Abstract
Context: Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility. The selection of first-line therapies for ovulation induction is empiric.
Objective: The aim of the study was to develop a clinically useful predictive model of live birth with varying ovulation induction methods.
Design, Setting, and Participants: We built four prognostic models from a large multicenter randomized controlled infertility trial of 626 women with PCOS performed at academic health centers in the United States to predict success of ovulation, conception, pregnancy, and live birth, evaluating the influence of patients’ baseline characteristics.
Interventions: Ovulation was induced with clomiphene, metformin, or the combination of both for up to six cycles or conception.
Main Outcome Measure: The primary outcome of the trial was the rate of live births.
Results: Baseline free androgen index, baseline proinsulin level, interaction of treatment arm with body mass index, and duration of attempting conception were significant predictors in all four models. History of a prior loss predicted ovulation and conception, but not pregnancy or live birth. A modified Ferriman Gallwey hirsutism score of less than 8 was predictive of conception, pregnancy, and live birth (although it did not predict ovulation success). Age was a divergent predictor based on outcome; age greater than 34 predicted ovulation, whereas age less than 35 was a predictive factor for a successful pregnancy and live birth. Smoking history had no predictive value.
Conclusions: A live birth prediction chart developed from basic clinical parameters (body mass index, age, hirsutism score, and duration of attempting conception) may help physicians counsel and select infertility treatments for women with PCOS.
The probability of a live birth after ovulation induction in PCOS women can be estimated based on their age, body mass index, hirsutism, and duration of attempted conception.
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility (1) and affects approximately 6.6% of reproductive-aged women (2). Women with PCOS are phenotypically diverse; diagnostic criteria include clinical signs of hyperandrogenism or biochemical hyperandrogenemia, menstrual irregularities, and sonographic evidence of polycystic ovaries, but obesity and impaired glucose tolerance are also common manifestations (3,4,5). The first step in infertility treatment is to restore ovulation (6), although this by itself does not guarantee a live birth, the ultimate treatment goal.
Overall, there is a lack of evidence-based recommendations for infertility treatment, leading to expert-based opinions or meta-analyses of small and sometimes poorly designed trials. Although both the selective estrogen receptor modulator clomiphene citrate (CC) and the insulin sensitizer metformin have been used for ovulation induction, recent randomized controlled trials have shown that metformin alone is inferior to CC at achieving live births (7,8), but meta-analyses have supported a beneficial effect of the combination of CC and metformin on pregnancy outcomes (9,10). The current expert-based recommendation by the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine for first-line treatment of PCOS-related infertility is ovulation induction with CC for up to six ovulatory cycles, with the addition of metformin only for glucose intolerance (11,12).
Determining which baseline characteristics are associated with the highest chance of achieving a successful pregnancy and live birth with ovulation induction would be beneficial in counseling patients and planning infertility treatment. Patients with a low chance of success could be potentially fast-tracked to more aggressive therapies such as laparoscopic ovarian diathermy, exogenous gonadotropins, or in vitro fertilization. Alternatively, a pretreatment intervention could focus on improving modifiable predictive factors (such as BMI or hirsutism) before attempting first-line therapy.
The Pregnancy in Polycystic Ovary Syndrome (PPCOS) study is a prospective, multicenter, randomized clinical trial sponsored by the National Institutes of Health/National Institute of Child Health and Human Development (7). It is the largest published study examining CC and metformin treatment in women with PCOS; 626 infertile women were enrolled, with 133 pregnancies and 118 live births achieved in the three study arms. Here we use the results from the randomized controlled trial to examine baseline patient characteristics related to ovulation, conception, pregnancy, and live birth and to formulate predictive clinically relevant models of treatment success in women with PCOS.
Subjects and Methods
Study design
From November 2002 to December 2004, 626 infertile women with PCOS were randomized to one of three study groups (CC plus placebo, metformin plus placebo, or combination CC plus metformin) as part of a multicenter double-blind, double-dummy randomized clinical trial (7). The institutional review board at each center approved the protocol, and all subjects gave written informed consent.
All subjects were diagnosed with PCOS, defined as oligomenorrhea (history of no more than eight spontaneous menses per year) and hyperandrogenemia (elevated testosterone level documented within the previous year in an outpatient setting on the basis of local laboratory results, with a predetermined cutoff level set by the principal investigator at each site). Although it was not an inclusion criterion, 90% had polycystic ovary morphology, and the mean volume of each ovary was 10 cm3 or more (13), consistent with the recommended ultrasound diagnostic features of PCOS (14). All subjects, however, fulfilled the subsequent Rotterdam diagnostic criteria published in 2004 (3,4). Exclusion criteria included hyperprolactinemia, congenital adrenal hyperplasia, thyroid disease, other causes of amenorrhea such as premature ovarian failure, and clinically suspected Cushing’s syndrome and androgen-secreting neoplasm. Hirsutism was not an exclusion or inclusion criterion but was assessed in all subjects using the modified Ferriman Gallwey Score (15), a measure of midline, primarily androgen-dependent hair growth. Other causes of infertility were excluded by documentation of a normal uterine cavity, at least one patent fallopian tube, and a normal semen sperm concentration of each woman’s current partner. All subjects were in good health, with no major medical disorders.
