Abstract
The HGF/c-Met signaling pathway is involved in the progression of a number of cancers and associated with increased tumor invasion and metastatic potential. We previously determined that the polyphenol epigallocatechin-3-gallate (EGCG) inhibited HGF-induced c-Met phosphorylation in a variety of tumor cell-lines, in part by disrupting lipid rafts. Fatty acid synthase (FASN) is implicated in cancer progression and may regulate lipid raft function. We therefore examined the effects of luteolin, a potent FASN inhibitor, on c-Met signaling. Luteolin blocked HGF-induced c-Met phosphorylation and scattering of DU145 prostate cancer cells, but inhibition required at least a 4 hour preincubation time. Western blot analysis indicated that inhibition of HGF-induced scattering by luteolin occurred coincident with reduction of total c-Met protein in DU145 cells. In addition, luteolin-induced c-Met downregulation was mimicked by a pharmacological inhibitor of FASN, C75, or shRNA knockdown of FASN. Consistent with a role for FASN, loss of c-Met in cells treated with C75 or luteolin was prevented by exogenous addition of palmitate. Luteolin-induced loss of c-Met primarily occurred at a post-transcriptional level and involved cell surface internalization, but did not involve translation inhibition, nor was it dependent on the activity of the 26S proteosome or acidic lysosomes. Taken together, our study demonstrates a novel connection between FASN activity and c-Met protein expression, and suggests that luteolin could act as a novel HGF/c-Met inhibitor by reducing expression of this receptor.
Keywords: c-Met, Fatty Acid Synthase, Luteolin
INTRODUCTION
Metastatic cancer is the primary cause of patient mortality, and therapeutic approaches to block this process are urgently needed. A major step of the malignant process is the transition of the stationary cancer cells to a motile mesenchymal-like phenotype (1). This epithelial to mesenchymal transition is thought to be important in the loss of cell-cell adhesions and eventual invasion of the basement membrane — a prerequisite for metastasis (2). A major contributor to the promotion of a mesenchymal phenotype is the HGF/c-Met signaling axis. Hepatocyte Growth Factor (HGF) is the only known ligand for the receptor tyrosine kinase (RTK) c-Met. Binding of HGF to c-Met leads to autophosphorylation of tyrosine residues within the cytoplasmic domain which function as docking sites for downstream effectors that mediate a number of cellular responses including proliferation, cell survival, actin remodeling, and motility (3,4). Prolonged induction of these pathways accounts for the cancer-promoting properties of the HGF/c-Met signaling axis. Overexpression of c-Met and activating mutations are seen in a number of cancers, including prostate and breast, and are strongly associated with aggressive disease (5-8).
Recently the interplay between distinct receptor tyrosine kinases has become better understood. For instance, c-Met overexpression leads to tumor cell resistance to EGFR-targeted therapy through heterodimerization that reestablishes downstream signaling (9). Because of these unique RTK partnerships, it has become clear that single target therapies will not be sufficient to inhibit tumor progression, and that multi-modality therapy, targeting several stimulatory pathways simultaneously, will prove more efficacious.
Phytochemicals, such as flavonoids, represent a source of relatively nontoxic, orally available and affordable compounds that are known to affect a number of different cancer-related pathways. Epidemiological studies have demonstrated a correlation between increased dietary intake of flavonoids with reduced risk of heart disease and cancer (10,11). Several anti-cancer properties attributed to these compounds include acting as anti-oxidants, the ability to interfere with various cancer promoting signaling pathways, and inhibition of growth factor receptors (11). We and others have also found that EGCG appears to inhibit c-Met activation and EGFR activation by disrupting lipid rafts (12).
Lipid rafts are important plasma membrane regions which regulate cellular signaling in part through compartmentalization of growth factor receptors. We have demonstrated that the active form of c-Met resides in lipid rafts (Duhon et al., 2008 manuscript submitted), suggesting that disruption of lipid rafts may lead to inhibition of c-Met signaling and its downstream effects. FASN is the sole enzyme responsible for synthesis of long-chain saturated fatty acids and may play a role in regulating the activity of lipid rafts. In addition, many human cancers exhibit increased FASN expression correlating with advanced disease (13). FASN activity has been proposed to maintain membrane microdomain integrity and may promote cell survival involving a regulatory loop with the PI3K pathway (14,15). Brusselmann et al. ranked the inhibitory effects of a series of flavonoids on LNCap prostate cancer cell lipogenesis. These effects correlated with growth arrest, induction of apoptosis, reduced synthesis of phospholipids and cholesterol, and selective cytotoxicity of malignant cells. Of the compounds investigated by the Swinnen laboratory, the flavonoid luteolin was determined to have the greatest inhibitory effect on lipogenesis (16). In addition, luteolin shares structural homology with known PI3K inhibitors, has been shown to inhibit FASN activity directly, and has strong antioxidant activity (16,17).
Given the possible role of FASN activity in regulating lipid raft function, and the localization of active c-Met in lipid rafts, we investigated the effects of luteolin on the HGF/c-Met signaling axis. In this report we demonstrate that luteolin blocks HGF-induced scattering and motility of DU145 prostate cancer cells, and is a very potent inhibitor of the PI3K pathway. Moreover, we show that luteolin can downregulate c-Met expression through FASN inhibition, demonstrating a novel link between FASN activity and c-Met protein expression.
Methods and Materials
Cell Culture
DU145, PC-3, H460, and MDA-MB-231 cell lines were obtained from ATCC and maintained in RPMI-1640 media, or DMEM/F-12 for 231s, (Cellgro, Herndon, VA, USA) containing 10% FBS (Gemini, CA, USA) and Penicillin/Streptomycin (Cellgro). Cells were maintained in a 37°C incubator with 5.0% CO2.
