Abstract
We have recently shown that disrupting the expression and postsynaptic clustering of gephyrin in cultured hippocampal pyramidal cells, by either gephyrin RNAi (RNA interference) or overexpression of a dominant negative gephyrin-EGFP fusion protein, leads to decreased number of postsynaptic gephyrin and GABAA receptor clusters and to reduced GABAergic innervation of these cells. On the other hand, increasing gephyrin expression led to a small increase in the number of gephyrin and GABAA receptor clusters and to little or no effect on GABAergic innervation. We are now reporting that altering gephyrin expression and clustering affects the size but not the density of glutamatergic synaptic contacts. Knocking down gephyrin with gephyrin RNAi, or preventing gephyrin clustering by overexpression of the dominant negative gephyrin-EGFP fusion protein, leads to larger postsynaptic PSD-95 clusters and larger presynaptic glutamatergic terminals. On the other hand, overexpression of gephyrin leads to slightly smaller PSD-95 clusters and presynaptic glutamatergic terminals. The change in size of PSD-95 clusters were accompanied by a parallel change in the size of NR2-NMDA receptor clusters. It is concluded that the levels of expression and clustering of gephyrin, a protein that concentrates at the postsynaptic complex of the inhibitory synapses, not only has homotypic effects on GABAergic synaptic contacts, but also has heterotypic effects on glutamatergic synaptic contacts. We are proposing that gephyrin is a counterpart of the postsynaptic glutamatergic scaffold protein PSD-95 in regulating the number and/or size of the excitatory and inhibitory synaptic contacts.
Keywords: GABAA receptor, GABA, glutamate, synapse formation, gephyrin, RNA interference
INTRODUCTION
Gephyrin is the main postsynaptic scaffold protein that accumulates at the postsynaptic complex of GABAergic and glycinergic synapses. It forms submembranous cytoplasmic lattices associated with postsynaptic clusters of GABAA receptors (GABAARs) and glycine receptors (Luscher and Keller 2004). Gephyrin is essential for the postsynaptic clustering of the glycine receptors in glycinergic synapses (Kirsch et al, 1993; Feng et al., 1998; Levi et al., 2004; Sola et al., 2004), and for the clustering of a significant population of GABAARs at the GABAergic synapses, particularly the ones containing the γ2 and α2 subunits (Kneussel et al., 1999, Levi et al., 2004; Yu et al., 2007). Moreover, postsynaptic gephyrin clustering is required for maintaining the normal innervation by presynaptic GABAergic contacts at GABAergic synapses (Yu et al., 2007).
It has recently been shown that overexpression in cultured hippocampal pyramidal cells of the postsynaptic glutamatergic scaffold protein PSD-95 (postsynaptic density protein 95) leads to increased size of both the postsynaptic PSD-95 clusters (El-Husseini et al., 2000; Prange et al., 2004) and the presynaptic glutamatergic terminals (El-Husseini et al., 2000; Graf et al., 2004; Prange et al., 2004, Levinson et al., 2005). Moreover, overexpression of PSD-95 also leads to a reduced number of GABAergic synapses on these pyramidal cells, thus increasing the E/I excitatory to inhibitory synaptic ratio (Prange et al., 2004; Levinson et al., 2005). On the other hand, knocking down PSD-95 expression with PSD-95 siRNA leads to a decreased number of excitatory glutamatergic synaptic contacts and an increased number of inhibitory GABAergic synaptic contacts, thus decreasing the E/I synaptic ratio (Prange et al., 2004). These results show that the glutamatergic postsynaptic scaffold protein PSD-95 plays an important role not only in the formation and/or maintenance of glutamatergic synapses, but also in GABAergic synapses.
In this communication we are reporting that affecting the expression and clustering of gephyrin, the main scaffold postsynaptic protein of GABAergic synapses, has heterotypic effects on glutamatergic synaptic contacts, in addition to the homotypic effects on GABAergic synapses described above. We are proposing that gephyrin is a counterpart of the postsynaptic glutamatergic scaffold protein PSD-95 in regulating the number and/or size of the excitatory and inhibitory synaptic contacts.
MATERIALS AND METHODS
All the animal protocols have been approved by the Institutional Animal Care and Use Committees (IACUC) of the University of Connecticut and followed the National Institutes of Health guidelines.
Antibodies
The rabbit anti-rat γ2 GABAAR subunit antibodies to amino acids 1–15 (QKSDDDYEDYASNKT) was raised in our laboratory and it has been thoroughly characterized elsewhere (Christie et al., 2002a, b; Christie and De Blas 2003; Chandra et al., 2005; Li et al., 2005b; Christie et al., 2006). The mouse mAb to gephyrin (clone mAb 7a) was from Cedarlane (Accurate Chemical & Scientific Corp., Westbury, NY). The rabbit anti-NR2, which recognizes NR2a-d, and the mouse anti-PSD-95 mAb were from Upstate Biotechnology (Lake Placid, NY). The guinea pig anti-vGlut1 (vesicular glutamate transporter 1) and the rabbit anti-GluR1 antibody were from Chemicon (Temecula, CA). The sheep anti-GAD was a gift from Dr. Irwin J. Kopin (NINDS, Bethesda, MD). All fluorophore-conjugated secondary antibodies (immunoadsorbed to eliminate cross-reactivity) were from Jackson ImmunoResearch (West Grove, PA).
Construction of various plasmid vectors
Two gephyrin small hairpin RNAs (shRNAs) were used, one targeting nucleotides 609–633 of the gephyrin coding region (Geph CR) (GeneBank accession number NM_022865.3; gi:145553981) and another one targeting nucleotides 2401–2425 of the gephyrin 3′ UTR (Geph UTR). The antisense strand of the shRNAs perfectly matched the target RNA sequences. Control shRNAs (Geph CR3m and Geph UTR3m, respectively) were made carrying three point mutations in the sense and antisense strands. These shRNA were inserted in the mU6 vector and synthesized under the control of RNA U6 polymerase III promoter (Yu et al., 2002; Li et al., 2005b). The construction of these plasmids has been described elsewhere (Yu et al., 2007). The GABAAR γ2 shRNAs targeting the coding region (γ2 CR) and the 3′ UTR region (γ2 UTR) of the γ2 mRNA, and their corresponding control shRNAs, γ2 CR3m and γ2 UTR3m, each containing 3 point mutations, have been described elsewhere (Li et al., 2005b).
The human gephyrin cDNA clone FJ06168 (GenBank name KIAA1385) was kindly provided by Dr. Nobumi Kusuhara, Kazusa DNA Research Institute, Japan. This gephyrinisoform, without the c5 cassette, is involved in clustering GABAARs (Meier and Grantyn, 2004). The subcloning of this gephyrin cDNA in pcDNA3.1(+) and pEGFP-N1 has been described elsewhere (Yu et al., 2007). The quality of the constructs was confirmed by DNA sequencing. The expression of gephyrin and gephyrin-EGFP was detected by both immunofluorescence and immunobloting after transfection of HEK293 cells and hippocampal neurons.
Transfection of cultured hippocampal neurons
Primary hippocampal cultures were prepared from embryonic day 18 (E18) Sprague-Dawley rat brains by the method of Goslin et al., (1998) as described previously (Christie et al., 2002b). The cultured neurons were maintained in glial cell conditioned medium containing 1% N2 supplement (Invitrogen, Carlsbad, CA). Cultured hippocampal neurons (10 day old in culture) were co-transfected with 3 μg of a shRNA plasmid and 1 μg of the pEGFP-N1 (in a molar ratio of 4:1), or with 3 μg of gephyrin plasmid and 1 μg of the pEGFP-N1 (in a molar ratio of 2:1), or with 4 μg of gephyrin-EGFP plasmid, using the CalPhos Mammalian Transfection Kit (BD Biosciences, San Jose, CA), according to the instructions provided by the manufacturer. Fluorescence immunocytochemistry was performed 5 days after transfection with shRNAs, gephyrin-EGFP or gephyrin.