Baseline laboratory testing was performed after an overnight fast, and all blood specimens were analyzed in a core laboratory by established assays (13). Subjects were treated for up to six cycles, or 30 wk. Metformin was administered as 500-mg tablets and was increased to four daily; CC was administered as 50-mg tablets, one to three daily, dependent on ovulatory response. Progesterone was measured weekly or every other week, and ovulation was documented by an elevated progesterone level. All study medication was discontinued if a pregnancy test was positive, and pregnant subjects were followed until fetal viability was documented on ultrasound evaluation. After delivery, investigators reviewed all obstetrical records to obtain data on birth outcomes. The primary outcome of the trial was the rate of live births. Secondary outcomes included the rate of pregnancy loss, singleton birth, and ovulation. Data were analyzed according to the intention-to-treat principle. More detailed information on power analysis, study design, main statistical methods, baseline characteristics, study medications, and outcomes has been published previously (7,13,16).
Data analysis
We built four prognostic models to predict success of ovulation, conception, pregnancy, and live birth. Ovulation was defined as a serum progesterone level above 5 ng/ml during a cycle. Conception was defined as any positive serum human chorionic gonadotropin level. Clinical pregnancy was defined as an intrauterine pregnancy with fetal heart motion as detected by transvaginal ultrasound. Live birth was defined as the delivery of a viable infant. The influence of patient’s baseline characteristics, including clinical and laboratory variables, on these outcomes was evaluated and included treatment, age, weight, body mass index (BMI), hirsutism score, race, waist measurement, waist/hip ratio, ethnic group, duration of attempting conception, pregnancy history, prior loss of pregnancy, prior parity, history of smoking, baseline total testosterone, baseline free androgen index (FAI), baseline glucose, baseline insulin, baseline proinsulin, baseline SHBG, and baseline white blood cell count.
Generalized estimating equations with logistic regression were used for analysis of repeated outcomes such as ovulation to account for correlation of multiple ovulation outcomes in multiple cycles within a patient. Logistic regression was used for the analysis of the other outcomes, such as conception, pregnancy, and live birth. Stepwise selection at a significance level of 0.10 was applied for model selection where both age and treatment variables are retained in the final models regardless of significance. For each covariate selected from the stepwise procedure, the interaction effects between the treatment and the selected covariates were then assessed, and those interactions that were significant at the 0.10 level were then included in the final models. For each outcome, the final models are presented in a table with odds ratios and the corresponding 90% confidence intervals for all covariates. The interaction effect of the treatment with a covariate was displayed by presenting the odds ratios for the treatment effect given the value of the covariate.
To provide clinical utility of the prediction model, we also created a live birth prediction chart with rapidly assessable clinical parameters only. Specifically, the estimated chance of live birth was calculated from a modified logistic regression model for live birth by removing the laboratory variables from the original final model. The estimated chances of live birth were then categorized by every 10 percentage points until it reached the maximum estimated chance of live birth. The color-coded chart was then created with the categorized chance of live birth by possible combinations of the clinical variables. All analyses were performed with SAS software, version 8.2 (SAS Institute, Cary, NC). All data entry, data management, and analyses were performed at the Data Coordinating Center at the Duke Clinical Research Institute.
Results
Baseline characteristics of the 626 randomized patients used as covariates in the prediction models are listed in Tables 1 and 2 (some values as previously reported) (7,13). The number of subjects was 209 in the CC group, 208 in the metformin group, and 209 in the combination therapy group. There were no statistically significant differences in the baseline characteristics among the treatment arms.
Table 1.
