Abstract
Low bone mass is a determinant of fractures in healthy children. Small studies provide limited evidence on the association between ethnicity, birth weight, family size, socioeconomic status, dietary calcium intake, or physical activity and fracture incidence. No studies have investigated whether these determinants of fracture risk act through affecting bone mass or through other mechanisms. The aim of this study was to use a population-based birth cohort to confirm which variables are determinants of fracture risk and to further study which of these risk factors act independently of bone mass. Children from the Avon Longitudinal Study of Parents and Children have been followed up from birth to 11 yr of age. Maternal self-reported data have been collected contemporaneously on early life factors, diet, puberty, and physical activity. These were linked to reported fractures between 9 and 11 yr of age. Multivariable logistic regression techniques were used to assess whether these potential determinants were independent of, or worked through, estimated volumetric BMD or estimated bone size relative to body size measured by total body DXA scan at 9.9 yr of age. A total of 2692 children had full data. One hundred ninety-three (7.2%) reported at least one fracture over the 2-yr follow-up period. Children who reported daily or more episodes of vigorous physical activity had double the fracture risk compared with those children who reported less than four episodes per week (OR, 2.06; 95% CI, 1.21–1.76). No other independent determinants of fracture risk in healthy children were found. In conclusion, reported vigorous physical activity is an independent risk factor for childhood fracture risk. However, the interrelationship between physical activity, bone mass, and childhood fracture risk suggests that the higher bone mass associated with increased physical activity does not compensate for the risk caused by increased exposure to injuries.
Keywords: fractures, children, physical activity, epidemiology, BMD, ALSPAC
INTRODUCTION
Fractures in healthy children are an important but neglected public health issue; 1.2%(1) to 3.6%(2) of children fracture a bone each year, and the lifetime risk of sustaining a fracture in childhood for boys is 42–64% and for girls is 27–40%.(1,3) The incidence of childhood fractures also seems to be increasing over time,(3) although this may now be reaching a plateau.(4)
Studying the determinants of fracture risk in healthy children is important, not only because fractures in healthy children are a neglected public health issue, but also because there is a concern that childhood fractures may be a marker of low peak bone mass acquisition and hence persistent skeletal fragility.(5) There is also a suggestion that the effect of increasing childhood bone mass by 10% would be to delay postmenopausal osteoporosis by ~13 yr.(6)
The most rigorously studied determinant of fracture risk in healthy children is bone mass. The evidence from both prospective(7) and case-control studies(8) strongly suggests that low BMD and low bone size relative to body size are risk factors for fractures in healthy children. Other determinants have also consistently shown an association with fractures in healthy children, particularly sex and age. The peak age of incidence of all childhood fractures is fairly consistent across the literature, with a peak at ~14 yr of age for boys, and ~11 yr of age for girls,(3,9,10) with a sharp decline in rate afterward. Overall, boys have a higher fracture rate compared with girls at all ages.(10)
There are small studies providing limited or contradictory evidence on the association between ethnicity,(11,12) birth weight,(13,14) family size,(12,15) socioeconomic status,(16,17) dietary calcium intake,(18,19) or physical activity(13,20,21) and fracture incidence in healthy children.
However, there are no studies that we could find that investigated which of these many determinants of fracture risk in healthy children are independent risk factors, particularly which act through affecting bone mass and which affect fracture risk through other mechanisms. Therefore, the aim of this study was to use a population-based birth cohort study to confirm which variables are determinants of fracture risk in healthy children and to further study which of these act independently of bone mass.
MATERIALS AND METHODS
Study population
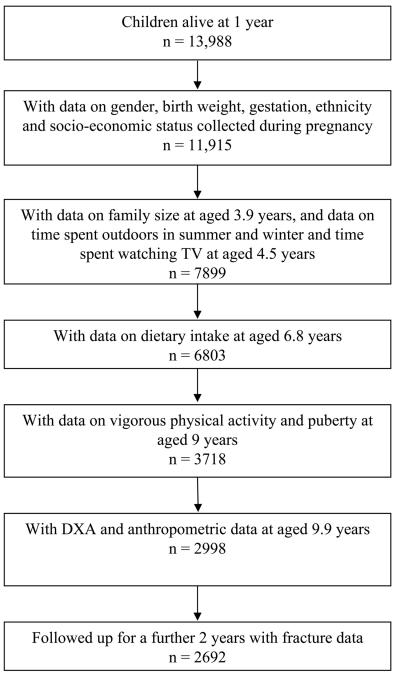
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a geographically based UK cohort that recruited pregnant women residing in Avon (southwest England) with an expected date of delivery between April 1, 1991 and December 31, 1992.(22) A total of 14,541 pregnancies were enrolled, with 14,062 children born (see www.alspac.bris.ac.uk for more information). Of these births, 13,988 children were alive at 12 mo (Fig. 1). This study is based on 2692 children with complete data. Ethical approval was obtained from the ALSPAC Law and Ethics committees and the Local Research Ethics Committees. Parental consent and child's assent was obtained for all measurements made.
FIG. 1.
Flow diagram showing the children from ALSPAC included in this analysis.
Early life factors
The mother's, partner's, and grandparent's race and ethnic group was recorded by the mother on self-reported questionnaires sent out at ~32 wk of gestation and was categorized as white or nonwhite. Sex was obtained from birth notifications. Data on birth weight were collected from obstetric data, from birth notifications, or as recorded by the ALSPAC measurers. Low birth weight was defined as <2500 g. Gestational age was also recorded and used as a binary variable: <37 or ≥37 wk.
Socioeconomic status
Mothers highest educational qualifications were also assessed at 32 wk of gestation and were coded on a five-point ascending scale where levels 1, 2, and 3 refer to educational qualifications generally gained at school by 16 yr of age, level 4 to qualifications gained at school at 18 yr of age, and level 5 to university degrees. Other measures of socioeconomic status such as paternal education, maternal and paternal social class, and housing tenure were not used in this analysis because they gave similar results to maternal education alone, as shown in a previous study on this cohort.(23) Data on the total number of persons in the household were collected when the child was 3.9 yr of age. This family size variable was categorized as one to three people, four people, and more than four people in the household.
