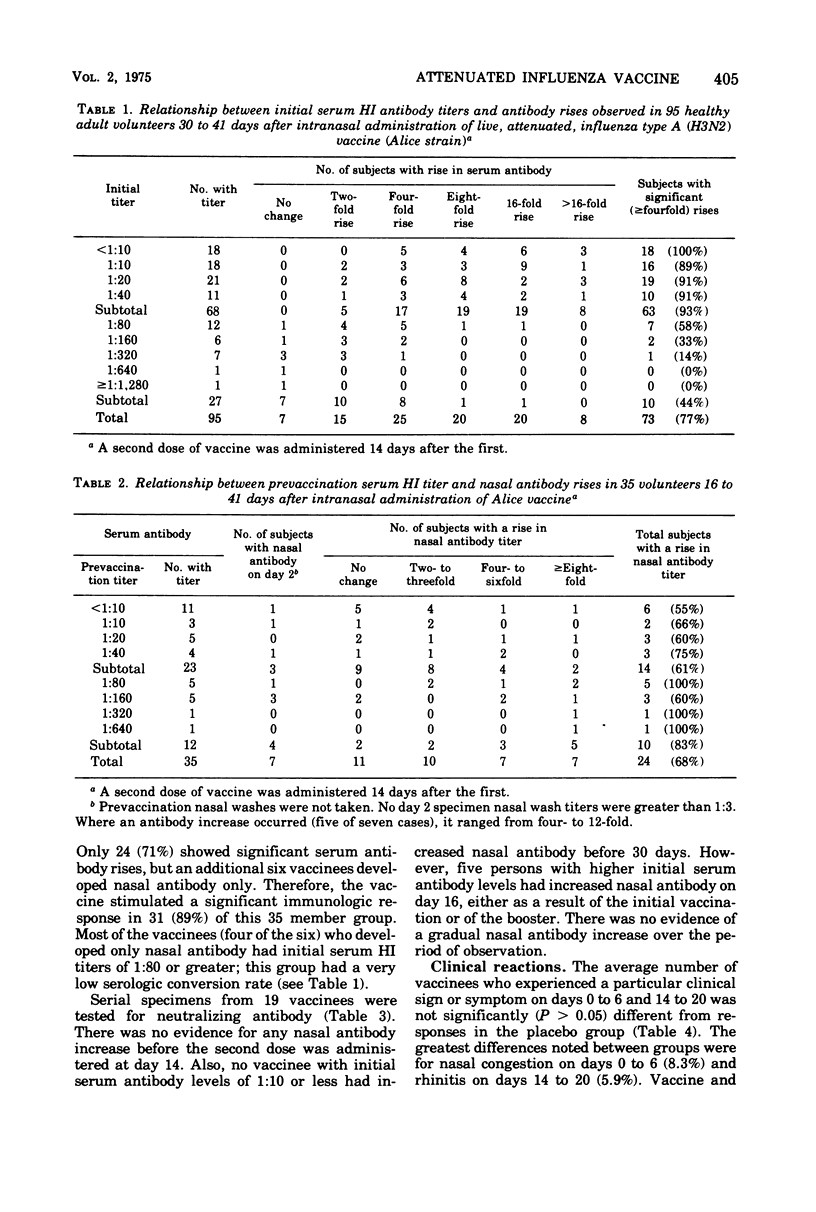
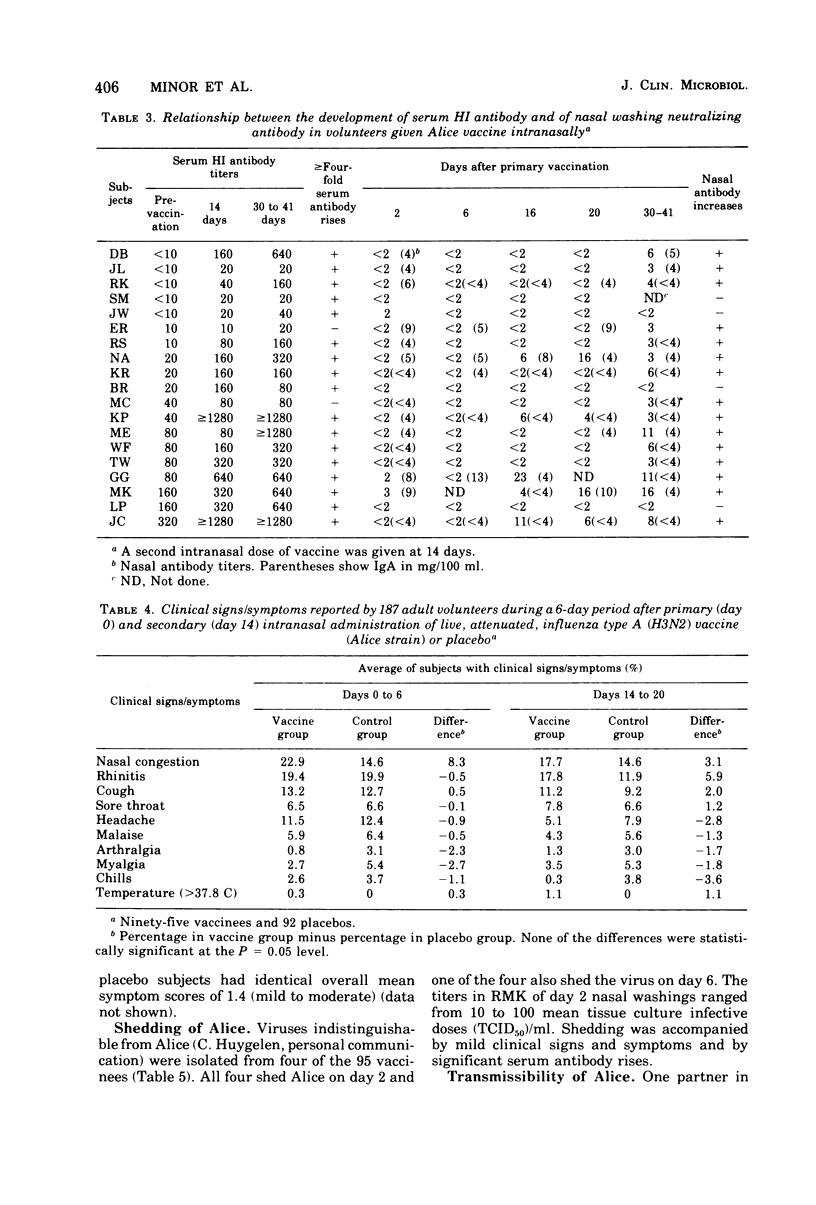
Abstract
Ninety-five healthy adults, ages 18 to 56 years, received two intranasal doses, 2 weeks apart, of a live, attenuated, influenza type A (H3N2) vaccine (an inhibitor-resistant recombinant strain of A/England/42/72 named "Alice"). Ninety-two persons were given placebos similarly. Ninety-three percent of 68 subjects with initial serum hemagglutination-inhibition (HI) titers of greater than or equal to 1:40 to influenza A (H3N2) had a fourfold or greater antibody increase in postvaccination sera. Forty-four percent of 27 subjects with an initial HI titer of greater than or equal to 1:80 had similar increases. Overall, 77% of vaccinees had fourfold or greater antibody titer increases. Vaccinees had geometric mean serum HI titers (GMT) of 1:26, 1:123, and 1:166 at 0, 14, and 30 days, respectively. The GMTs for placebos were 1:21, 1:22, and 1:21. Thirty-five vaccinees were examined for both serum and nasal antibody; 89% had significant increases in one or both. Nasal antibody response was directly related to the level of initial serum HI titer in that 83% of 12 persons with prevaccination HI titers of 1:80 greater than or equal to 1:80 showed significant nasal antibody rises, whereas only 61% of the remaining 23 subjects with prevaccination HI titers of less than or equal to 1:40 did so. The number and severity of clinical signs and symptoms reported by vaccinees and placebos did not differ significantly. The greatest differences noted between groups were for nasal congestion on days 0 to 6 (8.3%) and rhinitis on days 14 to 20 (5.9%). Four vaccinees shed Alice after primary vaccination, but viral titers were low (10 to 100 tissue culture-infective doses/ml). One member in each of 15 cohabiting male-female couples received Alice while the other received a placebo; one of the placebo members had significant increases in serum and nasal antibody, indicating a possible transmission.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- D'Alessio D. J., Cox P. M., Jr, Dick E. C. Failure of inactivated influenza vaccine to protect an aged population. JAMA. 1969 Oct 20;210(3):485–489. [PubMed] [Google Scholar]
- Dick E. C., Blumer C. R., Evans A. S. Epidemiology of infections with rhinovirus types 43 and 55 in a group of university of Wisconsin student families. Am J Epidemiol. 1967 Sep;86(2):386–400. doi: 10.1093/oxfordjournals.aje.a120749. [DOI] [PubMed] [Google Scholar]
