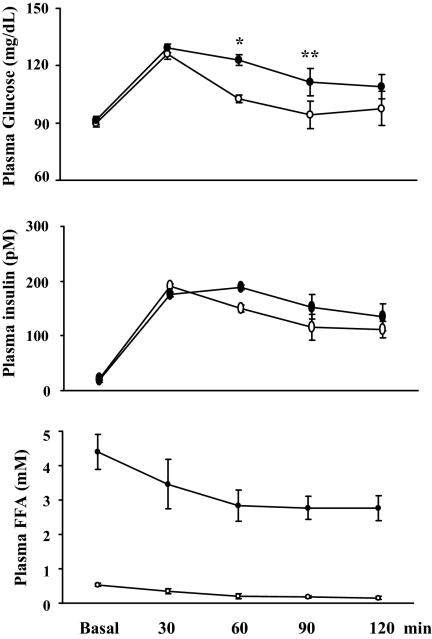
Figure 1 shows the time course of plasma glucose, insulin, and FFAs at baseline and after the ingestion of the mixed meal. During the control admission, plasma glucose levels rose above basal (90 ± 2 vs. 126 ± 7 mg/dl; P < 0.001) by 30 min and then declined toward the basal levels by 90 min. Similarly, meal ingestion raised plasma insulin concentrations from 21 ± 6 to 192 ± 24 pm (P < 0.001), a midphysiological concentration seen typically postprandially, which remained elevated 2 h after the meal ingestion (112 ± 26 pm; P < 0.01). This was accompanied by a marked and progressive decrease in plasma FFA concentrations (P < 0.001, ANOVA). Although meal ingestion did not change plasma triglyceride levels (59.4 ± 7.6 vs. 60.4 ± 10 mg/dl, basal vs. meal; P = 0.9), it decreased both low-density lipoprotein-cholesterol (98.8 ± 5.5 vs. 77.9 ± 3.6 mg/dl, basal vs. meal; P < 0.001) and HDL-cholesterol (53.3 ± 4.4 vs. 40.9 ± 2.1 mg/dl, basal vs. meal; P = 0.005), leading to a significant decrease in total plasma cholesterol concentrations (to 128.8 ± 4.9 mg/dl; P < 0.002).
Figure 1.
Changes in biochemical parameters after meal ingestion in protocol 1 subjects. Open circles, Saline infusion; closed circles, Intralipid infusion. *, P < 0.04; **, P = 0.01, compared with saline infusion. Data are presented as mean ± sem.
Infusion of Intralipid and heparin for 5 h raised plasma levels of FFAs from basal values of 0.15 ± 0.02 to 2.8 ± 0.7 mm (P < 0.0005). Ingestion of a mixed meal during Intralipid infusion resulted in a similar rise in plasma insulin (to 177 ± 22 pm; P < 0.001 vs. basal) and glucose (to 129 ± 7 mg/dl; P = 0.001 vs. basal) levels by 30 min as in the saline (control) study. However, when compared with control study values, plasma glucose concentrations were significantly higher at 60 (P < 0.04) and 90 (P = 0.01) min. As a result, the area under the curve for plasma glucose after mixed meal feeding was significantly higher during Intralipid infusion (12,498 ± 704 vs. 13,910 ± 479 mg/dl/120 min; P < 0.04). Similar to control study, plasma FFA concentrations declined continuously after ingestion of the mixed meal (P < 0.001, ANOVA) despite continued infusion of Intralipid and heparin.
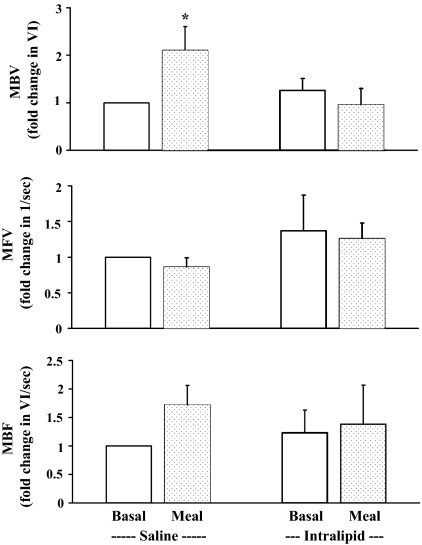
As shown in Fig. 2, mixed meal significantly increased forearm muscle MBV by approximately 2-fold (P = 0.03), and this increase was abolished by acute elevation in plasma FFA levels. MFV did not change after meal ingestion in the presence or absence of the Intralipid infusion. Total MBF, which was derived from the product of MBV and MFV, rose marginally (P = 0.08) with the meal during control admission but did not change during Intralipid infusion.
