Abstract
Context: GLUT4 is the predominant glucose transporter isoform expressed in fat and muscle. In GLUT4 null mice, insulin-stimulated glucose uptake into muscle was diminished but not eliminated, suggesting that another insulin-sensitive system was present.
Objective: This study was intended to determine whether insulin caused GLUT12 translocation in muscle.
Design: Six normal volunteers had muscle biopsies before and after euglycemic insulin infusions.
Setting: Infusions and biopsies were performed in an outpatient clinic.
Participants: Subjects were nonobese, young adults with no family history of diabetes.
Main Outcome Measures: GLUT12, GLUT4, and GLUT1 proteins were quantified in muscle biopsy fractions. Cultured myoblasts were used to determine whether GLUT12 translocation was phosphatidyl inositol-3 kinase (PI3-K)-dependent.
Intervention. Insulin was infused at 40 mU/m2 · min for 3 h.
Results: In human muscle, insulin caused a shift of a portion of GLUT12 from intracellular low-density microsomes to the plasma membrane (PM) fraction (17% in PM at baseline, 38% in PM after insulin). Insulin increased GLUT4 in PM from 13 to 42%. GLUT1 was predominantly in the PM fractions at baseline and did not change significantly after insulin. L6 myoblasts in culture also expressed and translocated GLUT12 in response to insulin, but inhibiting PI3-K prevented the translocation of GLUT12 and GLUT4.
Conclusions: Insulin causes GLUT12 to translocate from an intracellular location to the plasma membrane in normal human skeletal muscle. Translocation of GLUT12 in cultured myoblasts was dependent on activation of PI3-K. GLUT12 may have evolutionarily preceded GLUT4 and now provides redundancy to the dominant GLUT4 system in muscle.
Insulin induces sequestered GLUT12 to move to the muscle cell surface.
Either increments in insulin concentration or muscle contraction cause the insulin-responsive glucose transporter, GLUT4, to move from sequestered intracellular locations to the muscle cell surface and t-tubules where this membrane protein allows glucose to enter the muscle cell (1).
More than a decade ago, a GLUT4 knockout mouse was developed, and its phenotype characterized. To the surprise of many of us, the GLUT4 null mouse did not develop diabetes, and glucose uptake into soleus muscle was still modestly insulin-responsive (2,3). We have recently identified seven GLUT isoforms that are expressed in human skeletal muscle, but three of these (GLUT4, GLUT5, and GLUT12) account for 98% of the mRNA (4). GLUT4 and GLUT12 are expressed more in type 1 (red) muscle fibers, and both have amino termini that contain a dileucine motif suggesting a predominantly intracellular expression site (5). GLUT8 also has the dileucine motif in the amino terminus, but the motif was expressed at a much lower level than GLUT12 (4), making GLUT12 a more likely backup isoform to the GLUT4 translocation system.
To test the hypothesis that GLUT12 worked in concert with GLUT4 in augmenting glucose uptake into muscle in response to insulin, we performed euglycemic insulin clamp studies in six normal volunteers and quantified changes in GLUT4 and GLUT12 in plasma membrane (PM)-enriched fractions of muscle. Our data suggested that as much as 12% of the insulin-translocatable GLUTs were accounted for by GLUT12.
Subjects and Methods
Materials
GLUT4 antibodies (AB1049, goat antihuman) were purchased from Chemicon (Temecula, CA). Rabbit anti-hGLUT12 antibodies (RDI-GLUT12abrx) were purchased from Research Diagnostics (Flanders, NJ). Alpha Diagnostic International was the source of anti-hGLUT1 antibodies (GT12-A). The corresponding immunization peptides were obtained from each of the primary antibody suppliers. SuperSignal west pico chemoluminescence substrate was purchased from Pierce (Rockford, IL). LY294002 was obtained from Sigma (St. Louis, MO). All other chemicals were reagent grade. L6 cells (rat-derived myocytes) were obtained from American Type Culture Collection (Manassas, VA).
Subject selection
Six volunteers of normal weight who had no family history of diabetes were recruited. Subject characteristics are shown in Table 1. The protocol and the consent document were approved by the Institutional Review Board of East Tennessee State University.
Table 1.
Patient characteristics and euglycemic insulin clamp data
| Subject | Gender | Age (yr) | BMI (kg/m2) | Insulin baseline (pmol/liter) | Insulin postinfusion (pmol/liter) | Glucose infusion rate (mg/kg · min) |
|---|---|---|---|---|---|---|
| G1 | F | 24 | 24.9 | 21 | 420 | 8.37 |
| G2 | M | 29 | 21.3 | 21 | 336 | 9.31 |
| G3 | M | 18 | 24.5 | 63 | 294 | 4.33 |
| G4 | F | 23 | 27.9 | 14 | 301 | 11.18 |
| G5 | F | 26 | 20.1 | 42 | 371 | 7.00 |
| G6 | M | 27 | 27.6 | 21 | 329 | 13.00 |
| Mean ± se | 25 ± 2 | 24.4 ± 1.3 | 28 ± 7 | 343 ± 21 | 8.87 ± 1.25 |
F, Female; M, male; BMI, body mass index.
