Abstract
Context: Increased body fat is a risk factor for cardiovascular and metabolic disease, yet it is uncertain whether obesity protects against osteoporosis or adiposity is harmful to bone.
Objective: The aim of the study was to assess whether the pattern of adipose tissue deposition influences bone structure and strength.
Design: The relations between sc and visceral adiposity and the cross-sectional dimensions and polar and principal moments of the femur in 100 healthy women ages 15 to 25 years were obtained using computed tomography.
Results: Multiple linear regression analyses indicated that, after adjusting for leg length and thigh musculature, both sc and visceral fat had strong and independent associations with femoral cross-sectional area, cortical bone area, principal moment maximum, principal moment minimum, and polar moment (all P values < 0.03). However, whereas sc fat had a positive predictive value with all femoral bone phenotypes, a similar but negative effect was observed between visceral fat and these measures (all P values < 0.01).
Conclusions: We found that visceral and sc fat have opposite effects on the appendicular skeleton; whereas sc fat is beneficial to bone structure and strength, visceral fat serves as an unique pathogenic fat depot.
While subcutaneous fat is beneficial to bone structure and strength, visceral fat serves as a unique pathogenic fat depot.
The pervasive negative health consequences of obesity involve many organ systems and a large proportion of the population. However, despite the dire cardiovascular and metabolic repercussions of obesity, the traditional paradigm is that adiposity is not detrimental to bone health, but rather protects against osteoporosis (1). Low body weight and recent weight loss are well-known risk factors for osteoporosis and fractures, and most studies have revealed a positive relation between body weight or body mass index (BMI) and bone mass (2,3,4). Moreover, women with more body fat are known to have lower rates of bone resorption, an independent predictor of fracture, than leaner women (5). The contention for a positive fat-bone association is credited not only to stresses from mechanical loading, but also to the metabolic effects of bone-regulator hormones secreted or regulated by adipocytes (1,6,7). Challenging this widely held view, recent information by us and other investigators suggests that fat mass is not associated with, or is negatively related to, bone mass (8,9,10,11,12). Given these discrepancies in results, a greater phenotypic characterization, beyond total fat accumulation, is needed to explain the complex relation between bone and adipose tissue.
A consistent body of literature suggests the pattern of regional fat deposition into the sc and visceral compartments to be a stronger predictor of disease risk than overall fat mass (13,14,15,16). Secretions from visceral adiposity pass through the liver before entering general circulation, uniquely implicating intraabdominal fat in the pathogenesis of insulin resistance and type 2 diabetes (13). In contrast, there is evidence that sc fat, unlike visceral adiposity, may serve in a protective role in the prevention of atherosclerosis (17). However, whether the conflicting data regarding the fat-bone link is related to differences in fat distribution in the previous population studied is unknown. To our knowledge, no studies have investigated the possible independent effects of sc abdominal fat (SAF) and visceral abdominal fat (VAF) on bone health.
The purpose of this study was to determine whether bone structure and/or bone strength in the appendicular skeleton are related to the distribution of adipose tissue. We chose to study young women because the amount of bone gained during young adulthood is the main contributor to peak bone mass, which, in turn, is a major determinant of osteoporosis and fractures in the elderly (17).
Subjects and Methods
Subjects
The institutional review board for clinical investigations approved the investigational protocol, and informed consent was obtained from all participants and/or their parents. This study included 100 healthy females between the ages of 15 to 25 yr who were recruited from schools and colleges in Los Angeles County. Study subjects had no known diagnosis of any chronic illness, no history of medical disorders resulting in a period of illness that interrupted their usual physical activity or nutritional status for more than 1 month in the 2 yr before enrollment, no intake of any medications including oral contraceptives, and no hospitalization since birth. Subjects who were pregnant or had ever been pregnant were also excluded.
Participants underwent a physical examination by an endocrinologist. Measurements of height and weight were obtained to the nearest 0.1 cm and 0.1 kg, respectively. Only subjects between the 3rd and 97th percentiles for height and weight according to the National Center for Health Statistics were included; reference standards for 20 yr olds were used for all subjects aged 20 yr or older. Trunk height was also measured to the nearest 0.1 cm. Leg length was determined by subtracting trunk height from the total height of each subject. BMI was calculated as weight (kilograms) divided by height (meters) squared. The Tanner stage of sexual development, determined by an endocrinologist, was used to assess sexual maturity; only subjects who had achieved sexual maturity (Tanner V) were included (18). Skeletal maturation was assessed from radiographs of the left hand and wrist, and only those who had reached skeletal maturity as defined as physeal closure in the phalanges and metacarpals using the method of Greulich and Pyle were included in this study (19).
