Abstract
Context: Repeated hypoglycemia is associated with hypoglycemia-associated autonomic failure (HAAF), a syndrome of defective counterregulation.
Objective: HAAF increases the risk of severe hypoglycemia in diabetes, although its mechanism remains unresolved. Because β-endorphin influences the autonomic response to hypoglycemia via opioid receptor activation, we hypothesized that it is also involved in the pathogenesis of HAAF.
Research Design and Methods: We asked whether opioid receptor blockade during antecedent hypoglycemia (60 mg/dl) on d 1 would prevent development of HAAF on d 2 in eight nondiabetic subjects (five males, 3 females; age, 28 ± 3.5 yr; body mass index, 24.2 ± 2.1 kg/m2). On four occasions, d 1 was: 1) two 90-min hypoglycemic clamps (N−); 2) two 90-min hypoglycemic clamps plus naloxone (N+); 3) two euglycemic 90-min clamps (C); or 4) two euglycemic 90-min clamps plus naloxone (C+).
Results: Day 1 hypoglycemia caused marked deterioration of d 2 hormonal responses to hypoglycemia, consistent with HAAF—i.e. decreased plasma epinephrine, norepinephrine, and glucagon compared to control (C) (374 ± 71 vs. 810 ± 94, 307 ± 65 vs. 686 ± 98, and 71 ± 9 vs. 93 ± 4 pg/ml, respectively, P < 0.01), as well as in endogenous glucose production (24 vs. 163%; P < 0.01). In contrast, naloxone on d 1 completely prevented the defective counterregulatory responses; epinephrine, norepinephrine, and glucagon (852 ± 82, 769 ± 77, and 98 ± 7 pg/ml) and endogenous glucose production recovery (167%) were identical to those after d 1 euglycemia (P < NS for all). Infusion of naloxone alone during euglycemia on d 1 (C+) had no effect on d 2 responses.
Conclusions: These data suggest that the opioid signaling system is a promising target for further studies to prevent HAAF.
Opioid receptor blockade with naloxone during hypoglycemia prevents subsequent hypoglycemia associated autonomic failure in non-diabetic subjects.
The Diabetes Control and Complications Trial (DCCT) demonstrated the benefits of glycemic control vis-à-vis the complications of diabetes (1). Achievement of near-normal glycemia, however, carries the risk of hypoglycemia. In the DCCT, episodes of biochemical and symptomatic hypoglycemia were more frequent during intensive therapy, and the risk of severe hypoglycemia increased 3-fold (2). Patients with type 1 diabetes suffer from compromised counterregulatory hypoglycemic responses—viz., blunted glucagon and catecholamine responses, which ultimately result in poor recovery from hypoglycemia (3,4). Despite recent advances, imperfect insulin treatment remains the mainstay of therapy and continues to be the source of iatrogenic hypoglycemia. Frequent hypoglycemia is associated with hypoglycemia-associated autonomic failure (HAAF), a deterioration in counterregulation. Clinically, HAAF may also be associated with compromised hypoglycemia awareness (5). Various studies have shown that a single episode of hypoglycemia in nondiabetic subjects is sufficient to induce HAAF, emphasizing its clinical relevance to the intensive diabetes management (6,7). Altered counterregulation after recurrent/antecedent experimental hypoglycemia has also been demonstrated in type 1 diabetes (8,9), providing a robust physiological model of HAAF in humans. The precise mechanisms of HAAF, however, have not yet been clarified, although central nervous system (CNS) signals that mediate the counterregulatory responses may be of importance in its pathogenesis (10). Studies in animals and humans demonstrate that various stresses, including hypoglycemia and exercise, are associated with central release of endogenous opioids that, in turn, play a significant role in modulating the autonomic/sympatho-adrenomedullary system (11,12). Blockade of opioid receptors with infusion of naloxone enhanced hypoglycemia counterregulation, suggesting that central release of endogenous opioids during hypoglycemia (13) may have suppressive effects on counterregulatory responses. However, it is not known whether blocking the effects of endogenous opioids during antecedent hypoglycemia can prevent the development of HAAF (i.e. normalize the counterregulatory responses to subsequent hypoglycemia). Based on our prior data that the counterregulatory response to hypoglycemia can be pharmacologically modulated in humans (14,15), we hypothesized that blocking the effects of endogenous opioids with naloxone during recurrent hypoglycemia may prevent HAAF and identify a novel mechanism for further study.
Subjects and Methods
Subjects
We studied eight nondiabetic volunteers (five men, three women; age 28 ± 3 yr; body mass index, 24.2 ± 2.1 kg/m2; glycosylated hemoglobin, 5.6 ± 0.6%). All were in good health, taking no medications, and had no family history of diabetes. Each participated in three sets of studies in random order, with an interval of 5 wk between each set of studies. All studies were performed after an overnight fast.
Every set of studies consisted of 2 consecutive days (d 1 and d 2). Day 2 was identical in all studies and included a stepped hyperinsulinemic hypoglycemia clamp, with quantification of hormonal responses and glucose kinetics. Day 1 in each set of studies consisted of one of the following: 1) two 90-min hyperinsulinemic hypoglycemia clamps (N−); 2) two 90-min hyperinsulinemic hypoglycemia clamps with coinfusion of naloxone (N+); or 3) two 90-min hyperinsulinemic euglycemia clamps (C) with no other infusions. This design enabled us to examine the counterregulatory responses and glucose kinetics during stepped hypoglycemia clamps (d 2), after each experimental manipulation performed on d 1. To examine whether naloxone on d 1 could have directly influenced the counterregulatory responses on d 2, we performed an additional set of control studies as follows: d 1 was identical to the control studies but with concomitant naloxone infusion (C+). We performed this additional control after analyzing the results of the above three protocols that demonstrated that naloxone infusion on d 1 had a significant effects on d 2 hypoglycemia counterregulation. Thus, these additional control experiments (C+) were performed 12 months after the completion of the N−, N+, and C studies.