Assessment of activity levels
Data on physical activity were collected by self-completion questionnaire at two time points: at 4.5 and 9 yr of age. At 4.5 yr of age, questions were asked about average time spent per week watching TV, time spent outdoors in summer, and time spent outdoors in winter. These were categorized into tertiles or binary variables. At 9 yr of age, a question was asked about the average number of times the child had participated in vigorous physical activity over the last month. Vigorous physical activity was defined as activities such as running, dance, gymnastics, netball, swimming, aerobics, or similar. This variable was reduced to three categories: less than four episodes per week; four to six episodes per week; or daily or more vigorous physical activity.
Other explanatory variables measured
At 6.8 yr of age, a food frequency questionnaire was sent to all mothers. The answers to this were calibrated against the dietary record information obtained from a random 10% subsample when the child was 7 yr of age to calculate nutrient intakes. For this study, the dietary variables studies were calcium, vitamin D, and total energy intake. An approximate daily nutrient intake for each child was calculated by multiplying the weekly frequency of consumption of a food by the nutrient content obtained from the 5th Edition of McCance and Widdowson's The Composition of Foods(24) and dividing by seven. Puberty was assessed by self-completion questionnaires at 9 yr of age using diagrams based on Tanner staging of pubic hair distribution for boys and girls and breast development for girls. Daily calcium, vitamin D, and total energy intake were divided into quartiles.
Measure of height, weight, and DXA-derived parameters
At the research clinic at 9.8 yr of age, height was measured to the last complete millimeter using a Harpenden stadiometer. Weight was measured to the nearest 50 g using a Tanita Body Fat Analyzer (model TBF 305). Total body less head bone area (TBLH BA) and total body less head BMC (TBLH BMC) were measured using a Lunar Prodigy DXA in 7444 of the 7725 children. Total body DXA scans were not used because the head is not responsive to environmental stimuli such as exercise.(25) A total of 111 DXA scans were not interpretable because of the presence of large movement artifacts or other anomalies, yielding 7333 (98.5%) usable scans. The CV for TBLH BMC was 0.8% based on 120 repeat scans. To estimate TBLH vBMD, TBLH BMC was adjusted for height, weight, and TBLH BA using linear regression. To estimate TBLH bone size relative to body size, TBLH BA was adjusted for height and weight using linear regression. For all the DXA and anthropometric measurements, Z-scores were calculated by subtracting the cohort mean from the individual measurements and dividing by the cohort SD.
Fracture incidence and descriptions of events surrounding the injury
DXA results were linked to results of a questionnaire administered on subsequent attendance at research clinics ~12 and 24 mo later, where children were asked if they had broken a bone since they visited for their DXA scan. Children who indicated they had sustained a fracture were sent a further questionnaire to collect information on the nature and circumstances of the injury and for consent to obtain a copy of their X-ray report. The time interval between the injury and return of the questionnaire ranged from weeks to many months. In those where radiology reports were available (~40%), 87% confirmed a fracture. For the rest of this study, it is reported fracture, not verified fracture, that is used as the outcome.
Statistical analyses
All statistical analyses were performed using STATA 8.0. The outcome measure was presence or absence of reported fracture over the 2-yr time period as a binary outcome. Odds of exposure to the putative risk factors in those with fractures compared with those without were calculated. Logistic regression was used to calculate ORs and 95% CIs to describe the association between risk factor and presence or absence of fracture. The OR test for trend was calculated by treating the categorical variables as continuous variables in the regression models. Z-scores were produced for the DXA and anthropometric variables, so ORs are for fracture risk per SD unit change. All analyses using DXA parameters were adjusted for age at measurement. Models were initially run separately for boys and girls, but because no difference was seen, results are presented for both sexes combined. Models were also run using birth weight, family size, time spent watching TV, in a vehicle, and outside, vigorous physical activity at 9 yr of age, and calcium or vitamin D intake as continuous or categorical variables, but because no difference was seen, results are presented using categorical variables for ease of interpretation. For dietary variables, total energy intake was included in the regression models. To look for independent determinants of fracture risk, all variables found to be associated with fractures during minimally adjusted analyses were included in a multivariable regression model.
RESULTS
Of the 2692 children included in this study, 1276 (47.4%) were male, 83 (3.1%) were of nonwhite ethnicity, 105 children (3.9%) were of low birth weight, and 127 (4.7%) were premature. Five hundred fifteen (19.1%) had mothers educated to degree level or higher. Six hundred sixty-one children (24.6%) lived in families with more than four members. A total of 1965 (73.0%) of children spent more than 28 h/wk outside in the summer. Three hundred eighty-four (14.3%) did daily or more episodes of vigorous physical activity. The majority of the children (2201, 81.8%) were in Tanner stage 1 of puberty at 9 yr of age. The mean daily calcium, vitamin D, and total energy intake for the children were 898 ± 295 mg, 2.87 ± 1.30 μg, and 7689 ± 1744 kJ, respectively. The mean height, weight, and BMI of the children were 139.6 ± 6.3 cm, 34.4 ± 7.2 kg, and 17.5 ± 2.8 kg/m2, respectively. One hundred ninety-three (7.2%) reported at least one fracture over the 2-yr follow-up period. As previously reported,(7,26) 71.4% of fractures occurred in the upper limb.
In unadjusted analyses, boys had a higher risk of fractures than girls (Table 1), with girls having a 26% reduced risk (OR, 0.74; 95% CI, 0.55–0.99). Children of normal gestation had three times the fracture rate of children born prematurely, although the CI was wide (OR, 3.31; 95% CI, 1.04–10.49). Children who spent more that 28 h/wk outdoors in summer had a doubled risk of fracture than children who spent less time outdoors, with an OR of fracture risk of 2.01 (95% CI, 1.36–2.99; Table 3). Children who did daily or more episodes of vigorous physical activity also had a doubling of fracture risk compared with those children who did less than four episodes per week (OR, 2.06; 95% CI, 1.21–1.76; Table 3). There was no association between birth weight, ethnicity, socioeconomic status (maternal education), family size, dietary intake of calcium, vitamin D, or energy, time spent watching TV, pubertal status, or any anthropometric measure and childhood fracture risk (Tables 1-4). Similar results were obtained when treating birth weight and dietary intake as continuous variables.