Immunofluorescence of hippocampal cultures
The immunofluorescence procedure has been described elsewhere (Christie et al., 2002a, b; Charych et al., 2004a, b, 2006; Li et al., 2005a, b). Except where mentioned, all steps were done at room temperature. Briefly, neurons were fixed in 4% paraformaldehyde/4% sucrose/phosphate-buffered saline (PBS) pH 7.4 for 15 minutes, followed by washing with PBS and incubation with 50 mM NH4Cl in PBS for 10 minutes and by permeabilization with 0.25% Triton X-100 in PBS for 10 minutes at 4°C. Cells were incubated with 5% normal donkey serum in PBS for 30 min followed by incubation with a mixture of primary antibodies (in 0.25% Triton X-100 in PBS) overnight at 4°C. After washing, cells were incubated with a mixture of secondary antibodies (anti-species specific IgG all made in donkey, Jackson Immunochemicals, West Grove, PA) conjugated to fluorescein isothiocyanate (FITC), Texas Red, or aminomethylcoumarin (AMCA) fluorophores (1:200 dilution in 0.25% Triton X-100 in PBS) for one hour at 37°C. The coverslips were washed with PBS followed by washing with PBS pH8.5 and mounting using Prolong Gold anti-fade mounting solution (Molecular Probes, Eugene, OG).
Image acquisition and analysis
Digital images from hippocampal cultures were collected using a 60 × pan-fluor objective on a Nikon Eclipse T300 microscope with a Sensys KAF 1401E CCD camera driven by IPLab 3.0 acquisition software (Scanalytics, Fairfax, VA). Images obtained from different fluorescence channels were processed and merged with PhotoShop 7.0 (Adobe) for analysis. Brightness and contrast were adjusted, the image was changed from 16 bits/channel to 8 bits/channel(1315×1035 pixel resolution), sharpened using the unsharp mask tool (setting: amount=125%, radius=1.5 pixel, threshold = 0 level), color was added to each channel and the images were merged for color co-localization. Fluorescence images in figures were presented before subtraction of the diffuse background signal in dendrites.
Quantification of clusters
For quantifying cluster density in neurons, the background fluorescence of each channel seen in the dendrites was subtracted and the maximum intensities of the fluorophore channels were normalized. Three independent immunofluorescence experiments were performed for each combination of antibodies. A total of 30 dendritic fields were analyzed from 20–30 randomly selected pyramidal neurons. Each measurement was taken from a 25-μm long, 4-μm wide dendritic segment. Density values were calculated (mean±SEM) as number of clusters per 100μm2 of dendritic surface. The number of clusters analyzed were 428–525 for PSD-95, 384–441 for vGlut1, 481–525 for GluR1, and 551–639 for NR2.
Measurement of the cluster size was performed using IPLab 3.5 software. Unprocessed 12-bit/channel TIFF image files, acquired with IPLab 3.0 software, were exported to IPLab 3.5 and images were segmented based on fluorescence intensity levels, to create a binary mask that maximized the number of clusters for analysis, while minimizing the coalescence of individual clusters. Calculated values for size (mean±SEM) were obtained from the analysis of the following number of clusters: 301–766 for PSD95, 396–626 for vGlut1, 428–582 for GluR1 and 444–506 for NR2, which were obtained from six neurons in three different experiments.
RESULTS
Knocking down gephyrin in pyramidal cells with gephyrin shRNAs leads to increased size, but not density, of both postsynaptic PSD-95 clusters and presynaptic glutamatergic boutons
We have recently shown (Yu et al., 2007) that knocking down gephyrin expression and clustering in hippocampal pyramidal cells with Geph CR shRNA (targeting the coding region) or Geph UTR shRNA (targeting the 3′ UTR) led to significant decreased density of gephyrin clusters (down to 22.3±2.2% and 36.3±3.0% respectively), γ2-GABAAR clusters (down to 27.4±1.7% and 40.1±2.3%), and GABAergic innervations (down to 49.2±4.2% and 51.9±4.0%) when compared with sister non-transfected cells (100%) or when compared with cells transfected with the control shRNAs Geph CR3m or Geph UTR3m respectively, whose values were not significantly different from that of non-transfected cells. These control shRNAs carry three point mutations. Fig 1A shows an illustrative example of a pyramidal cell transfected with Geph CR in which both the gephyrin cluster density (Fig 1A, red, arrowheads) and GABAergic innervation are reduced as shown by decreased number of GAD+ presynaptic boutons (Fig 1A, blue, arrowhead), when compared to pyramidal cells transfected with the control Geph CR3m shRNA (Fig 1B). The reduced number of GAD+ boutons showed a high degree of apposition to the remaining gephyrin clusters (Fig 1A, arrowhead), as in the normally innervated non-transfected cells or cells transfected with the control Geph CR3m (Fig 1B, arrowheads). The gephyrin clustering and GABAergic innervation of the pyramidal cells transfected with Geph UTR was rescued by an exogenous gephyrin mRNA from which the 3′ UTR was removed (Yu et al. 2007). These and other experiments showed that gephyrin plays a critical role in the stability of GABAergic synapses (Yu et al. 2007)
Fig 1. Gephyrin knock down by shRNAs reduces the density of gephyrin clusters and GABAergic innervation.
A–B, Cultured hippocampal neurons were co-transfected with pEGFP-N1 and Geph CR (A) or Geph CR3m (B) shRNAs. Triple-label immunofluorescence was done using the mouse mAb to gephyrin (red color) and the sheep anti-GAD (blue color) antibodies. EGFP fluorescence of transfected neurons is shown in green color. The smaller panels at the right side of each figure show at higher magnification the corresponding boxed area. Arrowheads in A and B show presynaptic GAD+ boutons apposed to postsynaptic gephyrin clusters. Note that the pyramidal cell transfected with Geph CR shRNAs (A) shows lower density of gephyrin clusters and GAD+ boutons than the cell transfected with Geph CR3m shRNA (B). Scale bar: 10 μm for large panels; 5 μm for the small panels.
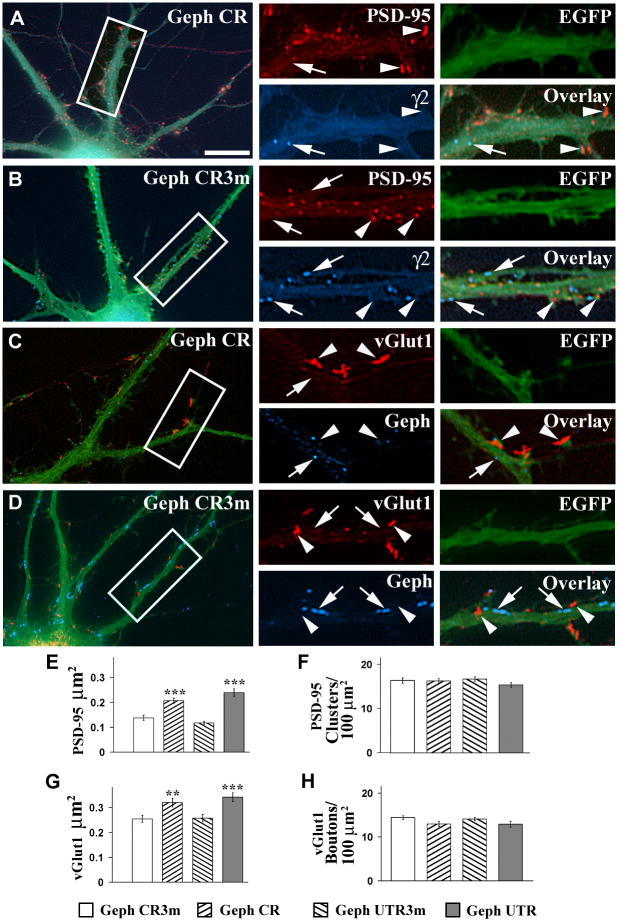
We are now reporting that knocking down gephyrin has not only homotypic effects on GABAergic synapses, but also heterotypic effects on glutamatergic synapses. Pyramidal cell transfected with Geph CR shRNA showed not only decreased number of γ2-GABA AR clusters (arrow, Fig 2A, blue) and gephyrin clusters (arrow, Fig 2C, blue) when compared with pyramidal cells transfected with Geph CR3m shRNA (arrows, Fig 2B and D, blue respectively), but the same cells also showed increased size of both postsynaptic PSD-95 clusters (arrowheads, Fig 2A, red) and presynaptic vGlut1-containing (vGlut1+) glutamatergic terminals (arrowheads, Fig 2C, red) when compared to pyramidal cells transfected with Geph CR3m shRNA (Fig 2B and D respectively). The PSD-95 clusters or vGlut1+ boutons did not colocalize with γ2-GABAAR clusters or gephyrin clusters.