Clinical parameters used as covariates in prediction models
| Parameter | CC | Metformin XR | Combined | All patients | P value across three groups |
|---|---|---|---|---|---|
| Age | 27.9 ± 4.0 | 28.1 ± 4.0 | 28.3 ± 4.0 | 28.1 ± 4.0 | 0.691 |
| Weight (kg) | 96.3 ± 25.9 | 95.5 ± 24.5 | 91.2 ± 23.3 | 94.3 ± 24.7 | 0.073 |
| BMI (kg/m2) | 36.0 ± 8.9 | 35.6 ± 8.5 | 34.2 ± 8.4 | 35.2 ± 8.7 | 0.115 |
| <30 | 57/209 (27.3) | 57/207 (27.5) | 65/209 (31.1) | 179/625 (28.6) | 0.723 |
| 30-34 | 42/209 (20.1) | 45/207 (21.7) | 48/209 (23.0) | 135/625 (21.6) | |
| ≥35 | 110/209 (52.6) | 105/207 (50.7) | 96/209 (45.9) | 311/625 (49.8) | |
| Waist circumference(cm) | 105.0 ± 22.3 | 102.4 ± 17.6 | 100.2 ± 18.2 | 102.5 ± 19.6 | 0.122 |
| Waist/hip ratio | 0.874 ± 0.098 | 0.857 ± 0.077 | 0.859 ± 0.097 | 0.864 ± 0.091 | 0.117 |
| Hirsutism score | 14.7 ± 8.2 | 14.3 ± 8.0 | 14.4 ± 7.4 | 14.4 ± 7.9 | 0.803 |
| <8 | 44/209 (21.1) | 36/208 (17.3) | 41/209 (19.6) | 121/626 (19.3) | 0.602 |
| 8-15 | 84/209 (40.2) | 96/208 (46.2) | 82/209 (39.2) | 262/626 (41.9) | |
| ≥16 | 81/209 (38.8) | 76/208 (36.5) | 86/209 (41.1) | 243/626 (38.8) | |
| Race | 0.932 | ||||
| White | 147/208 (70.7) | 140/207 (67.6) | 148/208 (71.2) | 435/623 (69.8) | |
| Black or African American | 37/208 (17.8) | 40/207 (19.3) | 32/208 (15.4) | 109/623 (17.5) | |
| Asian | 5/208 (2.4) | 5/207 (2.4) | 7/208 (3.4) | 17/623 (2.7) | |
| American Indian or Alaska Native | 21/208 (10.1) | 27/207 (13.0) | 24/208 (11.5) | 72/623 (11.6) | |
| Native Hawaiian or Pacific Islander | 1/208 (0.5) | 0 (0.0) | 0 (0.0) | 1/623 (0.2) | |
| Ethnic group | 0.433 | ||||
| Not Hispanic or Latino | 156/209 (74.6) | 147/208 (70.7) | 159/209 (76.1) | 462/626 (73.8) | |
| Hispanic or Latino | 53/209 (25.4) | 61/208 (29.3) | 50/209 (23.9) | 164/626 (26.2) | |
| Length of attempting conception (months) | 41.4 ± 39.4 | 39.0 ± 31.9 | 40.7 ± 36.0 | 40.4 ± 35.8 | 0.961 |
| Prior conception | 77/209 (36.8) | 66/208 (31.7) | 67/209 (32.1) | 210/626 (33.5) | 0.467 |
| Prior pregnancy loss | 53/209 (25.4) | 40/208 (19.2) | 45/209 (21.5) | 138/626 (22.0) | 0.314 |
| Prior parity | 76/209 (36.4) | 66/208 (31.7) | 67/209 (32.1) | 209/626 (33.4) | 0.536 |
| History of smoking | 88/209 (42.1) | 80/208 (38.5) | 79/209 (37.8) | 247/626 (39.5) | 0.626 |
Data are presented as mean ± sd or number of subjects/total number (percentage).
Table 2.
Baseline laboratory parameters used as covariates in prediction models
| Parameter | CC | Metformin XR | Combined | All patients | P value across three groups |
|---|---|---|---|---|---|
| Total testosterone (ng/dl) | 61.3 ± 32.0 | 61.6 ± 25.0 | 63.1 ± 28.4 | 62.0 ± 28.6 | 0.495 |
| FAIa | 9.4 ± 7.1 | 9.9 ± 6.2 | 9.4 ± 6.8 | 9.5 ± 6.7 | 0.157 |
| Glucose (mg/dl) | 89.2 ± 16.5 | 88.8 ± 17.1 | 88.9 ± 18.6 | 89.0 ± 17.4 | 0.895 |
| Insulin (μU/ml)b | 22.6 ± 20.7 | 24.0 ± 28.4 | 22.4 ± 30.0 | 23.0 ± 26.6 | 0.430 |
| Proinsulin (pmol/liter)b | 25.4 ± 24.3 | 25.5 ± 27.5 | 23.9 ± 25.7 | 24.9 ± 25.8 | 0.361 |
| SHBG (nmol/liter) | 29.8 ± 18.7 | 27.5 ± 14.4 | 31.8 ± 20.3 | 29.7 ± 18.1 | 0.150 |
| White blood cells (103/μl) | 7.4 ± 2.0 | 7.7 ± 4.0 | 7.3 ± 3.7 | 7.5 ± 3.3 | 0.480 |
Data are presented as mean ± sd.
FAI was calculated from the following formula: FAI = [total testosterone (nmol/liter)/SHBG (nmol/liter)] × 100.
Conversion to SI units: glucose × 0.0551 (mmol/liter); insulin × 7.175 (pmol/liter).
Of the 2925 total cycles, there were 1340 ovulatory cycles. In the multivariable model for ovulation, predictors for success included interaction of treatment and BMI (CC or combination treatment were predictive of success over metformin monotherapy within each BMI category, with the largest effect occurring in the comparison of combined group vs. metformin group for subjects with BMI ≥35 kg/m2), age above 34 yr, a history of prior pregnancy loss, a lower baseline proinsulin level (<23 pmol/liter), a lower FAI (<10), and a shorter duration of attempting conception (<1.5 yr) (Table 3). When the treatment arms were stratified by BMI, both CC and combined therapy were more successful in all three treatment arms with respect to ovulation (Table 3). Hirsutism did not factor into the final model for ovulation success.
Table 3.
