Abstract
Context: Factors associated with the high prevalence of vitamin D deficiency in China are not well described, especially among Chinese adolescents.
Objectives: The aim of the study was to examine important environmental or sociodemographic factors influencing 25-hydroxyvitamin D [25(OH)D] levels and estimate its heritability.
Design: A sample of 226 male and female adolescent twins aged 13–20 yr from a large prospective twin cohort of rural Chinese children and adolescents that has been followed for 6 yr were evaluated.
Main Outcome Measure(s): Blood level of 25(OH)D was measured using tandem mass spectrometry methodology.
Results: The overall mean (sd) 25(OH)D level was 18.0 (9.4) ng/ml, with wide variation by gender and season. In males (47.4% of subjects), the mean (sd) 25(OH)D level was 12.1 (4.2) ng/ml in non-summer and 27.4 (8.8) ng/ml in summer; in females, it was 10.1 (4.1) ng/ml in non-summer and 19.5 (6.3) ng/ml in summer. A multivariate model that included gender, age, season, physical activity, and student status demonstrated that male gender, summer season, and high physical activity significantly increased 25(OH)D levels. Summer season and male gender also significantly decreased the risk of being in the lowest 25(OH)D tertile. Overall, 68.9% of the variability in 25(OH)D level was attributable to additive genetic influence. Stratification by gender found that in males, 85.9% of the variability in 25(OH)D level was attributable to such influence, but in females, it was only 17%.
Conclusion: In this sample of rural Chinese adolescents, 25(OH)D level was influenced by gender, season, and physical activity level. There was a strong genetic influence on 25(OH)D level in males only.
25(OH)D level was influenced by gender, season, physical activity level, and student status; there appeared to be a strong genetic influence on 25(OH)D levels in males only.
Vitamin D adequacy plays a critical role in the overall body economy of calcium and phosphorus, and concentrations of circulating 25-hydroxyvitamin D [25(OH)D] in the blood less than 10 ng/ml have been found to lead to rickets among children and osteomalacia in adults (1,2,3). Although vitamin D deficiency-related rickets is relatively uncommon in western countries, it is the second most common nutritional disease among children in China (4). However, rickets may be reemerging as a global public health problem (5,6), as evidenced by an increasing number of case reports in developed countries. Moreover, vitamin D deficiency is suspected to play an etiological role in a widening spectrum of diseases, including select cancers, autoimmune disorders, type 1 diabetes, heart disease, and obesity (7,8).
Prior studies have demonstrated that race/ethnicity, season, latitude, and various chronic conditions may influence 25(OH)D levels during all stages of human development (7,9). Nonetheless, factors influencing vitamin D levels in Chinese populations are not well studied, particularly among children and adolescents. Almost 20% of the world’s population resides in China (10), and cardiovascular diseases and cancer are leading causes of morbidity and mortality in this population (11). The role of vitamin D in a broad spectrum of diseases is growing, thereby providing impetus for examining its association with current disease trends in China. As such, examining factors influencing vitamin D status in populations undergoing nutrition and lifestyle transitions, such as China (12), would be illuminating particularly from a public health perspective.
Studies in adult twins have demonstrated that 25(OH)D levels are somewhat under genetic control (13,14). However, genetic influence has not yet been estimated in children or adolescents. This is important because the physiology in children and adolescents is different from that in adults, particularly during adolescence, which is a transitional period characterized by epiphyseal fusion, attainment of adult stature and muscle mass (especially in males), attainment of adult size of the pelvic inlet (in females), fertility, peak bone mass, and physiological homeostasis (15,16). Genetic influences at work during adolescence could be different from those at work in adulthood. Estimating the genetic influence on vitamin D status in twin populations is an important first step to subsequent genetic research regarding the genetic influences on the covariation of vitamin D and disease. This study elucidates factors strongly associated with 25(OH)D levels and estimates the genetic influence on 25(OH)D levels in a cohort of male and female adolescent twins in rural China.
Subjects and Methods
Study sample
The study subjects are participants of an ongoing study of a large Chinese twin cohort (17). Twins were recruited in eight counties of the Anqing region in 1998–2000 and were followed up in 2005–2006. Anqing is located at 33.5° N latitude and stretches for about 80 km along the north bank of the Yangtze River in Anhui Province, China. It has one city, two suburbs, and eight rural counties, with a total population of 6.1 million (90% rural) (18). Twins were chosen to participate in the study based on the following criteria: 1) they were 6 yr or older at the first visit; 2) both were available; and 3) both twins assented to participate. Pregnant or nursing twins were excluded from the study. Eligible twins were invited to a central office to complete a questionnaire interview and physical examination and to undergo peripheral venipuncture.