Fig 2. The knockdown of gephyrin leads to increased size of the PSD-95 clusters and the vGlut1-containing boutons .
A–DCultured hippocampal neurons were co-transfected with EGFP and Geph CR (A, C) or Geph CR3m (B, D) shRNAs. Triple-label immunofluorescence was done using the mAb to PSD-95 (A, B red color) and the rabbit anti-γ2 GABAAR subunit (A and B, blue color), or the guinea pig anti-vGlut1 (C and D, red color) and the mouse mAb to gephyrin (C and D, blue color) antibodies. Transfected neurons show EGFP green fluorescence. The smaller panels at the right side of each figure show the corresponding boxed area at higher magnification. Arrowheads in A and C (red color) show large PSD-95 clusters (A) and vGlut1+ boutons (C) in neurons transfected with Geph CR shRNA when compared with the smaller PSD-95 clusters (B, arrowheads) or vGlut1+ boutons (D, arrowheads) respectively, which are present in neurons transfected with Geph CR3m shRNA. The same neurons that showed increased size of PSD-95 clusters or vGlut1+ terminals showed decreased density of γ2 -GABAAR clusters (A, arrow, blue color), and gephyrin clusters (C, arrow, blue color) when compared with B and D (arrows, blue color) respectively. E–F, Quantification of PSD-95 cluster size (E) and cluster density (F). G–H, Quantification of vGlut1+ bouton size (G) and bouton density (H). Quantification values (mean±SEM) are given in the text. There is a significant increase in the size of PSD-95 and vGlut1+ boutons in the cells transfected with Geph CR or Geph UTR shRNA over cells transfected with Geph CR3m or Geph UTR3m shRNA respectively. Significant differences with the corresponding mutated shRNA-treated cells are indicated by asterisks (**, p<0.01, ***, p<0.001 in Student’s t-test). Comparisons between groups using one-way ANOVA Tukey test at p<0.05 showed that the neurons transfected with gephyrin shRNAs (Geph CR, Geph UTR, Geph CR3m, Geph UTR3m) or non-transfected neurons had no significant difference in the density of PSD-95 clusters or the density of vGlut1+ boutons. Scale bar: 10 μm for the large panels; 5 μm for the small panels.
Quantification showed that knocking down gephyrin in pyramidal cells with gephyrin shRNAs significantly (p<0.001) increased the size of PSD-95 clusters (0.21±0.01 μm2 for Geph CR and 0.24±0.02 μm2 for Geph UTR, Fig 2E) when compared with that of pyramidal cells transfected with the corresponding mutated gephyrin shRNAs (0.14±0.01 μm2 for Geph CR3m and 0.12±0.01 μm2 for Geph UTR3m, Fig 2E) or with that of sister non-transfected cells (0.12±0.01 μm2). All values are given in mean±SEM. However, there was no significant effect on the density of PSD-95 clusters in these cells (16.4±0.6 for Geph CR, p=0.90 and 16.7±0.5 clusters/100 μm2 for Geph UTR, p=0.09) when compared to pyramidal cells transfected with Geph CR3m or Geph UTR 3m (16.3±0.5 and 15.4±0.5 clusters/100 μm2 respectively) or sister non-transfected cells (17.5±0.7 clusters/100 μm2) as shown in Fig 2F. Comparison between groups using one-way ANOVA Tukey test showed that there was no significant difference in PSD-95 cluster size between pyramidal cells transfected with the mutated Geph-CR3m or Geph UTR3m shRNAs and sister non-transfected pyramidal cells.
Knocking down gephyrin in pyramidal cells with gephyrin shRNAs also led to enlarged presynaptic vGlut1+ boutons (0.32±0.02 μm2 for Geph CR, p=0.004, and 0.34±0.02 μm2 for Geph UTR, p<0.001) when compared with that of cells transfected with mutated shRNAs (0.25±0.01 μm2 for Geph CR3m and 0.26±0.02 μm2 for Geph UTR3m, Fig 2G) or with that of sister non-transfected pyramidal cells (0.25±0.02 μm2). However, there was no significant effect on the density of vGlut1+presynaptic terminals (14.4±0.4 for Geph CR, p=0.063 and 14.1±0.4 boutons/100 μm2 for Geph UTR shRNAs, p=0.139, respectively, Fig 2H) when compared to that of neurons transfected with Geph CR3m or Geph UTR3m shRNAs (13.0±0.6 and 12.9±0.7 boutons/100 μm2 respectively) or when compared with that of sister non-transfected cells (13.4±0.5 boutons/100 μm2) as shown in Fig 2H.
These results show that knocking down gephyrin not only decreases the number of gephyrin clusters, GABAAR clusters and GABAergic synapses, but also increases the size of the glutamatergic synaptic contacts as shown by increased size of both the postsynaptic PSD-95 clusters and the presynaptic glutamatergic terminals. However, knocking down gephyrin has no effect on the density of the glutamatergic synaptic contacts.
Knocking down the γ2 GABAAR subunit with γ2 shRNAs in pyramidal cells leads to increased size, but not density, of both the postsynaptic PSD-95 clusters and the presynaptic glutamatergic boutons
In previous work we have shown that knocking down the γ2-GABAAR subunit in pyramidal cells with γ2 shRNAs reduced the density of the postsynaptic γ2 subunit-containing GABAARs (γ2-GABAARs) and gephyrin clusters and of the presynaptic GABAergic innervation that these cells received (Li et al., 2005b). These effects were similar to the ones obtained after knocking down gephyrin (Yu et al., 2007). We have now tested whether knocking down the γ2 GABAAR subunit also leads to increased size of the PSD-95 clusters and vGlut1+ glutamatergic boutons, as when gephyrin was knocked down.
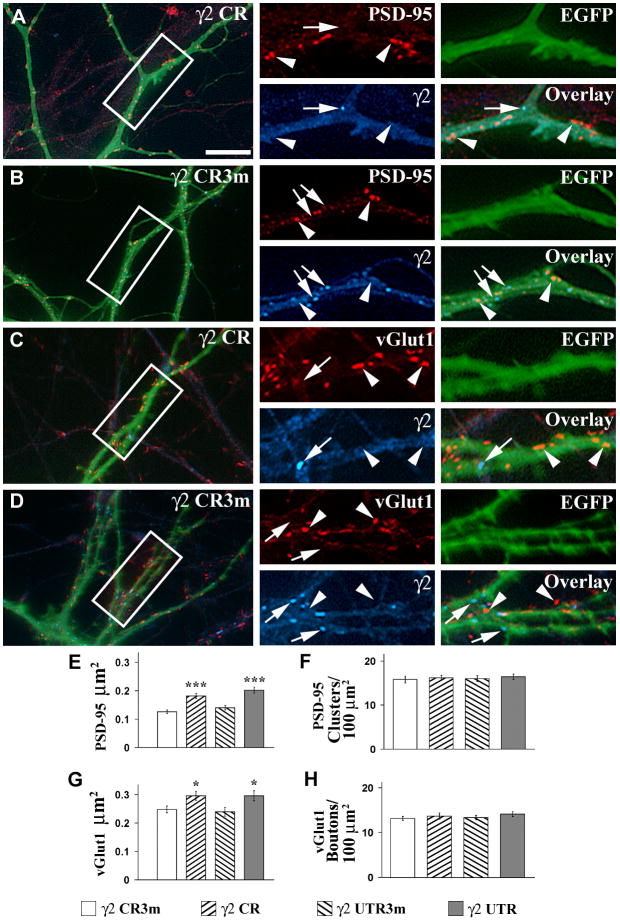
Transfection of pyramidal cells with γ2 CR shRNA highly decreased the density of γ2- GABAAR clusters (arrow, Fig 3A and C blue) and the density of gephyrin clusters to 15.5 ± 1.8% and 21.8±1.9% respectively as we have reported in Li et al., 2005b, when compared to that of non-transfected cells or cells transfected with γ2 CR3m shRNA (Fig 3B and D, blue). Similar reductions in the density of γ2-GABAAR clusters and gephyrin clusters were obtained (to 11.4±1.7% and 15.9±1.8% respectively, Li et al., 2005b) after transfecting pyramidal cell with γ2 UTR when compared with that of non-transfected cells or cells transfected with γ2 UTR3m. Fig 3A–D now shows that the pyramidal cells transfected with γ2 CR shRNA, which had reduced density of γ2-GABAAR clusters (arrows, Fig 3A and C, blue), also showed increased size of the PSD-95 clusters (arrowheads, Fig 3A, red) and vGlut1+ boutons (arrowheads, Fig 3C, red) when compared with that of cells transfected with Geph CR3m shRNA (arrowheads, Fig 3B and D, red, respectively).