Models to determine predictive factors for success
| Effect | Ovulation | Conception | Pregnancy | Live birth |
|---|---|---|---|---|
| Baseline BMI ≥35 (kg/m2) | ||||
| Metformin (reference) | 1.0 | 1.0 | 1.0 | 1.0 |
| CC | 3.17 (2.20, 4.56) | 6.28 (2.67, 14.8) | 5.97 (2.31, 15.4) | 5.22 (2.01, 13.6) |
| Combined | 5.12 (3.55, 7.40) | 11.70 (4.98, 27.5) | 11.50 (4.48, 29.5) | 8.84 (3.42, 22.8) |
| Baseline BMI 30–34 (kg/m2) | ||||
| Metformin (reference) | 1.0 | 1.0 | 1.0 | 1.0 |
| CC | 2.03 (1.26, 3.27) | 2.31 (0.93, 5.79) | 1.63 (0.59, 4.50) | 2.01 (0.70, 5.79) |
| Combined | 1.96 (1.18, 3.27) | 2.57 (1.07, 6.17) | 1.59 (0.61, 4.18) | 1.96 (0.71, 5.40) |
| Baseline BMI <30 (kg/m2) | ||||
| Metformin (reference) | 1.0 | 1.0 | 1.0 | 1.0 |
| CC | 2.23 (1.40, 3.56) | 2.38 (1.15, 4.95) | 4.01 (1.72, 9.34) | 5.96 (2.34, 15.1) |
| Combined | 3.77 (2.43, 5.83) | 2.85 (1.41, 5.75) | 4.52 (2.00, 10.23) | 5.77 (2.32, 14.3) |
| Age (yr) | ||||
| >34 (reference) | 1.0 | 1.0 | 1.0 | 1.0 |
| ≤34 | 0.61 (0.41, 0.92) | 2.06 (0.95, 4.44) | 5.86 (1.69, 20.3) | 5.05 (1.46, 17.52) |
| History of prior loss | 1.70 (1.33, 2.14) | 1.49 (1.02, 2.18) | N.A. | N.A. |
| Baseline proinsulin (pmol/liter) | ||||
| ≥23 (reference) | 1.0 | 1.0 | 1.0 | 1.0 |
| <23 | 1.42 (1.13, 1.79) | 1.56 (1.04, 2.25) | 1.72 (1.09, 2.70) | 1.71 (1.07, 2.74) |
| Baseline FAI | ||||
| ≥10 (reference) | 1.0 | 1.0 | 1.0 | 1.0 |
| <10 | 1.37 (1.11, 1.68) | 1.96 (1.33, 2.88) | 1.72 (1.34, 2.85) | 1.55 (1.44, 3.12) |
| Hirsutism score | N.A. | |||
| ≥16 (reference) | 1.0 | 1.0 | 1.0 | |
| 8–15 | 1.29 (0.88, 1.89) | 1.45 (0.95, 2.23) | 1.40 (0.89, 2.18) | |
| <8 | 1.69 (1.06, 2.68) | 2.45 (1.49, 4.04) | 2.51 (1.50, 4.17) | |
| Duration of attempting conception | ||||
| ≥1.5 yr (Reference) | 1.0 | 1.0 | 1.0 | 1.0 |
| <1.5 yr | 1.43 (1.15, 1.77) | 1.77 (1.25, 2.51) | 1.95 (1.34, 2.85) | 2.12 (1.44, 3.12) |
Odds ratios, point estimates, and 90% Wald confidence limits for models displayed. N.A., Not applicable.
In the main study, 167 of 626 women conceived. As in the model of ovulation, success in the conception model (Table 3) was predicted by history of prior loss, baseline proinsulin level, baseline FAI, and duration of attempting conception. Although hirsutism was not predictive of ovulation, it was predictive of conception when comparing women with a normal score (<8) vs. hirsute women with a score of at least 16. There was interaction effect of treatment and BMI where CC and combined treatment was predictive of a higher chance of conception over metformin monotherapy for a given BMI category, except for the comparison of CC vs. metformin therapy in the intermediate BMI group (30–34 kg/m2) which was not statistically significant. Although age greater than 34 was predictive of ovulation, in the model for conception, women age 34 or younger had a higher, but nonsignificant, odds of conception (odds ratio, 2.06) over the older group.
There were 133 pregnancies, of which 118 resulted in a live birth. The predictive models for both pregnancy and live birth included baseline proinsulin level, baseline FAI, duration of attempting conception, and hirsutism score for the less than 8 vs. at least 16 groups (Table 3). Age of 34 yr or less was predictive of a successful pregnancy as well as live birth (Table 3). Again, there was significant interaction effect of treatment with BMI, where CC and combined therapy were significantly more predictive of pregnancy and live birth in both the lowest and highest BMI categories (<30 kg/m2 and ≥35 kg/m2, respectively), with a trend toward greater pregnancy success in the intermediate category (BMI, 30–34 kg/m2). Although history of prior loss factored in the models for ovulation and conception, it was not predictive of an intrauterine pregnancy or live birth.