Computed tomography (CT) measures of fat, bone, and muscle
Measurements of fat, bone, and muscle phenotypes were obtained by the same technologist using the same CT scanner (CT Highlite Advantage; General Electric Co., Milwaukee, WI), the same mineral reference phantom (CT-T bone densitometry package; General Electric Co.), and a graphical user interface created with Matlab 2006b (Mathworks, Natick, MA).
Determinations of waist circumference, SAF, and VAF were obtained at the umbilicus. For the purposes of this study, SAF was defined as the area in square centimeters of adipose tissue located between the skin and the rectus muscles of the abdomen, the external oblique muscles, the broadest muscle of the back, and the erector muscles of the spine at the level of the umbilicus. VAF was defined as the intraabdominal adipose tissue surrounded by the rectus muscles of the abdomen, the external oblique muscles, the lumbar quadrate muscle, the psoas muscles, and the lumbar spine at the same site. The same researcher did all manual tracing for the VAF measures. Previous studies have established that a single slice CT predicts 87% of total VAF (20).
Measurements in the appendicular skeleton were made at the midshaft of both femurs. A separate graphical user interface was created with Matlab 2006b to automatically extract endosteal and periosteal contours of the femur and calculate geometric parameters, and the contours of the femur were found via edge detection and were used to calculate cross-sectional area (CSA), cortical bone area (CBA), and polar moment of inertia and maximum and minimum principal moments of inertia. Values for femoral bone phenotypes represent the mean of both femurs.
From the same CT images, measures of thigh musculature were obtained. Thigh musculature was defined as the sum of areas of the rectus femoris, vastus, biceps femoris, semitendinosus, semimembranosus, gracilis, and adductors. Muscle area was determined by separating the image, with bone and air voxels removed, into adipose and muscle tissue. The threshold, which separates adipose from muscle, was found by taking the average of their peaks from an image histogram. All areas were calculated by converting the number of voxels to square centimeters.
The coefficients of variation for CT measures of SAF and VAF, femoral CSA and CBA, and thigh musculature have been reported to be between 0.6 and 3.5% (21,22,23,24). Intraclass correlations for the polar and minimum and maximum principal moments were calculated using right and left femoral values for the 100 subjects to range between 0.94 and 0.96. The time required for this procedure was approximately 10 min and was localized at the umbilicus and midshaft of the femur (radiation exposure of this limited CT examination was 100–150 mrem); the effective radiation dose was approximately 4 mrem (25).
Statistical analysis
Statistical analyses were conducted using Statview (version 5.0.1; SAS Institute Inc., Cary, NC). Power calculations were based on the addition of a variable to an existing regression model with an R2 of 0.4 to 0.5. A sample size of 100 provides 84–90% power at the 5% significance level for detecting an increase in the R2 of 0.05 or greater.
Pearson correlations were used to examine associations between variables, and multiple regression analyses were used to determine the influence of SAF and VAF on bone phenotypes. For these analyses, dependent variables were femoral CSA, CBA, principal moment maximum, principal moment minimum, and polar moment, and the independent variables were SAF, VAF, leg length, and thigh musculature.
In building this model, we excluded weight to avoid multicollinearity among covariates, but we included leg length and thigh musculature as covariates in the model to adjust for the confounding effects of femoral length and mechanical stress on the cross-sectional dimensions and strength of the bone. To further exclude any possibility of multicollinearity on the final model, postestimation procedures were used to calculate a condition number for the regression models and to compare the condition number to the suggested cutoff value of 15 (26). Models with condition numbers less than 15 were judged not to have any substantial collinearity problems that would affect the results of the conclusions. The goodness of fit for the regression models was evaluated using the postestimation procedures of STATA. All models that were presented passed the following goodness of fit criteria: residuals appeared random, and no strong influence or leverage points were present, based on both a graphical and a distribution evaluation. All models were repeated after deleting suspicious points to be sure the coefficients remained similar, indicating that the suspicious points were not affecting the results. In these models, standardized regression coefficients were used to directly compare the effects of independent variables on the dependent variable, regardless of differences in the scale of the variables involved. The standardized coefficients were calculated by performing the linear regression on the standardized independent variables.