Fig 3. The knock down of the γ2 GABAAR subunit leads to increased size of both PSD-95 clusters and vGlut1+ boutons.
A–DCultured hippocampal neurons were co-transfected with EGFP and γ2 CR (A, C) or γ2 CR3m (B, D) shRNAs. Triple-label immunofluorescence was done by using a combination of the rabbit anti-γ2 GABAAR subunit (A–D, blue color) and the mouse mAb to PSD-95 (A and B, red color) or the guinea pig anti-vGlut1 (C and D, red color) antibodies. Neurons transfected with γ2 CR shRNA show enlarged PSD-95 clusters (A, arrowheads) and vGlut1+ boutons (C, arrowheads) when compared with neurons transfected withγ2 CR3m shRNA (B and D, respectively, arrowheads), as shown in the red color panels. Arrows in A and C show γ2 GABAAR clusters (blue color), which are reduced in number in the neurons transfected with γ2 CR shRNA when compared with that of neurons transfected with γ2 CR3m shRNA (B and D, respectively). E–F, Quantification of PSD-95 cluster size (E) and cluster density (F). G–H, Quantification of vGlut1+ bouton size (G) and bouton density (H). Quantification values (mean±SEM) are given in the text. There is a significant increase in the size of PSD-95 clusters and vGlut1+ boutons in the pyramidal cells transfected with γ2 CR or γ2 UTR shRNA over cells transfected with γ2 CR3m or γ2 UTR3m shRNA respectively. Significant differences with the corresponding mutated control shRNA-treated cells are indicated by asterisks (*, p<0.05; ***, p<0.001 in Student’s t-test). Comparisons between groups using one-way ANOVA Tukey test at p<0.05 showed that the neurons transfected with γ2 shRNAs (γ2 CR, γ2 UTR, γ2 CR3m, γ2 UTR3m) or non-transfected neurons had no difference in the density of PSD-95 clusters or in the density of vGlut1+ boutons. Scale bar: 10 μm for large panels; 5 μm for small panels.
Quantification showed that the size of the PSD-95 clusters was significantly increased (p<0.001) in pyramidal cells transfected with γ2 shRNAs (0.18±0.01 μm2 for γ2 CR and 0.20±0.01 μm2 for γ2 UTR) when compared with that of cells transfected with mutated γ2 shRNAs (0.13±0.01 μm2 for γ2 CR3m and 0.14±0.01 μm2 for γ2 UTR3m) or sister non-transfected cells (0.13±0.01 μm2) as shown in Fig 3E. The size of vGlut1+ boutons was also increased in pyramidal cells transfected with γ2 shRNAs (0.30±0.02 μm2 for γ2 CR, p=0.020, and 0.30±0.02 μm2 for γ2 UTR, p=0.022) when compared with that of cells transfected with mutated γ2 shRNAs (0.25±0.01 μm2 for γ2 CR3m or 0.24±0.02 μm2 for γ2 UTR3m)or sister non-transfected cells (0.25±0.02 μm2) as shown in Fig 3G. However, cells transfected with γ2 shRNAs showed no significant change in the density of PSD-95 clusters compared with the controls (15.8±0.8 for γ2 CR, p=0.674 and 16.0±0.7 for γ2 UTR, p=0.630 vs. 16.2±0.5 for γ2 CR3m, and 16.4±0.6 for γ2 UTR3m and 17.0±0.9 clusters/100 μm2 for non-transfected cells) or the density of vGlut1+ boutons (13.2±0.4 for γ2 CR, p=0.518 and 13.4±0.5 for γ2 UTR, p=0.137 vs. 13.7±0.7 for γ2 CR3m and 14.5±0.6 for γ2 UTR3m shRNAs, and 14.7±0.8 boutons/100 μm2 for non-transfected cells) as shown in Fig 3F and H respectively).
Therefore, our results show that knocking down either gephyrin or the γ2 GABAAR subunit, two conditions leading to decreased clustering of both gephyrin and γ2-GABAARs and to decreased GABAergic innervation, also leads to increased size, with no change in density, of the glutamatergic synaptic contacts.
Overexpression of a gephyrin-EGFP dominant negative construct, a condition that leads to reduced number of gephyrin and GABAAR clusters and to reduced GABAergic innervation, leads to increased size of both PSD-95 clusters and vGlut1+ boutons
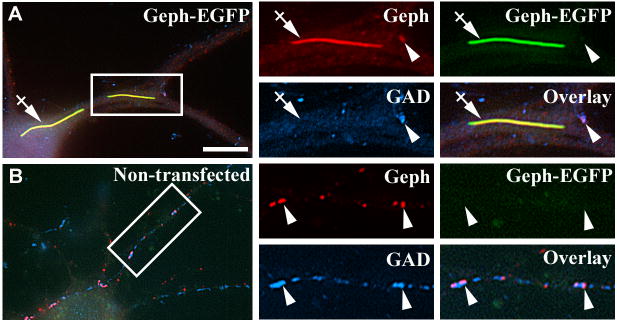
We have previously shown (Yu et al., 2007) that overexpression in pyramidal cells of a gephyrin-EGFP fusion protein that acts as a dominant negative of gephyrin clustering, leads to the formation of filamentous aggregates of gephyrin-EGFP in the soma and some proximal dendrites (Fig 4A, crossed arrow) and to a significant decrease of gephyrin clusters (to 14.6±1.9%), γ2-GABAAR clusters (to 22.4±2.5%), and GABAergic innervation (to 44.6±2.6%) of these cells. Presumably, the gephyrin-EGFP aggregates “trapped” the endogenous gephyrin thus preventing the formation of the endogenous gephyrin clusters. An illustrative example is shown in Fig 4. The pyramidal neurons overexpressing gephyrin-EGFP showed decreased number of endogenous gephyrin clusters (arrowhead, Fig 4A, red) and decreased GABAergic innervation, as shown by decreased numbers of GAD+ boutons contacting these cells (arrowhead, Fig 4A, blue), when compared with that of non-transfected cells (arrowheads, Fig 4B, red and blue respectively). The anti-gephyrin mAb recognizes both the endogenous gephyrin and gephyrin-EGFP as revealed by immunofluorescence of HEK293 cells transfected with gephyrin-EGFP (not shown). Thus, clusters of endogenous gephyrin are identified because they are recognized by the anti-gephyrin antibody but show no EGFP fluorescence (anti-Geph+/EGFP−, Fig 4A, arrowhead). On the other hand, the gephyrin-EGFP aggregates are recognized by the anti-gephyrin antibody and show EGFP fluorescence (anti-Geph+/EGFP+, Fig 4A, crossed arrow).
Fig 4. Overexpression of the dominant negative gephyrin-EGFP construct decreases the density of endogenous gephyrin clusters and GABAergic innervation.
Cultured hippocampal neurons were transfected with gephyrin-EGFP. A–B, Triple-label immunofluorescence, with the mouse mAb to gephyrin (red color), the sheep anti-GAD antibody (blue color) and EGFP fluorescence (green color). Note that the pyramidal cell overexpressing gephyrin-EGFP forms large filamentous aggregates of gephyrin-EGFP in the some proximal dendrites (A, crossed arrows). These abnormal aggregates are anti-Geph+/EGFP+. There is a significant reduction of both endogenous gephyrin clusters of normal aspect, which are anti-Geph+/EGFP− (A, red color, arrowhead) and GAD+ boutons contacting the pyramidal neurons (A, blue color, arrowhead), when compared with the sister non-transfected cells from the same culture (B), which shows no gephyrin-EGFP green fluorescence. Arrowheads also show some presynaptic GAD+ boutons apposed to postsynaptic gephyrin clusters. There is no apposition of gephyrin-EGFP aggregates with GAD+ boutons. Scale bar: 10 μm for the large panels; 5 μm for the small panels.