Euglycemic insulin clamp studies
Insulin infusion studies were performed essentially as previously described (6,7), except no isotope or somatostatin was used in this study. After an overnight fast, subjects were maintained in quiet recumbency for 2 h to minimize the effects of muscular activity on protein subcellular localization. After a baseline muscle biopsy, a constant insulin infusion was performed at 40 mU/m2 · min for 3 h. A second muscle biopsy was performed on the contralateral side in the last 10 min of the insulin infusion period.
Muscle biopsies
Percutaneous needle biopsies of vastus lateralis were performed using a 5-mm Bergstrom-Stille needle under suction after an overnight fast and 2 h of quiet recumbency as previously described (4). A 50- to 100-mg specimen was quickly blotted, and the entire sample was frozen in liquid nitrogen for later analysis.
Fractionation of muscle microsamples
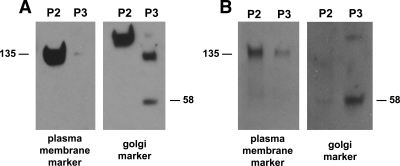
We have made minor modifications to our previously described techniques (8). Briefly, 25 mg muscle is removed from the −80 C freezer and slowly thawed on ice. The muscle is homogenized in 1.0 ml Buffer A (250 mm sucrose, 20 mm HEPES, pH 7.4) with 5 μl protease cocktail (cocktail kit no. 78410; Pierce) added. Initial homogenization is 30 sec with a Pellet Pestle Motor (Kimble-Kontes, Vineland, NJ). The homogenate is passed through a BD Falcon 100 μm cell strainer (BD Diagnostics, Franklin Lakes, NJ) and, after the addition of 100 μl Buffer B (3 m KCl, 250 mm sodium pyrophosphate), is spun at 227,000 × g for 30 min. The pellet is resuspended in 1.0 ml of Buffer A with protease cocktail using the Pellet Pestle Motor again for 30 sec. This sample is then spun at 12,000 × g for 20 min. The pellet is resuspended in 200 μl Triton Lysis Buffer (Boston Bioproducts Inc., Worcester, MA) with Protease Inhibitor Cocktail Kit (Thermo Scientific, Rockford, IL) using the Pellet Pestle Motor. This sample is designated “PM,” the plasma membrane-enriched fraction. The supernate is removed and spun at 227,000 × g for 30 min. The resulting pellet is also resuspended in 200 μl Boston Bioproducts Triton Lysis Buffer and is designated “LDM,” the low-density microsomes fraction. PM protein content averaged 139 ± 7 μg, and LDM contained 265 ± 8 μg protein. In studies performed on human control muscle sample fractions, the PM fraction contained 83% of the immunological signal for the PM marker pan Cadherin (ab6529 from Abcam, Inc., Cambridge, MA). LDM contained 75% of the Golgi protein marker (ab5820 from Abcam). Typical immunoblots for determining the PM (P2) and LDM (P3) fraction enrichment for these markers are shown in Fig. 1.
Figure 1.
PM and Golgi marker expression in fractions from human muscle biopsies (A) and from L6 myoblasts (B). Shown here are typical immunoblots of fractions from the muscle and cultured cells used in these studies. The PM marker is pan Cadherin and is seen at 135 kDa. The Golgi marker is FTCD (an enzyme involved in formiminoglutamate metabolism) and is seen at 58 kDa. P2 and P3 are the second and third pellets in the fractionation procedure. P2 is PM-enriched, and P3 contains the LDM.
Fractionation of L6 muscle cells
L6 cells were obtained from the American Tissue Culture Collection (Manassas, VA) and cultured in DMEM with 10% fetal bovine serum, with subculturing every 3 to 5 d. For the studies described below, cells were grown to near confluence in 100-mm dishes. Cells were harvested in 1 ml homogenization Buffer A containing 5 μl protease inhibitors using a rubber policeman. The remainder of the technique is the same as muscle microsamples. The yield of protein averaged 199 ± 14 μg for PM and 419 ± 22 μg for LDM. Figure 1 also includes immunoblots showing L6 PM and LDM enrichment for the PM and Golgi markers.