Western Blot Analysis
Western blot analysis was performed as previously described (18). Primary antibodies used were: phospho-Met (Y1234/1235), phospho-Met (Y1349), phospho-Met (Y1003), phospho-Akt (S473), phospho-Akt (S308), Akt, phospho-Erk, 4EBP1, phospho-JNK, FASN (Cell Signaling Technology, Beverly, MA, USA), phospho-S6K, total Erk, total c-Met (C-28) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and phospho-FAK (BD Transduction Laboratories, Franklin Lakes, NJ, USA), Actin (Sigma Aldrich, St. Louis, MO, USA), and Tubulin (Neomarkers, Fremont, CA, USA) were used as load controls. Blots were probed with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences, Pittsburgh, PA), and ECL (Amersham Biosciences) was used for protein detection. Recombinant Hepatocyte Growth Factor and LY294002 were obtained from EMB Biosciences (San Diego, CA, USA). Luteolin, C75, Apigenin, EGCG, Quercetin, Taxifolin, Lactocystin and Concanamycin A were obtained from Sigma Aldrich. MG132 was obtained from Axxora. (San Diego, CA, USA). Palmitate (Sigma) was complexed to bovine serum albumin (Fischer) as described (16,18,19). In short, palmitate was dissolved in ethanol to 150 mM and 1 volume was added to 4 volumes of a 4% bovine serum albumin solution in .9% NaCl and incubated for 1 hr at 37% for a 30 mM stock of BSA-complexed palmitate.
RT-PCR
DU145 cells were plated and allowed to grow to confluency on a 10 cm dish in serum-containing media. Cells were incubated ± 25 μM luteolin for 8 hours. Cells were homogenized in Trizol (Invitrogen Corp., Carlsbad, CA, USA), and total RNA was isolated according to manufacturer's protocol. RNA was subjected to reverse-transcriptase PCR for a range of cycles. The primer set for c-Met were purchased from Integrated DNA Technologies (Coralville, IA, USA): Forward 5′ – AGG CAC TAG CAA AGT CCG AGA TGA – 3′, Reverse 5′ – GGA AAC AAT CTG GGT GTT CCA GCA – 3′. PCR products were run on a 1% agarose gel. GAPDH PCR products were used as a loading control.
Immunofluorescence Microscopy
DU145 were grown on 4-well plastic slides (NalgeNunc, Rochester, NY, USA) in serum-containing media. Following treatment, cells were fixed in 4% PFA for 20 min and incubated for 1 hour at room temperature with primary tubulin, phosphotyrosine (Cell Signaling), or c-Met (R&D Systems, MN, USA) antibody suspended in BSP (Bovine Serum Albumin, Saponin, PBS). Wells were washed with PBS and Texas Red-dye conjugated secondary antibody was applied suspended in BSP along with phalloidin. Wells were washed again and Antifade/DAPI (Invitrogen) was added prior to setting coverslips. Fluorescent images were taken using an Olympus BX-50 epifluorescence microscope using MetaMorph software.
RNAi
DU145 cells stably expressing shRNAs targeting FASN or non-target shRNA were generated using Mission Lentiviral Transduction Particles from Sigma-Aldrich. Five separate cell lines were established using five lentivirus clones according to the manufacturer's protocol.
Scattering and Motility Assays
For the scattering assay, 4×104 DU145 cells, plated on a 24-well plate and grown in serum-containing media, were pretreated with the indicated concentrations of luteolin for the appropriate time. HGF was then spiked into wells to give a final concentration of 33 ng/ml and incubated overnight. For the motility assay, 4.5×105 DU145 cells were plated on a 6-well plate to form a confluent monolayer in serum-containing media. Cells were pretreated for 1 hr ± 25 μM luteolin. Intersecting scratches were made across the plate with a pipette tip and washed with PBS to remove cell debris. Serum-free media ± 15 μM luteolin ± 33 ng/ml HGF was then added to the cells and incubated overnight. The scratch for a representative T0 was made immediately prior to fixing. For each experiment, cells were fixed with PFA and stained with phalloidin, and representative images were taken using an Eclipse TE300 inverted microscope (Nikon, Japan).
RESULTS
Pretreatment with Luteolin Prevents HGF-Induced Scattering and Motility of Prostate Cancer Cells
We have demonstrated that the addition of the flavonoid EGCG blocks the HGF-induced signaling and scattering of DU145 tumor cells, via disruption of lipid rafts (Duhon et al., 2008; manuscript submitted). To determine if the flavonoid luteolin, a known FASN inhibitor, also affected the HGF-induced phenotypic change, DU145 cells were pretreated overnight with a range of luteolin concentrations prior to stimulation with HGF (33 ng/ml) for 18 hours. Luteolin blocked HGF-induced scattering in a dose-dependent fashion, and this inhibition of scattering was more pronounced with overnight pretreatment compared to one hour pretreatment (Fig. 1A). To address this pretreatment time-dependent effect, we pretreated cells with 25 μM luteolin for varying periods of time from 1-12 hours prior to HGF addition. An increase in inhibition of HGF-induced scattering was observed beginning at 4 hours of luteolin pretreatment with a greater percentage of cells remaining adherent in colonies with 8 hours or more of pretreatment time (Fig. 1B).
Figure 1. Luteolin pretreatment blocks HGF-induced cell scattering.
(A) DU145 prostate cancer cells were pretreated with DMSO (i and ii) or increasing luteolin concentrations (iii—10 μM; iv—15 μM; v and vi —30 μM) for one hour (vi) or overnight (iii-v) prior to HGF stimulation (ii-vi—33 ng/mL) for 18 hours. (B) DU145 cells were incubated with DMSO (i and ii) or 25 μM luteolin for 1 hour (iii), 4 hours (iv), 8 hours (v), or 12 hours (vi) prior to HGS stimulation (ii-vi). Cells were fixed, stained for actin, and representative images captured.
In addition to blocking HGF-induced scattering, luteolin caused a change in cell morphology (Fig. 1; compare panel Ai with Av). Cells appeared flatter and larger in diameter (Fig. 2A). To more closely examine the effects of luteolin on cell morphology, we performed immunofluorescence (I.F.) microscopy on DU145 cells treated with 25 μM luteolin for 9 hours. Cells were fixed and stained to visualize actin and tubulin. Figure 2A demonstrates that luteolin caused a reduction in the number of F-actin stress fibers, but had no apparent effect on microtubule distribution.