Fig 5A–D shows that the pyramidal cells overexpressing gephyrin-EGFP not only had decreased density of γ2-GABAARs clusters (arrows, Fig 5A, blue) and gephyrin clusters (arrow, Fig 5C, blue) when compared with sister non-transfected cells from the same culture (arrows, Fig 5B and D), but they also had PSD-95 clusters (arrowheads, Fig 5A, red) and vGlut1+ boutons (arrowheads, Fig 5C, red) that were significantly larger in size than that of the corresponding non-transfected sister cells (arrowheads, Fig 5B and D, respectively) as shown in the red panels. Quantification showed that the size of the PSD-95 clusters was significantly increased in pyramidal transfected with gephyrin-EGFP (0.25±0.02 μm2, p<0.001) when compared with that of sister non-transfected cells (0.14±0.01 μm2) as shown in Fig 5E. However, there was no significant change in PSD-95 cluster density compared with that of sister non-transfected neurons from the same culture (14.3±0.6 vs 15.6±0.8 clusters/100 μm2, p=0.203, Fig 5F). Gephyrin-EGFP overexpression in pyramidal cells also led to increased size of vGlut1+ boutons when compared with that of non-transfected sister neurons (0.32±0.02 vs 0.27±0.02 μm2, p=0.039, Fig 5G). However it had no significant effect on the density of vGlut1+ boutons (13.4±0.6 vs. 13.9±0.5 boutons/100 μm2, p=0.532, Fig 5H).
Fig 5. Overexpression of the gephyrin-EGFP construct leads to increased size of PSD-95 clusters and vGlut1+ presynaptic boutons.
A–DCultured hippocampal neurons were transfected with gephyrin-EGFP (A, C). Triple-label immunofluorescence was done using the mouse mAb to PSD-95 (A and B, red color) and the rabbit anti-γ2 GABAAR subunit (A and B, blue color), or the guinea pig anti-vGlut1 (C and D, red color) and the mouse mAb to gephyrin (C and D, blue color). Neurons transfected with gephyrin-EGFP show enlarged PSD-95 clusters (A, arrowheads, red color) and vGlut1+ boutons (C, arrowheads, red color) compared with non-transfected neurons (B and D, respectively, arrowheads, red color). Arrows (blue color) show the γ2 GABAAR (A) and the gephyrin clusters (C), which are reduced in number in the gephyrin- EGFP transfected neurons when compared with non-transfected neurons (B and D, respectively). E–F, Quantification of PSD-95 cluster size (E) and cluster density (F). G–H, Quantification of vGlut1+ bouton size (G) and bouton density (H). Quantification values (mean±SEM) are given in the text. There is a significant increase in the size of PSD-95 clusters and vGlut1+ boutons in the pyramidal cells transfected with Geph-EGFP over sister non-transfected cells. Significant differences with the corresponding non-transfected sister cells are indicated by asterisks (*, p<0.05; ***, p<0.001 in Student’s t-test). Gephyrin-EGFP expression had no significant effect on the density of PSD-95 clusters or on the density of vGlut1+ boutons. Scale bar: 10 μm for the large panels; 5 μm for the small panels.
These results, obtained with an alternative experimental approach, further support the notion derived from the shRNA experiments that reducing gephyrin expression and clustering leads to increased size of the glutamatergic synaptic contacts but it has no effect on the density of these contacts.
Disrupting gephyrin expression and clustering, by either knocking down gephyrin or by overexpressing a dominant negative gephyrin-EGFP, increases the cluster size but not the density of NR2-NMDA receptors
We have shown above that disrupting gephyrin expression and clustering, by either knocking down gephyrin or by overexpressing the dominant negative gephyrin-EGFP construct, leads to increased size of PSD-95 clusters and vGlut1+ boutons. We also tested whether this is accompanied by increased size of the NR2-NMDA receptor clusters, since PSD-95 interacts with the NR2 subunits of the NMDA receptors (Kornau et al. 1995, O’Brien et al. 1998).
Fig 6A and B shows an illustrative example of a pyramidal cell transfected with Geph CR. This cell shows reduced density of gephyrin clusters (arrow, Fig 6A, blue) when compared to a cell transfected with Geph CR3m (arrows, Fig 6B, blue). The same Geph CR-transfected cell showed increased size of the NR2-NMDA receptor clusters when compared to the cell transfected with Geph CR3m (arrowheads, Fig 6A vs B, red). Quantification showed that the size of NR2 clusters was significantly increased in pyramidal cells transfected with Geph CR (0.14±0.01 μm2, p=0.010) or Geph UTR (0.15±0.01 μm2, p=0.024) when compared with that of neurons transfected with mutated Geph shRNAs (0.11±0.01 μm2 for Geph CR3m and 0.11±0.01 μm2 for Geph UTR3m) or when compared with non-transfected cells (0.10±0.01 μm2), as shown in Fig 6C. However, there was no significant change in the density of NR2-NMDA receptor clusters induced by the shRNAs when compared with that of the cells transfected with the mutated shRNAs (18.7±0.7 for Geph CR, p=0.142 and 20.0±0.8 for Geph UTR, p=0.097 vs 19.3±0.6 for Geph CR3m and 20.1±0.7 clusters/100 μm2 for Geph UTR3m shRNAs respectively) or when compared with sister non-transfected cells (20.1±0.7 clusters/100 μm2) as shown in Fig 6D.
Fig 6. Disrupting gephyrin expression and clustering, by either knocking down gephyrin or by overexpressing the dominant negative gephyrin-EGFP, increases the cluster size but not the density of NR2-NMDA receptors.
Cultured hippocampal neurons were co-transfected with EGFP and Geph CR (A) or Geph CR3m (B) shRNAs or were transfected with gephyrin-EGFP (E). Triple-label immunofluorescence in A, B, E and F, was done using the rabbit anti-NR2 (red color) and the mouse mAb to gephyrin (blue color). Transfected neurons show EGFP green fluorescence. The smaller panels at the right side of each figure show the corresponding boxed area at higher magnification. A–B, Arrowheads in A (red color) show large NR2 clusters in the neurons transfected with Geph CR shRNA when compared with the smaller NR2 clusters (B, arrowheads) that are present in the neurons transfected with Geph CR3m shRNA. The same neuron that showed increased size of NR2-NMDA clusters showed decreased density of gephyrin clusters (A, arrow, blue color) when compared with B (arrows, blue color). C–D, Quantification of NR2 cluster size (C) and cluster density (D). Quantification values (mean±SEM) are given in the text. There is a significant increase in the size of the NR2 clusters in the cells transfected with Geph CR or Geph UTR shRNA over cells transfected with Geph CR3m or Geph UTR3m shRNA respectively. However, there is no change in the density of NR2 clusters. Significant differences with the corresponding mutated shRNA-treated cells are indicated by asterisks (*, p<0.05; **, p ≤ 0.01; in Student’s t-test). Comparisons between groups using one-way ANOVA Tukey test at p<0.05 showed that the neurons transfected with gephyrin shRNAs (Geph CR, Geph UTR, Geph CR3m, Geph UTR3m) or non-transfected neurons had no difference in the density of NR2 clusters. E–F, Neurons transfected with gephyrin-EGFP show enlarged NR2 clusters (E, arrowheads) compared with non-transfected neurons (F, arrowheads), as shown in red color. Arrows (blue color) show the endogenous gephyrin clusters (anti-Geph+/EGFP−), which are reduced in number in the neurons transfected with gephyrin-EGFP, when compared with the non-transfected neurons. The crossed arrow shows a perinuclear filament aggregate of gephyrin-EGFP that is anti-Geph+/EGFP+. G–H, Quantification of NR2 cluster size (G) and cluster density (H). There is a significant increase in the size of NR2 clusters (G) in the pyramidal cells transfected with Geph-EGFP over those of sister non-transfected cells. Significant differences with the corresponding non-transfected sister cells are indicated by asterisks (*, p<0.05; in Student’s t-test). Gephyrin-EGFP expression has no significant effect on the density of NR2 clusters compared with those of the non-transfected sister cells (H). Scale bar: 10 μm for the large panels; 5 μm for the small panels.