Results
Age and anthropometric characteristics, and fat, muscle, and bone measurements are described in Table 1. Age did not correlate with any anthropometric or CT measures. There were strong correlations between weight, BMI, and waist circumference and all fat, bone and muscle phenotypes—the strongest with SAF (Table 2). SAF and VAF were also significantly related to each other (r = 0.70; P < 0.001), and to thigh musculature (r = 0.57 and r = 0.38 respectively; P values < 0.01).
Table 1.
Age and anthropometric characteristics and CT measures of fat, muscle, and bone in 100 females
| Mean ± sd (range) | |
|---|---|
| Age (yr) | 17.9 ± 1.9 (15.1–24.9) |
| Height (cm) | 160.0 ± 4.8 (149.3–173.0) |
| Weight (kg) | 62.0 ± 11.9 (43.4–91.3) |
| BMI (kg/m2) | 24.2 ± 4.3 (16.8–34.2) |
| Trunk height (cm) | 84.6 ± 3.5 (76.1–94.0) |
| Leg length (cm) | 75.4 ± 4.4 (64.9–84.5) |
| Waist circumference (cm) | 87.0 ± 10.4 (66.6–110.4) |
| Subcutaneous fat (cm2) | 238.6 ± 95.0 (70.9–462.8) |
| Visceral fat (cm2) | 19.40 ± 8.42 (5.63–45.70) |
| Thigh musculature (cm2) | 116.4 ± 29.6 (79.9–269.7) |
| Femoral CSA (cm2) | 4.81 ± 0.54 (3.53–6.28) |
| Femoral CBA (cm2) | 3.81 ± 0.43 (2.84–5.04) |
| Femoral principal moment maximum (cm4) | 2.20 ± 0.54 (1.13–3.86) |
| Femoral principal moment minimum (cm4) | 1.48 ± 0.33 (0.69–2.40) |
| Femoral polar moment (cm4) | 3.68 ± 0.83 (2.08–6.08) |
Table 2.
Correlations between age and anthropometric characteristics and CT measures of fat, muscle, and bone in 100 females
| Weight | BMI | WC | Age | Height | Trunk height | Leg length | |
|---|---|---|---|---|---|---|---|
| Subcutaneous fat | 0.87 | 0.90 | 0.97 | 0.12 | 0.14 | 0.17 | 0.02 |
| Visceral fat | 0.56 | 0.59 | 0.72 | 0.10 | 0.08 | 0.03 | 0.06 |
| Thigh musculature | 0.77 | 0.74 | 0.59 | −0.04 | 0.24 | 0.14 | 0.16 |
| Femoral CSA | 0.66 | 0.53 | 0.48 | −0.02 | 0.51 | 0.35 | 0.28 |
| Femoral CBA | 0.65 | 0.57 | 0.49 | 0.02 | 0.38 | 0.30 | 0.18 |
| Femoral principal moment maximum | 0.63 | 0.51 | 0.44 | −0.08 | 0.47 | 0.32 | 0.27 |
| Femoral principal moment minimum | 0.65 | 0.53 | 0.48 | 0.03 | 0.47 | 0.37 | 0.23 |
| Femoral polar moment | 0.67 | 0.54 | 0.48 | −0.04 | 0.50 | 0.36 | 0.27 |
WC, Waist circumference. Correlations r > 0.20 are significant at P < 0.05.
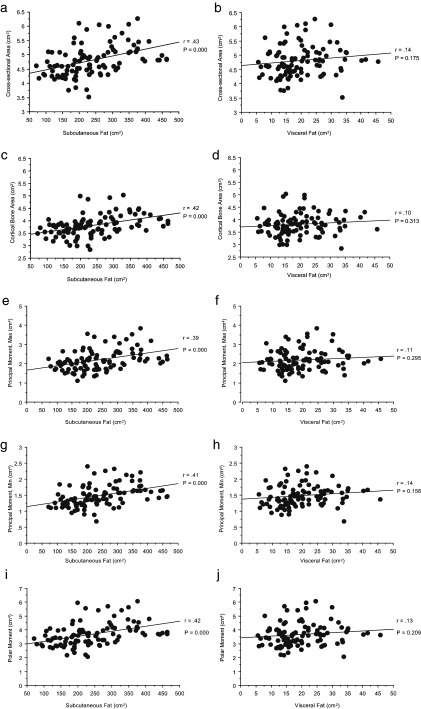
Figure 1 shows the relations between measures of SAF and VAF and femoral cross-sectional dimensions and moments of inertia. Positive associations were present between measures of SAF and all bone phenotypes in the appendicular skeleton. For visceral adiposity, none of the relations achieved statistical significance.