Figure 2. Luteolin disrupts actin stress fibers and blocks HGF-induced cell motility, but does not affect focal adhesions.
(A) DU145 cells were treated with DMSO or 25 μM luteolin for 12 hours. Cells were fixed, stained for actin, DAPI, and microtubules, and representative 40X and 60X images were captured. (B) Confluent DU145 monolayers were pretreated with DMSO (ii and iii) or 25μM luteolin (iv) for 1 hour. The monolayer was wounded, washed, and fresh serum-free media containing 15 μM luteolin (iv) or DMSO (ii and iii) was added. Cells were then incubated alone (ii) or with HGF (iii and iv—33 ng/mL) overnight. The following day, one monolayer was wounded as an untreated control (i), after which all cells were fixed and stained for actin. (C) DU145 cells were incubated with DMSO or 25μM luteolin for 12 hours. Cells were fixed, stained for actin or phosphotyrosine, and representative images were captured. Focal adhesions, represented by areas of actin and phosphotyrosine colocalization, are indicated by arrows.
Actin stress fiber formation is an important contributor to cell motility and accordingly we performed a wound-healing motility assay to determine the effects of luteolin on HGF-induced motility and wound closure. Confluent monolayers of DU145 cells were incubated with or without 25 μM luteolin for one hour, after which a scratch was made with a pipette tip to wound the monolayer. The media was aspirated and fresh serum-free media was added with or without 15 μM luteolin and HGF at 33 ng/ml. Serum-free media was used so that wound closure due to motility would not be obscured by closure due to normal cell growth and proliferation. A T0 scratch was also made immediately prior to fixing the cells in paraformaldehyde (Fig. 2Bi). The control cells, not treated with HGF, failed to migrate into the cleared area (Fig. 2Bii), while the HGF-stimulated cells almost entirely closed the wound area (Fig. 2Biii). Luteolin, however, blocked the HGF-stimulated wound-closure (Fig. 2Biv), suggesting that luteolin blocks HGF-mediated cell motility (Fig. 2B). Trypan blue staining revealed that luteolin had no affect on cell viability (results not shown).
Cell attachment to the substratum is mediated by focal adhesions, and dynamic focal adhesion turnover is essential for cell motility (20). To determine if luteolin inhibits cell migration by lowering the number of focal adhesion sites, DU145 cells were treated with 25 μM luteolin for 9 hours, and actin and phosphotyrosine residues were visualized by I.F. microscopy. Focal adhesions were identified as areas densely populated with phosphorylated proteins localized at the tips of actin fibers. As shown in Figure 2C, there was no difference in the size or quantity of focal adhesions between control and luteolin treated cells, suggesting that luteolin does not affect focal adhesion formation or stability.
Luteolin Pretreatment is required to Attenuate c-Met Activation by HGF, but Pretreatment is not required for Inhibition of Akt-phosphorylation
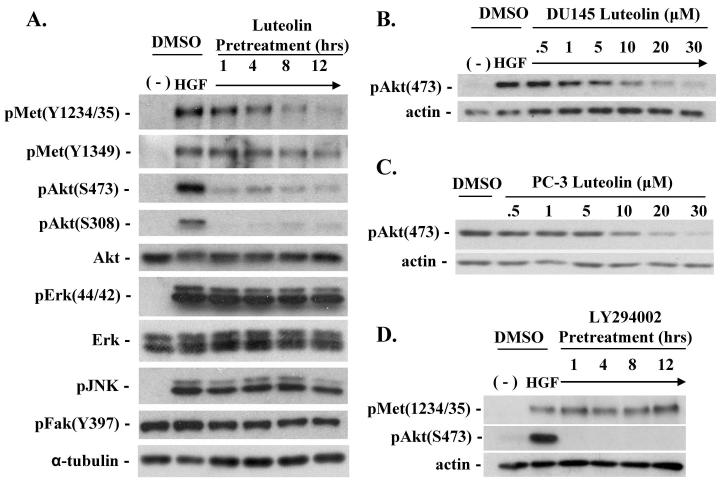
To determine if luteolin affected HGF/c-Met signaling pathways, thus blocking cell scattering and motility, we treated DU145 cells with 25 μM luteolin for 1, 4, 8, or 12 hours prior to HGF treatment for 20 minutes. As shown in Figure 3A, Western blot analysis of whole cell lysates using antibodies specific for c-Met phosphorylation showed that HGF-induced c-Met phosphorylation at tyrosine residues 1234/1235 (the kinase domain) and 1349 (an adaptor docking site) was significantly attenuated, but only after pretreatment with luteolin for greater than 1 hour (Fig. 3A); moreover attenuation increased with pretreatment time. However, luteolin blocked phosphorylation of Akt at two separate residues within one hour of pretreatment. Additional experiments demonstrated luteolin blocked phosphorylation of Akt when pretreated for only 10 minutes before the addition of HGF (results not shown). Conversely, HGF-mediated activation of two MAP kinases, JNK and Erk, along with focal adhesion kinase (FAK) was not affected by luteolin pretreatment (Fig. 3A).
Figure 3. Luteolin inhibits PI3K and blocks HGF-induced c-Met phosphorylation with prolonged pretreatment.
(A) DU145 cells were pretreated with DMSO or 25 μM luteolin for 1, 4, 8, or 12 hours prior to HGF (33ng/ml) stimulation for 20 minutes. (B) DU145 cells were pretreated with DMSO or indicated luteolin concentrations for 1 hour prior to HGF treatment for 20 minutes. (C) PC-3 prostate cancer cells were treated with DMSO or indicated concentrations of luteolin for 1 hour. (D) DU145 cells were pretreated with DMSO or 20 μM LY294002 for 1, 4, 8, or 12 hours prior to HGF stimulation. For each experiment, whole cell lysates were collected and probed by western blot analysis using indicated antibodies. Actin or tubulin was also probed as a load control.
