Abstract
Morphogen gradients play an important role in pattern formation during early stages of embryonic development in many bilaterians. In an adult hydra, axial patterning processes are constantly active because of the tissue dynamics in the adult. These processes include an organizer region in the head, which continuously produces and transmits two signals that are distributed in gradients down the body column. One signal sets up and maintains the head activation gradient, which is a morphogenetic gradient. This gradient confers the capacity of head formation on tissue of the body column, which takes place during bud formation, hydra's mode of asexual reproduction, as well as during head regeneration following bisection of the animal anywhere along the body column. The other signal sets up the head inhibition gradient, which prevents head formation, thereby restricting bud formation to the lower part of the body column in an adult hydra. Little is known about the molecular basis of the two gradients. In contrast, the canonical Wnt pathway plays a central role in setting up and maintaining the head organizer.
The morphology of the adult hydra is maintained by two signals continuously produced by an ”organizer” region in the head. These set up opposing gradients that restrict head and bud formation to distinct regions of the animal.
Morphogen gradients play a critical role in the early stages of embryogenesis in a number of metazoans in that they initiate and are involved in axial patterning processes. Such a gradient also plays a role in axial patterning in hydra, a primitive metazoan. However, unlike in most metazoans, this gradient is continuously active in an adult hydra as part of the tissue dynamics of the adult animal.
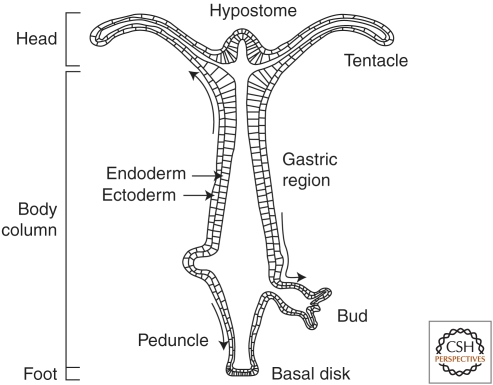
The structure of a hydra is fairly simple (Fig. 1). It consists of a single axis with radial symmetry, which contains a head, body column, and foot along the axis. The head consist of two parts: the hypostome in the apex, and the tentacle zone from which the tentacles emerge in the basal part of the head. The body column has three parts: the gastric region and peduncle in the apical, and basal parts with a budding zone between the gastric region and peduncle. Buds, hydra's mode of asexual reproduction, emerge from the budding zone between the gastric region and peduncle.
Figure 1.
Longitudinal cross section of an adult hydra. The multiple regions are labeled. The two protrusions from the body column are early and late stages of bud development. The arrows indicate the direction of tissue displacement. (Reprinted from Bode 2001.)
Three cell lineages are involved. The axis consists of a cylindrical shell that is made up of two concentric epithelial layers, the ectoderm and endoderm, which are separated by a basement membrane. Interspersed among the epithelial cells of both layers are the cells of the third lineage, the interstitial cell lineage. It consists of interstitial cells, which are multipotent stem cells (David and Murphy 1977), located primarily in the ectoderm throughout the body column. They give rise to neurons, secretory cells, and nematocytes, which are the stinging cells that are typical of cnidarians, as well as gametes when a hydra undergoes sexual reproduction (David and Murphy 1977).
In an adult hydra, the epithelial cells of both layers are constantly in the mitotic cycle (David and Campbell 1972; Campbell and David 1974). The expanding tissue in the upper part of the body column is continuously displaced apically into the head (Fig. 1). Once there, it is displaced onto and along the tentacles or into the hypostome, and eventually sloughed when reaching the extremities (Campbell 1967; Otto and Campbell 1977). Tissue in the remainder of the body column is displaced basally either onto developing buds, or further down onto the foot, where it is sloughed at the bottom of the foot. Thus, the tissues of an adult hydra are continuously in a steady state of production and loss. As a hydra has no defined lifetime (Martinez 1998), this activity goes on indefinitely.