Figure 2.
Muscle MBV (top), MFV (middle), and MBF (bottom) at baseline and at 120 min after a mixed meal ingestion in the presence of Intralipid + heparin infusion or saline infusion. *, P = 0.03, compared with baseline (n = 10). Data are presented as mean ± sem.
Insulin protocol
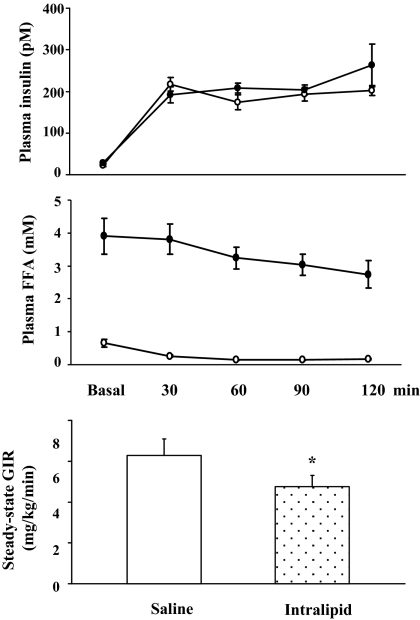
The biometric parameters of study subjects in the insulin protocol were very similar to values in the mixed meal protocol. Figure 3 shows the time course of plasma levels of insulin and FFAs at the baseline and during 120 min of insulin infusion. During saline (control) study, insulin infusion raised plasma insulin concentrations by more than 6-fold (from 29 ± 5 to 192 ± 19 pm; P < 0.0001) by 30 min, which remained elevated throughout insulin infusion. This was accompanied by a significant and progressive decrease in plasma FFA concentrations (P < 0.001, ANOVA).
Figure 3.
Changes in biochemical parameters during insulin clamp in protocol 2 subjects. Open circles, Saline infusion; closed circles, Intralipid infusion. *, P = 0.003, compared with saline infusion. Data are presented as mean ± sem.
Infusion of Intralipid and heparin for 5 h raised plasma levels of FFAs to 2.7 ± 0.4 mm (vs. saline study, 0.12 ± 0.02 mm; P < 0.001). This acute elevation in plasma FFA concentrations significantly decreased insulin-mediated whole body glucose disposal, as defined by steady-state glucose infusion rates (6.3 ± 0.8 vs. 4.8 ± 0.5 mg/kg · min, control vs. Intralipid; P = 0.003).
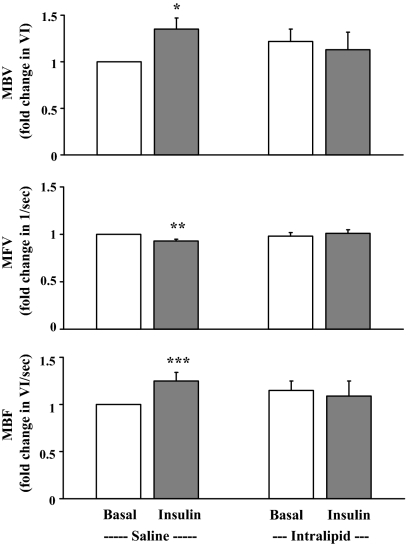
Figure 4 shows the forearm microvascular responses toward insulin in the absence or presence of high plasma concentrations of FFAs. Insulin infusion significantly increased forearm muscle MBV (P < 0.01) and total MBF (P = 0.02), with a small but significant decrease in MFV (P < 0.003). However, in the presence of acute elevation in the plasma concentrations of FFAs, these insulin-induced microvascular responses were completely abolished.