For this study, 117 same-sex twin pairs at Tanner stages III and IV between the ages of 13 and 20 yr at follow-up were randomly selected from the entire cohort of 309 twin pairs. The final sample consisted of 226 total subjects —51 complete same-sex dizygotic (DZ) pairs, five with one twin only, and 58 complete same-sex monozygotic (MZ) pairs, three with one twin only. The study protocol was approved by the Institutional Review Boards of Children’s Memorial Hospital and the Institute of Biomedicine, Anhui Medical University in Hefei, China.
Physical exam and anthropometrics
As detailed in previous reports (17), height and weight were measured using standard protocols. Height was measured to the nearest 0.1 cm on a portable, calibrated, stadiometer. Weight was measured to the nearest 0.1 kg with the subject standing motionless on a scale. Body mass index was calculated as weight (kilograms)/height (meters) squared. Tanner stage (III and IV) was determined by visual inspection by a physician as described by Marshall and Tanner (19,20).
Survey
Each participant completed a survey assessing demographic variables including age, gender, and occupation. Occupation categories consisted of: full-time student, farmer, laborer, professional, office clerk, waiter, homemaker, and other. Because there were so few non-students, occupation was later dichotomized to “student” and “non-student.” To assess physical activity level, the short version of the International Physical Activity Questionnaire was used (IPAQ-Short) (http://www.ipaq.ki.se/ipaq.htm). Briefly, the IPAQ-Short consists of seven items that collected information about three types of physical activity (walking, moderate activity, and vigorous activity) across various physical activity domains (leisure time, occupational, domestic, and transport), using the “last 7 days” as a reference period. IPAQ generates a categorical indicator (low, moderate, and high) of regular physical activity based on the total volume of activity and the number of activity days or sessions per week. Criteria for categorical indicators are detailed elsewhere (21).
25(OH)D assay
We measured total 25(OH)D and its components, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3, by HPLC tandem mass spectrometry as recently described (22). The sample preparation involves protein precipitation with acetonitrile containing a stable deuterium-labeled internal standard, d6–25-hydroxyvitamin D3. 25-Hydroxyvitamin D3 and D2 are separated by liquid-liquid extraction using hexane. 25-Hydroxyvitamin D2 and D3 are further separated by HPLC using a C-18 analytical column. Tandem mass spectrometry detection was performed in the multiple-reaction monitoring mode, and the transitions monitored are m/z 401→m/z 383, m/z 413→m/z 395, and m/z 407→m/z 389 for 25-hydroxyvitamin D3, 25-hydroxyvitamin D2 and, d6–25-hydroxyvitamin D3, respectively. We used a Micromass Quattro Micro triple-quadrupole mass spectrometer equipped with a Z-spray ion source and a Waters 2795 Alliance HT HPLC system (Waters Chromatography, Milford, MA) The tandem mass spectrometer is operated in the electrospray positive ionization mode. System operation and data processing are controlled by MassLynx NT 4.0 software (Waters Chromatography).
Blood samples collected in January and February were considered “non-summer” samples, and those collected in July to September were considered “summer” samples. Because there were only six subjects with detectable 25-hydroxyvitamin D2 levels ranging from 2.9–5.5 ng/ml after all the blood samples were collected, we did not analyze 25-hydroxyvitamin D2 further. These six subjects were retained in the analysis because they did not significantly alter the results.
Statistical analysis
Descriptive statistics were used to illustrate sample demographics and 25(OH)D levels [mean (sd)] in the overall sample. ANOVA, using generalized estimating equations (GEE) (23), was used to compare continuous variables between groups, and χ2 test was used to test categorical distributions between groups. For all analyses, a P value of <0.05 was considered statistically significant.
Tertiles of 25(OH)D level were created by stratifying the data by Tanner stage (III or IV), then creating tertiles within each Tanner stage strata. χ2 test was used to compare tertile distributions across gender within season and season within gender. GEE was used to model the relationship of important factors, such as gender and season, and 25(OH)D level in the twins (23). The 25(OH)D level was modeled both as a continuous and a binary variable, where the risk of being in the low tertile group was used as the outcome in the logistic models.
Finally, the genetic influence on 25(OH)D levels was estimated using a structural equation modeling (SEM) (24). In SEM, the twin model assumes that the variance of a phenotypic trait [in this case 25(OH)D level] is a function of multiple genes and environmental factors and can be decomposed into three latent, unmeasured, components: additive genetics (A), shared environment (C), and nonshared or unique environment (E)—referred to as the ACE model. MZ twins resemble one another because they share all of their genetic and shared environmental components, whereas DZ twins share (on average) half of their genetics. The shared environmental variance is assumed to be the same for MZ and DZ pairs. Akaike’s information criterion (AIC) was used to compare models (25). Intraclass correlations (ICC) are estimated using random effects regression models (26). GEE and SEM operate under assumptions of normality in the outcome variable. To test for deviation from normality, a Kolmogorov-Smirnov test was used. Although 25(OH)D level in its raw measure did deviate significantly from normal, the natural log of 25(OH)D level was improved in terms of skew and kurtosis and was therefore used. The data analysis for this study was performed using SAS software, version 8.2 (SAS Institute Inc., Cary, NC) and Mx software (27).