AXIAL PATTERNING PROCESSES
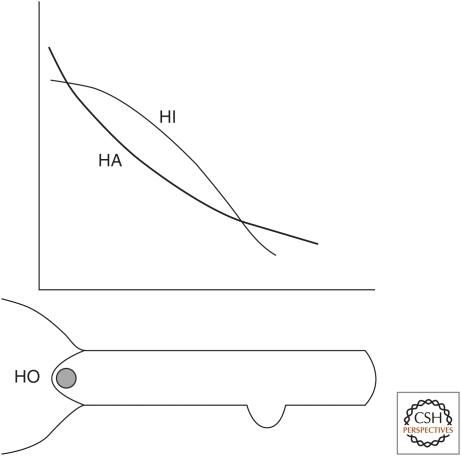
The maintenance of the structure of the head, body column, and foot in the context of tissue dynamics is mainly controlled by three patterning processes that are continuously active and in a steady state of production and loss (Fig. 2). One is the head organizer, which is located in the hypostome. A second is a morphogenetic gradient termed the head activation gradient, which runs the length of the axis of the animal. The third is a head inhibition gradient that also runs down the body column. The head organizer produces and transmits two signals that are transmitted to the body column. One sets up the head activation gradient, and the second sets up the head inhibition gradient. There is also evidence for an inhibition gradient produced in the foot that is active in the lower quarter of the body column (Cohen and MacWilliams 1975), but it has not been well characterized.
Figure 2.
Axial patterning processes in hydra. The head organizer (HO) is located in the hypostome. The head activation (HA) and head inhibition (HI) gradients are graded down the body column. The formation of new axis resulting in bud formation takes place in a location along the body axis where HA is greater than HI. A second inhibitor produced in the foot prevents bud formation in the lower part of the body column.
The head organizer has the characteristics of an embryonic organizer. When the hypostome of one hydra is transplanted to the body column of another hydra, it induces the formation of a second axis consisting of a head and body column (Browne 1909; MacWilliams 1983b; Broun and Bode 2002). The hypostome of this new axis is formed by the transplanted hypostome, while the rest of the head and the body column are formed by the host tissue (Broun and Bode 2002). Transplantation of a piece of body column tissue the size of the hypostome does not induce, or form, a second axis (Yao 1945; Broun and Bode 2002).
The head activation gradient is a morphogenetic gradient. It has a maximal value in the head and declines in a graded manner down the body column, and has two functions: (1) It confers the capacity to form a head organizer on tissue when the level of head activation rises above a threshold level and (2) as tissue is displaced from the body column into the head or foot, it affects the mitotic, differentiation, and morphogenetic activity of the epithelial cells. It also affects the differentiation activity of the interstitial cells along the body column.
The head activation gradient differs from the head organizer in that it provides the tissue of the body column with a capacity to form a head organizer, but does not have head organizer activity as such. This capacity is illustrated in two ways: (1) When a hydra is bisected anywhere along the body column, a head organizer forms at the apical end of the basal piece, resulting in the regeneration of a head, and (2) when an eighth of the body column is isolated and transplanted to the body column of another hydra, it forms a second axis. However, there is an important difference in the formation of this second axis and the one induced by transplantation of the hypostome. When an eighth of the body column is transplanted, a second axis forms in which the head and body column consist of the transplanted tissue (Broun and Bode 2002). Thus, there is no inductive effect because host tissue does not participate in the formation of the second axis. The head organizer and head activation gradient also differ in two other ways: (1) As measured during regeneration experiments, the head organizer has a half-life of 12 h, while the head activation gradient has a half-life of 36 h (MacWilliams 1983b), and (2) treatment of hydra with 0.5 mM LiCl lowers the level of the head activation gradient in the body column, but has no effect on the head organizer (Hassel and Berking 1990).
That this head activation capacity is graded down the body column was shown as follows. Transplantation of an eighth of the body column to a host results in a fraction of the axes forming a second axis. This fraction is highest for an eighth isolated from the top of the body column, and declines the further down the body column the origin of the piece of transplanted tissue is (MacWilliams 1983b). That this gradient is set up with a signal from the head was demonstrated by grafting a head onto the basal end of an isolated body column. This resulted in an inversion of the head activation gradient with the maximum near the head grafted to the original basal end of the body column (Wilby and Webster 1970b). Thus, the gradient is most likely a reflection of a graded distribution of a signal produced in the head organizer and transmitted down the body column.
Both the head organizer and the head activation gradient are associated with one or both of the two epithelial cell lineages. This was demonstrated by removing the interstitial cell lineage from a hydra, which results in animals consisting only of the two epithelial cell lineages. These animals have the same morphology as do normal animals, and when fed on a regular basis, reproduce by bud formation indefinitely (Marcum and Campbell 1978). Upon bisection, they undergo head and foot regeneration at the apical and basal ends of the upper and lower halves, as do normal animals. As the axial patterning behavior of these animals devoid of the interstitial cell lineage is the same as normal animals, the interstitial cell lineage most likely does not play a role in axial patterning.