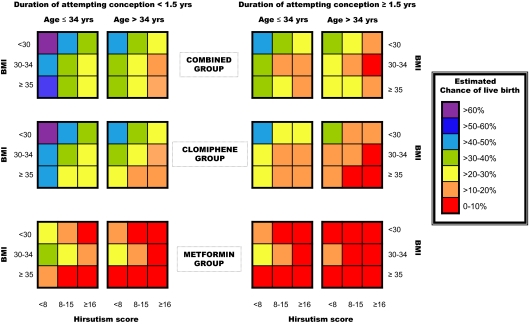
Given that the most clinically relevant outcome of ovulation induction is live birth, we used the model for live birth to create a prediction chart. Although proinsulin and FAI levels were significant in our model, we chose to remove these variables and use only the clinically evident predictors that could be rapidly assessed on the initial presentation of a PCOS patient for infertility management. Based on the model for live birth without proinsulin and FAI variables, the estimated chance of live birth ranged from 1.4 to 62.6%. Figure 1 demonstrates the estimated chance of live birth (categorized by every 10 percentage points) for a given PCOS patient for varying forms of therapy based on BMI, age, hirsutism, and duration of attempting conception.
Figure 1.
Live birth prediction chart for method of ovulation induction using clinical measures. How to use the chart: 1) choose the row of boxes depending on your desired treatment option; 2) choose the column of boxes that matches the time your patient has been attempting conception and her age; and 3) using her BMI and hirsutism score, find the cell that matches her chance of live birth success for each of the three treatment options.
Discussion
In this study, we built models to predict successful ovulation, conception, pregnancy, and most importantly live birth in women with PCOS undergoing ovulation induction, and we used these data to construct a clinically useful chart for prediction of live birth success. Expected predictors such as smoking history were not significant in our assessment. The factors that were persistently significant in all four models were interaction of treatment with baseline BMI, FAI, proinsulin level, and duration of attempting conception. However, we also noted unique and, in some cases, divergent predictors dependent on the outcome of interest. For instance, age 34 or younger was a predictive factor for a successful pregnancy and live birth, with a trend toward successful conception. Conversely, age greater than 34 yr was predictive only of successful ovulation. History of a prior pregnancy loss predicted only ovulation and conception, but not clinical pregnancy or live birth. The presence of hirsutism was noted to have an adverse prognosis when comparing both the extremes of less than 8 (nonhirsute) and 16 or greater (severely hirsute) for conception, pregnancy, and live birth, but not ovulation. These analyses further underscore the importance of following PCOS subjects participating in ovulation induction clinical trials until a live birth is achieved and not relying solely on ovulation as the primary end-point for such studies.
The divergent effects of age on ovulation and pregnancy are consistent with both the natural history of PCOS and the well-documented age-related decline in fertility. As women with PCOS age, the reproductive phenotype lessens, with an improvement in hyperandrogenemia (17) and menstrual regularity (18). As our model reflects, older women are more likely to ovulate in response to therapy, but likely with an age-related diminishment in oocyte competence that lowers the chance for pregnancy. This further supports one of the key findings of our clinical trial, that age-related quality of eggs, rather than frequency of ovulation, determines the chance for pregnancy and successful birth.
We expected smoking history to be adversely associated with pregnancy outcomes, given the well-known association between smoking and infertility (19). It may be, however, that smoking effects were diluted because such a low proportion were active smokers during the study. Smoking cessation should still be a part of preconceptional counseling for women with PCOS (11,12). Obesity confounds and worsens the PCOS phenotype, including the prognosis for fertility. Obese PCOS patients have more severe clinical features, including worse metabolic parameters, hyperandrogenemia, and menstrual abnormalities, than do normal-weight PCOS women (20). Obesity also negatively impacts stimulation in ovulation induction cycles, necessitating higher doses with longer periods of stimulation (21,22). The impact of obesity on fertility is further punctuated by the benefits of weight loss in obese PCOS women, with an improvement in metabolic parameters, menstrual bleeding patterns, and ovulation with lifestyle interventions (23).
However, BMI should be considered both according to severity and in the context of other predictive factors when counseling patients about the likelihood of pregnancy. The usual clinical (and expert panel) advice for obese women with PCOS is to “lose weight” (11,12). This recommendation must be tempered by the now known adverse effects of age and duration of infertility. Because for most patients it would take an extended period of time to change their BMI by the 5 or more units necessary to significantly improve their prognosis based on this model, it is possible that the delay may actually be counterproductive to the goal of achieving a successful pregnancy. In fact, we noted that there were some cases where a low BMI did not improve the estimated chance of live birth, such as in the case of overall very poor prognosis (0–10% estimated chance of live birth) in the metformin group, and in the case where all the other prognostic factors were poor (age >34 yr, duration of attempting conception ≥1.5 yr, and higher hirsutism scores) in the combined group. In all other scenarios, however, women with a BMI below 30 kg/m2 had a better chance of success than obese women with a BMI of at least 35 kg/m2.