Figure 4.
Muscle MBV (top), MFV (middle), and MBF (bottom) at baseline and at end of 120 min insulin infusion in the presence of Intralipid/heparin infusion or saline infusion. *, P < 0.01; **, P < 0.003; ***, P = 0.02, compared with baseline (n = 12). Data are presented as mean ± sem.
Discussion
Using the noninvasive CEU technique, we found that raising plasma insulin concentrations to midphysiological levels, through a systemic insulin infusion, potently recruits muscle microvasculature in healthy humans, and this effect is abolished by acute elevation of plasma concentrations of FFAs. Ingestion of a mixed meal likewise provokes an expansion of MBV. This effect may be secondary to meal-induced hyperinsulinemia, but given the complex neuroendocrine responses to feeding, we cannot exclude other contributions. Again, this effect is blocked by FFAs. These findings demonstrate that FFAs produce vascular insulin resistance in the human muscle microcirculation in addition to affecting insulin-mediated glucose disposal.
The finding that FFA elevation abolished mixed meal-induced microvascular recruitment is of particular interest. Meal feeding is a potent physiological stimulus of insulin secretion, yet, unlike simple insulin infusion, feeding provokes a complex, systemic response involving the changes in circulating concentrations of nutrients such as glucose; amino acids; FFAs; hormones such as insulin, glucagon, and incretins; and autonomic nervous system tone. We and others have previously reported that ingestion of a mixed meal induces significant increases in microvascular perfusion in both skeletal muscle (5) and cardiac muscle (27) and (using muscle microdialysis) that an oral glucose load increases the permeability surface for glucose and insulin in healthy humans (28). Because plasma insulin concentrations increased 8- to 10-fold in those studies, it is very likely that the microvascular responses observed after meal feeding or glucose load were mainly secondary to the effects of insulin. As such, a decrease in postprandial insulin-mediated muscle glucose uptake would be manifested by increased plasma glucose concentrations. Indeed, as shown in Fig. 1, we have in the current study observed that subjects receiving Intralipid infusion had significantly higher plasma glucose concentrations after the mixed meal, leading to a significantly higher area under the curve for plasma glucose (P < 0.04). It is thus possible that chronic insulin resistance in the muscle microvasculature contributes to prolonged postprandial hyperglycemia, which is one of the hallmarks of prediabetes and type 2 diabetes and has been implicated in the pathogenesis of diabetic complications.
Our finding that FFAs abrogated insulin-mediated muscle capillary recruitment using the euglycemic clamp is consistent with a previous report demonstrating a similar effect of FFAs in laboratory animals (14). The effect of FFA on meal-induced microvascular recruitment has not previously been examined. Considerable previous work has demonstrated an inhibitory role of FFAs on insulin-signaling pathways that may underlie the pathogenesis of insulin resistance in muscle and vasculature. In humans, high plasma concentrations of FFAs decrease insulin receptor substrate-1-associated PI3-kinase activity and glucose transport in skeletal muscle (29). Acute elevation of plasma FFAs via systemic lipid infusion induces oxidative stress, activates the nuclear factor-κB pathway, impairs endothelium-dependent vasodilation, and blunts insulin-mediated vasodilation and NO production in humans (22,23,30). In cultured endothelial cells, palmitate inhibits insulin-mediated tyrosine phosphorylation of insulin receptor substrate-1, serine phosphorylation of Akt and eNOS, and NO production while increasing IKKβ activity (16,31). Additionally, FFAs could also contribute to endothelial dysfunction by triggering endothelial cell apoptosis and inhibiting cell cycle progression (32,33).