Because any part of the body column has the capacity to form a head, there must be a mechanism for preventing head formation from occurring randomly along the body column. This is carried out by the head inhibition gradient (Fig. 2). The head organizer produces a signal, the head inhibitor, which is transmitted down the body column. That this signal is graded in a decreasing concentration down the body column was demonstrated by transplanting the apical eighth of the body column of donor animals to different locations along the body column of host animals. The fraction of transplants that formed a second axis increased the further down the body column of the host that the piece of tissue was implanted (Wilby and Webster 1970a; MacWilliams 1983a). Head inhibition can be transported in either direction along the body column, as shown by removing the head of a hydra and grafting it onto the basal end of the body column. This prevents a head from regenerating at the apical end (Wilby and Webster 1970a). This head inhibition signal is very labile with a half-life of 2–3 h, because removal of the head results in an immediate sharp increase of transplants, forming a second axis anywhere along the body column (MacWilliams 1983a). This short half-life is a relevant factor in the rapid initiation of head organizer formation at the apical tip during head regeneration following bisection.
A reaction–diffusion model developed by Gierer and Meinhardt (1972), involving the head organizer as well as the head activation and head inhibition gradients, has provided a useful framework for these biological observations. This is described in detail by Meinhardt (2009).
ROLE OF AXIAL PATTERNING PROCESSES IN THE DEVELOPMENTAL ACTIVITIES OF AN ADULT HYDRA
Tissue Dynamics in Adult Hydra
Given that the tissues of an adult hydra are continuously being produced, continuously displaced apically or basally along the body column, and continuously being sloughed at the extremities, the axial patterning mechanisms must also be continuously active to maintain the structure of the animal. The head organizer resides in the upper part of the hypostome. As tissue of the hypostome is displaced apically and sloughed at the tip (Dübel et al. 1987), the head organizer would be lost if it were not continuously renewed to maintain it in this dynamic context. Similarly, the head activation gradient would be diluted in the context of the ever-dividing, and thus, increasing number of epithelial cells of the body column, if the head organizer did not continuously produce and transmit a signal to the body column to maintain the graded distribution of head activation along the body column. The same is true for the head inhibition gradient. And, most likely even more so since head inhibition has a short half-life. Thus, to maintain the tissues and structure of an adult hydra in the steady state of these tissue dynamics, the head organizer must continuously renew itself and produce and transmit the two signals to maintain the head activation and head inhibition gradients.
Morphogenesis and Cell Differentiation
The head activation gradient plays an important role in morphogenesis in hydra. As tissue is displaced apically from the body column into the tentacle zone, most of the tissue is rearranged into the cylindrical shape of several tentacles. This is in response to a threshold level of head activation. This conclusion is supported by the following experiment. Treatment with 2 mM LiCl (Hassel et al. 1993) or alsterpaullone, which raises head activation to a high level throughout the body column (Broun et al. 2005), results in the formation of ectopic tentacles all along the body column (see Meinhardt [2009] for figure of this effect). Conversely, treatment with 0.5–1.0 mM LiCl lowers the level of the head activation gradient, and results in the conversion of pieces of the body column to feet (Hassel and Berking 1990).
Moving into the tentacle zone and onto tentacles also changes the activities of the epithelial cells. In both layers, these cells cease cell division and change their shape dramatically. Once on a tentacle, the ectodermal epithelial cells differentiate into battery cells (Holstein et al. 1991). In the hypostome, epithelial cells of both layers become involved in the head organizer (J. Hartig et al.; T. Lengfeld et al., both in prep.). At the basal end of a hydra, epithelial cells of both layers are displaced onto the basal disc (or foot), cease division, and differentiate into basal disc cells (Holstein et al. 1991).
The head activation gradient also affects the type of cell an interstitial cell differentiates into. For example, there are four types of nematocytes. Interstitial cells differentiate more frequently into desmonemes than into stenoteles in the upper part of the body column (Bode and Smith 1977). The reverse is the case in the lower part of the body column. Isolation of the lower half of the body column will result in head regeneration at the apical end, the rebuilding of the head activation gradient, and subsequently an increase in the desmoneme/stenotele ratio formed (Bode and Smith 1977). Since interstitial cells are continuously displaced along the body column with their neighboring epithelial cells, they are continuously exposed to changing levels of the head activation gradient, which will affect the differentiation choices undertaken by the interstitial cells.
Head Regeneration
Hydra is well known for its regeneration capacity. Bisect a hydra anywhere along the body column and a head will regenerate at the apical end of the basal piece, while a foot regenerates at the basal end of the upper piece. This directional regeneration behavior is also illustrated by isolating a piece of the body column anywhere along the axis. Invariably, a head regenerates at the apical end and a foot at the basal end. Thus, the entire body column has the capacity to form a head or a foot. This capacity is restricted to the body column because an isolated head does not regenerate a foot, nor does an isolated foot regenerate a head.