Figure 1.
Relations between SAF and VAF and measures of the cross-sectional dimensions (a–d) and strength (e–j) of the femur.
Multiple linear regression analyses indicated that, after adjusting for leg length and thigh musculature, both sc and visceral fat had strong and independent associations with femoral CSA, CBA, principal moment maximum, principal moment minimum, and polar moment. However, whereas SAF had a positive predictive value with all femoral bone phenotypes, a similar but negative effect was observed between VAF and theses measures (Table 3). Overall, the ratios of the standardized coefficients of sc to visceral fat were around 1.5 in all models. The calculated condition number of 12.85 for the model suggested multicollinearity not to be a concern.
Table 3.
Multiple linear regression models for CT measurements of femoral cross-sectional dimensions and moments of inertia using leg length, thigh musculature, sc fat, and visceral fat as independent variables
| Standardized coefficient | se | P value | 95% confidence interval | |
|---|---|---|---|---|
| Femoral CSA (cm2) | ||||
| Leg length (cm) | 0.239 | 0.08 | 0.004 | 0.08 to 0.40 |
| Thigh musculature (cm2) | 0.364 | 0.10 | <0.001 | 0.17 to 0.56 |
| Subcutaneous fat (cm2) | 0.437 | 0.13 | <0.001 | 0.19 to 0.69 |
| Visceral fat (cm2) | −0.323 | 0.11 | 0.005 | −0.54 to −0.10 |
| Femoral CBA (cm2) | ||||
| Leg length (cm) | 0.126 | 0.08 | 0.12 | −0.03 to 0.28 |
| Thigh musculature (cm2) | 0.450 | 0.10 | <0.001 | 0.26 to 0.64 |
| Subcutaneous fat (cm2) | 0.419 | 0.12 | 0.001 | 0.17 to 0.67 |
| Visceral fat (cm2) | −0.371 | 0.11 | 0.001 | −0.59 to −0.15 |
| Principal moment, maximum (cm4) | ||||
| Leg length (cm) | 0.222 | 0.08 | 0.008 | 0.06 to 0.39 |
| Thigh musculature (cm2) | 0.388 | 0.10 | <0.001 | 0.19 to 0.59 |
| Subcutaneous fat (cm2) | 0.400 | 0.13 | 0.002 | 0.15 to 0.65 |
| Visceral fat (cm2) | −0.336 | 0.11 | 0.004 | −0.56 to −0.11 |
| Principal moment, minimum (cm4) | ||||
| Leg length (cm) | 0.182 | 0.08 | 0.03 | 0.02 to 0.35 |
| Thigh musculature (cm2) | 0.376 | 0.10 | <0.001 | 0.17 to 0.58 |
| Subcutaneous fat (cm2) | 0.393 | 0.13 | 0.003 | 0.13 to 0.65 |
| Visceral fat (cm2) | −0.287 | 0.12 | 0.014 | −0.52 to −0.06 |
| Polar moment (cm4) | ||||
| Leg length (cm) | 0.219 | 0.08 | 0.007 | 0.06 to 0.38 |
| Thigh musculature (cm2) | 0.406 | 0.10 | <0.001 | 0.21 to 0.60 |
| Subcutaneous fat (cm2) | 0.420 | 0.12 | 0.001 | 0.17 to 0.67 |
| Visceral fat (cm2) | −0.336 | 0.11 | 0.003 | −0.55 to −0.12 |
Discussion
Increased fat is a major public health concern and a risk factor for many diseases, but determining whether it is beneficial or detrimental to bone health has been difficult. The results of this study indicate that sc and visceral adiposity have strong but opposing relations with bone structure and strength. Whereas sc fat had a positive relation with the CSA, the CBA, the principal moment maximum, principal moment minimum, and polar moment of the femurs, visceral fat had negative associations with all bone phenotypes. These reciprocal relations of SAF and VAF with the biomechanical properties of the appendicular skeleton of young healthy women were present after adjusting for key determinants of bone mass.
