Abstract
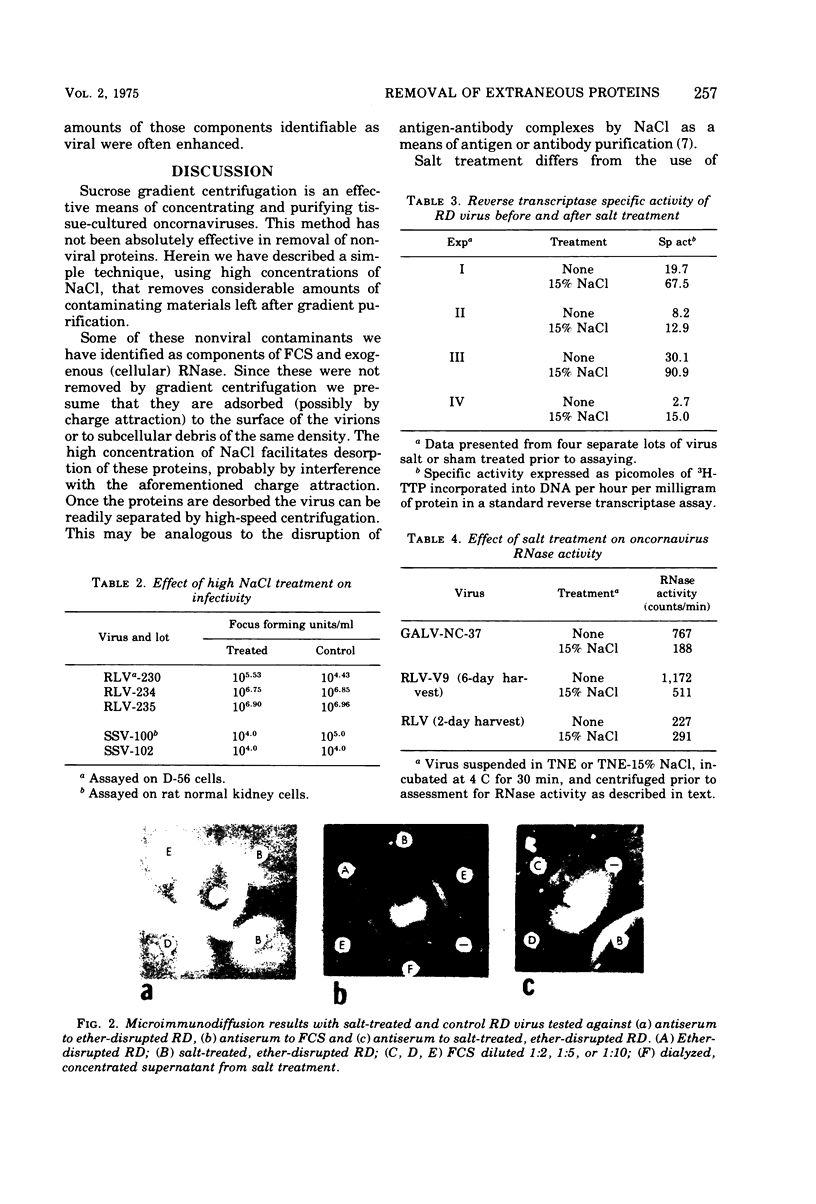
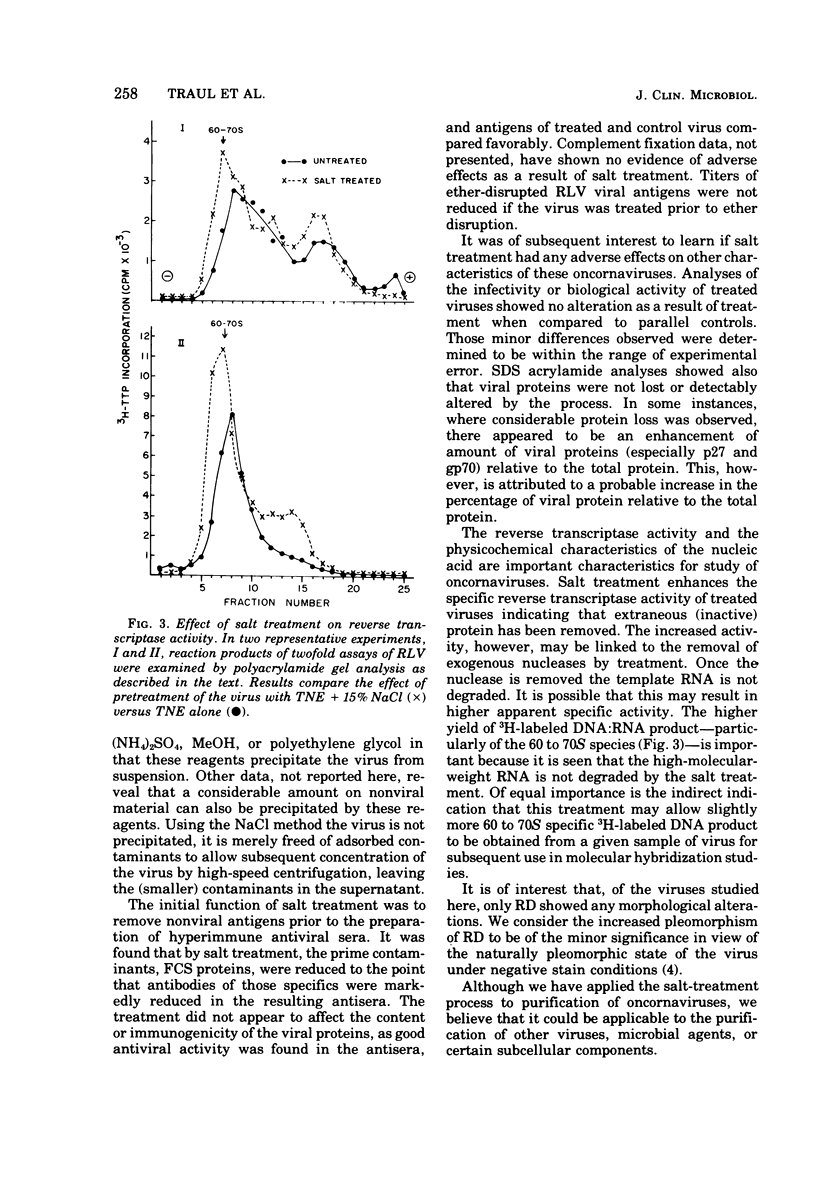
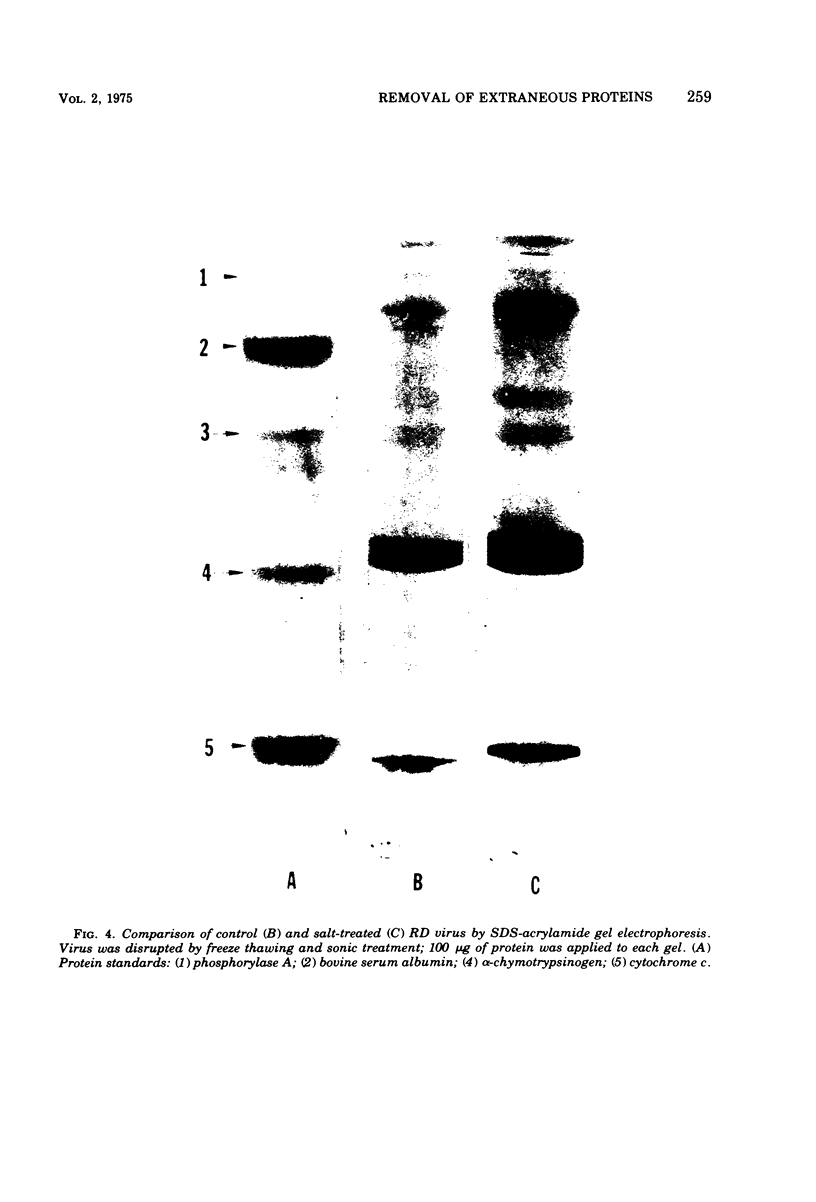
Conventional methods (i.e. gradient centrifugation) for the purification of oncornaviruses are usually not effective in complete removal of nonviral proteins. Such contaminants often prove to be a nuisance in subsequent immunological or biochemical studies. Hyperimmune sera prepared from these viruses must be absorbed to assure specificity; cell-derived proteins can be shown to interfere with studies of virus structural proteins, nucleic acids, or viral enzymes. Herein is described a method for removal of most of these contaminants. Viruses are diluted in a high concentration of NaCl to achieve a final concentration of 15%, incubated for 30 min, sedimented, and resuspended in buffer. This procedure results in reductions of up to 48% of the protein without affecting particle count. Immunological, biochemical, and biological properties are not adversely affected. Of the proteins removed, fetal calf serum components and a ribonuclease (presumably cell-derived) were identified. This technique differs significantly from other high-salt methods in that the virus is