These regeneration activities are a reflection of the head activation and head inhibition gradients. With the removal of the head, no more head inhibition is transmitted to the body column. Since the head inhibition signal has a short half-life of 2–3 h (MacWilliams 1983b), decapitation will lead to rapid reduction of the level of head inhibition throughout the body column. When the level of head inhibition drops below a critical level, which would be below the level of head activation in a reaction–diffusion model, head organizer formation begins. The higher the level of head activation, the more rapidly the head organizer would form and begin producing head inhibition, which would be transported down the column to prevent head organizer formation elsewhere along the body column. Further, injury also enhances head organizer formation. This was shown with reg-16, a mutant with a low head regeneration capacity upon decapitation (Kobatake and Sugiyama 1989). However, when reg-16 animals are decapitated and subsequently reinjured at the site of decapitation, most regenerated a head. Thus, the higher level of head activation coupled with the injury effect clearly favored the formation of a head organizer at the apical end of an isolated piece.
Bud Formation
Hydra reproduces asexually by bud formation. It starts with an evagination of the body column in the budding zone, which is located about two-thirds of the distance down the body column (Fig. 1). The evagination elongates into a cylindrical protrusion, and subsequently a head forms at the apex of the protrusion and foot at the other end. When both extremities are fully formed, the bud detaches from the adult. Upon feeding, the detached bud grows into an adult in 5–7 d. As the bud develops, it is displaced basally as part of the tissue dynamics of the adult. The next bud forms on the opposite side of the adult again at a distance two-thirds down the body column (Fig. 1).
The ability of the head activation gradient to confer head organizer activity on body column tissue is involved in the initiation of bud formation. An early stage bud already has head organizer activity in its apical end as measured by transplantation studies (Sanyal 1966). And, as described in a later section, HyWnt-3, the hydra ortholog of Wnt-3, and the canonical Wnt pathway are involved in the head organizer. HyWnt-3 is expressed in the spot in the budding zone where a bud will emerge, and continues to be expressed at the apical tip throughout bud development and subsequently in the apex of the hypostome of the adult (J. Hartig et al.; T. Lengfeld et al., both in prep.). This early formation of the head organizer leads to the formation of the head activation gradient along the axis of the developing bud, resulting in the formation of the head, body column, and foot.
Since any part of the body column can undergo head formation following bisection, why doesn't bud formation take place further up the body column? This is most likely due to the interaction of the head inhibition and head activation gradients. The level of head inhibition is assumed to be greater than the level of head activation in the upper part of the body column, thereby preventing the initiation of head organizer formation (Fig. 2). Measurement of the head inhibition gradient indicates that it declines more steeply than does the head activation gradient (MacWilliams 1983a). By the time the budding zone is reached, the level of head activation will be greater than the level of head inhibition, leading to the initiation of head organizer formation and subsequently bud formation, as described above. Some evidence indicates that the foot also produces an inhibitor of bud formation that extends from the foot up through the peduncle, the lower part of the body column (Cohen and MacWilliams 1975). Thus, the two inhibition gradients would restrict bud formation to the budding zone.
MOLECULAR BASIS OF THE AXIAL PATTERNING COMPONENTS
Head Inhibition and Head Activation Gradients
Of the three components governing axial patterning, little is known about the molecular basis of the two gradients. Although the molecular nature of the head inhibitor is unknown, it is probably a small diffusible molecule. The epithelial cells within each layer, and between the two layers, are strongly connected to one another by a high density of gap junctions (Wood 1977; West 1978). Blocking gap junction communication between epithelial cells blocked transmission of the head inhibitor (Fraser et al. 1987).
The molecular basis of the head activation gradient is unknown, but is probably also a diffusible molecule that is more stable than head inhibition since it has a half-life of 36 h (MacWilliams 1983b). How it is transported along the body column is not known. In addition to initiating the process that gives rise to the head organizer, this gradient affects the expression of genes in epithelial cells in different locations along the axis of the animal. For example, two genes, HyBMP5-8 and CnNK2, hydra orthologs of BMP5-8 and CNK2, are normally expressed in the lower quarter of the body column, where the level of head activation is at a low level (Grens et al. 1996; Reinhardt et al. 2004). When treated with 0.5 mM LiCl, which lowers the level of head activation in the body column (Hassel and Berking 1990), the expression of both genes was extended into the upper half of the body column (Grens et al. 1996; Reinhardt et al. 2004). Conversely, when the head activation level was raised throughout the body column by treatment with alsterpaullone, as will be described below, the expression of HyBMP5-8 was eliminated from the body column (Reinhardt et al. 2004). This treatment also significantly altered the expression of HyBra, an ortholog of Brachyury. Normally, HyBra is expressed only in the hypostome (Technau and Bode 1999), but, after treatment with alsterpaullone, it is expressed throughout the body column (Broun et al. 2005). These changes in expression reflect the role of the head activation gradient in affecting or controlling the differentiated state of epithelial cells along the body column.