Since Akt phosphorylation, indicative of PI3K activity, was sensitive to short preincubation times with luteolin, we further investigated the role of luteolin in HGF-dependent PI3K activity. To determine if luteolin affected the activity of PTEN, a phosphatase that negatively regulates PI3K signaling, a dose response experiment was performed using DU145 cells and the PTEN −/− prostate cancer cell line PC-3 (21,22). Western blot analysis indicated that luteolin inhibited phosphorylation of Akt similarly in both cell lines with an IC50 of approximately 5 μM, suggesting that luteolin acts directly as a PI3K inhibitor rather than by activating PTEN (Figures 3B and C). Since luteolin pretreatment caused an apparent reduction of PI3K activity within 1 hour, followed by inhibition of c-Met phosphorylation beginning at 1 hour, we tested if the loss of PI3K activity negatively regulated c-Met phosphorylation. To test this hypothesis, we used the PI3K inhibitor, LY294002, to determine if prolonged inhibition of PI3K resulted in the reduction of HGF-induced c-Met phosphorylation. As shown in Figure 3D, LY294002 pre-treatment over a time course similar to luteolin pre-treatment did not affect c-Met phosphorylation; despite inhibiting HGF-induced Akt phosphorylation. Therefore, the ability of luteolin to attenuate c-Met phosphorylation is independent of its ability to inhibit PI-3K.
Luteolin Pretreatment Induces a Post-Transcriptional Loss of c-Met Most Likely Through Inhibition of FASN
To further investigate the mechanism of luteolin-mediated decrease in HGF-mediated c-Met activity, we performed Western blot analysis using whole cell lysates from DU145 cells treated with luteolin for various times. Figure 4A shows that luteolin caused a reduction in the level of total c-Met protein beginning as early as 1 hour, leading to a greater than 90 percent loss by 12 hours. The loss of c-Met expression over time mirrors the loss of HGF-induced c-Met phosphorylation seen in Figure 3A, suggesting that the mechanism of luteolin-mediated downregulation of c-Met activity may be primarily through decreased receptor expression. In order to confirm the generality of these findings, we treated several additional cancer cell lines, including the prostate tumor cell-line PC-3, the breast cancer cell line MDA-MB-231, and the lung cancer cell line H460, with 25 μM luteolin for 9 hours. Luteolin treatment reduced c-Met expression in all cell lines (Fig. 4A, right panel), suggesting that the observed effects of luteolin on c-Met receptor levels are not cell context dependent.
Figure 4. Luteolin reduces c-Met levels through inhibition of fatty acid synthase.
(A, left panel) DU145 cells were treated with DMSO or 25 μM luteolin for 1, 4, 8, 12, or 24 hours. (A, right panel) DU145, PC-3, the lung cancer cell line H460, and breast cancer cell line MDA-MB-231 were treated for 9 hours with DMSO (−) or 25 μM luteolin (+). (B) DU145 cells were treated with DMSO or 25 μM C75 for 1, 4, 8, 12, or 24 hours. (C) DU145 cells were stably transduced with lentivirus expressing non-target shRNA (−) or one of four FASN-targeted shRNA clones (126,127, 128, or 129). (D, left panel) DU145 cells were pretreated with DMSO or 25 μM luteolin (right panel) or 25 μM C75 prior to incubation with palmitate-BSA for 9 hours. For each experiment, whole cell lysates were collected and probed by western blot analysis using c-Met or FASN-specific antibodies. Actin was also probed as a load control. Densitometry was performed on appropriate blots and shown as fold change of control.
Since luteolin is a known FASN inhibitor, we predicted that a specific FASN inhibitor, C75, might also induce a loss of c-Met expression (23). As seen in Figure 4B, the addition of C75 resulted in the loss of c-Met with kinetics similar to that observed for luteolin treatment. To more directly determine the role of FASN in controlling c-Met levels, we expressed FASN-specific shRNA via lentivirus to generate stable cell-lines. As demonstrated in Figure 4C, the FASN shRNA cell-lines contained greatly reduced FASN levels, and this was paralled by comparable decreases in c-Met levels. Finally, the addition of palmitate, the end product of FASN catalytic activity, prevented luteolin- (Fig. 4D, left panel) and C75-induced (right panel) c-Met loss in a dose-dependent manner. These experiments suggest a potential novel link between FASN activity and c-Met expression levels.
To determine if luteolin affected c-Met transcription, thus accounting for loss of c-Met protein, we performed reverse-transcriptase PCR on RNA prepared from DU145 cells cultured alone or in the presence of luteolin for 8 hours. Gel electrophoresis of PCR products at different cycle times indicated that luteolin treatment only reduced c-Met mRNA by approximately 20 percent in DU145 cells relative to GAPDH RNA controls (Fig. 5A); real-time PCR confirmed this 20 percent reduction (data not shown). The modest loss of c-Met mRNA compared to the greater than 90 percent loss of c-Met suggests a post-transcriptional level of control is involved. The PI3K/Akt pathway regulates cap-dependent translation in part by affecting 4EBP1 through mTOR activation, leading to eIF4E availability to initiate translation of mRNAs with complex 5′ UTRs (24). The c-Met mRNA is predicted to have a 5′ UTR that might be eIF4E responsive (unpublished results). Accordingly, we used rapamycin, a specific mTOR inhibitor, and LY294002 to inhibit the PI3K/Akt/mTOR pathway. Western blot analysis revealed that inhibition of this pathway, as indicated by the blocked phosphorylation of downstream S6 kinase and the shift of 4EBP1 to a hypo-phosphorylated form in cells exposed to HGF, did not result in a decrease in c-Met levels (Fig. 5B). This suggests that in DU145 cells c-Met mRNA translation is most likely not dependent on the mTOR pathway and that luteolin is not lowering c-Met protein levels through inhibition of cap-dependent translation.
Figure 5. Luteolin post-translationally regulates c-Met levels.
(A) DU145 cells were treated with DMSO (−) or 25 uM luteolin (+) for 8 hours. RNA was then isolated and RT-PCR was performed using primers for c-Met, and for GAPDH as control at different cycles. (B) DU145 cells were pretreated with DMSO, rapamycin (100 ng/ml), or LY294002 (20 μM) for indicated time periods prior to HGF stimulation (33ng/ml) for 20 minutes. Lysates were collected and probed by western blot analysis using indicated antibodies. Actin was used as a load control.