Results
There were no differences between DZ and MZ twins for any of the demographic and anthropometric characteristics, except for student status, where there were fewer students among the DZ twins, 70.1 vs. 82.4% (P = 0.02) (Table 1). Of note, there were more females among the MZ pairs, 57.1 vs. 47.7%, but this was not significantly different.
Table 1.
Subject characteristics of DZ and MZ Chinese adolescent twins (n = 226)a
| DZ and MZ twins | DZ twins | MZ twins | |
|---|---|---|---|
| n | 226 | 107 | 119 |
| Age (yr) | 16.4 (1.5) | 16.5 (1.5) | 16.4 (1.5) |
| Height (cm) | 157.8 (7.5) | 158.6 (7.3) | 157.1 (7.6) |
| Weight (kg) | 48.6 (6.4) | 49.0 (6.0) | 48.1 (6.7) |
| Body mass index (kg/m2) | 19.5 (2.0) | 19.5 (2.0) | 19.5 (2.1) |
| Gender (% females) | 52.7 | 47.7 | 57.1 |
| Tanner (% stage III) | 53.1 | 53.3 | 52.9 |
| Season (% summer)b | 56.2 | 53.3 | 58.8 |
| Student (% students)c | 76.6 | 70.1d | 82.4 |
| Physical activity (IPAQ), n (%) | |||
| Low | 117 (52.2) | 51 (48.5) | 66 (55.5) |
| Moderate | 61 (27.2) | 26 (24.8) | 35 (29.4) |
| High | 46 (20.5) | 28 (26.7) | 18 (15.1) |
Data represent mean (sd) unless otherwise described.
Sample restricted to Tanner stages III and IV; five DZ pairs had only one twin; three MZ pairs had only one twin.
Serum collected in January and February = non-summer; and serum collected in July, August, and September = summer.
Non-students consisted of farmers, laborers, professional, office clerk, waiter, homemaker, and other.
DZ twins significantly different from MZ twins, P = 0.03.
The mean (sd) 25(OH)D level for the total study population was 18.0 (9.4) ng/ml, and 90.3% were less than 32 ng/ml. In the low tertile, the median 25(OH)D level was 8.8 ng/ml, and the range was 4.3–14.2 ng/ml; in the middle tertile, the median value was 16.3 ng/ml, and the range was 10.1–23.0 ng/ml; and in the high tertile, the median value was 26.9 ng/ml, and the range was 18.7 to 52.7 ng/ml. There were no differences between DZ and MZ twins in the mean or tertile distributions of 25(OH)D levels (Table 2).
Table 2.
25(OH)D levels in DZ and MZ Chinese adolescent twins (n = 226)a
| DZ and MZ twins | DZ twins | MZ twins | |
|---|---|---|---|
| n | 226 | 107 | 119 |
| 25(OH)D (ng/ml) | 18.0 (9.4) | 18.5 (10.9) | 17.5 (8.9) |
| 25(OH)D tertiles, n (%)b | |||
| Low median = 8.8 ng/ml (range, 4.3–14.2 ng/ml) | 75 (33.2) | 34 (31.8) | 41 (34.5) |
| Middle median = 16.3 ng/ml (range, 10.1–23.0 ng/ml) | 75 (33.2) | 35 (32.7) | 40 (33.6) |
| High median = 26.9 ng/ml (range, 18.7–52.7 ng/ml) | 76 (33.6) | 38 (35.5) | 38 (31.9) |
| 25(OH)D <32 ng/ml, n (%) | 204 (90.3) | 96 (89.7) | 108 (90.8) |
Sample restricted to Tanner stages III and IV; five DZ pairs had only one twin; three MZ pairs had only one twin.
Tertiles are Tanner stage independent.