Head Organizer
More is known about the genes involved in the head organizer. The canonical Wnt pathway plays a central role in the head organizer. Components of the pathway, including orthologs of Wnt-3, Dishevelled, APC, GSK-3β, β-catenin, and Tcf, have been identified in hydra (Hobmayer et al. 2000). HyWnt-3 is expressed only and continuously in the apex of the hypostome of an adult hydra, where it is expressed in both the ectoderm and endoderm (J. Hartig et al.; T. Lengfeld et al., both in prep.). This gene is probably involved in the formation of the head organizer, as indicated by the following: (1) It begins and continues expression in the apical tip within 2 h following bisection and the initiation of head regeneration (Hobmayer et al. 2000), and (2) it is expressed as a spot in the budding zone, and subsequently at the apical tip throughout the development of the bud. This indicates the gene is involved in setting up and maintaining the head organizer (Hobmayer et al. 2000; J. Hartig et al.; T. Lengfeld et al., both in prep.). In addition, the hydra Tcf ortholog, HyTcf, is strongly expressed in the head of an adult and developing bud (Hobmayer et al. 2000).
That this pathway plays a central role in the head organizer is further supported by four lines of evidence that indicate a role for Hyβ-cat, the hydra β-catenin ortholog, in the head organizer. First, since the epithelial cells of both layers are continuously in the mitotic cycle, and β-catenin also plays an important role in cell adhesion, the gene is continuously expressed throughout the body column in order to generate enough of this protein to maintain the adherence of these cells to one another in the body column (L. Gee et al., in prep.). However, only in the apex of the hypostome of an adult hydra is the protein located in the nuclei of epithelial cells as determined using an anti-β-catenin antibody (Broun et al. 2005), which is where one would expect it to be located if involved in the canonical Wnt pathway. Second, PKC is known to block the activity of GSK-3β (Goode et al. 1992), which would result in an elevation of the level of the β-catenin protein. In hydra, HyPKC2 is expressed only in the apical half of the hypostome, where the head organizer is located, and not in the body column (Hassel et al. 1998). Thus, HyPKC could contribute to maintaining a high level of Hyβ-cat in the region of the head organizer.
Third, two-day treatment with alsterpaullone, a specific inhibitor of GSK-3β (Loest et al. 2000), raises the level of β-catenin throughout the body column of a hydra (Broun et al. 2005). One measure of this is the presence of elevated levels of this protein in nuclei not only in the hypostome, but also in the body column. This elevated level of β-catenin confers head organizer capacity on tissue of the body column. When a piece of body column tissue of an alsterpaullone-treated animal similar in size to the hypostome is transplanted to the body column of a normal hydra, it induces a second axis in >95% of the samples (Broun et al. 2005). In addition, genes associated with the head organizer and the hypostome are expressed in the body column of such animals. HyWnt-3 is expressed in spots similar in size to the spot of expression in the hypostome that are fairly evenly distributed throughout the body column (Broun et al. 2005). HyTcf, which is strongly expressed only in the hypostome of the normal adult, is expressed strongly throughout the body column as well as in the head of these animals (Broun et al. 2005).
Fourth, recently transgenic hydra have been generated by injecting a plasmid containing a gene driven by a hydra actin promoter into 2–4-cell hydra embryos (Wittlieb et al. 2006). Modifying this construct, a transgenic hydra expressing Hyβ-cat, in which the GSK-3β binding site had been removed from the gene, were generated (L. Gee et al., in prep.). Normally, when a hydra embryo hatches, it has a morphology similar to that of an adult hydra. In contrast, these transgenic animals hatched with one or two secondary heads or axes emerging from the body column. Subsequently, they grew up into a single animal with multiple axes emerging from the initial axis, as well as additional axes emerging from these secondary axes. The population increased by occasional bud formation. In normal hydra, Hyβ-cat is expressed throughout the animal, although more strongly in the head and developing buds as determined by in situ hybridization on whole mounts (Hobmayer et al. 2000). In contrast, this gene is expressed very strongly throughout the β-catenin transgenic animal (L. Gee et al., in prep.). And, when a hypostome-sized piece of the body column of such an animal is transplanted, it induced a second axis in >85% of the samples. This provides fairly direct evidence for the role of β-catenin in the head organizer.
Thus, the expression patterns of HyWnt-3 and HyTcf, along with activity of Hyβ-cat, provide fairly strong evidence that the canonical Wnt pathway plays a critical role in the head organizer in hydra.