We were surprised to find proinsulin as a marker of success in ovulation, conception, pregnancy, and live birth because it has not been previously reported in this context. Furthermore, the purported insulin sensitizer metformin was relatively ineffective in terms of live births in this study (7). Fasting proinsulin indicates an advanced stage of β-cell dysfunction, is a marker for insulin resistance in type 2 diabetes (24), and is an independent predictor of the development of type 2 diabetes (25). Elevated proinsulin levels are found in women with PCOS (26,27) and are also noted in their first-degree relatives (28). In PCOS women, proinsulin is correlated positively with hyperinsulinemia (29) and indices of insulin resistance (26), but insulin levels dropped out of our model when proinsulin was included. It should be noted that in addition to insulin resistance, women with PCOS have been shown to have early and pronounced β-cell dysfunction (30). In a population-based study, nondiabetic nulliparous women had elevated levels of proinsulin as compared with parous women, suggesting that nulliparity was associated with β-cell dysfunction (31). Thus, our study further supports a correlation between elevated proinsulin levels and decreased reproductive ability in women with PCOS, and this level may reflect both decreased insulin sensitivity and β-cell dysfunction.
A low hirsutism score was not predictive of ovulation success, but was significant in the models of conception, pregnancy, and live birth. In a population-based cohort, women with oligomenorrhea and/or hirsutism had a higher risk of infertility and lower fecundability than asymptomatic women (32). Therefore, hirsutism may be a measurable bioassay for the extent and duration of androgen excess, reflecting similar changes in other androgen-responsive tissues, such as the ovary and endometrium. The following evidence suggests that intraovarian androgen excess may perturb oocyte development: 1) intrafollicular androstenedione and testosterone concentrations have been shown to be elevated in PCOS women after undergoing gonadotropin-stimulated cycles (33), which has been inversely correlated with oocyte maturation and developmental potential (34); and 2) a low follicular estradiol/testosterone ratio is associated with decreased pregnancy potential (35). In addition, factors other than androgens, including an excess of insulin and other growth factors, contribute to the recruitment and terminal maturation of hair follicles in women with PCOS (36) and may also contribute to the decreased pregnancy rates in hirsute women.
Our study involved a large cohort of women with PCOS and was conducted prospectively, and baseline characteristics were obtained before randomization, eliminating selection bias. We also evaluated clinically important end-points beyond ovulation such as pregnancy and live birth. However, we note the following limitations to the generalizability of our findings to clinical practice and other populations. Our population was primarily overweight or obese, although otherwise healthy and racially diverse. We did not monitor response with ultrasound; only physical symptoms and progesterone levels were monitored. Despite these caveats, our predictors overlap with those found in other studies.
In one study from Italy evaluating 80 infertile anovulatory PCOS patients treated with either metformin or CC monotherapy, it was found that BMI was the strongest predictor of both ovulation and pregnancy in the metformin group, and baseline FAI was the strongest predictor in the CC arm (37). In another study from Spain, predictors of pregnancy in 76 PCOS patients treated with CC or recombinant FSH were duration of infertility and use of FSH with 25 resultant pregnancies (38). The best previous study to predict the chance for live birth, based on 259 women with oligomenorrheic infertility (most with PCOS) is from The Netherlands and used a two-part clinical nomogram with factors to predict ovulation, and then if ovulation occurred, factors to predict live birth (39). These factors included FAI, BMI, cycle history, and age. With the exception of proinsulin, our results are similar, but our clinical predictive chart has the advantage that it is more user-friendly and uses information from the history and physical, easily obtained at the initial consult.
In summary, this is the largest prospective study of predictors of pregnancy after ovulation induction in women with PCOS and the only one to examine all four clinical outcomes of ovulation, conception, pregnancy, and live birth. Women with PCOS can be counseled on their likelihood for live birth with front-line infertility therapy by the use of the following clinical parameters: BMI, age, duration of attempting conception, and hirsutism score. For women with projected pregnancy success of 20% or less with 6 months of treatment (Fig. 1, red and orange boxes), the poor utility of this intended treatment should be discussed with the patient, and serious consideration should be given to the pursuit of a more aggressive initial therapy, such as ovarian diathermy, injectable gonadotropins, or in vitro fertilization. The proposed algorithm should prove of significant value to both primary care physicians and specialists as they evaluate and counsel PCOS patients on their infertility treatment plans and the chance of pregnancy and delivery. We look forward to the prospective validation of our predictive models on existing data sets or, preferably, properly designed prospective clinical trials.
Acknowledgments
In addition to the authors, other investigators of the National Cooperative Reproductive Medicine Network were as follows: University of Pennsylvania, K. Barnhart, L. Martino, and K. Timbers; Duke University, L. Lambe, R. DeWire, H. Yang, C. Bodine, and D. Mark; Wayne State University, E. Puscheck, K. Ginsburg, K. Collins, M. Brossoit, R. Leach, F. Yelian, and M. Perez; Baylor College of Medicine, J. Buster, P. Amato, and M. Torres; Pennsylvania State University, W. C. Dodson, C. Gnatuk, J. Ober, L. Demers, and A. Kunselman; University of Medicine and Dentistry of New Jersey, D. Heller, J. Colon, G. Weiss, and A. Solnica; University of Colorado, K. Gatlin and S. Hahn; University of Texas, Southwestern, M. Roark; University of Alabama, R. Blackwell, V. Willis, and L. Love; University of Pittsburgh, K. Laychak; Virginia Commonwealth University, M. Nazmy and D Stovall; University of Virginia, W. Evans; Stanford University, K. Turner; University of California San Diego, J. Chang and P. Malcolm; Denver Health Medical Center, C. Coddington; and Kaiser Permanente, K. Faber.