Our current findings are of physiological significance because the delivery of nutrients such as glucose, amino acids, and fatty acids, and hormones such as insulin to skeletal muscle is dependent on muscle blood flow, microvascular/capillary surface area, and vascular permeability. It is in the microcirculation where substrate extraction takes place, and the rate of substrate extraction is given by Fick’s law: ([V] − [A]) = ([I] − [A]) × (1 − e−PS/Q), where V is the venous plasma concentration, I is the interstitial concentration, A is the arterial plasma concentration, P is surface permeability, S is surface area, and Q is the plasma flow rate. Thus, a relatively small increase in the microvascular volume (i.e. microvascular surface area or substrate exchange surface area) could markedly increase substrate extraction by a given tissue. This is particularly important for organs or tissues with low resting blood flow such as skeletal muscle because an increase in total flow to muscle alone in the absence of exchange surface area expansion would not significantly augment tissue nutrient exchange within skeletal muscle (34,35). In contrast, expansion of the endothelial exchange surface area between the plasma compartment and the interstitial compartment within the tissue would significantly facilitate the extraction of nutrients and insulin. Thus, postprandially, muscle microvascular insulin resistance could significantly decrease muscle uptake of nutrients and insulin from plasma and lead to hyperglycemia (with compensatory increases of insulin) and high circulating concentrations of FFAs which could further contribute to insulin resistance seen in other tissues.
The lack of insulin- or meal-induced capillary recruitment during lipid infusion likely contributes to the observed decrease in insulin-stimulated glucose disposal (clamp) and elevated plasma glucose after meal feeding, respectively. Previous studies have shown that insulin-mediated capillary recruitment precedes insulin-stimulated glucose uptake in skeletal muscle (13), and blockade of insulin-mediated capillary recruitment with NOS inhibitor L-nitro-arginine methyl ester decreases glucose disposal by 40% (12,13). Thus, microvascular insulin resistance could significantly reduce muscle glucose disposal.
The insulin concentrations achieved during our insulin clamp study were lower than the previously reported levels of approximately 600 pm using a similar insulin infusion rate. This may have reflected the increased insulin assay specificity. Indeed, our basal levels of approximately 20 pm were also lower than the previously reported values of approximately 60 pm. These values are in line with a recent report from other investigators demonstrating a basal insulin concentration of 14 pm and a 2 mU/kg · min insulin clamp increasing plasma insulin concentrations to only 660 pm in lean subjects (36).
In conclusion, our findings demonstrate that raising plasma concentrations of FFAs by lipid infusion, compared with saline infusion, causes insulin resistance within skeletal muscle microvasculature in healthy, otherwise insulin-sensitive human adults, which manifests as loss of microvascular recruitment in response to physiological hyperinsulinemia. Because microvascular recruitment increases delivery of nutrients and hormones to muscle by expanding the endothelial surface exchange area, this FFA-induced muscle microvascular insulin resistance may contribute to the pathogenesis of overall muscle insulin resistance, prolonged postprandial hyperglycemia, and the microvascular complications of diabetes.
Footnotes
This work was supported by an American Diabetes Association clinical research award (7-07-CR-34, to Z.L.), and National Institutes of Health Grants DK-DK057878 and DK-073759 (to E.J.B.) and RR-00847 (to University of Virginia General Clinical Research Center).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 30, 2009
Abbreviations: CEU, Contrast-enhanced ultrasound; eNOS, endothelial NO synthase; FFA, free fatty acid; HDL, high-density lipoprotein; MBF, microvascular blood flow; MBV, microvascular blood volume; MFV, microvascular flow velocity; NO, nitric oxide; PI, pulsing interval; PI3-kinase, phosphatidylinositol 3-kinase.