THE HEAD ORGANIZER IN THE CONTEXT OF THE TISSUE DYNAMICS OF HYDRA
As mentioned above, the head organizer must be maintained in the context of the tissue dynamics of an adult hydra. And, it must be maintained indefinitely, because a hydra has no defined lifetime. With tissue continuously displaced apically along the hypostome and sloughed at the apex, the head organizer must be maintained in a steady state of production and loss. This may involve a positive feedback loop in the canonical Wnt pathway, as described in Drosophila (Heslip et al. 1997). In this model, Wnt acting through the canonical Wnt pathway raises the level of β-catenin, leading to the transcription of genes associated with the head organizer. It also leads to β-catenin activating the transcription of the Wnt gene, which in turn activates the canonical Wnt pathway again. Thus, in the context of the apical displacement of the hypostomal tissue, some of the newly produced HyWnt-3 protein would diffuse in a basal direction from the organizer. There, acting through the canonical Wnt pathway, it would initiate head organizer formation in this tissue immediately below the existing head organizer. Subsequently, as the hypostomal tissue containing the existing head organizer would be sloughed, the newly formed head organizer would take over the function. In this manner, the canonical Wnt pathway would maintain the head organizer in a steady state of loss and renewal.
A problem with this model concerns the rate of production of the new head organizer. If the continuous production of new head organizer tissue takes place faster than the loss of head organizer tissue at the apex, the continuous generation of head organizer could lead to its expansion and displacement in a basal direction through the entire hypostome into the body column. As head organizer activity is confined to the hypostome, this clearly does not take place. The head organizer could be confined to the apical part of the hypostome by interfering with the positive feedback loop of the canonical Wnt pathway. Three mechanisms could be involved in such a process.
The most obvious one would be that the level of head inhibition is higher than the level of head activation starting at the top of the body column, and would prevent head organizer formation. Although, the molecular basis of such a mechanism is not known. A second arises from information gained from a large hydra EST project (in preparation) that has revealed the presence of ten additional Wnt genes in hydra. Six of these are expressed only in the hypostome (J. Hartig et al.; T. Lengfeld et al., both in prep.). One of them, HyWnt-16, is the ortholog of the Wnt-16 gene, which is known to activate the JNK pathway (Teh et al. 2007). JNK blocks the accumulation of β-catenin in the nucleus (Liao et al. 2006). Unlike HyWnt-3 and the other five HyWnt genes that are expressed in the apex or upper half of the hypostome, HyWnt-16 is expressed throughout the hypostome. Thus, by activating the JNK pathway, HyWnt-16 could block the canonical Wnt pathway from acting in a positive feedback loop by preventing the nuclear accumulation of Hyβ-cat, and hence, the synthesis of HyWnt-3 in the lower part of the hypostome. This would prevent the basal extension of the head organizer. HyWnt-16 probably would not block head organizer activity in the apex because the combined concentration of the five other HyWnt genes is probably greater than that of HyWnt-16.
The third mechanism could involve DKK, which is known to act in a mutually antagonistic manner with Wnt signaling in the canonical Wnt pathway (Glinka et al. 1998). HyDKK1,2,4, a hydra ortholog of DDK, is expressed in gland cells throughout the body column (Augustin et al. 2006; Guder et al. 2006). It is expressed in a graded manner with the highest level just below the tentacle zone at the top of the body column. This could interfere with the possible basal displacement of head organizer tissue from the head into the body column. It could also interfere with the initiation of bud development since HyWnt-3 is expressed in a spot in the budding zone where a new head organizer forms to initiate this process. However, HyDKK1,2,4 is not expressed in the budding zone where the next bud will form (Augustin et al. 2006) and, thus, would not interfere with Wnt activity in the formation of the head organizer for the next bud to develop.
CONCLUSIONS
Thus, in the steady state context of the tissue dynamics of the adult hydra, these three processes control most of the axial patterning that goes on continuously. The head organizer is most likely acting in a positive feedback loop to maintain itself in the hypostome, coupled with mechanisms to keep its location restricted to the hypostome. While in this steady state, the head organizer continuously produces the two signals that are transmitted to the body column, each of which is distributed in graded manner down the body column. The head activation gradient affects the morphogenesis of the tissue approaching the head as well as differentiation capacities of cells displaced apically or basally along the body column. It also provides the capacity to initiate head organizer formation. And, the head inhibition gradient prevents the head activation gradient from carrying out this latter function along most of the body column. Less is known about the mechanisms controlling foot formation, although a foot inhibition gradient exists, which helps restrict bud formation to the budding zone. Continuing efforts to uncover the molecular components of these axial patterning processes will most likely provide more clarity as to how they operate.