- Fulk R. V., Fedson D. S., Huber M. A., Fitzpatrick J. R., Kasel J. A. Antibody responses in serum and nasal secretions according to age of recipient and method of administration of A2-Hong Kong-68 inactivated influenza virus vaccine. J Immunol. 1970 Jan;104(1):8–13. [PubMed] [Google Scholar]
- Gwaltney J. M., Jr, Edmondson W. P., Jr, Rothenberg R., White P. W. A comparison of subcutaneous, nasal, and combined influenza vaccination. I. Antigenicity. Am J Epidemiol. 1971 Jun;93(6):472–479. doi: 10.1093/oxfordjournals.aje.a121281. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Hume E. B., Fulk R. V., Togo Y., Huber M., Hornick R. B. Antibody responses in nasal secretions and serum of elderly persons following local or parenteral administration of inactivated influenza virus vaccine. J Immunol. 1969 Mar;102(3):555–562. [PubMed] [Google Scholar]
- Miller L. W., Hume E. B., O'Brien F. R., Togo Y., Hornick R. B. Alice strain live attenuated influenza (H3N2) vaccin in an elderly population. Am J Epidemiol. 1975 Apr;101(4):340–346. doi: 10.1093/oxfordjournals.aje.a112102. [DOI] [PubMed] [Google Scholar]
- Minor T. E., Dick E. C., DeMeo A. N., Ouellette J. J., Cohen M., Reed C. E. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974 Jan 21;227(3):292–298. [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Prévost J. M., Peetermans J., Lamy F., Huygelen C. Immune response to vaccination with a live influenza virus (H3N2) vaccine ("Ann" strain). Infect Immun. 1973 Sep;8(3):420–424. doi: 10.1128/iai.8.3.420-424.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben F. L., Jackson G. G. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis. 1972 Jun;125(6):656–664. doi: 10.1093/infdis/125.6.656. [DOI] [PubMed] [Google Scholar]
- Schiff G. M., Linnemann C. C., Jr, shea L., Lange B., Rotte T. Evaluation of a live, attenuated recombinant influenza vaccine in high school children. Infect Immun. 1975 Apr;11(4):754–757. doi: 10.1128/iai.11.4.754-757.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R. P., Hendley J. O., Sande M. A., Gwaltney J. M., Jr Revised (1972-1973) bivalent influenza vaccine. Serum and nasal antibody responses to parenteral vaccination. JAMA. 1973 Oct 22;226(4):435–438. [PubMed] [Google Scholar]