Immunoblot technique
Immunoblotting was performed essentially as previously described (9). In general, 10–20 μg protein from muscle homogenate or fractions was separated on a 10% polyacrylamide gel using the Laemmli system (10), transferred to a nitrocellulose membrane, subjected to blocking with 2.5% nonfat dry milk in PBS, incubated with a validated dilution of one of the anti-GLUT antibodies above including 1.25% milk, and developed with the enhanced chemiluminescence reagent and x-ray film. Image analysis was performed on scanned film digital files using Quantity One version 4.5.2 software from Bio-Rad (Hercules, CA). The specificity of the antibodies employed for GLUT1, GLUT4, and GLUT12 was empirically tested using blots that included control muscle homogenates and specific chimeric protein standards. Each antibody was tested with these blots in the absence and presence of the immunization peptide for GLUT1, GLUT4, and GLUT12 separately. For these specificity studies, each gel lane contained 20–40 μg homogenate, the antibodies were at 1:500 final dilution (2 μg/ml), and the immunization peptide was at 10 μg/ml. Before incubation with the blot, the antibodies and each peptide were incubated at 20 C for 20 min. In each case, the antibody specifically labeled a band in muscle homogenate with mobility of apparent molecular mass of 55, 45, and 58 kDa for GLUT1, GLUT4, and GLUT12, respectively. The apparent molecular mass for all of the specific chimeric protein standards was 41 kDa. The immunizing peptides only inhibited labeling with their specific antibody in muscle homogenate and the chimeric protein standards.
GLUT protein standards
The construct ova-GLUT1 was generated to express a soluble chimeric protein comprised of ovalbumin and a GLUT1 epitope tag. Chicken ovalbumin (nt 66–68, 81–1223; GenBank accession no. V00383) was amplified from pOV2 (generously provided by Dr. Michel Sanders of the University of Minnesota) using the primers ova 1F (5′-ATG GCA GCA AGC ATG GAA TTT TG) and ova glut1R (5′-TTA CAC TTG AGA ATC AGC GCC CAG TGG ATG GAA CAG CTC AGG GGA AAC ACA TCT GCC AAA G) and was TA-cloned into pCR T7 TOPO (Invitrogen, Carlsbad, CA). Because the ovalbumin coding region in pOV2 is incomplete, missing the first five codons and first base pair of the sixth codon, the sense primer (Ova F1) was designed to restore the initiator ATG and complete the sixth codon, while excluding the second through fifth codons. The antisense primer was complimentary to ovalbumin nt 1201–1222 and included sequence encoding the C-terminal 12 amino acids of human GLUT1 (GluLeuPheHisProLeuGlyAlaAspSerGlnVal; aa 481–492; SwissProt accession no. P11166). Codons were optimized for use of Escherichia coli for translation. Chimeric protein was expressed using a coupled in vitro transcription/translation system (Active Pro In Vitro Translation kit; Ambion, Inc.) according to the manufacturer’s protocol. The amount of chimeric protein generated was then quantified using electrophoresis with the Agilent Bioanalyzer 2100e (Agilent Technologies, Palo Alto, CA). The ova-GLUT1 fusion protein had a deduced molecular mass of 44,244 Da and migrated as a discrete band, which represented 3.5% of the total protein present. Further purification was not done when the product was used as a standard in gel electrophoresis and immunoblotting.
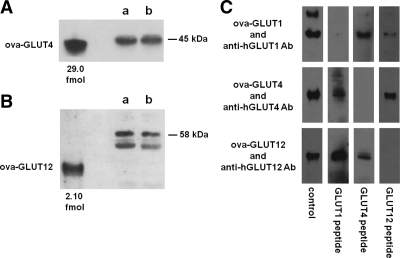
The other chimeric constructs used in this study (ova-GLUT4 and ova-GLUT12) were generated using the same protocol as for ova-GLUT1, with the exception that the antisense primer was changed to correspond to the appropriate carboxy-terminal sequence (primer ova glut4R, 5′-TCA ATC GTT CTC ATC CGG GCC TAA ATA TTC AAG TTC GGT AGG GGA AAC ACA TCT GCC AAA G for ThrGluLeuGluTyrLeuGlyProAspGluAsnAsp; and primer ova glut12R, 5′-TTA GGT CTC TGG AGA AAG CTG CCT GGA TTG ACC CCT ACC AGG GGA AAC ACA TCT GCC AAA G for GlyArgGlyGlnSerArgGlnLeuSerProGluThr). Figure 2, A and B, displays immunoblots that include the ova-GLUT4 and the ova-GLUT12 constructs.
Figure 2.