Informed written consent was obtained in accordance with policy of the Committee on Clinical Investigations of the Albert Einstein College of Medicine.
Procedures
Subjects were admitted to the Clinical Research Center for each experiment.
Day 1
At 0700 h on the study day, all subjects had two indwelling cannulae inserted, one placed in an antecubital vein for infusions and the second placed in a retrograde fashion in a distal hand vein of the contralateral forearm for blood sampling. To obtain arterialized venous blood samples, this hand was maintained at 65 C in a thermoregulated sleeve. At t = −30 min, a constant insulin infusion (Humulin Regular; Eli Lilly, Indianapolis, IN) was initiated at a rate of 1 mU/kg · min, and a variable infusion of 20% dextrose was administered to maintain the plasma glucose concentration at 60 mg/dl (N−) for a duration of 90 min. Blood samples were collected at 5-min intervals for measurements of plasma glucose. At the end of the 90-min clamp, insulin infusion was stopped, the subjects received a small snack (15 g of carbohydrate), and their plasma glucose was maintained at euglycemia by the use of an exogenous dextrose infusion (as needed) for an additional 2 h. At that point, insulin was restarted at a rate of 1 mU/kg · min, and a variable infusion of 20% dextrose was administered for a second 90-min hyperinsulinemic-hypoglycemic (N−) clamp. After completion of the second 90-min clamp, subjects consumed a large meal, had a bedtime snack, and were admitted for an overnight fast. All subjects received 5 ml/kg body water of 2H2O (99.9% 2H; Isotec, Miamisburg, OH), divided into three equal portions and given at 2000, 2300, and 0300 h. Additional water ingested during the fast was enriched to 0.5% with 2H2O to prevent dilution of the isotopic steady state.
The second set of studies was similar; however, naloxone (Narcan; Du Pont Pharmaceuticals, Wilmington, DE) was administered as a primed (4 μg/kg) continuous (0.4 μg/kg · min) infusion beginning at t = −30 min and continued throughout each of the hypoglycemia periods of the d 1 hypoglycemia clamps (N+). During the interval between the clamps, naloxone infusion was discontinued.
In the third set of studies (control), d 1 was similar to N−, however plasma glucose was maintained at euglycemia during the two 90-min hyperinsulinemic clamps (C).
In the fourth set of studies (C+), d 1 consisted of euglycemia during the two 90-min hyperinsulinemic clamps, together with naloxone (Narcan; Du Pont Pharmaceuticals) administered as a primed (4 μg/kg) continuous (0.4 μg/kg · min) infusion beginning at t = −30 min and continued throughout each of the two euglycemic periods. During the interval between the clamps, naloxone infusion was discontinued.
Day 2
The study conducted on d 2 was identical in all protocols. At 0700 h, subjects had two indwelling cannulae inserted. At t = −120 min, a primed-continuous infusion of HPLC-purified [3-3H]glucose was initiated with a bolus of 21.6 μCi, followed by continuous infusion of 0.15 μCi/min for the entire period of study. The specific activity of infused dextrose was kept equivalent to plasma glucose specific activity by addition of [3-3H]glucose to the infusate (16). At t = 0 min, a primed-continuous infusion of insulin was initiated at a rate of 0.5 mU/kg · min throughout the study, and a variable infusion of 20% dextrose was begun to maintain the plasma glucose concentration at 90 mg/dl for 50 min (step 1 of the clamp). At t = +50 min and every 50 min thereafter, the plasma glucose concentration was decreased by 10 mg/dl decrements for 50 min each by reducing the dextrose infusion rate accordingly. Plasma glucose was clamped at the desired range by varying the dextrose infusion according to plasma glucose measured at 5-min intervals with targets of 90, 80, 70, and 60 mg/dl.
Fasting blood for glycosylated hemoglobin was collected on the morning of each clamp study. During the clamps on d 2, blood samples were obtained for the determinations of plasma insulin, C-peptide, glucagon (collected in heparin-trasylol-treated tubes), epinephrine, and norepinephrine (collected in glutathione-treated tubes), β-endorphin (collected in EDTA-treated tubes), and cortisol, as well as for glucose kinetics and 2H enrichment of the hydrogen bound to C2 and C5 of blood glucose (for gluconeogenesis calculations). Symptoms of hypoglycemia were not measured.
Analytical methods
Plasma glucose was measured with a Beckman glucose analyzer (Beckman Coulter, Fullerton, CA), using the glucose oxidase method. Plasma [3-3H]glucose radioactivity was measured in duplicate on the supernatants of barium hydroxide-zinc sulfate precipitates of plasma samples, after evaporation to dryness to eliminate tritiated water.
The methods for measurement of plasma insulin, C-peptide, glucagon, cortisol, and their intra- and interassay variations have been previously reported (17). Plasma β-endorphin was measured using ELISA. Plasma epinephrine and norepinephrine levels were determined using the radioenzymatic assay described by Evans et al. (18).