Table 1.
Early Life and Socioeconomic Details of the Children in This Study
| Children without fractures (n = 2499) [N (%)] |
Children with fractures (n = 193) [N (%)] |
p value for difference between children with and without fractures |
Crude OR for fracture risk OR (95% CI) |
Adjusted OR for fracture risk OR (95% CI), p value* |
Adjusted OR for fracture risk† |
Adjusted OR for fracture risk‡ |
|
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 1171 (91.8) | 105 (8.2) | 0.043 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1328 (93.8) | 88 (6.2) | 0.74 (0.55, 0.99) | 0.80 (0.59, 1.10) | 0.80 (0.58, 1.09) | 0.75 (0.55, 1.03) | |
| Birth weight | |||||||
| Normal | 2397 (92.7) | 190 (7.3) | 0.081 | 1.0 | 1.0 | 1.0 | 1.0 |
| Low | 102 (97.1) | 3 (2.9) | 0.37 (0.12, 1.18) | 0.54 (0.15, 1.89) | 0.57 (0.16, 1.99) | 0.53 (0.15, 1.86) | |
| Gestational age | |||||||
| <37 wk | 124 (97.6) | 3 (2.4) | 0.031 | 1.0 | 1.0 | 1.0 | 1.0 |
| 37 wk or more | 2375 (92.6) | 190 (7.4) | 3.31 (1.04, 10.49) | 2.64 (0.76, 9.14) | 2.72 (0.78, 9.45) | 2.66 (0.77, 9.24) | |
| Ethnicity | |||||||
| White | 2418 (92.7) | 191 (7.3) | 0.088 | 1.0 | 1.0 | 1.0 | 1.0 |
| Nonwhite | 81 (97.6) | 2 (2.4) | 0.31 (0.08, 1.28) | 0.30 (0.07, 1.27) | 0.30 (0.07, 1.26) | 0.32 (0.08, 1.36) | |
| Maternal education | |||||||
| Level 1 | 228 (89.8) | 26 (10.2) | 0.194 | 1.0 | 1.0 | 1.0 | 1.0 |
| Level 2 | 170 (90.9) | 17 (9.1) | 0.88 (0.46, 1.67) | 0.86 (0.45, 1.68) | 0.86 (0.44, 1.68) | 0.84 (0.43, 1.63) | |
| Level 3 | 903 (93.2) | 66 (6.8) | 0.64 (0.40, 1.03) | 0.64 (0.39, 1.05) | 0.64 (0.39, 1.04) | 0.64 (0.39, 1.05) | |
| Level 4 | 720 (93.9) | 47 (6.1) | 0.57 (0.35, 0.95) | 0.58 (0.34, 0.98) | 0.57 (0.34, 0.97) | 0.57 (0.34, 0.97) | |
| Level 5 | 478 (92.8) | 37 (7.2) | 0.68 (0.40, 1.15) OR test for trend: 0.86 (0.79, 1.01) |
0.70 (0.40, 1.23) OR test for trend: 0.88 (0.78, 1.01) |
0.70 (0.40, 1.23) OR test for trend: 0.88 (0.77, 1.01) |
0.70 (0.40, 1.23) OR test for trend: 0.88 (0.77, 1.01) |
|
| Family size | |||||||
| 1–3 people | 388 (92.8) | 30 (7.2) | 0.394 | 1.0 | 1.0 | 1.0 | 1.0 |
| 4 people | 1505 (93.3) | 108 (6.7) | 0.93 (0.61, 1.41) | 0.85 (0.55, 1.31) | 0.82 (0.53, 1.27) | 0.86 (0.55, 1.32) | |
| >4 people | 606 (91.7) | 55 (8.3) | 1.17 (0.74, 1.87) OR test for trend: 1.11 (0.88, 1.41) |
1.01 (0.63, 1.63) OR test for trend: 1.04 (0.82, 1.33) |
0.98 (0.61, 1.58) OR test for trend: 1.03 (0.81, 1.31) |
1.04 (0.64, 1.67) OR test for trend: 1.06 (0.83, 1.35) |
Number and percentage for children with and without fractures are given, along with crude and adjusted ORs for fracture risk. Statistically significant relationships are shown in bold.
Table 3.
Physical Activity and Puberty Details for the Children in This Study
| Children without fractures (n = 2499) [N (%)] |
Children with fractures (n = 193) [N (%)] |
p value for difference between children with and without fractures |
Crude OR for fracture risk OR (95% CI) |
Adjusted OR for fracture risk OR (95% CI), p value* |
Adjusted OR for fracture risk† |
Adjusted OR for fracture risk‡ |
|
|---|---|---|---|---|---|---|---|
| Time outdoors in summer | |||||||
| <28 h/wk | 696 (95.7) | 31 (4.3) | <0.001 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥28 h/wk | 1803 (91.8) | 162 (8.2) | 2.01 (1.36, 2.99) | 2.39 (1.57, 3.63) | 2.37 (1.56, 3.62) | 2.38 (1.56, 3.62) | |
| Time outdoors in winter (tertiles) | |||||||
| 1 (least) | 1034 (91.7) | 94 (8.3) | 0.029 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 1166 (94.3) | 71 (5.7) | 0.67 (0.49, 0.92) | 0.53 (0.38, 0.75) | 0.54 (0.39, 0.76) | 0.53 (0.38, 0.75) | |
| 3(most) | 299 (91.4) | 28 (8.6) | 1.03 (0.66, 1.60) OR Test for Trend: 0.90 (0.72, 1.12) |
0.79 (0.50, 1.25) OR Test for Trend: 0.77 (0.61, 0.97) |
0.79 (0.50, 1.25) OR Test for Trend: 0.77 (0.61, 0.98) |
0.79 (0.50, 1.25) OR Test for Trend: 0.77 (0.61, 0.97) |
|
| Time watching TV (tertiles) | |||||||
| 1 (least) | 829 (92.0) | 72 (8.0) | 0.402 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 978 (93.6) | 67 (6.4) | 0.79 (0.56, 1.11) | 0.76 (0.53, 1.09) | 0.76 (0.53, 1.09) | 0.75 (0.52, 1.07) | |
| 3(most) | 692 (92.8) | 54 (7.2) | 0.90 (0.62, 1.30) OR test for trend: 0.94 (0.78, 1.13) |
0.77 (0.52, 1.14) OR test for trend: 0.86(0.71, 1.05) |
0.76 (0.51, 1.13) OR test for trend: 0.86 (0.70, 1.05) |
0.75 (0.51, 1.12) OR test for trend: 0.86 (0.70, 1.05) |
