Abstract
Objective
To determine the prevalence of HPV DNA in cervical specimens from treatment-naïve women initiating highly active antiretroviral therapy (HAART) and explore the longitudinal association of HPV DNA with CD4 count and HIV viral load (VL).
Methods
Women enrolled prior to HAART were evaluated at baseline, weeks 24, 48, and 96 with CD4 count, VL, and cervical swab for HPV DNA.
Results
The 146 subjects had a median CD4 count of 238 cells/μL and VL of 13,894 copies/mL. Ninety-seven (66%) subjects had HPV DNA detected in the baseline specimen including 90 subjects (62%) positive for one or more high risk HPV types. HPV DNA detection declined to 49% at week 96, and that of a high risk HPV type to 39%. The duration of follow-up was associated with decreased detection of HPV DNA of any type (p=0.045) and of high risk HPV types (p=0.003). There was at most a marginal association between HAART response and loss of detection of cervical HPV DNA.
Conclusions
Women initiating HAART had a high prevalence of cervical HPV DNA that declined over 96 weeks of HAART. The relationship of CD4 count and VL response to the decline of cervical HPV DNA was not strong.
Keywords: human papillomavirus, human immunodeficiency virus, cervical dysplasia, antiretroviral therapy
Introduction
Human immunodeficiency virus type 1 (HIV 1) infection is a significant risk factor for human papillomavirus (HPV) infection and the development of HPV-associated lesions in the female genital tract. Several large clinical studies have established that there are important differences in the characteristics of genital tract HPV in women with and without HIV infection. HPV DNA can be detected in genital specimens from HIV-infected women two to five times as frequently as in similar specimens obtained from HIV-negative women 1-4 and the HPV infection is more likely to persist 1, 5, 6, 7. HIV-infected women are three to five times as likely to develop cervical dysplasia as HIV-negative women 8-10. These findings suggest that HIV infection increases a woman's susceptibility to HPV infection, facilitates the ability of HPV to persist, and/or alters the natural history of preexisting HPV infection.
Certain HPV types are closely associated with the development of high-grade intraepithelial lesions and invasive carcinoma. There must be persistent genital tract infection with one or more of the high-risk HPV types for neoplastic changes to occur 11. A substantial proportion of HIV-negative women clear their HPV infection over time 12 while HIV-infected women are more likely to have a persistent HPV infection 1, 5.
Treatment with highly-active antiretroviral therapy (HAART) has been shown to result in significant increases in CD4 counts and partial reconstitution of the immune system 13, 14. Lower CD4 counts have been associated with HPV persistence and cervical dysplasia in several studies 3, 5, 6, 8, 15-17. It is not known whether treatment of HIV infection with potent antiretroviral regimens could affect the persistence of HPV infection and progression of cervical dysplasia. One study that evaluated changes in the incidence of AIDS-associated cancers in the HAART era failed to show any change in cervical cancer, although the number of cases was small 18. Several studies have attempted to evaluate the impact of HAART on genital tract HPV infection and dysplasia. The conclusions have been mixed, with some studies showing an effect and others showing no effect 19-26. These studies have generally been retrospective observations, case control studies, or small studies of limited duration.
The primary objectives of this study were to determine the prevalence of HPV DNA in cervical specimens from treatment-naïve women initiating HAART and to explore the trend of HPV DNA prevalence over time, and its association with CD4 and HIV viral load response after HAART. There were also a number of secondary objectives to examine the impact of HAART on specific HPV types and on cervical dysplasia.