2H2O method
Body water was calculated as 50% of body weight in women and 60% in men (19). Enrichment of the hydrogen at C2 and C5 of blood glucose was determined by procedures previously described (20). Briefly, blood was deproteinized and deionized, followed by glucose extraction. The resulting glucose was derivatized into monoacetone glucose (MAG) for nuclear magnetic resonance (NMR) analysis. The MAG sample was dissolved in 120 ml deuterium-depleted water and 420 ml HPLC acetonitrile in a 5-mm NMR tube. A 2H NMR spectrum was collected at 14.1 T (92 MHz for 2H) on a Brucker DRX600 system (Brucker BioSpin Corporation, Billerica, MA) equipped with a 5-mm deuterium probe with the ability to do 1H decoupling and 19F lock and with a z-gradient. Deuterium was tuned to the 2H carrier frequency. Shimming was performed on the 1H signal using the automated gradient shimming routine. 1H Waltz-16 decoupling was used during acquisition to remove proton-deuterium J coupling. Data were collected over a spectral width of 1.8 KHz at 47 C using the 90-degree pulse, at 1.1-sec acquisition time and an average of 5000–10,000 acquisition over 2–4 h. Peak areas were analyzed using the curve-fitting routine supplied with the NUTS PC-based NMR spectral analysis program (Acorn NMR Inc., Fremont CA). Given that the acquisition time was 1.1 sec and the longest T1 of any deuteron in MAG is less than 0.25 sec, no postacquisition correction of peak areas was necessary (21).
Analyses
The data are presented as the mean ± sem.
Steele’s equation was used for calculation of glucose turnover as described (22). Values for endogenous glucose production (EGP) and glucose uptake, obtained at 10-min intervals, were averaged over the final 30-min of each glucose step. The glycemic threshold for activation of a particular hormone was calculated as the glycemic level at which there was an increase of more than 2 sd values above the basal concentration (15). Statistical analyses were performed using repeated measures ANOVA for multiple comparisons and paired Student’s t test for comparing between two means (same subject) before and after an intervention (naloxone infusion). A value of P < 0.05 was considered significant.
Results
All results relate to the stepped hypoglycemia clamp studies performed on d 2.
Plasma glucose levels and glucose infusion rates (Fig. 1)
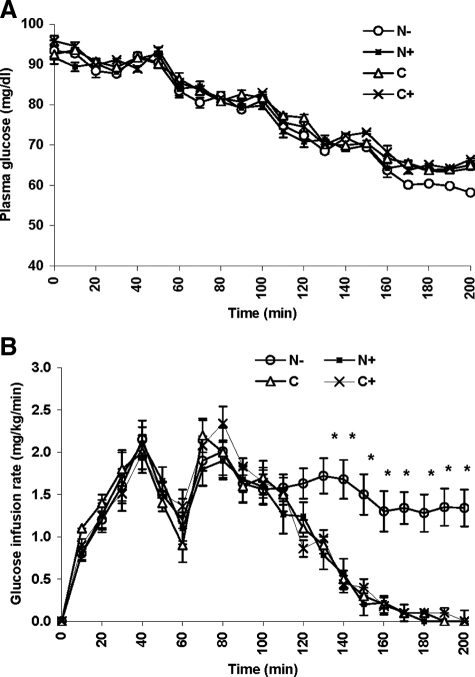
Figure 1.
Plasma glucose concentrations (d 2) at each glucose step in the studies with (▪, N+), and without (○, N−) coinfusion of naloxone on the previous day (d 1), C (▵), and C+ (×) studies. A, Hyperinsulinemic euglycemic clamp on d 1. B, Glucose infusion rates over time. *, P < 0.01.
Plasma glucose concentrations during the clamps (N−, N+, C, and C+) are shown in Fig. 1A. Plasma glucose concentrations at t = 0 were 95 ± 3 mg/dl in the N− studies, 94 ± 2 mg/dl in the N+ studies, 97 ± 4 mg/dl in the C, and 95 ± 5 mg/dl in the C+ studies [P = not significant (NS)]. No significant difference was noted between all studies during the 90, 80, and 70 mg/dl target glucose steps; however, during the 60 mg/dl target glucose step, plasma glucose could not be decreased to less than 64 ± 1 mg/dl in the N+, 65 ± 1 mg/dl in the C, and 64 ± 2 mg/dl in the C+ studies. The higher plasma glucose levels in the N+, C, and C+ studies during this glucose step occurred despite the fact that the glucose infusion was discontinued and coincided with elevated plasma concentration of epinephrine, norepinephrine, and glucagon (see below).
Glucose infusion rates are depicted in Fig. 1B. During the first (90 mg/dl) and second (80 mg/dl) glucose steps, average glucose infusion rates were comparable in all studies (1.3 ± 0.2 mg/kg · min in the N−, 1.3 ± 0.2 mg/kg · min in the N+, 1.4 ± 0.2 mg/kg · min in the C, and 1.3 ± 0.3 mg/kg · min in the C+ studies; P = NS). However, during the 70 mg/dl glucose step, the mean rate of glucose infusion was lower in the N+, C, and C+ studies (0.8 ± 0.2, 0.9 ± 0.1, and 0.8 ± 0.1 mg/kg · min, respectively, vs. 1.6 ± 0.2 mg/kg · min in the N− studies; P = 0.01). During the 60 mg/dl glucose step, the average glucose infusion rate was also significantly lower (0.1 ± 0.1 mg/kg · min) in the N+, C, and C+ studies vs. 1.3 ± 0.2 mg/kg · min in the N− studies (P < 0.001). These data complement our findings suggesting suppressed hypoglycemia counterregulation in the N− studies compared with the N+ and both control studies (C and C+).