Fig 6E and F illustrates that overexpression in pyramidal cells of the dominant negative gephyrin-EGFP, which leads to the formation of filamentous aggregates (located in this neuron around the nucleus, crossed arrow, Fig 6E), also leads to reduced density of gephyrin clusters (arrow, Fig 6E, blue), when compared to that of non-transfected cells (arrows, Fig 6F, blue). The same pyramidal cell overexpressing Geph-EGFP had larger NR2-NMDA receptor clusters than the non-transfected cells (arrowheads, Fig 6E and F respectively, red). Quantification (Fig 6G–H) showed that in pyramidal cells transfected with gephyrin-EGFP, the NR2-NMDA receptor cluster size (0.15±0.01 μm2, p=0.014) was increased when compared with non-transfected cells (0.12±0.01 μm2) as shown in Fig 6G. However, the cluster density was not changed (21.3±0.8 vs. 19.5±0.9 clusters/100 μm2, p=0.147), as shown in figure 6H.
Therefore, the results show that disrupting the expression and clustering of gephyrin leads to increased size, but not to changes in density, of the NR2-NMDA receptor clusters.
Disrupting gephyrin expression and clustering, by either knocking down gephyrin or by overexpressing a dominant negative gephyrin-EGFP, does not significantly affect the cluster size or density of the GluR1-AMPA receptors
Fig 7A illustrates a pyramidal cell transfected with Geph CR, which shows reduced density of gephyrin clusters (arrow, Fig 7A, blue) when compared to a cell transfected with Geph CR3m (arrows, Fig 7B, blue). The same Geph CR transfected cell shows no apparent change in the size or density of the GluR1-AMPA receptor clusters (arrowheads, Fig 7A vs B, red). Quantification showed that the size of GluR1-AMPA receptor clusters, although slightly larger, was not significantly different from those of the neurons transfected with mutated Geph shRNAs, (0.11±0.01 μm2 for Geph CR, p=0.138 and 0.11±0.01 μm2 for Geph UTR, p=0.191 vs. 0.09±0.01μm2 for Geph CR3m and 0.10±0.01 μm2 for Geph UTR3m, respectively) or non-transfected cells (0.10±0.01 μm2), as shown in Fig 7C.
Fig 7. Disrupting gephyrin expression and clustering, by either knocking down gephyrin or by overexpressing the dominant negative gephyrin-EGFP, doesn’t significantly affect the cluster size or density of the GluR1-AMPA receptors.
Cultured hippocampal neurons were cotransfected with EGFP and Geph CR (A) or Geph CR3m (B) shRNAs or were transfected with gephyrin-EGFP (E). Triple-label immunofluorescence in A, B, E and F was done using the rabbit anti-GluR1 (red color) and the mouse mAb to gephyrin (blue color). Transfected neurons show EGFP green fluorescence. The smaller panels at the right side of each figure show the corresponding boxed area at higher magnification. A–B, Arrowheads show GluR1 clusters (A and B, red color) while arrows show gephyrin clusters (blue color). There is a large reduction in the gephyrin cluster density in the neuron transfected with Geph CR shRNA (A, arrows, blue), compared with the neuron transfected with Geph CR3m shRNA (B, arrows, blue), but the same cell shows no apparent change in the GluR1 receptor clusters (A and B, arrowheads, red). C–D, Quantification of GluR1 cluster size (C) and cluster density (D). Quantification values (means±SEM) are given in the text. There are no significant differences in the size or density of the GluR1-AMPA receptors clusters between the cells transfected with Geph CR or Geph UTR and the cells transfected with the mutated Geph CR3m or Geph UTR3m respectively (p>0.05 in Student’s test). Comparisons between groups using one-way ANOVA Tukey test at p<0.05 showed that the neurons transfected with gephyrin shRNAs (Geph CR, Geph UTR, Geph CR3m, Geph UTR3m) or the non-transfected neurons had no difference in the cluster size or density of GluR1-AMPA receptors. E–F, Arrowheads show GluR1 clusters (red color) while arrows show gephyrin clusters (blue color). Note the large reduction in the number of endogenous gephyrin clusters (anti-Geph+/EGFP−) in the pyramidal cell transfected with gephyrin-EGFP (E) when compared with the non-transfected neurons (F). The crossed arrow shows a filament aggregate of gephyrin-EGFP (anti-Geph+/EGFP+). G–H, Quantification of GluR1-AMPA receptors cluster size (G), and cluster density (H). Quantification values (mean±SEM) are given in the text. There are no significant differences in the size and density of GluR1-AMPA receptor clusters between cells transfected with gephyrin-EGFP and sister non-transfected cells (p>0.05 in Student’s test). Scale bars: 10 μm for the large panels; 5 μm for the small panels.
There was no significant change in the density of GluR1-AMPA receptor clusters in the pyramidal cells transfected with the gephyrin shRNAs vs. the cells transfected with the mutated shRNAs (16.8±0.8 for Geph CR, p=0.546 and 17.5±0.6 for Geph UTR, p=271 vs 17.1±0.7 for Geph CR3m and 16.8±0.8 clusters/100 μm2 for Geph UTR3m shRNAs respectively) or vs. sister non-transfected cells (17.8±0.6 clusters/100 μm2), as shown in Fig 7D.
Pyramidal cells transfected with gephyrin-EGFP showed filamentous aggregates of gephyrin-EGFP (crossed arrows, Fig 7E, blue and green) and reduced density of gephyrin clusters (arrows, Fig 7E, blue), when compared to non-transfected cells (arrows, Fig 7F, blue). The same pyramidal cell showed no apparent change in the size or the density of GluR1-AMPA receptor clusters (arrowheads, Fig 7E vs. F, red). Quantification showed (Fig 7G–H) that in neurons transfected with gephyrin-EGFP, the GluR1-AMPA receptor cluster size (0.10±0.01 μm2, p=0.178) and density (14.8±0.5 clusters/100 μm2, p=0.601) were not significantly different from those of non-transfected cells (0.08±0.01 μm2 and 15.8±0.7 clusters/100 μm2 respectively).
Therefore, these results show that although disrupting the expression and clustering of gephyrin leads to a significant increase in the size of PSD-95 clusters, NR2-NMDA receptor clusters and glutamatergic boutons, it does not have a significant effect on the size of the GluR1-AMPA receptor clusters.
Overexpression of gephyrin, a condition that slightly increases the number of gephyrin clusters, leads to a slight decrease in size of both PSD-95 clusters and vGlut1+ boutons, but 16 it has no effect on their density
We have shown above that decreasing gephyrin expression and clustering leads to increased size of postsynaptic PSD-95 clusters and presynaptic vGlut1+ boutons. We have also investigated whether increasing gephyrin expression and clustering has an opposite effect leading to decreased size of PSD-95 clusters and vGlut1+ boutons. For this purpose we transfected pyramidal cells with non-tagged gephyrin aiming to avoid aggregation artifacts of the gephyrin-EGFP construct. We have recently shown that overexpression of non-tagged gephyrin in pyramidal cells led to a small but significant increase in the density of gephyrin clusters (to 113.8±3.8%, 5 days after transfection) and γ2-GABAAR clusters (to 112.4±4.1%) while there were no changes in the density of GABAergic innervation that these cells received, when compared with sister non-transfected cells (Yu et al., 2007). The small increase in density of γ2- GABAAR clusters (arrows, Fig 8A blue color) and gephyrin clusters (arrows, Fig 8C, blue color) in the transfected cells over that of non-transfected cells (Fig 8B,D) was not apparent by visual inspection, only after quantification (Yu et al. 2007).
Fig 8. Overexpression of non-tagged gephyrin slightly decreases the size of both PSD-95 clusters and vGlut1+boutons.
A–D, Cultured hippocampal neurons were transfected with non-tagged gephyrin (A and C). Triple-label immunofluorescence was done using the mouse mAb to PSD-95 (A and B, red color) and the rabbit anti-γ2 (A and B, blue color), or the guinea pig anti- vGlut1 (C and D, red color) and the mouse mAb to gephyrin (C and D, blue color). E–F, Quantification of PSD-95 cluster size (E) and cluster density (F). G–H Quantification of vGlut1+ bouton size (G), and bouton density (H). Quantification values (mean±SEM) are given in the text. There is a small but significant decrease in the size of the PSD-95 clusters and the vGlut1+ boutons in the pyramidal cells transfected with non-tagged gephyrin, when compared with those of the non-transfected cells. Significant differences with the corresponding non-transfected sister cells are indicated by asterisks (*, p<0.05; **, p<0.01, in Student’s t-test). Transfection of pyramidal cells with non-tagged gephyrin had no significant effect (p>0.05) on the density of the PSD-95 clusters or the vGlut1+ boutons when compared with those of non-transfected sister cells. Scale bar: 10 μm for the large panels; 5 μm for the small panels.