Ample data indicate that femoral cross-sectional growth is strongly driven by mechanical load associated with increasing weight. Because weight in young women is highly correlated to sc fat, to avoid multicollinearity, we constructed a model that excluded weight but included measures of adiposity, musculature, and height as covariates. Moreover, by constructing a model with leg length and thigh musculature as independent variables, we accounted for the effects of other important determinants of cross-sectional dimensions and strength of the femurs, allowing the analysis to be concentrated on the fat variables. Overall, using this mechanistic model, the beneficial effect of sc fat on bone structure and strength was slightly stronger than the detrimental effect of visceral fat.
The adverse visceral fat-bone association found in this study lends further support to the growing amount of data indicating that the allocation of adipose tissue in the body is an important predictor of disease risk. Increased VAF is associated with insulin resistance and dyslipidemia and is an independent risk factor for type 2 diabetes, myocardial infarction, hypertension, and all-cause mortality. Whereas insulin resistance, type 2 diabetes, and arteriosclerosis have previously been linked to osteopenia, the detrimental relationship of VAF to bone mass in nonobese, nondiabetic populations has not been carefully examined. We found, however, that even in healthy young women with weights between the 3rd and 97th percentiles, VAF is negatively correlated with the amount and strength of bone in the appendicular skeleton.
Our results, suggesting the influence of fat on bone to be foremost dependent on the site in which it accumulates, may help explain the large body of conflicting data on the link between body adiposity and bone mass. Previous research is inconclusive as to whether fat mass has no relation, an inverse association, or a positive association with bone density (8,11,12,27). These investigations were limited by the use of dual energy x-ray absorptiometry, an imaging technique that does not allow for the independent analysis of sc fat and visceral fat. Similarly, although waist circumference is frequently employed as a surrogate for visceral adiposity and is reported to be more closely associated with negative health outcomes than sc fat or BMI (28,29,30), this measure is also a composite of the sc and visceral fat depots (31,32). Indeed, like other investigators, we found waist circumference to have a stronger correlation with sc fat than with visceral fat, and, like sc adiposity, a positive relation to bone.
The basis for the differential effect of SAF and VAF on bone observed in this study is unknown. However, adipose tissue is the source of a number of hormones, cytokines, and inflammatory factors that modulate multiple biological functions and have depot-specific differences in gene expression (33). Proteins that are potentially protective against the development of osteoporosis, such as adiponectin, have lower levels of expression in visceral than sc fat tissue (34). Leptin, a satiety-regulating hormone that is produced by adipocytes, promotes the differentiation of osteoblasts, and affects bone resorption, is also supposedly less abundant in visceral tissue (35,36). Likewise, the expression of aromatase, which converts androgen to estrogen and results in reduced osteoclast activity is known to be lower in visceral adipocytes (6). In contrast, visceral adiposity is associated with increased levels of proinflammatory cytokines, (37,38,39) like TNF-α and IL-6, both of which increase bone resorption and promote osteoporosis (40).
The inclusion of healthy subjects with weights between the 3rd and 97th percentiles to minimize the confounding effects of obesity and the independent evaluations of the influence of SAF and VAF on both the structure and strength of bone are positive attributes of this study. This study has, however, several notable limitations. We chose to study young women, and our findings cannot be extrapolated to the elderly or to the male skeleton. This limits the generalizability of the results but should not affect the internal validity. Moreover, available data indicate that greater increases in VAF than SAF occur in the perimenopausal years (41), concomitant to a decline in circulating estradiol decreases; postmenopausal women have higher total and abdominal fat mass than premenopausal women (42), and those on hormone replacement therapy have less VAF than estrogen nonusers (43,44). Knowledge that both bone loss and abdominal fat mass increase after menopause supports the notion that the deleterious effect of visceral fat spans throughout life. Similarly, in spite of marked gender differences in the amount and distribution of fat, VAF is associated with the same constellation of comorbidities in men and women (13,29), and it is unlikely that our findings are only relevant to women. Most importantly, the associations between SAF and VAF and bone do not prove causality; it is possible that environmental, inherited, or biological factors, such as estrogen secretion, that influence bone formation could also affect sc and intraabdominal fat accumulation differently.