Plasma insulin and C-peptide concentrations (Fig. 2)
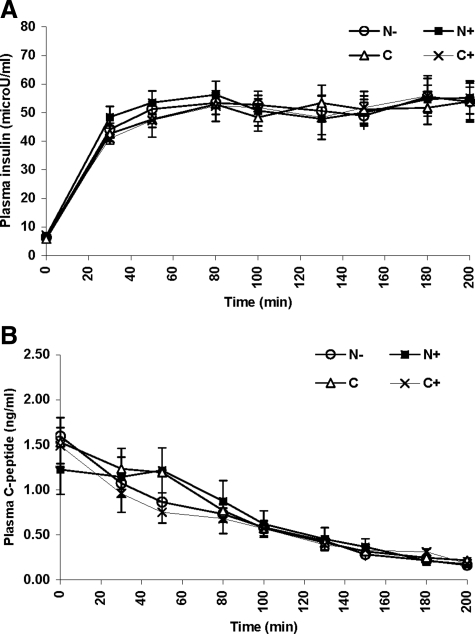
Figure 2.
Plasma insulin concentrations (A) and C-peptide concentrations (B) in the N+ (▪), N− (○), C (▵), and C+ (×) on d 2 of the studies.
Basal plasma insulin concentrations were nearly identical in all studies, averaging 5.9 ± 0.8 μU/ml in the N−, 6.3 ± 0.5 μU/ml in the N+, 5.5 ± 0.8 μU/ml in the C, and 6.2 ± 0.9 μU/ml in the C+ studies (P = NS). Similarly, there was no significant difference in plasma insulin concentration during all clamp steps averaging 52.3 ± 4.2 μU/ml in the N−, 51.7 ± 1.4 μU/ml in the N+, 53.7 ± 1.0 μU/ml in the C, and 48.8 ± 3.3 μU/ml in the C+ studies (Fig. 2A). Plasma C-peptide concentrations were comparable in all studies at baseline (1.6 ± 0.2, 1.3 ± 0.4, 1.5 ± 0.4, and 1.3 ± 0.3 ng/ml in the N−, N+, C, and C+ studies, respectively; P = NS). C-peptide values remained very similar during the clamp, suppressed in all studies to 0.2 ± 0.03 ng/ml at the hypoglycemic nadir, at t = 200 min (Fig. 2B).
Counterregulatory hormones (Fig. 3)
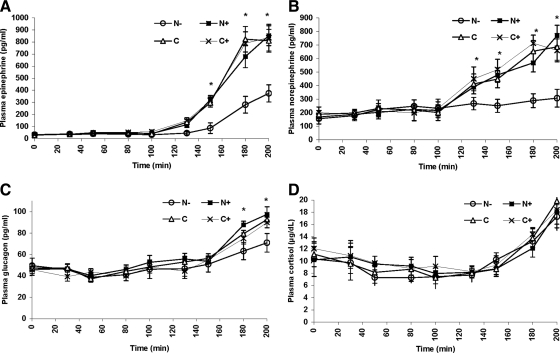
Figure 3.
Plasma epinephrine (A), norepinephrine (B), glucagon (C), and cortisol (D) concentrations over time in the N+ (▪), N− (○), C (▵), and C+ (×) on d 2 of the studies. *, P < 0.05.
During the 90 and 80 mg/dl glucose steps, plasma epinephrine concentrations remained near or at basal values and were similar in all studies (35 ± 12, 37 ± 14, 38 ± 15, and 34 ± 18 pg/ml in the N−, N+, C, and C+ studies, respectively, P = NS). Further reduction in plasma glucose to 70 and 60 mg/dl was associated with appropriate increases in plasma epinephrine in the control studies (C and C+) to 810 ± 94 and 765 ± 110 pg/ml, respectively (P = NS). However, in the N− studies, plasma epinephrine was only modestly increased to 374 ± 71 pg/ml (P < 0.01 vs. C and C+), demonstrating blunting of epinephrine response after antecedent hypoglycemia. Finally, in the N+ studies, the plasma epinephrine increase was comparable to that in control studies (852 ± 82 pg/ml), suggesting normalization of epinephrine release despite recurrent antecedent hypoglycemia. The amplification of the epinephrine response in the N+ studies was demonstrated by the plasma glucose needed to trigger epinephrine release (higher threshold for epinephrine release) as well as by significantly greater peak and plateau levels of plasma epinephrine that were similar to the control studies (Fig. 3A and Table 1).
Table 1.
Plasma epinephrine, norepinephrine, and glucagon concentrations on d 2 studies in the control (C and C+), N+, and N− studies at basal (time 0), peak, and their respective glycemic thresholds
| C | C+ | N+ | N− | |
|---|---|---|---|---|
| Epinephrine | ||||
| Basal (pg/ml) | 38 ± 15 | 34 ± 18 | 37 ± 14 | 35 ± 12 |
| Peak (pg/ml) | 810 ± 94 | 765 ± 110 | 852 ± 82 | 374 ± 71a |
| Glycemic threshold (mg/dl) | 73 ± 7 | 71 ± 5 | 75 ± 4 | 63 ± 6a |
| Norepinephrine | ||||
| Basal (pg/ml) | 196 ± 42 | 174 ± 29 | 180 ± 33 | 170 ± 32 |
| Peak (pg/ml) | 686 ± 98 | 719 ± 83 | 769 ± 77 | 307 ± 65a |
| Glycemic threshold (mg/dl) | 71 ± 4 | 69 ± 5 | 69 ± 5 | 62 ± 4a |
| Glucagon | ||||
| Basal (pg/ml) | 45 ± 5 | 41 ± 6 | 47 ± 3 | 46 ± 4 |
| Peak (pg/ml) | 93 ± 4 | 89 ± 5 | 98 ± 7 | 71 ± 9a |
| Glycemic threshold (mg/dl) | 68 ± 4 | 70 ± 3 | 68 ± 4 | 65 ± 3a |
P < 0.05 vs. C, C+, and N+.