|
| Vigorous physical activity | |||||||
| <4 episodes | 1414 (94.5) | 82 (5.5) | <0.001 | 1.0 | 1.0 | 1.0 | 1.0 |
| 4–6 episodes | 742 (91.4) | 70 (8.6) | 1.63 (1.17, 2.27) | 1.68 (1.19, 2.37) | 1.70 (1.21, 2.40) | 1.71 (1.21, 2.41) | |
| daily or more | 343 (89.3) | 41 (10.7) |
2.06 (1.39, 3.05) OR test for trend: 1.46(1.21, 1.76) |
2.07 (1.37, 3.14) OR test for trend: 1.45 (1.19, 1.77) |
2.13 (1.40, 3.23) OR test for trend: 1.47 (1.20, 1.79) |
2.19 (1.44, 3.33) OR test for trend: 1.48 (1.22, 1.81) |
|
| Puberty (pubic hair distribution) | |||||||
| Tanner stage 1 | 2037 (92.6) | 164 (7.4) | 0.480 | 1.0 | 1.0 | 1.0 | 1.0 |
| Tanner stage 2 | 409 (94.0) | 26 (6.0) | 0.79 (0.52, 1.21) | 0.85 (0.54, 1.31) | 0.85 (0.54, 1.31) | 0.84 (0.54, 1.31) | |
| Tanner stage 3 | 53 (94.6) | 3 (5.4) | 0.70 (0.22, 2.27) OR test for trend: 0.81 (0.56, 1.15) |
0.70 (0.21, 2.35) OR test for trend: 0.83 (0.57, 1.20) |
0.72 (0.21, 2.43) OR test for trend: 0.83 (0.58, 1.20) |
0.74 (0.22, 2.49) OR test for trend: 0.84 (0.58, 1.21) |
Number and percentage for children with and without fractures are given, along with crude and adjusted ORs for fracture risk. Statistically significant relationships are shown in bold.
Table 4.
Anthropometric Details of the Children in This Study
| Children without fractures (n = 2499) [N (%)] |
Children with fractures (n = 193) [N (%)] |
p value for difference between children with and without fractures |
Crude OR for fracture risk OR (95% CI) |
Adjusted OR for fracture risk OR (95% CI), p value* |
Adjusted OR for fracture risk† |
Adjusted OR for fracture risk‡ |
|
|---|---|---|---|---|---|---|---|
| Height (cm) | 139.6 (6.3) | 139.5 (6.2) | 0.742 | 0.98 (0.84, 1.13) | 1.14 (0.47, 2.77) | 1.07 (0.43, 2.65) | 1.16 (0.48, 2.78) |
| Weight (kg) | 34.4 (7.2) | 34.2 (7.1) | 0.616 | 0.96 (0.83, 1.12) | 0.70 (0.09, 5.37) | 0.80 (0.10, 6.35) | 0.67 (0.09, 4.98) |
| BMI (kg/m2) | 17.5 (2.8) | 17.5 (2.8) | 0.679 | 0.84 (0.83, 1.13) | 1.35 (0.28, 6.52) | 1.23(0.25,6.11) | 1.40 (0.30, 6.58) |
Mean and SD for children with and without fractures are given, along with crude and adjusted ORs for fracture risk. Statistically significant relationships are shown in bold.
To assess whether these associations were confounded by any of the other variables, the analyses were rerun including all other variables in the four tables. No real change was seen for the association between sex and fracture risk (Table 1). There was a reduction in the strength of association between gestation and fracture risk, with the CIs now crossing zero (OR, 2.64; 95% CI, 0.76–9.14). The magnitude of the association between time spent outdoors in summer and fracture risk increased (OR changed from 2.01 to 2.39), as did the association between time spent outdoors in winter and fracture risk (OR changed from 0.90 to 0.77; Table 3). Children who spent the most time outdoors in winter, after adjusting for all other variables, had a 23% reduced risk of fracture compared with those children who spent the least time outside in winter (adjusted OR, 0.77; 95% CI, 0.61–0.97). There was no change in the association between vigorous physical activity and fracture risk. All other associations remained null.
To study whether these variables were determinants of fracture risk independent of estimated volumetric density, the regression analyses were rerun including estimated TBLH vBMD (Tables 1-4). No change was seen in either the magnitude or direction of any association found, suggesting that sex, time spent outdoors in summer or winter, and vigorous physical activity are risk factors for childhood fracture risk, irrespective of BMD. Similar results were seen after adjusting for estimated TBLH bone size relative to body size.
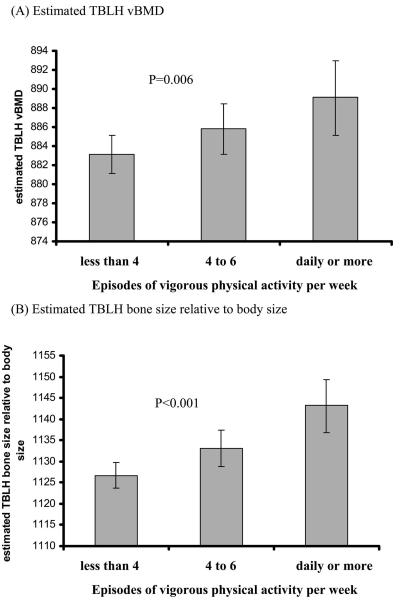
To further study the association between reported vigorous physical activity, bone mass, and childhood fracture risk, the association between vigorous physical activity and TBLH vBMD and bone size relative to body size was assessed (Fig. 2). Analyses showed a strong positive association between reported vigorous physical activity and both estimated volumetric BMD and bone size relative to body size (i.e., children who reported daily or more vigorous physical activity has a higher volumetric BMD and bone size relative to body size compared with children who reported less than four episodes).