not precipitated from suspension. It is believed that absorbed proteins are desorbed and left in solution (or suspension) as the virus is sedimented by centrifugation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Biologic characterization of mammalian cells transformed by a primate sarcoma virus. Virology. 1973 Apr;52(2):562–567. doi: 10.1016/0042-6822(73)90351-6. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Dessev G. N., Grancharov K. Precipitation of RNA, DNA, and nucleoprotein particles from very dilute solutions. Anal Biochem. 1973 May;53(1):269–271. doi: 10.1016/0003-2697(73)90428-4. [DOI] [PubMed] [Google Scholar]
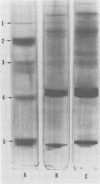
- Harewood K., Wolff J. S., 3rd A rapid electrophoretic procedure for the detection of SDS-released oncorna-viral RNA using polyacrylamide-agarose gels. Anal Biochem. 1973 Oct;55(2):573–581. doi: 10.1016/0003-2697(73)90146-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayol R. F., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XXXVI. Measurement of virus-specific proteins during a normal cycle of infection. J Virol. 1970 Sep;6(3):310–319. doi: 10.1128/jvi.6.3.310-319.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
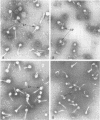
- Monroe J. H., Brandt P. M. Rapid semiquantitative method for screening large numbers of virus samples by negative staining electron microscopy. Appl Microbiol. 1970 Aug;20(2):259–262. doi: 10.1128/am.20.2.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Boyse E. A., de Harven E., Geering G. Group-specific viral antigens in the milk and tissues of mice naturally infected with mammary tumor virus or Gross leukemia virus. Virology. 1968 Apr;34(4):617–629. doi: 10.1016/0042-6822(68)90083-4. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Stephens R., Traul K., Lowry G., Zelljadt I., Mayyasi S. Differential morphology of the RD virus from the human rhabdomyosarcoma, RD-114B cell line demonstrated by negative staining electron microscopy. Nat New Biol. 1972 Dec 13;240(102):212–214. doi: 10.1038/newbio240212a0. [DOI] [PubMed] [Google Scholar]
- Traul K. A., Mayyasi S. A., Garon C. E., Schidlovsky G., Bulfone L. M. Antigenic comparison of Rauscher murine leukemia virus cultivated in human embryo and mouse cells. Proc Soc Exp Biol Med. 1972 Jan;139(1):10–14. doi: 10.3181/00379727-139-36065. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]