Basal and stimulated plasma norepinephrine concentrations were equivalent during the 90 and 80 mg/dl glucose steps in all studies (Fig. 3B). However, release of norepinephrine was significantly blunted in the N− studies during the 70 and 60 mg/dl glucose steps, and the threshold for norepinephrine release occurred at a significantly lower plasma glucose concentration. The threshold for release and peak levels of norepinephrine were no different in controls (C and C+) and N+ studies, suggesting correction of the blunted norepinephrine response after recurrent hypoglycemia by naloxone administration (Fig. 3B and Table 1).
Plasma glucagon was equivalent in all studies during the 90 and 80 mg/dl glucose steps (Fig. 3C), but the increase in glucagon was blunted in the N− studies during the 60 mg/dl glucose step (71 ± 9 pg/ml in the N− vs. 93 ± 4 and 89 ± 5 pg/ml in the C and C+ studies, respectively; P < 0.01 for both). Naloxone administration, however, restored the glucagon response to that observed in control studies (98 ± 7 pg/ml), with normalization of both the amplitude and glycemic threshold for glucagon release (Table 1).
Finally, plasma cortisol levels were similar in all studies (Fig. 3D) and unaffected by either antecedent hypoglycemia or antecedent naloxone.
Glucose kinetics and fractional rates of gluconeogenesis (Fig. 4)
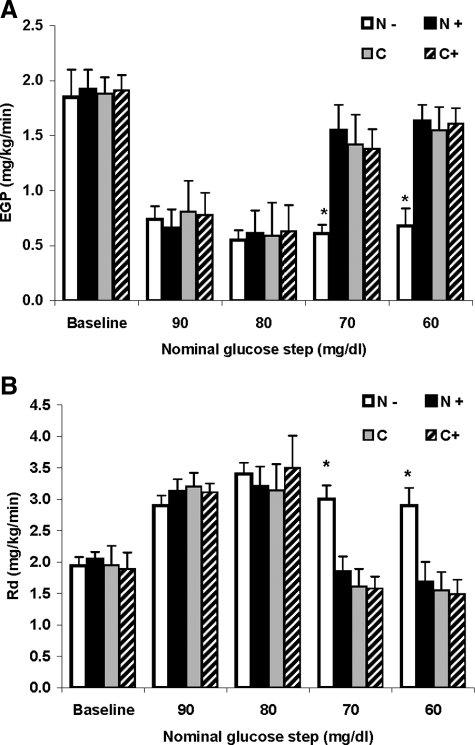
Figure 4.
A, EGP (d 2) at baseline (after 120 min of tracer infusion, at t = 0), and averaged for the final 30 min of each glucose step. B, Glucose Rd averaged for the final 30 min of each glucose step. Studies included previous (d 1) coinfusion of naloxone (N+), without coinfusion of naloxone (N−), and controls studies with previous (d 1) coinfusion of naloxone (C+), and without coinfusion of naloxone (C). *, P < 0.05.
Mean fasting EGP, measured at the end of the 120 min of tracer infusion (t = 0), was similar in all studies (1.8 ± 0.2, 1.9 ± 0.2, 1.9 ± 0.1, and 2.0 ± 0.3 mg/kg · min in the N−, N+, C, and C+ studies, respectively; P = NS). During the 90 and 80 mg/dl glucose steps, EGP was equally suppressed by approximately 65% compared with baseline across all studies. During the next two clamp steps (70 and 60 mg/dl), EGP rose by 11 and 24% from nadir, respectively, in the N− studies, showing only modest EGP recovery. In contrast, EGP rose by 154 and 167% from nadir, respectively, in the N+ studies (P < 0.001) and to a similar extent (141 and 163%, and 158 and 171%, respectively; P < 0.001) in the C and C+ studies, demonstrating normalization of EGP recovery in the N+ studies to that observed during the control studies (C and C+, Fig. 4A).
Glucose disposal rates (Rd) were similar before insulin infusion in all studies and increased equally with the initiation of insulin infusion by approximately 64% from baseline (to 3.4 ± 0.2, 3.2 ± 0.3, 3.1 ± 0.4, and 3.5 ± 0.5 mg/kg · min in the N−, N+, C, and C+ studies, respectively; P = NS, during the 80 mg/dl glucose step). Subsequently, during the 70 and 60 mg/dl glucose steps, glucose Rd decreased by approximately 15% in the N− studies, and by 48, 51, and 53% in the N+ and control (C and C+) studies, respectively (P < 0.05), promoting glucose recovery in the N+ studies similar to the controls (Fig. 4B).
The contribution of gluconeogenesis to EGP averaged 44.2 ± 11.9% in the N−, 50.0 ± 8.8% in the N+, and 48.7 ± 5.7% in the C studies before the initiation of the clamps (P = NS), and decreased significantly to 37.1 ± 7.4, 34.6 ± 5.2, and 32.2 ± 3.9%, respectively (P < 0.05, vs. baseline for all), at the 60 mg/dl glucose step, however, with no significant differences between the three protocols. Glycogen turnover measurements were not performed in the C+ studies.