FIG. 2.
Bar charts showing mean ± 95% CI for (A) estimated TBLH vBMD and (B) estimated TBLH bone size relative to body size according to category of reported vigorous physical activity. p values are the test for trend. Results are adjusted for all variables in Tables 1-4.
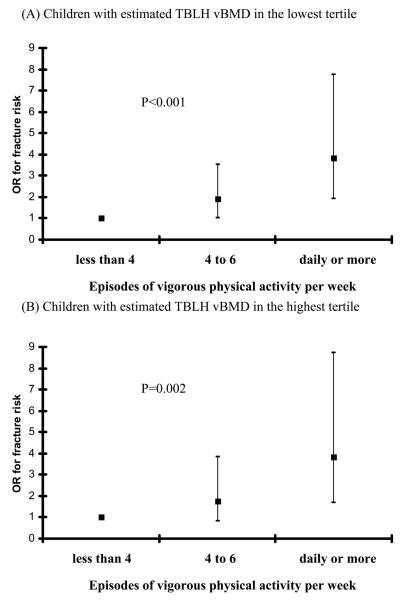
However, after the children were stratified into those with an estimated volumetric BMD in the lowest tertile and those in the highest tertile (Fig. 3), similar fracture risks were seen in those children with the lowest BMD who reported daily or more vigorous physical activity compared with those with the highest BMD who reported similar amounts of activity (ORs of 3.84 compared with 3.85). Similar stratification for the associations between sex, time spent outdoors in summer, or time spent outdoors in winter and fracture risk suggested residual confounding by BMD. Similar results were seen for vigorous physical activity in children with the lowest versus highest tertiles of estimated TBLH bone size relative to body size.
FIG. 3.
Box plots of OR for fracture risk ±95% CI for children who did less than four episodes of vigorous physical activity per week, those who did four to six, and those who did daily or more in (A) children with an estimated TBLH vBMD in the lowest tertile and (B) children with an estimated TBLH vBMD in the highest tertile. p values are the test for trend. Results are adjusted for all variables in Tables 1-4. For box plot A, 27 children had fractures in the less than four episodes of vigorous physical activity per week category, 23 in those who did four to six, and 18 in those who did daily or more. For box plot B, 19 children had fractures in the less than four episodes of vigorous physical activity per week category, 14 in those who did four to six, and 15 in those who did daily or more.
DISCUSSION
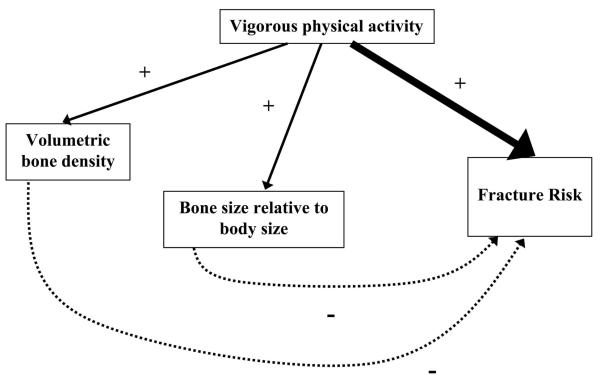
Our study showed that, despite being associated with both higher volumetric BMD and bone size relative to body size, daily or more vigorous physical activity increases fracture risk, presumably through increased exposure to injuries: even among those with an estimated volumetric BMD or bone size relative to body size in the highest tertile, daily or more episodes of vigorous physical activity resulted in a tripling of fracture risk. This suggests that the higher bone mass associated with increased physical activity in children does not compensate for the increased exposure to injuries (Fig. 4). To the best of our knowledge, this is the first time this association between physical activity, bone mass, and fracture risk in children has been explored in a prospective study.
FIG. 4.
Proposed relationships between reported vigorous physical activity at 9 yr of age, bone mass at 9.9 yr of age, and fracture risk over the following 2 yr. The higher bone mass associated with increased physical activity does not compensate for the increased exposure to injuries (solid arrows, positive associations; dashed arrows, negative associations).
Studies on the epidemiology of childhood fractures suggest that 36%(2) to 50%(27) of fractures in children are related to sporting activities, many of which may involve vigorous physical activity, suggesting that exposure to injuries is an important determinant of childhood fracture risk. Other studies also agreed with this finding and reported that higher levels of sports participation increases the risk of fracture in children,(13,20) although these did not assess the additional association between the physical activity and bone mass.
However, two studies on the same cohort of children from Tasmania have shown that time spent watching TV or videos or playing on a computer was associated with increased upper limb fractures in both boys and girls,(20,28) which was not replicated here. Likely reasons for this discrepancy include study design issues such as smaller sample size, but also the use of time spent watching TV as a proxy for less physical activity. A recent meta-analysis(29) suggests that the relationship between TV viewing and physical activity in children is negative but small, and watching television may replace other sedentary activities such as reading rather than replacing physical activity.
Results from this study of a positive association between vigorous physical activity and childhood fracture risk that is independent of bone mass can perhaps help explain the contradictory literature. Data from randomized controlled trials show that physical activity in children increases volumetric BMD in the bones that are involved in the exercise,(30-33) and this agrees with reports that light physical activity reduces fracture risk in children.(20) However, the type of activity may be important. Physical activity with a small chance of injury probably increases bone mass and may reduce fracture risk,(20) whereas vigorous physical activity or contact sports participation probably also increases bone mass, but because of increased numbers of injuries, also increases fracture risk. The current literature is likely to be contradictory because the interrelated effects of physical activity, bone mass, and injury exposure have not been fully explored.
Our finding that, despite having an estimated volumetric BMD or bone size relative to body size in the highest tertile, daily or more episodes of vigorous physical activity increases fracture risk in children perhaps suggests that, to reduce childhood fractures, we should advise children to do less vigorous physical activity. However, this is clearly in-appropriate. Discipline-specific advice such as results of this pediatric epidemiological study should not be used in isolation to formulate public health policy, because there are many gains to be had from childhood physical activity. However, there may be a case for increased supervision by adults and awareness of the potential increased fracture risk for children doing sports, because our results suggest that exposure to injuries is a more important determinant of fracture risk in healthy children than BMD.