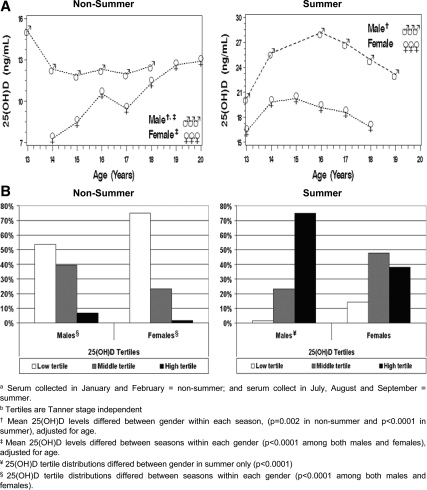
Gender differences in 25(OH)D levels across age were examined within each season (Fig. 1A). In the non-summer season, male 25(OH)D levels tended to remain flat across age, approximately 13 ng/ml, but, in females, 25(OH)D levels increased with increasing age, and by the late teens, appeared to catch up with males. On average, in non-summer, 25(OH)D levels were higher in males than in females, i.e. 12.1 vs. 10.1 ng/ml (P = 0.002). In summer, the relationship of 25(OH)D levels to age was similar between males and females; however, the overall mean 25(OH)D level in males (27.4 ng/ml) was higher than in females (19.5 ng/ml) (P < 0.0001). Interestingly, summer 25(OH)D levels peaked at age 15 in both males and females, with significant (P < 0.0001) seasonal differences also present within males and females.
Figure 1.
A, Gender and seasonal differences in 25(OH)D blood levels across age. B, Gender and seasonal differences by percentage of individuals in 25(OH)D tertiles.
In non-summer, the tertile distribution of 25(OH)D for males and females did not significantly differ (P = 0.06), with 53% of males and 75% of female subjects within the lower tertile (Fig. 1B). In contrast, there was a notable gender difference in tertile distributions in summer (P < 0.0001), with 75% of the males compared with only 38% of the females in the high tertile, and with the greatest proportion of females in the middle tertile. Seasonal differences were observed within both genders (P < 0.0001, in males and females). We concluded that males have higher 25(OH)D levels regardless of season and there are strong seasonal differences regardless of gender. Moreover, females were much more likely to fall in the low tertile group.
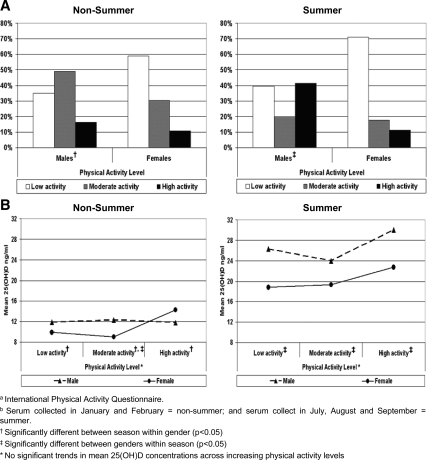
To investigate whether or not physical activity may account for seasonal and gender differences in 25(OH)D level, we first examined physical activity levels stratified by gender and season (Fig. 2A). In non-summer, the physical activity levels in males and females did not differ (P = 0.06). The majority of females (58.9%) engaged in low physical activity, and the majority of males engaged in low (34.9%) to moderate (48.8%) physical activity. However, in summer, significantly more males (41.3%) than females (11.3%) engaged in high physical activity (P = 0.002). Physical activity in males also differed between seasons, with a greater percentage of high physical activity occurring in the summer (41.3%) compared with non-summer (16.3%) (P = 0.0002). There was no seasonal difference within females, with low levels of physical activity both in non-summer (58.9%) and summer (71.0%) (P = 0.27).
Figure 2.
A, Gender and seasonal differences by percentage of individuals in physical activity (IPAQ) categories. B, Gender and seasonal differences in 25(OH)D blood levels across physical activity (IPAQ) categories.
To further investigate the impact of physical activity on 25(OH)D levels, mean 25(OH)D levels across physical activity levels were examined by season and gender (Fig. 2B). In non-summer, mean 25(OH)D levels in males were higher than females, but only among those engaged in moderate physical activity: 12.3 vs. 9.0 ng/ml (P = 0.005). However, in summer, males had higher mean 25(OH)D levels than females at every level of physical activity: 26.3 vs. 18.8 ng/ml in the high physical activity group (P = 0.001), 24.0 vs. 19.3 ng/ml in the moderate group (P = 0.005), and 30.0 vs. 22.7 ng/ml in the low group (P = 0.0002). Moreover, there were seasonal differences in 25(OH)D levels at every level of physical activity, regardless of gender (P < 0.0001 among both males and females). Although there were differences between seasons, there were no significant trends in 25(OH)D levels across physical activity, indicating that physical activity is unlikely to confound the relationship of gender or season to measured 25(OH)D levels.
The univariate analysis of factors influencing 25(OH)D levels (Table 3) showed that 25(OH)D levels were lower in females (P = 0.0006) during the non-summer season (P < 0.0001), among non-students (P = 0.05), and among those with high physical activity (P = 0.01). Females were at higher risk of being in the low tertile [odds ratio (OR) = 2.4; 95% confidence interval (CI), 1.1–5.0], and non-summer increased the risk of being in the low 25(OH)D tertile (OR = 21.6; 95% CI, 8.2–52.1). The multivariate analysis (Table 3), where gender, season, occupation, and physical activity were included, showed 25(OH)D levels to be lower in females (P < 0.0001), during the non-summer season (P < 0.0001) and significantly higher in those engaging in high physical activity (P = 0.02). The relative risk of being in the low 25(OH)D tertile dramatically increased for females (OR = 5.0; 95% CI, 1.8–13.9) and non-summer (OR = 37.9; 95% CI, 12.3–116) in this model. Student status and physical activity were not associated with the risk of being in the low tertile. Gender, season, and physical activity were found to be the environmental factors that affected 25(OH)D levels, whereas only gender and season appeared to affect the risk of being in the low tertile.