Quantification indicated that the PSD-95 clusters of the cells overexpressing gephyrin (arrowheads, Fig 8A, red), were slightly but significantly smaller in size than those of the non-transfected sister cells from the same culture (0.10±0.01 vs. 0.12±0.01 μm2, p= 0.030) as shown in Fig 8E. Quantification also revealed that gephyrin overexpression also led to a small but statistically significant decrease in the size of vGlut1+ terminals when compared with that of non-transfected neurons (0.18±0.01 vs. 0.22±0.01 μm2, p=0.005) as shown in Fig 8G. Some illustrative examples are given in Fig 8A,B (arrowheads, red) for PSD-95 clusters and Fig 8C,D (arrowheads, red) for vGlut1+ boutons. The small but statistically significant effects described above were not revealed by visual inspection (Fig 8A–D). Quantification showed that there was no significant effect of gephyrin overexpression on the density of PSD-95 clusters (16.1±0.6 vs. 16.8±0.6 clusters/100 μm2, p=0.143, Fig 8F) or the density of vGlut1+ boutons (13.0±0.4 vs. 13.5±0.4 boutons/100 μm2, p=0.116) when compared with non-transfected sister cells (Fig 8H).
These results show that although knocking down gephyrin leads to a large decrease in gephyrin cluster density and a robust increase in the size of PSD-95 clusters, NR2-NMDA receptor clusters and vGlut1+ terminals, overexpression of gephyrin only leads to a slight increase in gephyrin cluster density and slight decrease in size in PSD-95 clusters and vGlut1+ terminals. These results also indicate that the maximum number of gephyrin clusters in pyramidal cells is tightly regulated by other factors besides gephyrin expression (Yu et al. 2007). Nevertheless, Lardi-Studler et al. (2007) have recently reported that overexpression of an EGFP-gephyrin fusion protein in hippocampal neurons is accompanied by decreased density of PSD-95 clusters. We do not know yet whether this difference is due to their use of a gephyrin fusion protein with an EGFP tag at the N-terminus, whereas we used a non-tagged gephyrin.
DISCUSSION
It has been shown that gephyrin clustering is essential for the postsynaptic clustering of many γ2-GABAARs (Kneussel et al., 1999, 2001; Levi et al., 2004; Jacob et al. 2005; Yu et al., 2007). In turn, the γ2 subunit of the GABAARs is essential for the postsynaptic clustering of both the GABAARs and gephyrin (Essrich et al., 1998; Li et al., 2005b; Fang et al. 2006). We have also shown that the postsynaptic co-clustering of γ2-GABAARs and gephyrin is important for the stability and maintenance of the presynaptic GABAergic contacts (Li et al., 2005b; Yu et al., 2007).
In the present communication we have shown that the aforementioned homotypic effects on GABAergic synapses, derived from altering the expression and clustering of gephyrin and GABAARs, are also accompanied by heterotypic effects on glutamatergic synapses. Therefore, disruption of the postsynaptic clustering of gephyrin and GABAARs in pyramidal cells, a condition that leads to reduced GABAergic innervation, is accompanied by an increase in the size of the postsynaptic PSD-95 clusters and by an increase in the size of the presynaptic vGlut1+ glutamatergic terminals contacting these cells. However, there was no change in the density of PSD-95 clusters or vGlut1+ glutamatergic terminals. The increased size of the vGlut1+ glutamatergic terminals and postsynaptic PSD-95 clusters is consistent with the notion that the synaptic glutamatergic contacts became enlarged. We have also measured a 19±1% increase (p<0.001) in the fluorescence intensity of the PSD-95 clusters in pyramidal cells transfected with Geph CR and Geph UTR over the cells transfected with the corresponding control shRNA or non-transfected cells (not shown). The increased PSD-95 cluster size and florescence intensity is interpreted as corresponding to increased number and density respectively of PSD-95 molecules in the postsynaptic PSD-95 clusters. No clear changes in the fluorescence intensity of the other glutamatergic markers, including vGlut1+ glutamatergic terminals, were detected (not shown).
The increased size of the PSD-95 clusters was accompanied by a significant increase in the cluster size of NR2-NMDA receptors but not of GluR1-AMPA receptors. This is explained because the NR2-NMDA receptor subunits interact with PSD-95 (Kornau et al. 1995, O’Brien et al. 1998) and therefore, the postsynaptic accumulation of PSD-95 molecules would bring additional NMDA receptors to the synapse.
On the other hand, overexpression of gephyrin leads to a slight reduction in the size of both PSD-95 clusters and vGlut1+ terminals. Therefore, downregulating and upregulating gephyrin expression and clustering has opposite effects on the size of the glutamatergic synaptic contacts.
We have also found that although manipulating the expression and clustering of gephyrin affected the size of the glutamatergic synaptic contacts, it did not affect the number of these contacts. It is worth pointing out that our observations in cultured hippocampal neurons need to be verified in the intact brain.
Our results are in agreement with previous studies on the geph −/− mutant mouse, which show that there is no change in the cluster density of various postsynaptic glutamatergic proteins including PSD-95, NR1, GluR1, or GluR2/3 when compared with geph +/+ or geph +/− littermates (Feng et al., 1998; Kneussel et al., 1999). There are also in agreement with another study in which it was reported that knocking down gephyrin with a shRNA had no significant effect on the density of synaptic GluR1-AMPA receptor clusters (Jacob et al., 2005). None of these studies have addressed whether manipulating the levels of gephyrin expression and clustering affected the size of the PSD-95 clusters and the vGlut1+ presynaptic glutamatergic terminals.
By manipulating gephyrin expression and clustering we have revealed a relationship between decreased number of GABAergic synapses and increased size of glutamatergic synapses. A relationship between increased size of glutamatergic synapses and decreased number of GABAergic synapses has also been observed by others using a different experimental paradigm. In these studies, overexpression of PSD-95 led to increased size of both the postsynaptic PSD-95 clusters and presynaptic glutamatergic terminals and to reduced number of GABAergic synapses, thus increasing the E/I excitatory to inhibitory synaptic ratio (El-Husseini et al., 2000; Graf et al., 2004; Prange et al., 2004; Levinson et al., 2005). Therefore, similar outcomes are observed by overexpressing PSD-95 or by knocking down gephyrin expression and clustering. The aforementioned experiments, in which the enlargement of the postsynaptic PSD-95 clusters is accompanied by the enlargement of the presynaptic glutamatergic terminals, indicate that there is a transynaptic regulation of the size of the synaptic contacts. On the other hand, it has also been shown that knocking down PSD-95 expression leads to decreased number of glutamatergic contacts and increased number of GABAergic contacts, thus decreasing the E/I synaptic ratio (Prange et al., 2004; Ehrlich et al., 2007).
PSD-95 overexpression, which as indicated above, leads to increased size of glutamatergic synapses and to decreased number of GABAergic synapses, also leads to an accumulation of various neuroligins (NLs) to glutamatergic synapses, including NL-2, which otherwise preferentially concentrates at GABAergic synapses (Prange et al., 2004; Graf et al., 2004; Levinson et al., 2005). On the other hand, the knock down of PSD-95, which leads to a decreased number of glutamatergic synapses and an increased number of GABAergic synapses, leads to a shift of the NL-1, which is normally present at glutamatergic synapses, to the GABAergic contacts (Gerrow et al. 2006). These results support the notion that postsynaptic NLs mediate the effects on the E/I synaptic ratio resulting from manipulating the expression levels of PSD-95 including the transynaptic interaction of NLs with the presynaptic terminals.