The implications of our results for adult bone health are more interesting. Because it is now well recognized that low bone mass is a risk factor for childhood fractures,(7,8) there has been interest as to whether childhood fractures are a marker of bone fragility that may be carried through to adulthood. However, recent work on the European Vertebral Osteoporosis Study (EVOS) does not support this,(34) because no association was seen between a history of childhood fracture and postmenopausal vertebral fracture, although recall bias may explain the null result. However, our results suggest it is probable that children who experience a fracture are likely to be the children who do more physical activity and contact sports. Consequently, a fracture in childhood may actually be a marker of higher levels of activity and therefore higher peak bone mass through increased loading(35) and a reduced risk of osteoporotic fracture in later life.
Our results initially suggested that sex, time spent outdoors in summer, and time spent outdoors in winter were risk factors for fracture risk independent of bone mass, but stratification by estimated volumetric BMD or bone size relative to body size did not confirm this independent association. This suggests these three factors do have some action through bone mass, which is not surprising. What is particularly interesting about these data, however, is the fact that physical activity reported at 4.5 yr of age seems to have a similar, albeit weaker association, as that between reported vigorous physical activity at 9 yr of age and fracture risk between 10 and 12 yr of age. Perhaps this suggests that children who are physically active at 4.5 yr of age are the children who are physically active at 9 yr of age, supporting the notion of tracking of behavioral characteristics throughout life.(36)
Our study also showed a lack of evidence that other variables are important determinants for fracture risk in healthy children. We found no association between family size and fracture risk, and because our study is the largest, does not provide support for the hypothesis that larger families results in reduced adult supervision, more rough and tumble play, and an increased fracture risk.(15) However, our study also found no association between birth weight and fracture risk in childhood. At first sight, this seems inconsistent with the literature on the positive association between birth weight and neonatal bone mass(37,38) but may be explained by the fact that, by adolescence, children of low birth weight seem to have “caught-up” and have a bone mass appropriate for their size.(39)
Our results also showed no association with pubertal status. All longitudinal surveillance of childhood fractures showed a peak fracture incidence at 14 yr of age for boys and 11 yr of age for girls,(9) and potential explanations for this peak include the endocrine and metabolic changes that occur during puberty, a temporary dissociation between skeletal expansion and skeletal mineralization during the growth spurt,(40) or increased risk-taking behavior associated with changes in psychosocial makeup. Other literature on the association between puberty and childhood fractures is small and contradictory, and our findings suggest that the peak of fractures at this age is unlikely to be caused by the endocrine or metabolic changes of puberty. However, our measure of puberty was by self-assessment of pubic hair distribution (boys self-assessment of genital development in ALSPAC is unreliable), which may have introduced measurement error and make a null result more likely. Also, because pubertal status was only measured at 9 yr of age, the majority of the children were prepubertal or only in early puberty, and this may also explain the null association seen.
Our results showed no association between dietary intake of calcium, vitamin D, or total energy intake and fracture risk. Previous studies assessing calcium intake as a determinant of childhood fracture risk are contradictory,(18,19,27,41,42) perhaps because the method of measuring calcium intake varied between studies, and total energy intake was not assessed or adjusted for in some of the studies. In this study, calcium intake was assessed at 6.8 yr of age, and perhaps this lag between assessment of diet and fracture data collected between 10 and 12 yr of age is an explanation for the null result, because changes in dietary patterns between 7 and 10 yr of age cannot be excluded. Alternatively, this null result between dietary intake of calcium, vitamin D, or total energy intake and fracture risk is not that surprising in a generally well-nourished population such as ours. Additionally, sunlight is an important source of vitamin D, and our study did not measure this.
Other limitations in this study include the use of questionnaire data for vigorous physical activity, because use of reported data on activity has been shown to suffer from measurement error.(43) However, previous work on the same ALSPAC cohort(44) has reported that moderate and vigorous physical activity as measured objectively using accelerometers is positively associated with bone mass, reinforcing the results of this study, although the questionnaire data for vigorous physical activity has not yet been compared directly with the objective accelerometer data. Another weakness of this study is the loss of a large proportion of the original ALSPAC cohort because of missing data. This may have introduced bias, for example, with a preferential dropout of children from families of lower socioeconomic status. In addition, although 87% of subjects in whom we were able to obtain X-ray reports were confirmed as having a fracture, we were not able to verify reported fractures in all cases, and it is inevitable that a small number of children were erroneously classified as having had a fracture. However, any such misclassification of our outcome is likely to have underestimated the association between physical activity, bone mass, and fractures, rather than produced a spurious association.
In conclusion, this is the first prospective study identifying independent risk factors for childhood fractures. We found a complex interrelationship between physical activity, bone mass, and childhood fracture risk, suggesting that the higher bone mass associated with increased physical activity in children only partially compensates for the increased exposure to injuries.
Table 2.