Table 3.
Factors associated with 25(OH)D blood levels and the risk of 25(OH)D deficiency (n = 226)
| Ln[25(OH)D] (continuous)
|
Lowest tertile (dichotomous)b OR (95% CI) | ||
|---|---|---|---|
| β (se) | P value | ||
| Univariatea | |||
| Gender (relative to males) | −0.31 (0.09) | 0.0006 | 2.4 (1.1–5.0) |
| Season (relative to summer) | −0.77 (0.05) | <0.0001 | 21.6 (8.2–52.1) |
| Student status (relative to students) | −0.25 (0.13) | 0.0505 | 1.9 (0.8–4.5) |
| Physical activity (IPAQ) | |||
| Low | − | − | − |
| Medium | −0.02 (0.06) | 0.7445 | 1.6 (0.8–2.9) |
| High | 0.15 (0.06)d | 0.0092 | 0.7 (0.4–1.2) |
| Multivariatec | |||
| Gender (relative to males) | −0.27 (0.06) | <0.0001 | 5.0 (1.8–13.9) |
| Season (relative to summer) | −0.76 (0.07) | <0.0001 | 37.9 (12.3–116.4) |
| Student status (relative to students) | 0.06 (0.10) | 0.5518 | 0.3 (0.08–1.3) |
| Physical activity (IPAQ) | |||
| Low | − | − | − |
| Medium | −0.002 (0.04) | 0.9773 | 1.8 (0.8–4.3) |
| High | 0.12 (0.05)d | 0.0185 | 0.9 (0.3–2.8) |
All models are age adjusted.
Tertiles are Tanner stage independent, and median value for the low tertile is 8.8 ng/ml.
All factors are included in the model. No significant interaction effects were found.
The trend was significant at Ptrend <0.05.
To estimate the genetic (heritable), shared, and unique environmental components that explain the variation in 25(OH)D levels in our study subjects, we present the overall and the gender-specific variance component models (Table 4). In the overall model, the observed higher ICC within MZ compared with DZ twins, ICCMZ = 0.87 and ICCDZ = 0.70, suggest that genes play a role in 25(OH)D levels in the blood. In the ACE model, adjusted for age only, the percentage of the 25(OH)D variance attributable to a genetic component is 36.5%; 51.0% is attributable to shared environmental components; and 12.5% is attributable to unique environmental components. However, adjusting for age, gender, physical activity, student status, and season improved the overall model fit of the model, where the AIC difference between the fully adjusted model and the age-only adjusted model is significantly smaller by 107.86 (P < 0.0001). In the full ACE model, the estimated genetic influence increased to 68.9% (95% CI, 25.9–79.3), the shared environmental component is removed, but the unique environmental component increased to 31.1%. We interpret this to indicate that the portion of the variance in 25(OH)D level unaccounted for by age, gender, physical activity, student status, and season is mediated strongly on a genetic basis consistent with those factors noted in our multivariate analysis.
Table 4.
Gender differences in 25(OH)D levels explained by variance components models (ACE)
| ICC (95% CI)
|
Variance components
|
Model comparison
|
||||||
|---|---|---|---|---|---|---|---|---|
| MZ | DZ | A | C | E | X2 | P value | AIC(X2−2) | |
| Overall | 0.87 (0.81–0.94) | 0.70 (0.59–0.87) | ||||||
| ACE modela | 36.5 (14.2–68.9) | 51.0 (18.8–71.7) | 12.5 (8.2–19.4) | − | − | − | ||
| ACE modelb | 68.9 (25.9–79.3) | 0 (0–35.4) | 31.1 (20.7–48.0) | 109.86 | <0.0001 | 107.86 | ||
| Malec | 0.95 (0.92–0.99) | 0.59 (0.41–1.00) | ||||||
| ACE model | 85.9 (62.3–92.8) | 0 (0–20.9) | 14.1 (7.2–30.9) | − | − | − | ||
| AE modeld | 85.9 (62.3–92.8) | − | 14.1 (7.2–30.9) | 0 | − | −2.00 | ||
| CE model | − | 41.7 (16.3–62.0) | 58.2 (38.0–83.7) | 16.15 | <0.0001 | 14.15 | ||
| Femalec | 0.77 (0.65–0.94) | 0.76 (0.62–0.97) | ||||||
| ACE model | 17.6 (0–73.1) | 38.2 (0–67.9) | 44.2 (25.4–70.1) | − | − | − | ||
| AE model | 59.3 (34.2–75.6) | − | 40.7 (24.4–65.8) | 1.30 | 0.26 | −0.70 | ||
| CE modeld | − | 51.4 (29.2–68.3) | 48.6 (31.7–70.8) | 0.24 | 0.63 | −1.76 | ||
ACE model: A = % variance explained by genetics; C = % variance explained by shared environment; E = % variance explained by unique environment.