To further address the possible mechanism of action accounting for luteolin-induced loss of c-Met, we compared the rate of c-Met loss upon treatment with the eukaryotic protein synthesis inhibitor, cycloheximide, alone or in combination with luteolin. Western blot analysis indicated an increased rate of c-Met loss in the presence of cycloheximide and luteolin as compared to treatment with either agent alone (data not shown). If luteolin was inhibiting c-Met protein synthesis like cycloheximide, we would not observe this increased rate of c-Met loss when combined with cycloheximide. Increased doses of cycloheximide did not increase the rate of c-Met loss suggesting the dose used was optimal (data not shown). Together, these data suggest luteolin promotes c-Met degradation of already synthesized c-Met protein.
Luteolin-induced Loss of c-Met is Independent of the Proteosomal and Lysosomal Degradation Pathways
When the c-Met receptor is activated by HGF, the juxtamembrane tyrosine residue 1003 is phosphorylated, which recruits the ubiquitin ligase Cbl. Ubiquitination prompts internalization of the receptor and trafficking for degradation via either the 26S proteosome or by trafficking to acidic lysosomes (25-29). Western blot analysis indicated that HGF treatment of DU145 cells resulted in loss of c-Met protein within 2 hours, preceded by phosphorylation at Y1003 (Fig. 6A, 6B). In contrast, luteolin treatment did not result in phosphorylation at Y1003 (Fig. 6B). We used specific pharmacological inhibitors to determine if luteolin-induced c-Met loss was dependent on the proteosomal or the lysosomal degradation pathway. MG132 and lactacystin are inhibitors of the 26S proteosome, and concanamycin A inhibits acidification of lysosomes, reducing proteolysis. DU145 cells were pretreated with the indicated inhibitors prior to HGF or luteolin treatment and probed for c-Met loss by Western blot analysis. HGF treatment resulted in a rapid loss of c-Met, which was partially prevented by inhibition of the 26S proteosome or by blocking lysosomal acidification (Fig. 6C). In contrast, luteolin-induced c-Met loss was not prevented in the presence of these inhibitors (Fig. 6C). In addition, inhibiting both the 26S proteosome and lysosomal acidification in combination prevented HGF-induced degradation (Fig. 6C bottom panel), but again luteolin-induced c-Met downregulation was not blocked by the combination of inhibitors (Fig. 6C bottom panel). These experiments suggest the proteosomal and lysosomal pathways do not play a major role in luteolin-induced c-Met loss, and suggest a potentially novel mechanism of action to account for the effects of this flavonoid on c-Met levels.
Figure 6. Luteolin downregulates c-Met levels independent of the lysosomal or proteosomal pathways.
(A) DU145 cells were incubated alone (−) or treated with HGF (33ng/ml) for 1, 2, 3, or 4 hours. (B) DU145 cells were incubated alone (−), treated with HGF for 45, 90, 135, or 180 minutes, or treated with 25 μM luteolin for 6 hours plus 45, 90, 135, or 180 minutes. (C top panel) Cells were pretreated with DMSO or luteolin for 5 hours prior to culture alone (−) or with 300 nM MG132 (MG) or 20 μM lactacystin (L) for 4 hours with or without HGF stimulation. (middle panel) Cells were treated as described above, but using 6 μM or 3 μM concanamycin A (ConA), or (bottom panel) 300nM MG132 and 6μM concanamycin A in combination (ConA-MG). For each, whole cell lysates were analyzed by western blot analysis using the indicated antibodies. Actin or tubulin was used as a load control. Densitometry was performed on appropriate blots and shown as fold change of control. (D) DU145 cells were treated with 25uM luteolin for 8 and 12 hours or with HGF for 1 or 2 hours to stimulate loss of total c-Met. Cells were then fixed and stained with a c-Met specific primary antibody and fluorescently-labelled secondary antibody. Representative images are shown. Arrowheads point out perinuclear distribution and arrows show the cell membrane periphery.
To confirm that a reduction in c-Met levels is the result of loss of c-Met from the cell surface, cells were treated with HGF or luteolin for the times indicated in Figure 6D. I.F. microscopy using a c-Met antibody indicated that HGF treatment rapidly resulted in redistribution of c-Met from the cell surface to vesicles residing near the nucleus. Cell surface c-Met was also reduced by luteolin treatment, but the intracellular distribution was less vesicular, although c-Met did accumulate near the nucleus during the time period levels were decreasing.
The 2,3 Double Bond in Ring C of Flavonoids is Important for c-Met Down Regulation
In order to determine if structurally related flavonoids have similar effects on c-Met levels when compared to luteolin, we treated DU145 cells for 9 hours with the flavones luteolin and apigenin, the flavonol quercetin, the flavanone taxifolin, and the flavanol EGCG. Western blot analysis indicated that luteolin, apigenin, and quercetin all reduced c-Met expression to similar levels, but that taxifolin and EGCG had no significant effect on c-Met expression at these concentrations (Supplemental Figure 1A). Interestingly, each compound effective at lowering c-Met levels (luteolin, apigenin, and quercetin) contained a double bond between carbons 2 and 3 of the center six-member oxygen-containing C ring. Comparatively, taxifolin, ineffective at lowering c-Met expression, lacks this double bond which is the only difference from quercetin's molecular structure (Supplemental Figure 1B). These data reveal that structurally-similar flavonoids have similar effects on c-Met expression, and that the 2, 3 double bond of the C-ring is important for this effect.
DISCUSSION
Some phytochemicals, such as EGCG from green tea extract, have potent inhibitory activity against growth factor signaling pathways, including HGF/c-Met (18,30). In this report, we investigated the effects of the flavonoid luteolin on the HGF/c-Met signaling axis, and found that it blocks c-Met signaling through a mechanism unique from EGCG. Our data suggest that one key mechanism of action is through inhibition of FASN, leading to a post-transcriptional reduction in c-Met protein levels.