Footnotes
Editors: James Briscoe, Peter Lawrence, and Jean-Paul Vincent
Additional Perspectives on Generation and Interpretation of Morphogen Gradients available at www.cshperspectives.org
REFERENCES
- Augustin R, Franke A, Khalturin K, Kiko R, Siebert S, Hemmerich G, Bosch TC 2006. Dickkopf related genes are components of the positional value gradient in Hydra. Dev Biol 296:62–70 [DOI] [PubMed] [Google Scholar]
- Bode HR 2001. The role of Hox genes in axial patterning in Hydra. Am Zool 41:621–628 [Google Scholar]
- Bode HR, Smith GS 1977. Regulation of interstitial cell differentiation in Hydra attenuata. II. Correlation of the axial position of the interstitial cell with nematocyte differentiation. Roux's Arch Dev Biol 181:203–213 [DOI] [PubMed] [Google Scholar]
- Broun M, Bode HR 2002. Characterization of the head organizer in hydra. Development 129:875–884 [DOI] [PubMed] [Google Scholar]
- Broun M, Gee L, Reinhardt B, Bode HR 2005. Formation of the head organizer in hydra involves the canonical Wnt pathway. Development 132:2907–2916 [DOI] [PubMed] [Google Scholar]
- Browne EN 1909. The production of new hydranths in hydra by the insertion of small grafts. J Exp Zool 7:1–37 [Google Scholar]
- Campbell RD 1967. Tissue dynamics of steady state growth in Hydra littoralis. II. Patterns of tissue movement. J Morphol 121:19–28 [DOI] [PubMed] [Google Scholar]
- Campbell RD, David CN 1974. Cell cycle kinetics and development of Hydra attenuata. II. Interstitial cells. J Cell Sci 16:349–358 [DOI] [PubMed] [Google Scholar]
- Cohen JE, MacWilliams HK 1975. The control of foot formation in transplantation experiments in Hydra viridis. J Theor Biol 50:87–105 [DOI] [PubMed] [Google Scholar]
- David CN, Campbell RD 1972. Cell cycle kinetics and development of Hydra attenuata. I. Epithelial cells. J Cell Sci 11:557–568 [DOI] [PubMed] [Google Scholar]
- David CN, Murphy S 1977. Characterization of interstitial stem cells in hydra by cloning. Dev Biol 58:372–383 [DOI] [PubMed] [Google Scholar]
- Dübel S, Hoffmeister SA, Schaller HC 1987. Differentiation pathways of ectodermal epithelial cells in hydra. Differentiation 35:181–189 [DOI] [PubMed] [Google Scholar]
- Fraser SE, Green CR, Bode HR, Gilula NB 1987. Selective disruption of gap junctional communication interferes with a patterning process in hydra. Science 237:49–55 [DOI] [PubMed] [Google Scholar]
- Gierer A, Meinhardt H 1972. A theory of biological pattern formation. Kybernetik 12:30–39 [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C 1998. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391:357–362 [DOI] [PubMed] [Google Scholar]
- Goode N, Hughes K, Woodgett JR, Parker PJ 1992. Differential regulation of glycogen synthase kinase-3β by protein kinase C isotypes. J Biol Chem 267:16878–16882 [PubMed] [Google Scholar]
- Grens A, Gee L, Fisher DA, Bode HR 1996. CnNK-2, an NK-2 homeobox gene, has a role in patterning the basal end of the axis in hydra. Dev Biol 80:473–488 [DOI] [PubMed] [Google Scholar]
- Guder C, Pinho S, Nacak TG, Schmidt HA, Hobmayer B, Niehrs C, Holstein TW 2006. An ancient Wnt-Dickkopf antagonism in Hydra. Development 133:901–911 [DOI] [PubMed] [Google Scholar]
- Hassel M, Berking S 1990. Lithium ions interfere with pattern control in Hydra vulgaris. Roux's Arch Dev Biol 198:382–388 [DOI] [PubMed] [Google Scholar]
- Hassel M, Albert K, Hofheinz S 1993. Pattern formation in Hydra vulgaris is controlled by lithium-sensitive processes. Dev Biol 156:362–371 [DOI] [PubMed] [Google Scholar]
- Hassel M, Bridge DM, Stover NA, Kleinholz H, Steele RE 1998. The level of expression of a protein kinase C gene may be an important component of the patterning process in Hydra. Dev Genes Evol 207:502–514 [DOI] [PubMed] [Google Scholar]
- Heslip TR, Theisen H, Walker H, Marsh JL 1997. SHAGGY and DISHEVELLED exert opposite effects on wingless and decapentaplegic expression and on positional identity in imaginal discs. Development 124:1069–1078 [DOI] [PubMed] [Google Scholar]