Adjusted for age.
Adjusted for age, gender, physical activity, student status, and season.
Adjusted for age, physical activity, student status, and season.
Best-fitting model.
The ICC in males indicated a high degree of genetic control over 25(OH)D levels because the ICCMZ = 0.95 and ICCDZ = 0.59, with a lesser degree observed in females because the ICCMZ = 0.77 and ICCDZ = 0.76. The ACE model in males estimated that 85.9% (95% CI, 62.3–92.8) of the variation in 25(OH)D levels was due to genetic control, 0% for shared environment, and 14.1% (95% CI, 7.2–30.9) for unique environment. However, in females, the ACE model estimated that only 17.6% (95% CI, 0–73.1) of the variation in 25(OH)D levels had a genetic component, and the shared and unique environmental components were 38.2% (95% CI, 0–67.9) and 44.2% (95% CI, 25.4–70.1), respectively. In the best fitting model among females, the genetic component was dropped from the ACE, and shared environment and unique environmental components were 51.4% (95% CI, 29.2–68.3) and 48.6% (95% CI, 31.7–70.8), respectively. In summary, blood levels of 25(OH)D appear to have a strong genetic mediation, but only in males; in females, the shared and unique environments appear more important in determining those levels.
Discussion
In our study of Chinese adolescent twin pairs, we found that non-summer season, female gender, and those reporting low physical activity had a decreased 25(OH)D level; and values in non-summer, and female gender were more likely to be in the lowest 25(OH)D tertile level. This is consistent with what has been shown to influence vitamin D status worldwide (9). Findings among the females in our study were also consistent with other general studies of vitamin D deficiency conducted among adolescent girls in Beijing, China (28,29), despite Beijing’s more northern latitude relative to Anqing. In one Beijing study, the 25(OH)D level of adolescent girls was 5.1 ng/ml in winter and 9.5 ng/ml in summer (28), much lower than the females in our study (10.1 ng/ml in winter and 19.5 ng/ml in summer). Furthermore, the prevalence of 25(OH)D levels below 5 ng/ml in the Beijing studies was 45.2% in winter and 6.7% in summer, whereas in our study there were only two girls (3.5%) in winter that had such levels. This is the first study to demonstrate that 25(OH)D level may be under strong genetic control during adolescence, especially among males. In females, 25(OH)D level was explained primarily by shared and unique environmental components.
Compared with developed countries, vitamin D deficiency in China is of prominent (30,31,32) and great concern. In China, particularly in the northern parts of the country, inadequate sunlight exposure and a low intensity of UV light during the winter are suspected causes of this deficiency in infants and young children. An assessment of UV exposure among adolescent girls in Beijing showed that sufficient exposure to UV light occurred only in summer (28). Few foods naturally contain vitamin D, with only oily fish such as salmon and cod liver oil providing excellent sources. Fortified food sources in some western countries include milk (100 IU per 8-ounce serving), orange juice (100 IU per 8-ounce serving) and other juice products, and some breads and cereals (33,34). A study in China showed that dietary vitamin D intake was only about 1 μg/d (mainly from eggs and some fortified milk) (35), so it is not surprising that our sample had virtually no 25-hydroxyvitamin D2. The main source of vitamin D in China is, therefore, dermal synthesis in response to sun exposure, where more than 90% of the vitamin D requirement for most people derives from casual exposure to sunlight (7). Our study adds to the evidence that children and adolescents have both inadequate intakes of vitamin D and exposure to sunlight to produce it.
To our knowledge, this is the first study to examine the genetic influence on 25(OH)D level among adolescents, particularly those in rural China. Two studies of adult twin pairs have provided initial estimates of the genetic influence on 25(OH)D levels. A study from the United Kingdom Adult Twin Registry (13), consisting primarily of female twins aged 18–71 yr, estimated a 43% heritability in vitamin D level. The Framingham Offspring Study (14), a cross-sectional study of 1762 adult males and females, found a 28.8% heritability in both genders. These studies demonstrate that, in adults, vitamin D nutrient levels are fractionally under some genetic control. Our estimate of the genetic influence on 25(OH)D level in adolescents was much higher overall (69%), which may have to do with differing complex physiological processes that occur in growing adolescents vs. adults.