In conclusion, our findings provide compelling evidence that visceral and sc fat have strong and opposite effects on the structure and strength of bone in young women. They support the hypothesis that sc fat is beneficial to peak bone mass, whereas visceral fat serves as a unique pathogenic fat depot. A greater understanding of the molecular and functional differences of visceral and sc adipocytes and how they interact with bone could likely lead to novel therapeutic approaches for obesity and osteoporosis.
Footnotes
This work was supported by grants from the National Institutes of Health (1R01 AR052744-01) and from the Department of the Army (DAMD17-01-1-0817).
Disclosure Summary: V.G., J.C., A.O.M., D.C.L., F.J.D., and S.D.M. have nothing to declare.
First Published Online June 16, 2009
Abbreviations: BMI, Body mass index; CBA, cortical bone area; CSA, cross-sectional area; CT, computed tomography; SAF, subcutaneous abdominal fat; VAF, visceral abdominal fat.
References
- Gnudi S, Sitta E, Fiumi N 2007 Relationship between body composition and bone mineral density in women with and without osteoporosis: relative contribution of lean and fat mass. J Bone Miner Metab 25:326–332 [DOI] [PubMed] [Google Scholar]
- Clark EM, Ness AR, Tobias JH 2006 Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab 91:2534–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ND, Pongchaiyakul C, Center JR, Eisman JA, Nguyen TV 2005 Abdominal fat and hip fracture risk in the elderly: the Dubbo Osteoporosis Epidemiology Study. BMC Musculoskelet Disord 6:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid IR 2002 Relationships among body mass, its components, and bone. Bone 31:547–555 [DOI] [PubMed] [Google Scholar]
- Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD 2000 Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15:1526–1536 [DOI] [PubMed] [Google Scholar]
- Klein KO, Larmore KA, de Lancey E, Brown JM, Considine RV, Hassink SG 1998 Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab 83:3469–3475 [DOI] [PubMed] [Google Scholar]
- Reid IR 2008 Relationships between fat and bone. Osteoporos Int 19:595–606 [DOI] [PubMed] [Google Scholar]
- Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V 2007 Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab 92:143–147 [DOI] [PubMed] [Google Scholar]
- Lazcano-Ponce E, Tamayo J, Cruz-Valdez A, Díaz R, Hernández B, Del Cueto R, Hernández-Avila M 2003 Peak bone mineral area density and determinants among females aged 9 to 24 years in Mexico. Osteoporos Int 14:539–547 [DOI] [PubMed] [Google Scholar]
- Lee K, Lee S, Kim YJ, Kim YJ 2008 Waist circumference, dual-energy X-ray absortiometrically measured abdominal adiposity, and computed tomographically derived intra-abdominal fat area on detecting metabolic risk factors in obese women. Nutrition 24:625–631 [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, Esche GR, Beck TJ, McKinlay JB 2008 Lean mass and not fat mass is associated with male proximal femur strength. J Bone Miner Res 23:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler HA, Janzen L, Green K, Grabowski J, Seshia MM, Yuen KC 2000 Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone 27:203–207 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB 2003 Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26:372–379 [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Abe M, Yahata K, Kajiyama N, Katsuma H, Saito N 2004 Contribution of visceral fat accumulation to the risk factors for atherosclerosis in non-obese Japanese. Intern Med 43:1138–1144 [DOI] [PubMed] [Google Scholar]
- Mori Y, Hoshino K, Yokota K, Itoh Y, Tajima N 2006 Differences in the pathology of the metabolic syndrome with or without visceral fat accumulation: a study in pre-diabetic Japanese middle-aged men. Endocrine 29:149–153 [DOI] [PubMed] [Google Scholar]
- Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Postano V, Buzzigoli E, Ghione S, Turchi S, Lombardi M, Ferrannini E 2004 Visceral fat in hypertension: influence on insulin resistance and β-cell function. Hypertension 44:127–133 [DOI] [PubMed] [Google Scholar]
- Loro ML, Sayre J, Roe TF, Goran MI, Kaufman FR, Gilsanz V 2000 Early identification of children predisposed to low peak bone mass and osteoporosis later in life. J Clin Endocrinol Metab 85:3908–3918 [DOI] [PubMed] [Google Scholar]