In the presence of HGF, DU145 prostate cancer cells lose cell-cell adhesions and acquire a motile phenotype. EGCG blocks HGF-induced scattering with no preincubation time required (18); however, we report here that luteolin had a minimal effect added with HGF, and that a preincubation time was required to block c-Met phosphorylation. This result suggests that the two compounds are working by different mechanisms. Also, luteolin caused changes in cell morphology (reduction in stress fibers and cell flattening) not observed with EGCG treatment. Similar effects by luteolin on the actin cytoskeleton were shown by Lee et al. in the context of HepG2 hepatoma cells (31). How luteolin effects these changes remains to be determined, but we speculate that luteolin disrupts actin-remodeling proteins, such as the Rho family of small GTPases and downstream effectors like Rho-kinase (ROCK). This hypothesis is supported by Hendricks et al. who have previously shown that luteolin can inhibit RhoA activity in a monocyte system (32). Actin stress fibers are important for cell motility, and disruption of actin stress fibers caused by luteolin treatment may account for the compound's ability to block HGF-induced scattering and motility, although this remains to be tested (33).
Luteolin also rapidly blocked HGF-induced Akt phosphorylation, while 1hour of pretreatment was required to inhibit c-Met phosphorylation. We conclude luteolin is working directly as a PI3K inhibitor, rather than blocking ligand-induced receptor activation. Consistent with this possibility, others have found that luteolin can inhibit PI3K in an in vitro assay (34). Furthermore, luteolin was equally effective at blocking Akt phosphorylation in a PTEN −/− prostate cancer cell line; thereby ruling out the possibility that luteolin activated this phosphatase to negatively regulate PI3K activity. Loss or inactivation of PTEN is common in a number of cancers including prostate cancer, and aberrant signaling through the PI3K pathway has been implicated in tumor cells becoming resistant to receptor tyrosine kinase-targeted therapies (21,22). Therefore, the ability of luteolin to target PI3K signaling independent of PTEN status emphasizes the potential therapeutic activity of this phytochemical.
Furthermore, the time-dependent attenuation of HGF-mediated c-Met phosphorylation by luteolin was independent of the effects on Akt phosphorylation, since prolonged treatment of cells with LY294002 did not block the ability of HGF to induce c-Met phosphorylation. Activation of the Erk and JNK signaling pathways were not affected by luteolin even after long preincubation when c-Met was no longer maximally activated by HGF. This suggests that sufficient active c-Met remained to induce phosphorylation of Erk and JNK. FAK was constitutively phosphorylated at high levels in DU145 cells, and phosphorylation was only minimally attenuated with prolonged luteolin preincubation.
Our results are consistent with Lee et al. who have shown that luteolin blocked HGF-induced Akt phosphorylation, but only partially affected MAP kinases in hepatoma cells (31). In addition, however, our results show a requirement for pretreatment greater than 1 hour to affect c-Met phosphorylation, whereas only a short pretreatment was required to block phosphorylation of Akt. We speculate that the requirement for long pretreatment could reflect the length of time required to alter the concentration of a factor(s) regulating c-Met. One of these factors could be levels of palmitate that regulates lipid raft activity or integrity. One can rule-out a delay in the compound crossing the cell membrane, because of the rapid effects on PI3K activity.
Interestingly, the reduction of HGF-induced phosphorylated of c-Met was not the result of luteolin blocking ligand-induced activation of the receptor, but instead appeared to be due to luteolin-mediated downregulation of total c-Met protein. RT-PCR analysis demonstrated no dramatic change in total c-Met mRNA levels, suggesting luteolin was acting primarily at a post-transcriptional level. This result was observed in multiple tumor cell-lines. Similar to our results, it has been reported that apigenin and luteolin downregulate HER2 expression in HER2/neu-overexpressing breast cancer cell lines, although the mechanism regulating this was not defined for luteolin (35). A subsequent report suggested that luteolin induced loss of HER2 at a post-transcriptional level (36). We have observed similar effects of luteolin and apigenin on HER2 expression in DU145 prostate cancer cells (unpublished data).
C75, a specific pharmacological inhibitor to FASN, as well as FASN-specific shRNA reduced the level of total c-Met protein in DU145 cells. FASN is the sole enzyme responsible for de novo synthesis of long-chain unsaturated fatty acids, primarily the 16 carbon fatty acid palmitate. The addition of exogenous palmitate to the system prevented the C75- and luteolin- induced loss of c-Met, further supporting a role or FASN in maintaining c-Met expression levels. Malignant cells have a much greater reliance on de novo synthesized fatty acids as opposed to exogenously-derived fatty acids, suggesting that FASN could be a good therapeutic target (37). The need of malignant cells for high expression of FASN has been attributed to maintenance of the lipid supply required by highly proliferative cells, regulation of stimulatory signaling pathways through palmitoylation of proteins and stabilizing membrane domains, as well as restoration of oxidation potential through consumption of NADPH under hypoxic conditions (13). A number of cellular receptors, including c-Met, require localization within ordered lipid microdomains (lipid rafts) for efficient signaling (38,39; Duhon et al., manuscript submitted). Lipid rafts are rich in cholesterol and sphingolipids, products generated in tumors cells by FASN (15,40). Our results suggest that higher FASN activity maintains lipid rafts which may help stabilize levels of c-Met.
Our results suggest that, in DU145 cells, translation of c-Met mRNA was not regulated by the PI3K/Akt/mTOR/eIF4E pathway, and therefore inhibition of PI3K is not the mechanism responsible for luteolin-induced c-Met loss. Our data also suggest that the loss of c-Met induced by luteolin occurred in part subsequent to c-Met synthesis, but was independent of an active proteosome or acidic lysosomes.
The c-Met receptor, similar to other RTKs, has been reported to undergo a mechanism of ligand-induced negative regulation through internalization and degradation (26,28,29). Using inhibitors to both proteosomal activity and acidification of lysosomes, we show that neither of these pathways was significantly involved in luteolin-induced c-Met loss. In contrast, Chiang et al. concluded that luteolin induced loss of HER2 in breast carcinoma cells was blocked by the addition of proteosomal inhibitors (36).