References
- Segal SS 2005 Regulation of blood flow in the microcirculation. Microcirculation 12:33–45 [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ 2006 Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55:1436–1442 [DOI] [PubMed] [Google Scholar]
- Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E 2001 Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50:2682–2690 [DOI] [PubMed] [Google Scholar]
- Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ 2004 Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53:447–453 [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ 2006 Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290:E1191–E1197 [DOI] [PubMed] [Google Scholar]
- Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ 2000 Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101:1539–1545 [DOI] [PubMed] [Google Scholar]
- Li G, Barrett EJ, Wang H, Chai W, Liu Z 2005 Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146:4690–4696 [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ 2006 Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113:1888–1904 [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H, Utriainen T 1998 Insulin-induced vasodilatation: physiology or pharmacology? Diabetologia 41:369–379 [DOI] [PubMed] [Google Scholar]
- Baron AD, Steinberg H, Brechtel G, Johnson A 1994 Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol 266:E248–E253 [DOI] [PubMed] [Google Scholar]
- Rattigan S, Clark MG, Barrett EJ 1997 Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes 46:1381–1388 [DOI] [PubMed] [Google Scholar]
- Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S 2003 Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285:E123–E129 [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ 2004 Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53:1418–1423 [DOI] [PubMed] [Google Scholar]
- Clerk LH, Rattigan S, Clark MG 2002 Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51:1138–1145 [DOI] [PubMed] [Google Scholar]
- Liu Z 2007 Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 293:E1250–E1255 [DOI] [PubMed] [Google Scholar]
- Kim JA, Koh KK, Quon MJ 2005 The union of vascular and metabolic actions of insulin in sickness and in health. Arterioscler Thromb Vasc Biol 25:889–891 [DOI] [PubMed] [Google Scholar]
- Li G, Barrett EJ, Barrett MO, Cao W, Liu Z 2007 Tumor necrosis factor-α induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology 148:3356–3363 [DOI] [PubMed] [Google Scholar]
- Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark AD, Clark MG 2002 Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 51:3492–3498 [DOI] [PubMed] [Google Scholar]
- Rattigan S, Clark MG, Barrett EJ 1999 Acute vasoconstriction-induced insulin resistance in rat muscle in vivo. Diabetes 48:564–569 [DOI] [PubMed] [Google Scholar]
- Youd JM, Rattigan S, Clark MG 2000 Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-α. Diabetes 49:1904–1909 [DOI] [PubMed] [Google Scholar]
- de Jongh RT, Serné EH, Ijzerman RG, de Vries G, Stehouwer CD 2004 Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes 53:2873–2882 [DOI] [PubMed] [Google Scholar]
- Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P 2003 Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52:2882–2887 [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD 1997 Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 100:1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A, Simonsen L, Bülow J, Christensen NJ 1988 Measurement of forearm oxygen consumption: role of heating the contralateral hand. Am J Physiol 255:E572–E578 [DOI] [PubMed] [Google Scholar]
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S 1998 Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97:473–483 [DOI] [PubMed] [Google Scholar]
- Wei K, Ragosta M, Thorpe J, Coggins M, Moos S, Kaul S 2001 Noninvasive quantification of coronary blood flow reserve in humans using myocardial contrast echocardiography. Circulation 103:2560–2565 [DOI] [PubMed] [Google Scholar]
- Scognamiglio R, Negut C, De Kreutzenberg SV, Tiengo A, Avogaro A 2005 Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation 112:179–184 [DOI] [PubMed] [Google Scholar]
- Gudbjörnsdóttir S, Sjöstrand M, Strindberg L, Wahren J, Lönnroth P 2003 Direct measurements of the permeability surface area for insulin and glucose in human skeletal muscle. J Clin Endocrinol Metab 88:4559–4564 [DOI] [PubMed] [Google Scholar]
- Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI 1999 Effects of free fatty acids on glucose transport and IRS-1–associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD 2000 Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 49:1231–1238 [DOI] [PubMed] [Google Scholar]
- Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW 2005 Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKβ. Arterioscler Thromb Vasc Biol 25:989–994 [DOI] [PubMed] [Google Scholar]
- Artwohl M, Roden M, Waldhäusl W, Freudenthaler A, Baumgartner-Parzer SM 2004 Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J 18:146–148 [DOI] [PubMed] [Google Scholar]
- Chai W, Liu Z 2007 p38 Mitogen-activated protein kinase mediates palmitate-induced apoptosis but not inhibitor of nuclear factor κB degradation in human coronary artery endothelial cells. Endocrinology 148:1622–1628 [DOI] [PubMed] [Google Scholar]
- Renkin EM 1985 B. W. Zweifach award lecture: Regulation of the microcirculation. Microvasc Res 30:251–263 [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ 2004 The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev 20:3–12 [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Jani R, Chavez A, Molina-Carrion M, Tripathy D, Defronzo RA 2009 Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia 52:574–582 [DOI] [PubMed] [Google Scholar]