We also acknowledge the substantial contributions of the Ligand Assay and Analysis Core Laboratory at the University of Virginia Center for Research and Reproduction, under the direction of D. Hasenleider.
Finally, we also acknowledge and thank members of the RMN Advisory Board: J. Hogan, F. Howard, M. Schiff, J. Wactawski-Wende, and N. Santoro (Chair); and we express special gratitude for careful and devoted oversight of our trial to the Data and Safety Monitoring Committee: E. Thom, J. Peipert, J. Zhang, P. Cato, C. Henderson, and R. Rebar (Chair).
Footnotes
This work was supported by National Institutes of Health/National Institute of Child Health and Human Development Grants U10 HD27049 (to C.C.), U10 HD38992 (to R.S.L.), U01 HD38997 (to E.R.M.), U10 HD39005 (to M.P.D.), U10 HD27011 (to S.A.C.), U10 HD33172 (to M.P.S.), U10 HD38988 (to B.R.C.), U10 HD38998 (to W.D.S.), U10 HD38999 (to P.M.G.), and U54-HD29834 to University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core; General Clinical Research Center Grants MO1RR00056 to the University of Pittsburgh, and MO1RR10732 and Construction Grant C06 RR016499 to Pennsylvania State University. Glucophage XR (Metformin) and matching placebo were provided by Bristol-Myers Squibb.
Trial Registration: ClinicalTrials.gov number: NCT00068861.
Disclosure Summary: R.S.L. reports receiving lecture fees from Serono and grant support from Solvay. J.E.N. reports equity ownership/stock options in Bristol Myers Squibb. P.C.L., E.R.M., and H.X.B. report grant support from Tap; H.X.B. also reports grant support from Ortho Biotech; and E.R.M. has received research support from Merck and is also a consultant. W.D.S. has grant support from Organon and Wyeth. N.A.C. consults for Organon. P.G.M. has grant support from Ferring and Serono. M.P.D. reports grant support from Serono, Tap, Glaxo Smith Klein, and Merck, and has served as a consultant for Tap and Serono. S.A.C. is a medical advisor to EMD Serono, Ferring, Columbia Labs, and FUJIREBIO. M.E.R., C.C., L.C.G., M.P.S., G.G.G., and B.R.C. report no disclosures.
First Published Online June 9, 2009
For editorial see page 3183
Abbreviations: CC, Clomiphene citrate; FAI, free androgen index; PCOS, polycystic ovary syndrome.
References
- Hull MG 1987 Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol 1:235–245 [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO 2004 The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25 [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- Legro RS 2007 Pregnancy considerations in women with polycystic ovary syndrome. Clin Obstet Gynecol 50:295–304 [DOI] [PubMed] [Google Scholar]
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER 2007 Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 356:551–566 [DOI] [PubMed] [Google Scholar]
- Zain MM, Jamaluddin R, Ibrahim A, Norman RJ 2009 Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril 91:514–521 [DOI] [PubMed] [Google Scholar]
- Creanga AA, Bradley HM, McCormick C, Witkop CT 2008 Use of metformin in polycystic ovary syndrome: a meta-analysis. Obstet Gynecol 111:959–968 [DOI] [PubMed] [Google Scholar]
- Moll E, van der Veen F, van Wely M 2007 The role of metformin in polycystic ovary syndrome: a systematic review. Hum Reprod Update 13:527–537 [DOI] [PubMed] [Google Scholar]
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2008 Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 23:462–477 [DOI] [PubMed] [Google Scholar]
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2008 Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 89:505–522 [DOI] [PubMed] [Google Scholar]
- Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, McGovern PG, Cataldo NA, Steinkampf MP, Nestler JE, Gosman G, Guidice LC, Leppert PC 2006 The Pregnancy in Polycystic Ovary Syndrome study: baseline characteristics of the randomized cohort including racial effects. Fertil Steril 86:914–933 [DOI] [PubMed] [Google Scholar]
- Balen AH, Laven JS, Tan SL, Dewailly D 2003 Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 9:505–514 [DOI] [PubMed] [Google Scholar]
- Hatch R, Rosenfield RL, Kim MH, Tredway D 1981 Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 140:815–830 [DOI] [PubMed] [Google Scholar]
- Myers ER, Silva SG, Hafley G, Kunselman AR, Nestler JE, Legro RS 2005 Estimating live birth rates after ovulation induction in polycystic ovary syndrome: sample size calculations for the pregnancy in polycystic ovary syndrome trial. Contemp Clin Trials 26:271–280 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Talbott E, Guzick DS, Zborowski J, McHugh KP 2000 Serum testosterone levels decrease in middle age in women with the polycystic ovary syndrome. Fertil Steril 73:724–729 [DOI] [PubMed] [Google Scholar]
- Elting MW, Korsen TJ, Rekers-Mombarg LT, Schoemaker J 2000 Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum Reprod 15:24–28 [DOI] [PubMed] [Google Scholar]