Direct comparison of muscle content of GLUT4 and GLUT12. A and B, Representative blots for GLUT4 and GLUT12 content in homogenates of muscle biopsies obtained from the vastus lateralis from normal volunteers. Both of the ova-GLUT constructs shown here had mobility consistent with a molecular size of 40 kDa. The lanes designated “a” and “b” contained 10 μg of muscle homogenate. The GLUT4 in the muscle samples exhibited a mobility suggesting 45 kDa, and the GLUT12 mobility suggested molecular size of 58 kDa. The GLUT12 blot contains strong nonspecific signals at about 52 kDa. In the presence of the immunization peptide, the bands labeled ova-GLUT12 and 58 kDa were absent, but the 52-kDa bands were not diminished, indicating that the 58-kDa band was specifically labeled by the anti-GLUT12 antibody. C, Strip immunoblots of ova-GLUT1, ova-GLUT4, and ova-GLUT12 incubated with the specific antibody directed against hGLUT1, hGLUT4, or hGLUT12 as indicated. At the bottom is noted the immunizing peptide that was incubated with the antibody and strip blot. Thus, each construct was incubated with its corresponding antibody and each of the different peptides. Control indicates that no immunizing peptide was employed. These blots demonstrate that each ova-GLUT is specific for its corresponding antibody.
The specificity of the ova-GLUT constructs used in these studies is demonstrated in Fig. 2C. Each of the 12 blots shown represents incubation of strip blots of the indicated ova-GLUT (1, 4, or 12) with its specific antibody in the presence of one of the immunizing peptides used to generate each of the three antibodies. Because more than 90% of the proteins present in each ova-GLUT preparation are native E. coli proteins, some of the blots contain nonspecific labeling at locations other than the 41-kDa specific bands.
Statistics
All data are presented as mean ± sem. Significant differences were inferred when P < 0.05 using t test or paired t test. Statistical procedures were performed using SigmaStat version 3.11 from Systat Software (San Jose, CA).
Results
Euglycemic insulin clamp
Six healthy young adults (three males, three females) achieved a physiological increment in plasma insulin concentration (Table 1). From a baseline insulin concentration of 28 ± 7 pmol/liter, the infusion of insulin at 40 mU/m2 · min caused an increase to 343 ± 21 pmol/liter. To maintain a blood glucose concentration of 85 mg/dl in the steady-state period of the insulin infusion, a mean glucose infusion rate of 8.87 mg/kg · min was necessary.
Insulin-induced increase in GLUT4 and GLUT12 in the PM-enriched muscle fraction
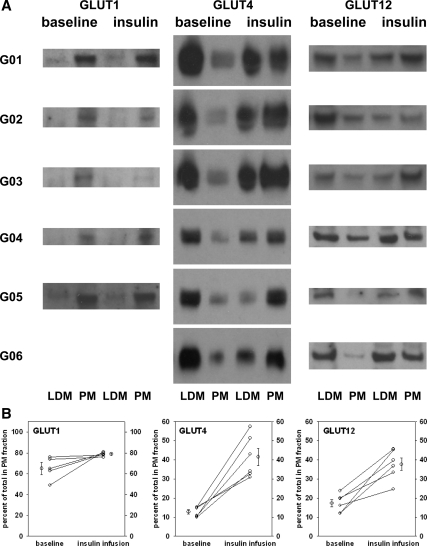
In the baseline muscle biopsies, GLUT4 protein was distributed 13% in the PM-enriched fractions. At the end of the insulin infusion, the GLUT4 distribution had changed dramatically to 42% in the PM-enriched fractions. Figure 3A shows a composite of several representative immunoblots of the two fractions, LDM- and PM-enriched, from the baseline and the insulin infusion muscle samples for each subject. Immunoblots typically included 10 μg of protein in each lane. The calculation for percentage of GLUT4 content in the PM fraction used the image analysis-generated femtomoles per lane multiplied by the total protein content of the fraction for each subject.
Figure 3.
Expression of GLUT1, GLUT4, and GLUT12 in normal skeletal muscle LDM and PM fractions before and after insulin infusion. Percutaneous muscle biopsies were obtained before and at the end of 3-h euglycemic insulin infusion in six normal volunteers. Muscle homogenates were divided into LDM- and PM-enriched fractions by centrifugation as described in Subjects and Methods. Representative blots are shown for each isoform in panel A. LDM and PM are designated at the bottom and “baseline” and “insulin” infusion samples are indicated at the top. The blots for GLUT1, GLUT4, and GLUT12 were each performed three times for each subject shown. The band intensities were quantified and compared with a known GLUT1, GLUT4, or GLUT12 standard using image analysis of the digitized films. The total amounts of GLUT1, GLUT4, and GLUT12 were estimated in each fraction using the quantification per gel lane (10 or 20 μg) and the total protein in the LDM and PM fractions. In the baseline samples, a mean of 13% of the GLUT4 was in the PM-enriched fractions, and insulin infusion increased the amount in the PM fractions to an average of 42%. Similarly, analysis of the GLUT12 blots revealed an average of 17% in the PM fraction at baseline and 38% in the PM fraction at the end of the insulin infusion. PM content of GLUT1 was 65 ± 6% in the baseline samples and 78 ± 2% after insulin. Each subject’s muscle biopsies were fractionated into LDM- and PM-enriched fractions by differential sedimentation. Immunoblots containing 10 μg per lane were probed with polyclonal rabbit anti-hGLUT1, anti-hGLUT4, or anti-hGLUT12 in the presence of a known standard of chimeric ovalbumin-GLUT1, ovalbumin-GLUT4, or ovalbumin-GLUT12 containing the peptide epitope that was used in generating the antibody. Each fraction sample was quantified by image analysis of digitized films in at least three separate studies. B, The data displayed represent the means of at least three separate estimates of the GLUT isoform protein content adjusted for the total amount of protein in each fraction. The difference between the baseline and the sample at the end of the insulin infusion was significant for both GLUT4 and GLUT12 at P < 0.01 by paired t test. There was a 20% average increase in GLUT1 content in PM that did not achieve statistical significance (P = 0.16).