Another mechanism of c-Met downregulation is receptor shedding, which is mediated by metalloproteinases that cleave the extracellular domain of the receptor promoting degradation of the cytoplasmic domain (41). Our results speak against this possibility for three reasons. Firstly, no c-Met protein was detected in the media following luteolin treatment (data not shown). Secondly, similar trends of c-Met loss were detected with antibodies to both a cytoplasmic domain and an extracellular domain (data not shown). Finally, I.F. microscopy indicated that reduction of c-Met levels was accompanied by internalization of the receptor into intracellular compartments.
An additional possibility for c-Met loss is that luteolin is stimulating an apoptotic response resulting in activation of caspases that subsequently degrade the receptor. However, no significant apoptosis was observed in treated cells during these time points by microscopy or protein analysis (data not shown). We conclude that luteolin is acting through a novel mechanism of receptor degradation unique from that induced by ligand or other known stimuli; although the mechanism remains to be defined.
We determined, using a series of structurally similar compounds, that the 2,3 double bond of the C ring of luteolin conveys the activity required for downregulation of c-Met. We show that the flavones luteolin and apigenin along with the flavonol quercetin, each containing the 2-3 double bond and having the most potent inhibitory activity toward FASN, as shown by Swinnen et al., have the greatest effect on c-Met levels (16). Apigenin has previously been reported to downregulate HER2 by proteosomal degradation in breast cancer cells (42). We hypothesize that by targeting FASN these compounds can downregulate the expression of a number of cancer-associated growth factor receptors that require membrane microdomains stabilized by FASN activity.
Our study provides evidence for a potential link between FASN activity controlling levels of c-Met perhaps by stabilization, and that luteolin could be a potential therapeutic agent to down regulate c-Met levels through inhibition of FASN. Overexpression of FASN has been reported to increase activity of the HER1 and HER2 receptors in breast cancer cells and may be important in resistance to Trastuzumab (43,44). We hypothesize that increased expression of FASN could stabilize growth factor receptors localized in lipid rafts, such as c-Met, thereby promoting cancer progression. Our results, consistent with other published studies, demonstrate that luteolin inhibits PI3K activity in prostate cancer cells. FASN expression is, in part, controlled by a regulation loop with PI3K, and is inversely correlated with PTEN expression (14,45). Consequently, luteolin has the potential to target both PI3K and FASN activities to disrupt growth factor receptor stability and cancer progression, suggesting it might be more effective than either PI3K inhibitors or FASN inhibitors given alone.
Supplementary Material
(A) DU145 cells were cultured alone (−) or with 25 μM each of luteolin (Lut), quercetin (Que), apigenin (Api), taxifolin (Tax), or 65 μM EGCG for 9 hours. Densitometric analysis was also performed to determine fold change. (B) Schematic of the chemical structures of these phytochemicals. Arrows indicate the double bond common to luteolin, quercetin, and apigenin; but not taxifolin.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Jared Snider and Josh Steffan for their critical reading of the manuscript. This work was supported in part by a grant from the National Institutes of Health (NIH R01 CA104242-01).
Abbreviations list
- FASN
fatty acid synthase
- HGF
hepatocyte growth factor
- PI3-K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- EGCG
epigallocatechin 3-gallate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- eIF4E
eukaryotic translation initiation factor 4E
- 4EBP1
4E binding protein 1
- UTR
untranslated region
REFERENCES CITED
- 1.Gupta GP, Massagué J. Cancer Metastasis: Building a Framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 3.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 4.Gentile A, Trusolino L, Comoglio P. The Met tyrosine kinase receptor in development and cancer. Cancer and Metastasis Reviews. 2008;27:85–94. doi: 10.1007/s10555-007-9107-6. [DOI] [PubMed] [Google Scholar]
- 5.Ernst Lengyel DP, Resau James H., Gauger Katja, Welk Anita, Lindemann Kristina, Salanti Georgia, Richter Thomas, Knudsen Beatrice, Vande Woude George F., Harbeck Nadia. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. International Journal of Cancer. 2005;113:678–682. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 6.Jeffers M, Schmidt L, Nakaigawa N, Webb CP, Weirich G, Kishida T, et al. Activating mutations for the Met tyrosine kinase receptor in human cancer. Proceedings of the National Academy of Sciences. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knudsen BS, Gmyrek GA, Inra J, Scherr DS, Vaughan ED, Nanus DM, et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60:1113–1117. doi: 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 8.Humphrey PA, Zhu X, Zarnegar R, Swanson PE, Ratliff TL, Vollmer RT, et al. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol. 1995;147:386–396. [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 10.Erdman JW, Jr., Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, et al. Flavonoids and Heart Health: Proceedings of the ILSI North America Flavonoids Workshop; J Nutr; Washington, DC. May 31-June 1, 2005; 2007. pp. 718S–737. [DOI] [PubMed] [Google Scholar]
- 11.Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 12.Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, et al. The Inhibitory Effect of (−)-Epigallocatechin Gallate on Activation of the Epidermal Growth Factor Receptor Is Associated with Altered Lipid Order in HT29 Colon Cancer Cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 13.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 14.Wang HQ, Altomare DA, Skele KL, Poulikakos PI, Kuhajda FP, Di Cristofano A, et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24:3574–3582. doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- 15.Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochemical and Biophysical Research Communications. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 16.Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of Cancer Cell Apoptosis by Flavonoids Is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity. J Biol Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 17.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 18.Bigelow RLH, Cardelli JA. The green tea catechins, (−)-Epigallocatechin-3-gallate (EGCG) and (−)-Epicatechin-3-gallate (ECG), inhibit HGF//Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene. 2006;25:1922–1930. doi: 10.1038/sj.onc.1209227. [DOI] [PubMed] [Google Scholar]