We were not able to determine why there is such a strong gender difference in the genetic influence for 25(OH)D level. Our gender stratified estimates showed that the level of 25(OH)D was under high genetic control in males only, who had a heritability estimate of 85.9%. This implies a distinct gender-specific genetic architecture. There is certainly a teleological plausibility that the female skeleton is protected to maintain reproductive viability, whereas males would not need this capacity. Therefore, when 25(OH)D level is low, a genetic component does not contribute further to variation among individual adolescent females as it does among males. It is also possible that there are strong gender-specific hormonal effects that regulate 25(OH)D levels, such as in one animal study where estradiol up-regulated the expression of vitamin D receptors (36). Further studies are needed to examine these possibilities. We speculate that, perhaps with a greater vitamin D receptor function, 25(OH)D levels in females may still be as functional as in males, although lower numerically.
Limitations
There are some limitations to our study. The sample is small and mostly from adolescents in rural China, where lifestyle is quite different from urban China, particularly in physical activity and diet. Therefore, our results may not be generalizable to adolescents in urban China. Second, although we did not obtain any information on vitamin D intake, the absence of 25-hydroxyvitamin D2 suggests that very little intake occurs. We did not obtain data with regard to clothing or socioeconomic status, which were shown to be important determinants of vitamin D status in Lebanon (37). However, our twin sample was quite homogeneous in terms of socioeconomic status and lifestyle, so these factors may not be as applicable.
Conclusions
Our data showed a high prevalence of vitamin D deficiency in a group of rural Chinese adolescents. We found that the 25(OH)D level was significantly influenced by season, gender, and physical activity level. Our heritability estimation indicated that 25(OH)D level was under a strong genetic influence in adolescent males. Our data underscore the importance of conducting larger and more in-depth epidemiological and genetic studies that could define the extent of vitamin D deficiency in a rural Chinese population and elucidate important factors related to vitamin D status. Such information will be critical for developing appropriate public health policies and cost-efficient clinical interventions.
Acknowledgments
We acknowledge the assistance and cooperation of the faculty and staff of the Anhui Institute of Biomedicine, Anhui Medical University, and would like to thank the study participants for their support, and the following team investigators for their valuable input to the study, including Drs. Katherine Kaufer Christoffel, Wendy Brickman, Donald Zimmerman, and Fengxiu Ouyang.
Footnotes
This work was supported by Grant R01 HD049059 from the National Institute of Child Health and Human Development; Grant R01 HL0864619 from the National Heart, Lung, and Blood Institute; Grant R01 AG032227 from the National Institute of Aging; the Food Allergy Project; and grants and endowments from the Zell Family Foundation and the McNulty Family Foundation.
Disclosure Summary: The authors have nothing to declare.
First Published Online June 23, 2009
Abbreviations: AIC, Akaike’s information criterion; CI, confidence interval; DZ, dizygotic; GEE, generalized estimating equations; ICC, intraclass correlation; IPAQ, International Physical Activity Questionnaire; MZ, monozygotic; 25(OH)D, 25-hydroxyvitamin D; OR, odds ratio; SEM, structural equation modeling.
References
- Basha B, Rao DS, Han ZH, Parfitt AM 2000 Osteomalacia due to vitamin D depletion: a neglected consequence of intestinal malabsorption. Am J Med 108:296–300 [DOI] [PubMed] [Google Scholar]
- Wharton B, Bishop N 2003 Rickets. Lancet 362:1389–1400 [DOI] [PubMed] [Google Scholar]
- Scharla SH, Scheidt-Nave C, Leidig G, Woitge H, Wüster C, Seibel MJ, Ziegler R 1996 Lower serum 25-hydroxyvitamin D is associated with increased bone resorption markers and lower bone density at the proximal femur in normal females: a population-based study. Exp Clin Endocrinol Diabetes 104:289–292 [DOI] [PubMed] [Google Scholar]
- Strand MA, Perry J, Jin M, Tracer DP, Fischer PR, Zhang P, Xi W, Li S 2007 Diagnosis of rickets and reassessment of prevalence among rural children in northern China. Pediatr Int 49:202–209 [DOI] [PubMed] [Google Scholar]
- Weisberg P, Scanlon KS, Li R, Cogswell ME 2004 Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr 80:1697S–1705S [DOI] [PubMed] [Google Scholar]
- Mughal MZ 2005 Resurgence of vitamin D deficiency rickets in the UK. Osteoporos Rev 13:10–13 [Google Scholar]
- Holick MF 2004 Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80:1678S–1688S [DOI] [PubMed] [Google Scholar]