Plasma β-endorphin levels (Table 2)
Table 2.
Plasma β-endorphin levels at baseline and peak during d 1 and d 2 in the N+, N−, C, and C+ studies
| N− | N+ | C | C+ | |
|---|---|---|---|---|
| Day 1 Baseline (ng/ml) | 2.5 ± 0.8 | 5.1 ± 1.3 | 4.3 ± 1.8 | 2.7 ± 1.4 |
| Day 1 Peak (ng/ml) | 16.7 ± 2.3a | 14.4 ± 3.6a | 5.3 ± 1.8 | 3.7 ± 2.2 |
| Day 2 Baseline (ng/ml) | 2.4 ± 1.3 | 4.2 ± 0.9 | 3.1 ± 0.6 | 2.6 ± 1.5 |
| Day 2 Peak (ng/ml) | 11.8 ± 2.5a | 15.2 ± 2.7a | 13.8 ± 3.3a | 14.4 ± 3.7a |
P < 0.001 vs. baseline.
On d 1, plasma β-endorphin levels at baseline averaged 2.5 ± 0.8, 5.1 ± 1.3, 4.3 ± 1.8, and 2.7 ± 1.4 ng/ml in the N−, N+, C, and C+ studies, respectively (P = NS). At the end of d 1 experiments, plasma β-endorphin concentrations increased significantly in the hypoglycemia studies (16.7 ± 2.3 and 14.4 ± 3.6 ng/ml in the N− and N+, respectively; P < 0.001 in both), however, in the control (C and C+) studies, plasma β-endorphin levels did not change significantly (5.3 ± 1.8 and 3.7 ± 2.2 ng/ml, respectively; P = NS).
On d 2, plasma β-endorphin levels at baseline averaged 2.4 ± 1.3, 4.2 ± 0.9, 3.1 ± 0.6, and 2.6 ± 1.5 ng/ml in the N−, N+, C, and C+ studies, respectively (P = NS). At the end of the hypoglycemia clamp, plasma β-endorphin concentrations increased significantly in all studies to 11.8 ± 2.5, 15.2 ± 2.7, 13.8 ± 3.3, and 14.4 ± 3.7 ng/ml in the N−, N+, C, and C+ studies, respectively (P = NS between studies; P < 0.001 vs. baseline in all studies).
Discussion
Our data demonstrate that in nondiabetic humans, two episodes of mild hypoglycemia (∼60 mg/dl) are sufficient to induce deterioration of the counterregulatory response to hypoglycemia on the following day. Compared with antecedent hyperinsulinemic euglycemia clamps (controls), the counterregulatory response in the N− studies was characterized by lower epinephrine, norepinephrine, and glucagon responses, with parallel defects in glucose kinetics and impaired glucose recovery. These findings support previous reports demonstrating experimental HAAF using similar designs (6,8,9,23,24). Most importantly, we found that an opioid receptor blockade during d 1 hypoglycemia completely prevented the deterioration of the counterregulatory response to hypoglycemia on d 2. Epinephrine, norepinephrine, and glucagon concentrations and the corresponding glucose recovery rates in these studies were comparable to control studies, demonstrating no deterioration in the counterregulatory systems that are used as surrogate evidence of autonomic failure (i.e. HAAF).
For the control studies [euglycemia on d 1 (C) and euglycemia with concomitant naloxone on d 1 (C+)], we used identical clamps to exclude any potential effects of hyperinsulinemia. Thus, plasma insulin concentrations during all studies were comparable. Furthermore, the fact that the counterregulatory responses were identical in the C and C+ studies indicates that naloxone infusion per se, in the absence of hypoglycemia, had no influence on the counterregulatory responses to subsequent hypoglycemia (d 2).
The stepped hypoglycemia clamps (on d 2) were identical in all studies with the exception that during the final (60 mg/dl) step plasma, glucose could not be further decreased in the N+ and control studies because glucose infusion had been already terminated. This may be explained by the fact that adequate glucose counterregulation had occurred. We intentionally chose a modest insulin infusion rate (0.5 mU/kg · min) to only partially suppress EGP during the hypoglycemic clamps, and this enabled us to quantify EGP recovery with counterregulation. In parallel with the changes in counterregulatory hormones, we observed the decrease in EGP and a corresponding increase in glucose disposal (Rd) induced by HAAF in the N− studies. In contrast, the increase in the counterregulatory responses in the N+ and control studies resulted in recovery of the suppressed EGP (due to hyperinsulinemia) and a decrease in Rd—fluxes that are responsible for recovery from hypoglycemia. Thus, these findings further demonstrate that the increase in counterregulation in the N+ studies was associated with normalization of hypoglycemia recovery.
To examine whether specific defects in the counterregulatory responses to hypoglycemia in HAAF are associated with abnormal glycogenolysis/gluconeogenesis during hypoglycemia recovery, we used the 2H2O method. The decrease in the contribution of gluconeogenesis to EGP during hypoglycemia recovery supports our and others’ previous studies showing that glycogen breakdown is the initial source for glucose-6-phosphate for EGP during the first several hours of hypoglycemia (25,26). Although the recovery from hypoglycemia was defective in the N− studies, the distribution in the pathways responsible for the increase in EGP (i.e. glycogen breakdown vs. gluconeogenesis) did not differ.