Figure 3A also displays GLUT12 immunoblots for each individual subject. Image analysis quantification of GLUT12 in the PM fraction, adjusted for protein content in the baseline samples, averaged 17%. After insulin infusion, GLUT12 protein was shifted to 38% in the PM-enriched fractions. The distribution of GLUT4 and GLUT12 in the baseline and postinsulin infusion were very similar.
The same fractions that were used for GLUT4 and GLUT12 immunoblots were subjected to SDS-PAGE, transfer to membranes, and incubation with anti-hGLUT1 antibodies to determine whether insulin infusion caused evidence of a shift of the GLUT1 content from one pool to another. Figure 3A also shows images of typical immunoblots for GLUT1 expression in each subject. In contrast to the results with GLUT4 and GLUT12, there was no change in the intensity of the GLUT1 signal in either the LDM or PM fractions after the insulin infusion. Adjusting for the protein content of the fractions, the baseline PM fractions contained 65 ± 6% of the GLUT1, and the postinsulin infusion PM fractions contained 78 ± 2%. These data are the mean ± se for subjects 1–5. Subject 6 had inadequate samples remaining to quantify the GLUT1 content after the insulin infusion.
Figure 3B (left panel) displays the mean data from image analysis of immunoblots from three separate determinations for each individual’s baseline and insulin infusion PM-enriched fraction content of GLUT1, GLUT4, and GLUT12. In each subject, the GLUT4 content of the PM fraction at least doubled. The GLUT12 content of the PM fraction more than doubled in four of the six subjects, with the minimum response being an increase of 60%. Unlike GLUT4 and GLUT12, GLUT1 was predominantly in the PM fractions at baseline. The increase from 65 to 78% after insulin infusion was not statistically significant.
Comparison of muscle expression of GLUT4 and GLUT12 proteins
Figure 2 displays representative immunoblots of muscle homogenates from gel electrophoresis that included known standards for GLUT4 (top blot) and GLUT12 (lower blot). From image analysis of digitized films of at least three separate experiments using muscle homogenates from the same six normal volunteers, the absolute amount of each of these two isoforms was quantified and compared. Muscle homogenates yielded approximately 600 μg protein from 25 mg muscle. These six normal subjects’ muscle biopsies were quantified to contain GLUT4 at 3.74 ± 0.33 fmol/mg muscle, and GLUT12 was expressed at 0.51 ± 0.12 fmol/mg muscle. These data suggest that GLUT12 represents about 12% of the potentially translocatable GLUTs in normal skeletal muscle. The ratio of GLUT4 to GLUT12 protein (8:1) was less than the ratio of mRNAs (12:1) previously reported (4). The data from muscle fractions gave similar ratios of GLUT4 and GLUT12 protein. Adding the amount of GLUT4 or GLUT12 in LDM and PM together also gave a ratio of expression of 8:1.
Insulin-stimulated translocation of GLUT4 and GLUT12 in L6 myoblasts is prevented by the phosphatidyl inositol-3 kinase (PI3-K) inhibitor, LY294002
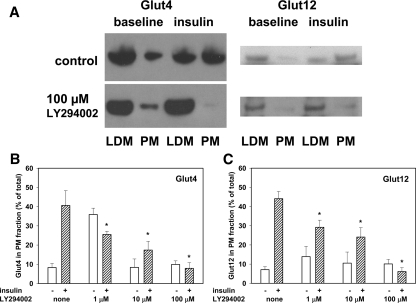
To determine whether the mechanism of GLUT12 movement to the cell surface of muscle was similar to the mechanism of GLUT4 translocation, we evaluated insulin-stimulated translocation of these proteins in a rat skeletal muscle-derived cell line previously demonstrated to respond to insulin (11). L6 cells have been used extensively to investigate translocation of GLUT4 and have been demonstrated to have PI3-K activation as an essential step in the insulin intracellular pathway leading to stimulation of glucose uptake (12,13). After having undergone serum starvation for 4 h, L6 cells were pretreated with PI3-K inhibitor LY294002 at concentrations of 0, 1, 10, and 100 μm for 20 min and were then incubated in the presence or absence of 10 ng/ml insulin for an additional 20 min. Cells were then harvested using a rubber scraper in Buffer A and homogenized using a hand-held Kontes homogenizer. After centrifugation (as described in Subjects and Methods) to separate each sample into LDM- and PM-enriched fractions, 10 or 20 μg protein aliquots were subjected to polyacrylamide electrophoresis and immunoblotting. Figure 4A displays representative immunoblots of the fractions. These immunoblots demonstrate that insulin caused translocation of both GLUT4 and GLUT12 to the PM-enriched fractions and that the PI3-K inhibitor prevented this from happening. Figure 4B shows summary data of dose-response studies for both GLUT4 and GLUT12 translocation inhibition by LY294002. The bars represent the mean and se data from three to five separate experiments. We found that LY294002 at lower concentrations actually stimulated translocation in the absence of insulin, perhaps acting as a partial agonist at 1 μm. However, at each concentration used (including 1 μm), there was inhibition of insulin-induced translocation.