Daily Dietary Intake for the Children in This Study
| Children without fractures (n = 2499) [N (%)] |
Children with fractures (n = 193) [N (%)] |
p value for difference between children with and without fractures |
Crude OR for fracture risk OR (95% CI) |
Adjusted OR for fracture risk OR (95% CI), p value* |
Adjusted OR for fracture risk† |
Adjusted OR for fracture risk‡ |
|
|---|---|---|---|---|---|---|---|
| Calcium intake | |||||||
| 1 (lowest) | 619 (92.0) | 54 (8.0) | 0.231 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 636 (94.5) | 37 (5.5) | 0.67 (0.43, 1.03) | 0.53 (0.33, 0.86) | 0.53 (0.33, 0.85) | 0.53 (0.33, 0.85) | |
| 3 | 625 (92.9) | 48 (7.1) | 0.88 (0.59, 1.32) | 0.65 (0.40, 1.05) | 0.67 (0.41, 1.09) | 0.65 (0.40, 1.06) | |
| 4 (highest) | 619 (92.0) | 54 (8.0) | 1.00 (0.68, 1.48) OR test for trend: 1.02 (0.90, 1.17) |
0.68 (0.39, 1.17) OR test for trend: 0.90(0.75, 1.08) |
0.68 (0.40, 1.18) OR test for trend: 0.90 (0.76, 1.08) |
0.67 (0.39, 1.16) OR test for trend: 0.90 (0.75, 1.08) |
|
| Vitamin D intake | |||||||
| 1 (lowest) | 622 (92.4) | 51 (7.6) | 0.105 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 637 (94.7) | 36 (5.3) | 0.69 (0.44, 1.07) | 0.72 (0.46, 1.13) | 0.72(0.45, 1.13) | 0.71 (0.45, 1.11) | |
| 3 | 614(91.2) | 59 (8.8) | 1.17 (0.79, 1.73) | 1.11 (0.73, 1.69) | 1.11 (0.72, 1.69) | 1.10 (0.72, 1.68) | |
| 4 (highest) | 626 (93.0) | 48 (7.0) | 0.92(0.61, 1.8) OR test for trend: 1.03 (0.90, 1.17) |
0.84 (0.53, 1.33) OR test for trend: 0.98 (0.84, 1.13) |
0.82(0.51, 1.31) OR test for trend: 0.97 (0.84, 1.13) |
0.82 (0.52, 1.31) OR test for trend: 0.97 (0.84, 1.13) |
|
| Energy intake | |||||||
| 1 (lowest) | 634 (94.2) | 39 (5.8) | 0.293 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 623 (92.6) | 50 (7.4) | 1.31 (0.85, 2.01) | 1.59 (0.98, 2.56) | 1.57 (0.98, 2.54) | 1.62 (1.01, 2.60) | |
| 3 | 626 (93.0) | 47 (7.0) | 1.22 (0.79, 1.89) | 1.53 (0.89, 2.64) | 1.51 (0.87, 2.62) | 1.58 (0.91, 2.72) | |
| 4 (highest) | 616(91.5) | 57 (8.5) | 1.50 (0.99, 2.29) OR test for trend: 1.12 (0.98, 1.28) |
1.85 (1.01, 3.39) OR test for trend: 1.19 (0.98, 1.44) |
1.85 (1.01, 3.41) OR test for trend: 1.19 (0.98, 1.44) |
1.93 (1.05, 3.54) OR test for trend: 1.20 (0.99, 1.45) |
All dietary variables are divided into quartiles with 1 representing the lowest and 4 the highest. Number and percentage for children with and without fractures are given, along with crude and adjusted ORs for fracture risk. Statistically significant relationships are shown in bold.
ACKNOWLEDGMENTS
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The UK Medical Research Council, the Wellcome Trust, and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, and EC will serve as guarantor for the contents of this paper. This research was specifically funded by the Wellcome Trust.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Moustaki M, Lariou M. Cross country variation of fractures in the childhood population. Is the origin biological or “accidental”? Inj Prev. 2001;7:77. doi: 10.1136/ip.7.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons RA, Delahunty AM, Kraus D, Heaven M, McCabe M, Allen H, Nash P. Children's fractures: A population based study. Inj Prev. 1999;5:129–132. doi: 10.1136/ip.5.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landin LA. Fracture patterns in children. Acta Orthop Scand. 1983;54(Suppl 202):1–109. [PubMed] [Google Scholar]
- 4.Tiderius CJ, Landin LA, Duppe H. Decreasing incidence of fractures in children. Acta Orthop Scand. 1999;70:622–626. doi: 10.3109/17453679908997853. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari S, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: An early marker of persistent bone fragility? J Bone Miner Res. 2006;21:501–507. doi: 10.1359/jbmr.051215. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and the menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 7.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: A prospective cohort study. J Bone Miner Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: A systematic review and meta-analysis. Pediatrics. 2006;117:e291–e297. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: A study using the GP Research Database. J Bone Miner Res. 2004;19:1976–1981. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 10.Jones IE, Williams SM, Dow N, Goulding A. How many children remain fracture free during growth? A longitudinal study of children and adolescents participating in the Dunedin Multidisciplinary Health and Development Study. Osteoporos Int. 2002;13:990–995. doi: 10.1007/s001980200137. [DOI] [PubMed] [Google Scholar]
- 11.Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. J Bone Joint Surg Am. 1999;81:500–509. doi: 10.2106/00004623-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Rowe R, Maughan B, Goodman R. Childhood psychiatric disorder and unintentional injury: Findings from a national cohort study. J Pediatr Psychol. 2004;29:119–130. doi: 10.1093/jpepsy/jsh015. [DOI] [PubMed] [Google Scholar]
- 13.Ma DQ, Jones G. Clinical risk factors but not bone density are associated with prevalent fractures in prepubertal children. J Pediatr Child Health. 2002;38:497–500. doi: 10.1046/j.1440-1754.2002.00037.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones IE, Williams SM, Goulding A. Associations of birth weight and length, childhood size and smoking with bone fractures during growth: Evidence from a birth cohort study. Am J Epidemiol. 2003;159:343–350. doi: 10.1093/aje/kwh052. [DOI] [PubMed] [Google Scholar]
- 15.Loder RT, Warschausky S, Schwartz EM, Hensinger RN, Greenfield ML. The psychosocial characteristics of children with fractures. J Pediatr Orthop. 1995;15:41–46. doi: 10.1097/01241398-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Stark AD, Bennet GC, Stone DH, Chishti P. Association between childhood fractures and poverty: Population based study. BMJ. 2002;324:457. doi: 10.1136/bmj.324.7335.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyons RA, Delahunty AM, Heaven M, McCabe M, Allen H, Nash P. Incidence of childhood fractures in affluent and deprived areas: Population based study. BMJ. 2000;320:149. doi: 10.1136/bmj.320.7228.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlebna-Sokol D, Blaszczuk A, Trafalska E, Grzybowski A. Bone mineralisation in children with skeletal system abnormalities in relation to dietary intake of some nutrients. Przegl Lek. 2003;60(Suppl 6):60–64. [PubMed] [Google Scholar]