Extensive research on the biology of endogenous opioids has provided a framework through which the current studies can be viewed (27). Opioid receptor activation occurs in response to a variety of stressors including hypoglycemia (11,13) and exercise (28), resulting in increased CNS release of endogenous opioids. The release of endogenous opioids is associated with inhibition of the central component of the sympatho-adrenomedullary system, principally by μ- and κ-receptor activation (29), whereas opioid antagonists enhance catecholamine release (30). In light of our results, we propose a heretofore unappreciated mechanism that may explain the development of HAAF after repeated hypoglycemia, and we suggest that acute hypoglycemia-induced CNS μ-opioid receptor activation results in an alteration of its activity, which ultimately results in a decrease in the sympathoadrenal response.
In vivo studies in animals and humans demonstrate that opioid receptor blockade with naloxone during hypoglycemia resulted in an enhanced counterregulatory response (31) and a significant increase in EGP (13). Our study indicates that the effect of blocking endogenous opioids with naloxone is prolonged and disrupts normal counterregulatory responses to hypoglycemia the following day. However, because naloxone infusion during euglycemia (d 1) had no effect on d 2 counterregulation (C+ studies), prevention of HAAF with naloxone administered during antecedent hypoglycemia is not due to a carryover effect of the drug. The fact that plasma β-endorphin concentrations increased during hypoglycemia supports other studies that demonstrate increases in plasma β-endorphin with stresses including hypoglycemia and minimal variations in peripheral plasma β-endorphin concentrations with naloxone infusion (32,33). Furthermore, our observation of nearly identical cortisol concentrations in all protocols suggests either that naloxone was infused at an insufficient concentration to induce an increase in cortisol or that there was a differential effect on the counterregulatory hormonal response. Interestingly, the effect of naloxone infusion on glucagon release during subsequent hypoglycemia suggests that glucagon secretion is mediated centrally, at least in part. Although there is considerable evidence that glucagon release during hypoglycemia is modulated at the islet level, several studies have shown that CNS manipulations (e.g. ventromedial hypothalamus) can affect glucagon secretion (reviewed in Ref. 34), suggesting the possibility of redundant regulation.
In summary, our data demonstrate that HAAF induced by antecedent hypoglycemia in nondiabetic subjects was prevented by opioid receptor blockade. Accumulating reports clearly demonstrate the beneficial effects of meticulous glycemic control in patients with diabetes (1,35). Such a therapeutic approach, however, carries a significant risk for developing recurrent hypoglycemia and HAAF (4), conditions that are associated with increased morbidity and mortality. Although it has been previously shown that meticulous avoidance from hypoglycemia can partially reverse HAAF (36), our data demonstrate that HAAF may be preventable pharmacologically. Further studies are required to examine whether opioid receptor blockade can prevent HAAF in patients with diabetes and whether other delivery methods (that can be used clinically) for opioid receptor blockade drugs have similar effects.
Acknowledgments
We are indebted to the staff of the Clinical Research Center and the Institute for Clinical and Translational Research. The authors thank Ms. Robin Sgueglia and Zhao Hu for laboratory determinations.
Footnotes
This work was supported by National Institutes of Health Grants DK 079974 (to I.G.), RR017313 (to I.G.), DK 20541 (to I.G. and H.S.), and DK020541, and the Clinical and Translational Science Awards (1UL1RR025750).
The results of this study were reported in abstract form at the Annual Scientific Sessions of the American Diabetes Association, San Francisco, CA, June 16, 2008.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 30, 2009
Abbreviations: CNS, Central nervous system; EGP, endogenous glucose production; HAAF, hypoglycemia-associated autonomic failure; MAG, monoacetone glucose; NMR, nuclear magnetic resonance; NS, not significant; Rd, disposal rate.
References
- 1993 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- 1991 Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med 90:450–459 [PubMed] [Google Scholar]
- Cryer PE 2002 The pathophysiology of hypoglycaemia in diabetes. Diabetes Nutr Metab 15:330–333; discussion 362 [PubMed] [Google Scholar]
- Cryer PE, Davis SN, Shamoon H 2003 Hypoglycemia in diabetes. Diabetes Care 26:1902–1912 [DOI] [PubMed] [Google Scholar]
- Cryer PE 2001 Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocrinol Metab 281:E1115–E1121 [DOI] [PubMed] [Google Scholar]
- Heller SR, Cryer PE 1991 Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 40:223–226 [DOI] [PubMed] [Google Scholar]
- Davis SN, Tate D 2001 Effects of morning hypoglycemia on neuroendocrine and metabolic responses to subsequent afternoon hypoglycemia in normal man. J Clin Endocrinol Metab 86:2043–2050 [DOI] [PubMed] [Google Scholar]