Figure 4.
Inhibition of translocation of GLUT4 and GLUT12 in L6 muscle cells in culture. L6 cells were cultured to near confluence in 35-mm dishes, and then the growth medium was replaced with medium without serum for 1 h. Cells were then incubated at 37 C for another hour in the presence or absence of insulin (10 ng/ml) with concentrations of the PI3-K inhibitor LY294002 at 0, 1, 10, and 100 μm. After harvesting and fractionating the cells, samples from the fractions were subjected to immunoblotting, and digital images were quantified by image analysis software. A, Typical immunoblots; B and C, summary graphs of the quantitative analysis. Portions of both GLUT4 and GLUT12 moved from the LDM fraction to the PM fraction in response to incubation with insulin. At 1 μm LY294002 in the absence of insulin, GLUT4 and GLUT12 were more in the PM fraction than in the absence of LY294002, suggesting a PI3-K agonist effect at the lowest concentration. The translocation induced by insulin was reduced by the presence of LY294002 in a dose-responsive manner over the full range of concentrations tested.
Discussion
GLUT4 is the predominantly expressed GLUT in normal muscle. Based on mRNA data, GLUT5 and GLUT12 are expressed in muscle at higher levels than GLUT1, GLUT3, GLUT8, and GLUT11 (4). Like GLUT4, GLUT12 is expressed at higher levels in type I muscle fibers (4) and is predominantly intracellular in its subcellular localization. We show here that in normal human muscle, both GLUT4 and GLUT12 move from a largely intracellular location to the PM fraction in response to a euglycemic increase in blood insulin concentration. In contrast, GLUT1 is predominantly in the PM fraction at baseline and does not change its distribution in response to insulin infusion. In vitro experiments using L6 myoblasts in culture demonstrated that insulin also caused a shift of GLUT4 and GLUT12 from an intracellular location to the PM fraction. This translocation was blocked by inhibiting PI3-K, suggesting, at least in this system, that the translocation of GLUT12 also requires PI3-K stimulation. These studies did not attempt to resolve the question whether GLUT4 and GLUT12 are in the same vesicle or separate vesicles that are translocated by similar pathways of insulin action.
Since 2000, seven members have been added to the family of mammalian facilitative GLUTs. These 14 proteins have been subdivided into three separate classes based on structure and function (14). Although there are big gaps in the knowledge about the novel GLUTs, several are expressed in human muscle. These are GLUT8, GLUT11, and GLUT12. GLUT5 is a fructose transporter and is a member of the class II of GLUTs that also includes GLUT7, GLUT9, and GLUT11. Of the novel GLUTs, only GLUT6, GLUT8, and GLUT11 have had the specificity of glucose transport evaluated. Two of these (GLUT6 and GLUT11) transport both fructose and glucose (5). Tissue expression has been determined in all but GLUT7, but subcellular localization of the novel GLUTs has been largely by deduction from the amino acid sequences containing dileucine motifs at the amino or carboxy termini. GLUT10 and GLUT11 have no dileucine motif, whereas it was deduced that GLUT6, GLUT7, GLUT8, GLUT9, and GLUT12 are predominantly intracellular in the basal state (5). Regulation by insulin was not known, other than for GLUT12, where it appears that insulin does not cause translocation in MCF-7 cells in culture (15). The transport specificity, tissue and subcellular localization, and the acute and chronic regulation of the novel transporters identified in the past 10 yr should be important new information over the next few years.