- Holick MF 2004 Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79:362–371 [DOI] [PubMed] [Google Scholar]
- Huh SY, Gordon CM 2008 Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord 9:161–170 [DOI] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs–Population Division 2001 Population, environment and development–the concise report. New York: United Nations; 69 [Google Scholar]
- Wang L, Kong L, Wu F, Bai Y, Burton R 2005 Preventing chronic diseases in China. Lancet 366:1821–1824 [DOI] [PubMed] [Google Scholar]
- Popkin BM, Horton S, Kim S, Mahal A, Shuigao J 2001 Trends in diet, nutritional status, and diet-related noncommunicable diseases in China and India: the economic costs of the nutrition transition. Nutr Rev 59:379–390 [DOI] [PubMed] [Google Scholar]
- Hunter D, De Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD 2001 Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res 16:371–378 [DOI] [PubMed] [Google Scholar]
- Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D'Agostino Sr RB, Ordovas JM, O'Donnell CJ, Dawson-Hughes B, Vasan RS, Booth SL 2009 Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr 63:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B 1999 Patterns of human growth. 2nd ed. Cambridge, UK: Cambridge University Press [Google Scholar]
- Largo RH, Gasser T, Prader A, Stuetzle W, Huber PJ 1978 Analysis of the adolescent growth spurt using smoothing spline functions. Ann Hum Biol 5:421–434 [DOI] [PubMed] [Google Scholar]
- Xu X, Niu T, Christiani DC, Weiss ST, Chen C, Zhou Y, Fang Z, Jiang Z, Liang W, Zhang F 1996 Occupational and environmental risk factors for asthma in rural communities in China. Int J Occup Environ Health 2:172–176 [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics of P.R. China 2007 China Statistical Yearbook. China Statistics Press [Google Scholar]
- Marshall WA, Tanner JM 1969 Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM 1970 Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Lu BS, Wang B, Wang H, Yang J, Li Z, Wang L, Liu X, Tang G, Xing H, Xu X, Zee PC, Wang X 2007 Short sleep duration and adiposity in Chinese adolescents. Sleep 30:1688–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM 2006 Quantification of serum 25-hydroxyvitamin D2 and D3 using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol 125:914–920 [DOI] [PubMed] [Google Scholar]
- Diggle P, Liang K-Y, Zeger SL 1994 The analysis of longitudinal data. New York: Oxford University Press [Google Scholar]
- Gielen M, Lindsey PJ, Derom C, Smeets HJ, Souren NY, Paulussen AD, Derom R, Nijhuis JG 2008 Modeling genetic and environmental factors to increase heritability and ease the identification of candidate genes for birth weight: a twin study. Behav Genet 38:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H 1987 Factor analysis and AIC. Psychometrika 52:317–332 [Google Scholar]
- McArdle JJ, Prescott CA 2005 Mixed-effects variance components models for biometric family analyses. Behav Genet 35:631–652 [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH 2002 Mx: statistical modeling. 6th ed. Richmond, VA: Virginia Commonwealth University [Google Scholar]
- Du X, Greenfield H, Fraser DR, Ge K, Trube A, Wang Y 2001 Vitamin D deficiency and associated factors in adolescent girls in Beijing. Am J Clin Nutr 74:494–500 [DOI] [PubMed] [Google Scholar]
- Foo LH, Zhang Q, Zhu K, Ma G, Trube A, Greenfield H, Fraser DR 2009 Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporos Int 20:417–425 [DOI] [PubMed] [Google Scholar]
- Rockell JE, Green TJ, Skeaff CM, Whiting SJ, Taylor RW, Williams SM, Parnell WR, Scragg R, Wilson N, Schaaf D, Fitzgerald ED, Wohlers MW 2005 Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5–14 y. J Nutr 135:2602–2608 [DOI] [PubMed] [Google Scholar]
- Docio S, Riancho JA, Pérez A, Olmos JM, Amado JA, González-Macías J 1998 Seasonal deficiency of vitamin D in children: a potential target for osteoporosis preventing strategies. J Bone Miner Res 13:544–548 [DOI] [PubMed] [Google Scholar]
- Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR 2002 Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777 [DOI] [PubMed] [Google Scholar]
- Calvo MS, Whiting SJ, Barton CN 2005 Vitamin D intake: a global perspective of current status. J Nutr 135:310–316 [DOI] [PubMed] [Google Scholar]
- Tangpricha V, Koutkia P, Rieke SM, Chen TC, Perez AA, Holick MF 2003 Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr 77:1478–1483 [DOI] [PubMed] [Google Scholar]
- Fraser DR 1995 Vitamin D. Lancet 345:104–107 [DOI] [PubMed] [Google Scholar]
- Gilad LA, Schwartz B 2007 Association of estrogen receptor β with plasma-membrane caveola components: implication in control of vitamin D receptor. J Mol Endocrinol 38:603–618 [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, Tannous R 2001 Hypovitaminosis D in healthy schoolchildren. Pediatrics 107:E53 [DOI] [PubMed] [Google Scholar]