- Davis MR, Mellman M, Shamoon H 1992 Further defects in counterregulatory responses induced by recurrent hypoglycemia in IDDM. Diabetes 41:1335–1340 [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack SE, Craft S, Cryer PE 1993 Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 91:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH 2002 CNS sensing and regulation of peripheral glucose levels. Int Rev Neurobiol 51:219–258 [DOI] [PubMed] [Google Scholar]
- Nakao K, Nakai Y, Jingami H, Oki S, Fukata J, Imura H 1979 Substantial rise of plasma β-endorphin levels after insulin-induced hypoglycemia in human subjects. J Clin Endocrinol Metab 49:838–841 [DOI] [PubMed] [Google Scholar]
- Goldfarb AH, Jamurtas AZ 1997 β-Endorphin response to exercise. An update. Sports Medicine 24:8–16 [DOI] [PubMed] [Google Scholar]
- Caprio S, Gerety G, Tamborlane WV, Jones T, Diamond M, Jacob R, Sherwin RS 1991 Opiate blockade enhances hypoglycemic counterregulation in normal and insulin-dependent diabetic subjects. Am J Physiol 260:E852–E858 [DOI] [PubMed] [Google Scholar]
- Gabriely I, Shamoon H 2005 Fructose normalizes specific counterregulatory responses to hypoglycemia in patients with type 1 diabetes. Diabetes 54:609–616 [DOI] [PubMed] [Google Scholar]
- Gabriely I, Hawkins M, Vilcu C, Rossetti L, Shamoon H 2002 Fructose amplifies counterregulatory responses to hypoglycemia in humans. Diabetes 51:893–900 [DOI] [PubMed] [Google Scholar]
- Finegood DT, Bergman RN, Vranic M 1987 Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 36:914–924 [DOI] [PubMed] [Google Scholar]
- Mellman MJ, Davis MR, Brisman M, Shamoon H 1994 Effect of antecedent hypoglycemia on cognitive function and on glycemic thresholds for counterregulatory hormone secretion in healthy humans. Diabetes Care 17:183–188 [DOI] [PubMed] [Google Scholar]
- Evans MI, Halter JB, Porte Jr D 1978 Comparison of double- and single-isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin Chem 24:567–570 [PubMed] [Google Scholar]
- Culebras JM, Moore FD 1977 Total body water and the exchangeable hydrogen. I. Theoretical calculation of nonaqueous exchangeable hydrogen in man. Am J Physiol 232:R54–R59 [DOI] [PubMed] [Google Scholar]
- Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC 1995 Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J Clin Invest 95:172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins RL, Malloy CR 2003 Measuring in-vivo metabolism using nuclear magnetic resonance. Curr Opin Clin Nutr Metab Care 6:501–509 [DOI] [PubMed] [Google Scholar]
- Steele R 1959 Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82:420–430 [DOI] [PubMed] [Google Scholar]
- Cryer PE 2005 Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 54:3592–3601 [DOI] [PubMed] [Google Scholar]
- Davis SN, Shavers C, Mosqueda-Garcia R, Costa F 1997 Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes 46:1328–1335 [DOI] [PubMed] [Google Scholar]
- Lecavalier L, Bolli G, Cryer P, Gerich J 1989 Contributions of gluconeogenesis and glycogenolysis during glucose counterregulation in normal humans. Am J Physiol 256:E844–E851 [DOI] [PubMed] [Google Scholar]
- Kishore P, Gabriely I, Cui MH, Di Vito J, Gajavelli S, Hwang JH, Shamoon H 2006 Role of hepatic glycogen breakdown in defective counterregulation of hypoglycemia in intensively treated type 1 diabetes. Diabetes 55:659–666 [DOI] [PubMed] [Google Scholar]
- Somogyi AA, Barratt DT, Coller JK 2007 Pharmacogenetics of opioids. Clin Pharmacol Ther 81:429–444 [DOI] [PubMed] [Google Scholar]
- Harbach H, Hell K, Gramsch C, Katz N, Hempelmann G, Teschemacher H 2000 β-Endorphin (1-31) in the plasma of male volunteers undergoing physical exercise. Psychoneuroendocrinology 25:551–562 [DOI] [PubMed] [Google Scholar]
- Shook JE, Kazmierski W, Wire WS, Lemcke PK, Hruby VJ, Burks TF 1988 Opioid receptor selectivity of beta-endorphin in vitro and in vivo: mu, delta and epsilon receptors. J Pharmacol Exp Ther 246:1018–1025 [PubMed] [Google Scholar]
- Samardzić R, Jovanović-Mićić D, Japindzić N, Beleslin DB 1990 β-Endorphin-induced behavioural effects: endorphin-catecholamines interactions. Acta Physiol Hung 75 Suppl:253–254 [PubMed] [Google Scholar]
- el-Tayeb KM, Brubaker PL, Lickley HL, Cook E, Vranic M 1986 Effect of opiate-receptor blockade on normoglycemic and hypoglycemic glucoregulation. Am J Physiol 250:E236–E242 [DOI] [PubMed] [Google Scholar]
- Tendzegolskis Z, Viru A, Orlova E 1991 Exercise-induced changes of endorphin contents in hypothalamus, hypophysis, adrenals and blood plasma. Int J Sports Med 12:495–497 [DOI] [PubMed] [Google Scholar]
- Radosevich PM, Lacy DB, Brown LL, Williams PE, Abumrad NN 1988 Effects of insulin-induced hypoglycemia on plasma and cerebrospinal fluid levels of ir-β-endorphins, ACTH, cortisol, norepinephrine, insulin and glucose in the conscious dog. Brain Res 458:325–338 [DOI] [PubMed] [Google Scholar]
- Taborsky Jr GJ, Ahren B, Mundinger TO, Mei Q, Havel PJ 2002 Autonomic mechanism and defects in the glucagon response to insulin-induced hypoglycaemia. Diabetes Nutr Metab 15:318–322; discussion 322–323 [PubMed] [Google Scholar]
- Bolli GB 2003 Treatment and prevention of hypoglycemia and its unawareness in type 1 diabetes mellitus. Rev Endocr Metab Disord 4:335–341 [DOI] [PubMed] [Google Scholar]
- Fanelli CG, Epifano L, Rambotti AM, Pampanelli S, Di Vincenzo A, Modarelli F, Lepore M, Annibale B, Ciofetta M, Bottini P, Porcellati F, Scionti L, Santeusanio F, Brunetti P, Bolli GB 1993 Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 42:1683–1689 [DOI] [PubMed] [Google Scholar]