- Practice Committee of American Society for Reproductive Medicine 2008 Smoking and infertility. Fertil Steril 90(5 Suppl):S254–S259 [DOI] [PubMed] [Google Scholar]
- Pasquali R, Gambineri A, Pagotto U 2006 The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG 113:1148–1159 [DOI] [PubMed] [Google Scholar]
- Dickey RP, Taylor SN, Curole DN, Rye PH, Lu PY, Pyrzak R 1997 Relationship of clomiphene dose and patient weight to successful treatment. Hum Reprod 12:449–453 [DOI] [PubMed] [Google Scholar]
- Balen AH, Platteau P, Andersen AN, Devroey P, Sørensen P, Helmgaard L, Arce JC; for the Bravelle Ovulation Induction Study Group 2006 The influence of body weight on response to ovulation induction with gonadotrophins in 335 women with World Health Organization group II anovulatory infertility. BJOG 113:1195–1202 [DOI] [PubMed] [Google Scholar]
- Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ 3 Dec 2008 Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 10.1016/j.fertnstert.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Pfützner A, Kunt T, Hohberg C, Mondok A, Pahler S, Konrad T, Lübben G, Forst T 2004 Fasting intact proinsulin is a highly specific predictor of insulin resistance in type 2 diabetes. Diabetes Care 27:682–687 [DOI] [PubMed] [Google Scholar]
- Hanley AJ, D'Agostino Jr R, Wagenknecht LE, Saad MF, Savage PJ, Bergman R, Haffner SM; Insulin Resistance Atherosclerosis Study 2002 Increased proinsulin levels and decreased acute insulin response independently predict the incidence of type 2 diabetes in the insulin resistance atherosclerosis study. Diabetes 51:1263–1270 [DOI] [PubMed] [Google Scholar]
- Panidis D, Macut D, Farmakiotis D, Rousso D, Kourtis A, Katsikis I, Spanos N, Petakov M, Bjekic J, Damjanovic S 2006 Indices of insulin sensitivity, β-cell function and serum proinsulin levels in the polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 127:99–105 [DOI] [PubMed] [Google Scholar]
- Maliqueo M, Atwater I, Lahsen R, Pérez-Bravo F, Angel B, Sir-Petermann T 2003 Proinsulin serum concentrations in women with polycystic ovary syndrome: a marker of β-cell dysfunction? Hum Reprod 18:2683–2688 [DOI] [PubMed] [Google Scholar]
- Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A 2008 Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care 31:1237–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway GS, Clark PM, Wong D 1993 Hyperinsulinaemia in the polycystic ovary syndrome confirmed with a specific immunoradiometric assay for insulin. Clin Endocrinol (Oxf) 38:219–222 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Breda E, Corcoran MC, Cavaghan MK, Imperial J, Toffolo G, Cobelli C, Polonsky KS 2004 Impaired β-cell compensation to dexamethasone-induced hyperglycemia in women with polycystic ovary syndrome. Am J Physiol Endocrinol Metab 287:E241–E246 [DOI] [PubMed] [Google Scholar]
- Hanley AJ, McKeown-Eyssen G, Harris SB, Hegele RA, Wolever TM, Kwan J, Zinman B 2002 Association of parity with risk of type 2 diabetes and related metabolic disorders. Diabetes Care 25:690–695 [DOI] [PubMed] [Google Scholar]
- Koivunen R, Pouta A, Franks S, Martikainen H, Sovio U, Hartikainen AL, McCarthy MI, Ruokonen A, Bloigu A, Järvelin MR, Morin-Papunen L; Northern Finland Birth Cohort 1966 Study 2008 Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod 23:2134–2139 [DOI] [PubMed] [Google Scholar]
- Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA 2006 Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab 91:2327–2333 [DOI] [PubMed] [Google Scholar]
- Cupisti S, Dittrich R, Mueller A, Strick R, Stiegler E, Binder H, Beckmann MW, Strissel P 2007 Correlations between anti-mullerian hormone, inhibin B, and activin A in follicular fluid in IVF/ICSI patients for assessing the maturation and developmental potential of oocytes. Eur J Med Res 12:604–608 [PubMed] [Google Scholar]
- Andersen CY 1993 Characteristics of human follicular fluid associated with successful conception after in vitro fertilization. J Clin Endocrinol Metab 77:1227–1234 [DOI] [PubMed] [Google Scholar]
- Landay M, Huang A, Azziz R 21 Aug 2008 Degree of hyperinsulinemia, independent of androgen levels, is an important determinant of the severity of hirsutism in PCOS. Fertil Steril 10.1016/j.fertnstert.2008.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomba S, Falbo A, Orio Jr F, Tolino A, Zullo F 2009 Efficacy predictors for metformin and clomiphene citrate treatment in anovulatory infertile patients with polycystic ovary syndrome. Fertil Steril 91:2557–2567 [DOI] [PubMed] [Google Scholar]
- López E, Gunby J, Daya S, Parrilla JJ, Abad L, Balasch J 2004 Ovulation induction in women with polycystic ovary syndrome: randomized trial of clomiphene citrate versus low-dose recombinant FSH as first line therapy. Reprod Biomed Online 9:382–390 [DOI] [PubMed] [Google Scholar]
- Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC 2002 A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril 77:91–97 [DOI] [PubMed] [Google Scholar]