Rogers and co-workers (15) first identified GLUT12 in MCF-7 human breast cancer cells by its homology with GLUT4. GLUT12 has 29% amino acid identity with GLUT4. They demonstrated GLUT12 in skeletal muscle, adipose tissue, small intestine, and placenta by immunoblotting (15,16). On the basis of perinuclear localization in MCF-7 cells and the presence of dileucine motifs near the amino and carboxy termini, Rogers speculated that GLUT12 might be part of a second insulin-responsive glucose transport system (15). Rogers’ group has shown GLUT12 expression to be present in prostate cancer and breast cancer, whereas it is absent in normal prostate and is expressed at very low levels in normal breast tissue (17,18). Although GLUT12 was identified early as a potential candidate for a second insulin-responsive GLUT in muscle and fat, there have not yet been any reports of insulin-induced translocation of GLUT12 either in cultured cells or in vivo.
We have previously quantified the mRNA expression of members of the GLUT family in normal human muscle (4). In these studies, we found detectable mRNA for GLUT1, GLUT3, GLUT8, and GLUT11, but much higher concentrations of message for GLUT4, GLUT5, and GLUT12. Immunohistochemistry of normal muscle showed strong expression of GLUT4 and GLUT12 in type 1 fibers, and GLUT5 was expressed more intensely in type 2 fibers (4). The muscle protein expression data in this report show a ratio of 8:1 for GLUT4:GLUT12. The previously reported data for mRNA in normal muscle (4) gave a somewhat higher ratio of 12:1, suggesting that disparate posttranslational processing or differential degradation may play a role in the observed protein concentrations. Because the patterns of expression in the immunohistochemistry studies were so similar for GLUT4 and GLUT12, and both are thought to be predominantly intracellular in the basal state, it was speculated at that time that GLUT4 and GLUT12 might even be colocalized to the same vesicles. In fact, in the studies we report here, the basal distribution and the postinsulin infusion muscle fraction distribution of GLUT4 and GLUT12 are very similar.
We performed euglycemic insulin infusions in six normal volunteers, and using modifications of Hirshman’s muscle microsample fractionation technique (19), we demonstrated a shift of GLUT4 from the intracellular LDM fractions to the PM-enriched fractions. In the baseline muscle samples, 13% of GLUT4 was in the PM fractions, whereas at the end of the insulin infusion, an average of 42% was in the PM fractions. These muscle GLUT4 translocation data are very similar to the human muscle GLUT4 translocation data reported by Kelley et al. (20), by Garvey et al. (21), and by Zierath et al. (22).
Two independent observations suggest that GLUT12 may have preceded GLUT4 in evolution. The human GLUT12 gene structure is much simpler than that of GLUT4. GLUT12 is a larger protein containing 617 amino acids, but the gene possesses only five exons and four introns (15), whereas GLUT4 has 509 amino acids and its human gene has 10 introns (23). The higher number of introns and exons in the GLUT4 gene is consistent with more complexity and more accumulated variations over millennia. Examining rat embryo ontogeny, GLUT12 message appears in heart at 14 d and the protein at 15 d (24), whereas GLUT4 mRNA appears at about 17 d and the protein is detectable at 21 d (25). In skeletal muscle both appear a little later, with GLUT12 protein at 17 d (24) and GLUT4 protein at 21 d (25).
The data presented here show that GLUT12 is present in human skeletal muscle and is distributed very similar to GLUT4 between intracellular and cell surface in the basal state. In response to euglycemic insulin infusions that achieved physiological insulin concentrations in blood, substantial amounts of GLUT12 moved to the muscle PM-enriched fractions in a manner that was quantitatively very similar to the effects on GLUT4. GLUT12 represents about 12% of the insulin-translocatable GLUTs in normal muscle. Our data from L6 muscle cells in culture suggest that the translocation of GLUT4 and GLUT12 both require activation of PI3-K. We conclude that GLUT12 translocates to the normal muscle cell PM in response to insulin. GLUT12 may provide a backup system for GLUT4 in skeletal muscle, and it likely preceded the evolutionary development of GLUT4.
Footnotes
This work was supported by National Institutes of Health Grants DK080488 (to C.A.S.) and DA020120 (to D.Y.) and a grant from Takeda Pharmaceuticals (to C.A.S.).
Disclosure Summary: C.A.S., M.E.A.H., Y.Z., and D.Y. have nothing to declare.
First Published Online June 23, 2009
Abbreviations: GLUT, Glucose transporter; LDM, low-density microsomes; PI3-K, phosphatidyl inositol-3 kinase; PM, plasma membrane.