- Tanner JM 1981 Growth and maturation during adolescence. Nutr Rev 39:43–55 [DOI] [PubMed] [Google Scholar]
- Greulich W, Pyle S 1959 Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Palo Alto, CA: Stanford University Press [Google Scholar]
- Schoen RE, Thaete FL, Sankey SS, Weissfeld JL, Kuller LH 1998 Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord 22:338–342 [DOI] [PubMed] [Google Scholar]
- Arfai K, Pitukcheewanont PD, Goran MI, Tavare CJ, Heller L, Gilsanz V 2002 Bone, muscle, and fat: sex-related differences in prepubertal children. Radiology 224:338–344 [DOI] [PubMed] [Google Scholar]
- Hangartner TN, Gilsanz V 1996 Evaluation of cortical bone by computed tomography. J Bone Miner Res 11:1518–1525 [DOI] [PubMed] [Google Scholar]
- Braillon PM 2002 Quantitative computed tomography precision and accuracy for long-term follow-up of bone mineral density measurements: a five year in vitro assessment. J Clin Densitom 5:259–266 [DOI] [PubMed] [Google Scholar]
- Steiger P, Block JE, Friedlander A, Genant HK 1988 Precise determination of paraspinous musculature by quantitative CT. J Comput Assist Tomogr 12:616–620 [DOI] [PubMed] [Google Scholar]
- Kalender WA 1992 Effective dose values in bone mineral measurements by photon absorptiometry and computed tomography. Osteoporos Int 2:82–87 [DOI] [PubMed] [Google Scholar]
- Belsley DA, Kuh E, Welsch RE 1980 Regression diagnostics. Hoboken, NJ: Wiley [Google Scholar]
- Harris SS, Dawson-Hughes B 1996 Weight, body composition, and bone density in postmenopausal women. Calcif Tissue Int 59:428–432 [DOI] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino Sr RB, O'Donnell CJ 2007 Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48 [DOI] [PubMed] [Google Scholar]
- Ohashi N, Yamamoto H, Horiguchi J, Kitagawa T, Hirai N, Ito K, Kohno N 2009 Visceral fat accumulation as a predictor of coronary artery calcium as assessed by multislice computed tomography in Japanese patients. Atherosclerosis 202:192–199 [DOI] [PubMed] [Google Scholar]
- Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V 2004 Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord 28:1018–1025 [DOI] [PubMed] [Google Scholar]
- Kuk JL, Lee S, Heymsfield SB, Ross R 2005 Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr 81:1330–1334 [DOI] [PubMed] [Google Scholar]
- Ross R, Rissanen J, Hudson R 1996 Sensitivity associated with the identification of visceral adipose tissue levels using waist circumference in men and women: effects of weight loss. Int J Obes Relat Metab Disord 20:533–538 [PubMed] [Google Scholar]
- Mackenzie SM, Huda SS, Sattar N, Fraser R, Connell JM, Davies E 2008 Depot-specific steroidogenic gene transcription in human adipose tissue. Clin Endocrinol (Oxf) 69:848–854 [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML 2006 Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43 [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G 2004 Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA 101:3258–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K 2001 Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf) 55:341–347 [DOI] [PubMed] [Google Scholar]
- Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després JP 2008 Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-α in men. J Clin Endocrinol Metab 93:1931–1938 [DOI] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney Jr JF, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS 2007 Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116:1234–1241 [DOI] [PubMed] [Google Scholar]
- Wood IS, Wang B, Jenkins JR, Trayhurn P 2005 The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFα in human adipocytes. Biochem Biophys Res Commun 337:422–429 [DOI] [PubMed] [Google Scholar]
- Morley JE, Baumgartner RN 2004 Cytokine-related aging process. J Gerontol A Biol Sci Med Sci 59:M924–M929 [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR 2008 Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horber FF, Gruber B, Thomi F, Jensen EX, Jaeger P 1997 Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition 13:524–534 [DOI] [PubMed] [Google Scholar]
- Haarbo J, Marslew U, Gotfredsen A, Christiansen C 1991 Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism 40:1323–1326 [DOI] [PubMed] [Google Scholar]
- Tchernof A, Poehlman ET, Despres JP 2000 Body fat distribution, the menopause transition, and hormone replacement therapy. Diabetes Metab 26:12–20 [PubMed] [Google Scholar]