- Hobmayer B, Rentzsch F, Kuhn K, Happel CM, von Laue CC, Snyder P, Rothbacher U, Holstein TW 2000. WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature 407:186–189 [DOI] [PubMed] [Google Scholar]
- Holstein TW, Hobmayer E, David CN 1991. Pattern of epithelial cell cycling in hydra. Dev Biol 148:602–611 [DOI] [PubMed] [Google Scholar]
- Kobatake E, Sugiyama T 1989. Genetic analysis of developmental mechanisms in hydra. XIX. Stimulation of regeneration by injury in the regeneration-deficient mutant strain, reg-16. Development 105:521–528 [DOI] [PubMed] [Google Scholar]
- Liao G, Tao Q, Kofron M, Chen JS, Schloemer A, Davis RJ, Hsieh JC, Wylie C, Heasman J, Kuan CY 2006. Jun NH2-terminal kinase (JNK) prevents nuclear β-catenin accumulation and regulates axis formation in Xenopus embryos. Proc Natl Acad Sci 103:16313–16318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zahaevitz DW et al. 2000. Paullones are potent inhibitors of glycogen synthase kinase-3β and cyclindependent kinase 5/p25. Eur J Biochem 267:5983–5994 [DOI] [PubMed] [Google Scholar]
- MacWilliams HK 1983a. Hydra transplantation phenomena and the mechanism of Hydra head regeneration. I. Properties of the head inhibition. Dev Biol 96:217–238 [DOI] [PubMed] [Google Scholar]
- MacWilliams HK 1983b. Hydra transplantation phenomena and the mechanism of Hydra head regeneration. II. Properties of the head activation. Dev Biol 96:239–257 [DOI] [PubMed] [Google Scholar]
- Marcum BA, Campbell RD 1978. Development of Hydra lacking nerve and interstitial cells. J Cell Sci 29:17–33 [DOI] [PubMed] [Google Scholar]
- Martinez DE 1998. Mortality patterns suggest lack of senescence in hydra. Exp Gerontol 33:217–225 [DOI] [PubMed] [Google Scholar]
- Meinhardt H 2009. Models for the generation and interpretation of gradients. Cold Spring Harb Perspect Biol 1:a001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto JJ, Campbell RD 1977. Tissue economics of hydra: Regulation of cell cycle, animal size and development by controlled feeding rates. J Cell Sci 28:117–132 [DOI] [PubMed] [Google Scholar]
- Reinhardt B, Broun M, Blitz IL, Bode HR 2004. HyBMP5-8b, a BMP5-8 orthologue, acts during axial patterning and tentacle formation in hydra. Dev Biol 267:43–59 [DOI] [PubMed] [Google Scholar]
- Sanyal S 1966. Bud determination in hydra. Indian J Exp Biol 4:88–92 [PubMed] [Google Scholar]
- Technau U, Bode HR 1999. HyBra1, a Brachyury homologue, acts during head formation in Hydra. Development 126:999–1010 [DOI] [PubMed] [Google Scholar]
- Teh MT, Blaydon D, Ghali LR, Briggs V, Edmunds S, Pantazi E, Barnes MR, Leigh IM, Kelsell DP, Philpott MP 2007. Role for WNT16B in human epidermal keratinocyte proliferation and differentiation. J Cell Sci 120:330–339 [DOI] [PubMed] [Google Scholar]
- West DL 1978. The epitheliomuscular cell of hydra: Its fine structure, three dimensional architecture and relation to morphogenesis. Tissue Cell 10:629–646 [DOI] [PubMed] [Google Scholar]
- Wilby OK, Webster G 1970a. Studies on the transmission of hypostome inhibition in hydra. J Embryol Exp Morphol 24:583–593 [PubMed] [Google Scholar]
- Wilby OK, Webster G 1970b. Experimental studies on axial polarity in hydra. J Embryol Exp Morphol 24:595–613 [PubMed] [Google Scholar]
- Wittlieb J, Khalturin K, Lohmann JU, Anton-Erxleben F, Bosch TC 2006. Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc Natl Acad Sci 103:6208–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RL 1977. The cell junctions of hydra as viewed by freeze-fracture replication. J Ultrastruct Res 58:299–315 [DOI] [PubMed] [Google Scholar]
- Yao T 1945. Studies on the organizer problem in Pelmatohydra oligactis. I. The induction potency of implants and the nature of the induced hydra. J Exp Biol 21:147–150 [Google Scholar]