The NLs are localized postsynaptically and they interact transynaptically with presynaptic neurexins (NRXs) and laterally with postsynaptic NRXs (Taniguchi et al., 2007). The interactions between NLs and NRXs play a central role in the formation and/or maintenance of both glutamatergic and GABAergic synapses (Scheiffele et al., 2000; Dean et al., 2003; Graf et al., 2004, 2006; Prange et al., 2004; Boucard et al., 2005; Chih et al., 2005, 2006; Levinson et al., 2005; Dean and Dresbach 2006; Varoqueaux et al., 2006; Taniguchi et al., 2007; Chubykin et al. 2007). These studies have shown that postsynaptic overexpression of NLs leads to increased number of GABAergic and glutamatergic synaptic contacts while knocking down the expression of NLs leads to decreased number of GABAergic and glutamatergic contacts. NL-1 preferentially localizes at glutamatergic synapses while NL-2 are preferentially localized at GABAergic synapses (Song et al., 1999; Graf et al., 2004; Varoqueaux et al., 2004; Boucard et al., 2005; Levinson et al., 2005; Chubykin et al. 2007) although alternative splicing of both forms seem to also play a role in their localization in GABAergic or glutamatergic synapses (Chih et al., 2005, 2006).
It is very likely that NLs mediate the effects of manipulating gephyrin expression and clustering on the GABAergic and glutamatergic synaptic contacts that we have reported in this communication. There is evidence in support of the notion that postsynaptic NLs directly or indirectly interact with gephyrin, GABAARs and PSD-95 at synapses: I) Antibody capping with NL antibodies induces co-aggregation of PSD-95 and/or gephyrin (Graf et al., 2004); II) NLs and NRXs interact at their C-terminus with PSD-95 (Irie et al., 1997); III) A preliminary meeting report suggests that accumulation of NL-2 at GABAergic synapses is strictly dependent on postsynaptic GABAARs (Luscher et al., unpublished observation); IV) NL-2 is critical in the molecular reconstitution of GABAergic synapses in HEK 293 cells (Dong et al. 2007).
Moreover, our observation, reported in this communication, that disrupting gephyrin or GABAAR clustering leads to enlarged postsynaptic PSD-95 clusters and presynaptic glutamatergic boutons can also be explained by the mediation of NLs. It has been shown that the splice form of NL-1 that binds only to β-NRXs stimulates the formation of new synapses, while the splice form of NL-1 that binds to both α and β NRXs promotes synapse enlargement (Boucard et al., 2005).
The homotypic transynaptic effects, that we and others have observed, which follow the disruption of the postsynaptic gephyrin clusters (Fig 1 and 4 and Yu et al., 2007), or the disruption of postsynaptic γ2-GABAAR clusters (Li et al, 2005b), or by preventing the trafficking of γ2-GABAARs to the postsynaptic membrane (Fang et al., 2006), all leading to the reduction of the presynaptic GABAergic innervation, could result from the simultaneous disruption of the postsynaptic clustering of NLs at GABAergic synapses. The unclustering of postsynaptic NLs would result in a weakened interaction of these molecules with the presynaptic NRXs at GABAergic synapses and the withdrawal of the presynaptic GABAergic innervation. In the absence of the normal complement of the GABAergic synapses, the otherwise postsynaptic GABAergic NLs would migrate to the existing postsynaptic glutamatergic synapses, thus promoting the enlargement of both the postsynaptic PSD-95 clusters and the presynaptic glutamatergic terminals. A model based on the migration of NLs between GABAergic and glutamatergic synapses for regulating the balance between excitatory and inhibitory synapses has been proposed by others (for review see Levinson and El-Husseini 2005). Our results based on the manipulation of gephyrin expression and clustering support this model.
Thus, we conclude that gephyrin, a scaffold postsynaptic protein of GABAergic synapses, plays an important role, as others have shown for the glutamatergic postsynaptic PDS-95 scaffold protein, in determining the number and/or size of GABAergic and glutamatergic synaptic contacts that a particular neuron receives. We are proposing that gephyrin and PSD-95 act in opposite directions maintaining the balance between GABAergic and glutamatergic synaptic contacts.
Acknowledgments
This work was supported by The National Institute of Neurological Disorders and Stroke grants NS38752 and NS39287. We would like to thank Dr. Nobumi Kusuhara for the gephyrin construct. We would also like to thank Dr. Irwin J. Kopin for the sheep anti-GAD antibody.
Abbreviations
- AIS
axon initial segment
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CR
coding region
- CR3m
coding region with three point mutations
- EGFP
enhanced green fluorescent protein
- E/I
excitatory to inhibitory synaptic ratio
- GABAAR
γ-aminobutyric acid type-A receptor
- GAD
glutamic acid decarboxylase
- Geph
gephyrin
- mAb
monoclonal antibody
- NL
neuroligin
- NRX
neurexin
- PBS
phosphate-buffered saline
- PSD-95
postsynaptic density protein 95
- RNAi
RNA interference
- shRNA
small hairpin RNA
- UTR
3′-untranslated region
- UTR3m
3′-untranslated region with three point mutations
- vGAT
vesicular GABA transporter
- vGlut1
vesicular glutamate transporter-1
References
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Chandra D, Korpi ER, Miralles CP, De Blas AL, Homanics GE. GABAA receptor gamma 2 subunit knockdown mice have enhanced anxiety-like behavior but unaltered hypnotic response to benzodiazepines. BMC Neurosci. 2005;6:30. doi: 10.1186/1471-2202-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Li R, Serwanski DR, Li X, Miralles CP, Pinal N, De Blas AL. Identification and characterization of two novel splice forms of GRIP1 in the rat brain. J Neurochem. 2006;97:884–898. doi: 10.1111/j.1471-4159.2006.03795.x. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Li R, Serwanski DR, Miralles CP, Li X, Yang BY, Pinal N, Walikonis R, De Blas AL. A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J Biol Chem. 2004a;279:38978–38990. doi: 10.1074/jbc.M405786200. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004b;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Christie SB, De Blas AL. GABAergic and glutamatergic axons innervate the axon initial segment and organize GABA(A) receptor clusters of cultured hippocampal pyramidal cells. J Comp Neurol. 2003;456:361–374. doi: 10.1002/cne.10535. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Riquelme R, Yang BY, Charych E, Yu W, Daniels SB, Cantino ME, De Blas AL. Synaptic and extrasynaptic GABAA receptor and gephyrin clusters. Prog Brain Res. 2002a;136:157–180. doi: 10.1016/s0079-6123(02)36015-1. [DOI] [PubMed] [Google Scholar]
- Christie SB, Miralles CP, De Blas AL. GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons. J Neurosci. 2002b;22:684–697. doi: 10.1523/JNEUROSCI.22-03-00684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non-clustered GABAA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2006;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Qi J, Chen G. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABA(A) receptors. Mol Cell Neurosci. 2007;35:14–23. doi: 10.1016/j.mcn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA-A receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2. MIT Press: Cambridge, MA; 1998. pp. 339–370. [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Lardi-Studler B, Smolinsky B, Petitjean CM, Koenig F, Sidler C, Meier JC, Fritschy JM, Schwarz G. Vertebrate-specific sequences in the gephyrin E-domain regulate cytosolic aggregation and postsynaptic clustering. J Cell Sci. 2007;120:1371–1382. doi: 10.1242/jcs.003905. [DOI] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–17319. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Levinson JN, El-Husseini A. Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron. 2005;48:171–174. doi: 10.1016/j.neuron.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Li RW, Serwanski DR, Miralles CP, Li X, Charych E, Riquelme R, Huganir RL, de Blas AL. GRIP1 in GABAergic synapses. J Comp Neurol. 2005a;488:11–27. doi: 10.1002/cne.20566. [DOI] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie SB, Miralles CP, Bai J, Loturco JJ, De Blas Al. Disruption of postsynaptic GABAA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J Neurochem. 2005b;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABA(A) receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Meier J, Grantyn R. A gephyrin-related mechanism restraining glycine receptor anchoring at GABAergic synapses. J Neurosci. 2004;24:1398–1405. doi: 10.1523/JNEUROSCI.4260-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Lau LF, Huganir RL. Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr Opin Neurobiol. 1998;8:364–369. doi: 10.1016/s0959-4388(98)80062-7. [DOI] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I, Saiyed T, O’Sullivan GA, Schmitt B, Betz H, Weissenhorn W. Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J. 2004;23:2510–1519. doi: 10.1038/sj.emboj.7600256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li R-w, Chen G, De Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007 doi: 10.1016/j.mcn.2007.08.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]