- 19.Briaud I, Harmon JS, Kelpe CL, Segu VBG, Poitout V. Lipotoxicity of the Pancreatic {beta}-Cell Is Associated With Glucose-Dependent Esterification of Fatty Acids Into Neutral Lipids. Diabetes. 2001;50:315–321. doi: 10.2337/diabetes.50.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 21.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative Regulation of PKB/Akt-Dependent Cell Survival by the Tumor Suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 22.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN Expression in Paraffin-embedded Primary Prostate Cancer Correlates with High Gleason Score and Advanced Stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 23.Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the Eukaryotic Translation Initiation Factor 4E for Cancer Therapy. Cancer Res. 2008;68:631–634. doi: 10.1158/0008-5472.CAN-07-5635. [DOI] [PubMed] [Google Scholar]
- 25.Abella JV, Peschard P, Naujokas MA, Lin T, Saucier C, Urbe S, et al. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol Cell Biol. 2005;25:9632–9645. doi: 10.1128/MCB.25.21.9632-9645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond DE, Urbe S, Vande Woude GF, Clague MJ. Down-regulation of MET, the receptor for hepatocyte growth factor. Oncogene. 2001;20:2761–2770. doi: 10.1038/sj.onc.1204475. [DOI] [PubMed] [Google Scholar]
- 27.Kermorgant S, Zicha D, Parker PJ. Protein Kinase C Controls Microtubule-based Traffic but Not Proteasomal Degradation of c-Met. J Biol Chem. 2003;278:28921–28929. doi: 10.1074/jbc.M302116200. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann KM, Tapia JA, Berna MJ, Thill M, Braunschweig T, Mantey SA, et al. Gastrointestinal Hormones Cause Rapid c-Met Receptor Down-regulation by a Novel Mechanism Involving Clathrin-mediated Endocytosis and a Lysosome-dependent Mechanism. J Biol Chem. 2006;281:37705–37719. doi: 10.1074/jbc.M602583200. [DOI] [PubMed] [Google Scholar]
- 29.Jeffers M, Taylor G, Weidner K, Omura S, Vande Woude G. Degradation of the Met tyrosine kinase receptor by the ubiquitin- proteasome pathway. Mol Cell Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.liShimizu M, Deguchi A, Lim JTE, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin Gallate and Polyphenon E Inhibit Growth and Activation of the Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor-2 Signaling Pathways in Human Colon Cancer Cells. Clin Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 31.Lee W-J, Wu L-F, Chen W-K, Wang C-J, Tseng T-H. Inhibitory effect of luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K-Akt pathways. Chemico-Biological Interactions. 2006;160:123–133. doi: 10.1016/j.cbi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Hendriks JJA, Alblas J, van der Pol SMA, van Tol EAF, Dijkstra CD, de Vries HE. Flavonoids Influence Monocytic GTPase Activity and Are Protective in Experimental Allergic Encephalitis. J Exp Med. 2004;200:1667–1672. doi: 10.1084/jem.20040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell Migration: Integrating Signals from Front to Back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 34.Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Rémésy C, Chap H, et al. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: A comparison with tyrosine kinase and protein kinase C inhibition. Biochemical Pharmacology. 1997;53:1649–1657. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- 35.Way T-D, Kao M-C, Lin J-K. Degradation of HER2/neu by apigenin induces apoptosis through cytochrome c release and caspase-3 activation in HER2/neuoverexpressing breast cancer cells. FEBS Letters. 2005;579:145–152. doi: 10.1016/j.febslet.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 36.Chiang C-T, Way T-D, Lin J-K. Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21WAF1/CIP1 expression with rapamycin. Mol Cancer Ther. 2007;6:2127–2138. doi: 10.1158/1535-7163.MCT-07-0107. [DOI] [PubMed] [Google Scholar]
- 37.Medes G, Thomas A, Weinhouse S. Metabolism of Neoplastic Tissue. IV. A Study of Lipid Synthesis in Neoplastic Tissue Slices in Vitro. Cancer Res. 1953;13:27–29. [PubMed] [Google Scholar]
- 38.Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K. Lipid Rafts/Caveolae Are Essential for Insulin-like Growth Factor-1 Receptor Signaling during 3T3-L1 Preadipocyte Differentiation Induction. J Biol Chem. 2003;278:11561–11569. doi: 10.1074/jbc.M211785200. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Zhao YF, Li YL, Xu YF, Xia Q, Ma KL. Effects of lipid rafts on signal transmembrane transduction mediated by c-Met. Zhonghua Gan Zang Bing Za Zhi. 2008;16:449–452. [PubMed] [Google Scholar]
- 40.Pike LJ. Growth factor receptors, lipid rafts and caveolae: An evolving story. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research Lipid Rafts: From Model Membranes to Cells. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Nath D, Williamson N, Jarvis R, Murphy G. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. J Cell Sci. 2001;114:1213–1220. doi: 10.1242/jcs.114.6.1213. [DOI] [PubMed] [Google Scholar]
- 42.Way T-D, Kao M-C, Lin J-K. Apigenin Induces Apoptosis through Proteasomal Degradation of HER2/neu in HER2/neu-overexpressing Breast Cancer Cells via the Phosphatidylinositol 3-Kinase/Akt-dependent Pathway. J Biol Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 43.Vazquez-Martin A, Colomer R, Brunet J, Menendez JA. Pharmacological blockade of fatty acid synthase (FASN) reverses acquired autoresistance to trastuzumab (Herceptin by transcriptionally inhibiting ‘HER2 super-expression’ occurring in high-dose trastuzumab-conditioned SKBR3/Tzb100 breast cancer cells. Int J Oncol. 2007;31:769–776. [PubMed] [Google Scholar]
- 44.Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proceedings of the National Academy of Sciences. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandyopadhyay S, Pai SK, Watabe M, Gross SC, Hirota S, Hosobe S, et al. FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene. 2005;24:5389–5395. doi: 10.1038/sj.onc.1208555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) DU145 cells were cultured alone (−) or with 25 μM each of luteolin (Lut), quercetin (Que), apigenin (Api), taxifolin (Tax), or 65 μM EGCG for 9 hours. Densitometric analysis was also performed to determine fold change. (B) Schematic of the chemical structures of these phytochemicals. Arrows indicate the double bond common to luteolin, quercetin, and apigenin; but not taxifolin.