- 19.Petridou E, Karpathios T, Dessypris N, Simou E, Trichopoulos D. The role of dairy products and non-alcoholic beverages in bone fractures among school age children. Scand J Soc Med. 1997;25:119–125. doi: 10.1177/140349489702500209. [DOI] [PubMed] [Google Scholar]
- 20.Ma DQ, Jones G. TV, computer and video viewing; physical activity; and upper limb fracture risk in children: A population based case control study. J Bone Miner Res. 2003;18:1970–1977. doi: 10.1359/jbmr.2003.18.11.1970. [DOI] [PubMed] [Google Scholar]
- 21.Loud KJ, Gordon CM, Micheli LJ, Field AE. Correlates of stress fractures among preadolescent and adolescent girls. Pediatrics. 2005;115:e399–e406. doi: 10.1542/peds.2004-1868. [DOI] [PubMed] [Google Scholar]
- 22.Golding J, Pembrey M, Jones R. ALSPAC—The Avon Longitudinal Study of Parents and Children I: Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 23.Clark EM, Ness AR, Tobias JH, ALSPAC Study Team Social position affects bone mass in childhood through opposing actions on height and weight. J Bone Miner Res. 2005;20:2082–2089. doi: 10.1359/JBMR.050808. [DOI] [PubMed] [Google Scholar]
- 24.McCrance RA, Widdowson EM. The Composition of Foods. Royal Society of Chemistry; Cambridge, UK: 1991. and the Ministry of Agriculture, Fisheries and Food, London, UK. [Google Scholar]
- 25.Taylor A, Konrad PT, Norman ME, Harcke HT. Total body BMD in young children: Influence of head BMD. J Bone Miner Res. 1997;12:652–655. doi: 10.1359/jbmr.1997.12.4.652. [DOI] [PubMed] [Google Scholar]
- 26.Clark EM, Ness AR, Tobias JH. Bone fragility contributes to the risk of fracture in children, even following moderate and severe trauma. J Bone Miner Res. 2008;23:173–179. doi: 10.1359/jbmr.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houshain S, Mehdi B, Larsen MS. The epidemiology of elbow fractures in children: Analysis of 355 fractures, with special reference to supracondylar humerus fractures. J Orthop Sci. 2001;6:312–315. doi: 10.1007/s007760100024. [DOI] [PubMed] [Google Scholar]
- 28.Ma DG, Jones G. Soft drink and milk consumption, physical activity, bone mass and upper limb fractures in children: A population based case-control study. Calcif Tissue Int. 2004;75:286–291. doi: 10.1007/s00223-004-0274-y. [DOI] [PubMed] [Google Scholar]
- 29.Marshall SJ, Biddle SJH, Gorely T, Cameron N, Murdey I. Relationships between media use, body fatness and physical activity in children and youth: A meta-analysis. In J Obesity. 2004;28:1238–1246. doi: 10.1038/sj.ijo.0802706. [DOI] [PubMed] [Google Scholar]
- 30.Caulton JM, Ward KA, Alsop CW, Dunn G, Adams JE, Mughal MZ. A randomised controlled trial of a standing programme on BMD in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131–135. doi: 10.1136/adc.2002.009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols DL, Sanborn CF, Love AM. Resistance training and BMD in adolescent females. J Pediatr. 2001;139:494–500. doi: 10.1067/mpd.2001.116698. [DOI] [PubMed] [Google Scholar]
- 32.Johansen N, Binkley T, Englert V, Neiderauer G, Specker B. Bone response to jumping is site specific in children: A randomised trial. Bone. 2003;33:533–539. doi: 10.1016/s8756-3282(03)00220-5. [DOI] [PubMed] [Google Scholar]
- 33.MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: A 2-year randomised controlled trial in girls. Pediatrics. 2003;112:447–452. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 34.Pye SR, Tobias JH, Silman A, Reeve J, O'Neill TW. Childhood fractures do not predict future fractures: Results from the European Prospective Osteoporosis Study (EPOS) Rheumatology. 2007;46:i132. doi: 10.1359/jbmr.090220. [DOI] [PubMed] [Google Scholar]
- 35.Bachrach L. Bone mineralization in childhood and adolescence. Curr Opin Paediatr. 1993;5:467–473. doi: 10.1097/00008480-199308000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 37.Dennison E, Wheeler T, Taylor P, Kellingray S, Cooper C. Determinants of neonatal bone mass. Bone. 1997;20:26S. [Google Scholar]
- 38.Avila-Diaz M, Flores-Huerta S, Martinez-Muniz I, Amato D. Increments in whole body BMC associated with weight and length in pre-term and full-term infants during the first 6 months of life. Arch Med Res. 2001;32:288–292. doi: 10.1016/s0188-4409(01)00291-0. [DOI] [PubMed] [Google Scholar]
- 39.Weiler HA, Yuen CK, Seshia MM. Growth and bone mineralisation of young adults weighing less than 1500g at birth. Early Hum Dev. 2002;67:101–112. doi: 10.1016/s0378-3782(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 40.Blimkie CJR, Lefevre J, Beunen GP, Renson R, Dequeker J, van Damme P. Fractures, physical activity and growth velocity in adolescent Belgium boys. Med Sci Sports Exerc. 1993;25:801–808. doi: 10.1249/00005768-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Goulding A, Rockell JE, Black RE, Grant AM, Jones IE, Williams SM. Children who avoid drinking cow's milk are at increased risk for prepubertal bone fractures. J Am Diet Assoc. 2004;104:250–253. doi: 10.1016/j.jada.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Wyshak G, Frisch RE. Carbonated beverages, dietary calcium, the dietary calcium/phosphorus ratio, and bone fractures in girls and boys. J Adolesc Health. 1994;15:210–215. doi: 10.1016/1054-139x(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari P, Friedenreich C, Matthews CE. The role of measurement error in estimating levels of physical activity. Am J Epidemiol. 2007;166:832–840. doi: 10.1093/aje/kwm148. [DOI] [PubMed] [Google Scholar]
- 44.Tobias JH, Steer CD, Mattocks CG, Riddoch C, Ness AR. Habitual levels of physical activity influence bone mass in 11-year old children from the UK: Findings from a large population-based cohort. J Bone Miner Res. 2007;22:101–109. doi: 10.1359/jbmr.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]