References
- Saltiel AR 1996 Diverse signaling pathways in the cellular actions of insulin. Am J Physiol 270(3 Pt 1):E375–E385 [DOI] [PubMed] [Google Scholar]
- Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ 1995 Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377:151–155 [DOI] [PubMed] [Google Scholar]
- Stenbit AE, Burcelin R, Katz EB, Tsao TS, Gautier N, Charron MJ, Le Marchand-Brustel Y 1996 Diverse effects of GLUT4 ablation on glucose uptake and glycogen synthesis in red and white skeletal muscle. J Clin Invest 98:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart CA, Yin D, Howell ME, Dykes RJ, Laffan JJ, Ferrando AA 2006 Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab 291:E1067–E1073 [DOI] [PubMed] [Google Scholar]
- Joost HG, Thorens B 2001 The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol 18:247–256 [DOI] [PubMed] [Google Scholar]
- Stuart CA, Nagamani M 1990 Insulin infusion acutely augments ovarian androgen production in normal women. Fertil Steril 54:788–792 [PubMed] [Google Scholar]
- Reeds DN, Stuart CA, Perez O, Klein S 2006 Adipose tissue, hepatic, and skeletal muscle insulin sensitivity in extremely obese subjects with acanthosis nigricans. Metabolism 55:1658–1663 [DOI] [PubMed] [Google Scholar]
- Stuart CA, Wen G, Williamson ME, Jiang J, Gilkison CR, Blackwell SJ, Nagamani M, Ferrando AA 2001 Altered GLUT1 and GLUT3 gene expression and subcellular redistribution of GLUT4 protein in muscle from patients with acanthosis nigricans and severe insulin resistance. Metabolism 50:771–777 [DOI] [PubMed] [Google Scholar]
- Stuart CA, Wen G, Jiang J 1999 GLUT3 protein and mRNA in autopsy muscle specimens. Metabolism 48:876–880 [DOI] [PubMed] [Google Scholar]
- Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Wilson CM, Mitsumoto Y, Maher F, Klip A 1995 Regulation of cell surface GLUT1, GLUT3, and GLUT4 by insulin and IGF-I in L6 myotubes. FEBS Lett 368:19–22 [DOI] [PubMed] [Google Scholar]
- Somwar R, Niu W, Kim DY, Sweeney G, Randhawa VK, Huang C, Ramlal T, Klip A 2001 Differential effects of phosphatidylinositol 3-kinase inhibition on intracellular signals regulating GLUT4 translocation and glucose transport. J Biol Chem 276:46079–46087 [DOI] [PubMed] [Google Scholar]
- Rudich A, Klip A 2003 Push/pull mechanisms of GLUT4 traffic in muscle cells. Acta Physiol Scand 178:297–308 [DOI] [PubMed] [Google Scholar]
- Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schürmann A, Seino S, Thorens B 2002 Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 282:E974–E976 [DOI] [PubMed] [Google Scholar]
- Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD 2002 Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab 282:E733–E738 [DOI] [PubMed] [Google Scholar]
- Gude NM, Stevenson JL, Rogers S, Best JD, Kalionis B, Huisman MA, Erwich JJ, Timmer A, King RG 2003 GLUT12 expression in human placenta in first trimester and term. Placenta 24:566–570 [DOI] [PubMed] [Google Scholar]
- Chandler JD, Williams ED, Slavin JL, Best JD, Rogers S 2003 Expression and localization of GLUT1 and GLUT12 in prostate carcinoma. Cancer 97:2035–2042 [DOI] [PubMed] [Google Scholar]
- Rogers S, Docherty SE, Slavin JL, Henderson MA, Best JD 2003 Differential expression of GLUT12 in breast cancer and normal breast tissue. Cancer Lett 193:225–233 [DOI] [PubMed] [Google Scholar]
- Hirshman MF, Wallberg-Henriksson H, Wardzala LJ, Horton ED, Horton ES 1988 Acute exercise increases the number of plasma membrane glucose transporters in rat skeletal muscle. FEBS Lett 238:235–239 [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mintun MA, Watkins SC, Simoneau JA, Jadali F, Fredrickson A, Beattie J, Thériault R 1996 The effect of non-insulin-dependent diabetes mellitus and obesity on glucose transport and phosphorylation in skeletal muscle. J Clin Invest 97:2705–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD 1998 Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest 101:2377–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath JR, He L, Gumà A, Odegoard Wahlström E, Klip A, Wallberg-Henriksson H 1996 Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia 39:1180–1189 [DOI] [PubMed] [Google Scholar]
- Bell GI, Burant CF, Takeda J, Gould GW 1993 Structure and function of mammalian facilitative sugar transporters. J Biol Chem 268:19161–19164 [PubMed] [Google Scholar]
- Macheda ML, Kelly DJ, Best JD, Rogers S 2002 Expression during rat fetal development of GLUT12—a member of the class III hexose transporter family. Anat Embryol (Berl) 205:441–452 [DOI] [PubMed] [Google Scholar]
- Santalucía T, Camps M, Castelló A, Muñoz P, Nuel A, Testar X, Palacin M, Zorzano A 1992 Developmental regulation of GLUT-1 (erythroid/Hep G2) and GLUT-4 (muscle/fat) glucose transporter expression in rat heart, skeletal muscle, and brown adipose tissue. Endocrinology 130:837–846 [DOI] [PubMed] [Google Scholar]