Subjects and methods
Subjects
Women were recruited and enrolled from 35 AIDS Clinical Trials Group (ACTG), Pediatric ACTG, and NICHD Clinical Trials Network sites in the United States and Puerto Rico. The enrollment period was from January 2001 to May 2003 and subject follow up ended in September 2005. All study subjects (or guardians) provided informed consent using documents and procedures approved by the ACTG and by each institution's Institutional Review Board or ethics committee. Subjects were at least 13 years old (post menarche), had confirmed HIV 1 infection, and had no history of antiretroviral treatment. Subjects were enrolled when they were about to begin HAART, either in a controlled clinical trial or (following a protocol amendment in late 2001) by prescription. The specific treatment regimen was not mandated by this study, but all regimens (or possible regimens) were reviewed and approved by the protocol team. HAART was defined for this protocol as a three (or more) drug combination therapy containing at least one protease inhibitor or non-nucleoside reverse transcriptase inhibitor or a triple nucleoside regimen containing abacavir. Because some subjects were participating in clinical treatment trials that included blinded antiretroviral regimens, the exact drugs received were not known for all subjects at the time of enrollment, but all possible regimens met the protocol definition of HAART. Women with a history of invasive cervical cancer or a hysterectomy were excluded from the study.
Evaluations on study
All women underwent a standard evaluation at baseline (within 14 days of beginning antiretroviral therapy), week 24, 48, and 96. In addition to a medical history and pelvic examination, the evaluation included CD4 count, HIV 1 plasma viral load, endocervical secretions for genital tract HIV 1 viral load, cervical cytology, and collection of a cervical swab specimen for HPV testing. The cytology specimens were read in a central laboratory and categorized using the Bethesda system 27. Women with abnormal cytology results were referred for clinical care. Behavioral information such as smoking and injecting drug use was only collected at the baseline visit. Information about sexual activity (yes/no) was collected at each visit.
HIV assays
The standard assays related to HIV infection (CD4 count and plasma HIV 1 viral load) were performed in laboratories at the ACTG sites using standardized techniques. The lower detection limit for plasma HIV 1 viral load was 50 copies per ml. All laboratories were certified by the NIAID Division of AIDS virology and immunology quality assurance programs. Genital tract HIV 1 viral load assays were performed on endocervical secretions collected on paper wicks at baseline and at the follow up visits. These assays were performed at the University of Washington Virology Laboratory as previously described 28. The lower limit of detection for this assay was 1500 copies/mL.
HPV DNA detection and typing
DNA was extracted from the cervical swab specimens as previously described 29. The Roche PCR/reverse blot strip assay was used to detect specific HPV types in the cervical swab specimens 30, 31. This assay uses nondegenerate biotinylated primer pairs to amplify 27 individual genital HPV types, including types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 73, 82, 83, and 84 and a β-globin target to determine specimen adequacy. Reactions were performed and interpreted as previously described 29, 32. We separated HPV types according to cancer risk based on the epidemiologic associations reported by Muñoz 11. Therefore, HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82 were considered to be high risk types and HPV types 6, 11, 40, 42, and 54 were considered low risk. HPV types 55, 83, and 84 were not included in the Muñoz report. Based on phylogenetic relationships to HPV types included in the epidemiologic study, those 3 types were included in the low risk group.
Statistical methods
The analysis investigated the prevalence of HPV DNA over time. Additionally, the relationship between HPV DNA, cervical cytological abnormalities, CD4 counts, HPV risk type and plasma HIV RNA level was also explored. Cervical cytological readings of atypical squamous cells of uncertain significance (ASCUS) were not counted as cervical cytological abnormalities.
Statistical significance was determined using Wilcoxon Rank Sum tests for continuous outcomes, Fisher's exact tests for discrete outcomes, Kruskal-Wallis tests for comparison of more than two groups and ordinal categories, ordered exact tests for ranked categories. No adjustment for multiple comparisons was made. Age was analyzed as a grouped variable (<17, 17-21, 22-35, 36-45, 46-55, and >55). Some analyses were also performed using a dichotomized age variable, above or below 35.
Missing data dropout pattern was examined through comparisons between the groups with vs. without HPV data at weeks 24, 48 and 96. Subjects with all visits (weeks 0, 24, 48 and 96) were also compared with subjects with at least 1 missing point. Baseline demographic (age, race/ethnicity and IV drug use at baseline) and health characteristics (baseline CD4 count and HIV 1 RNA) were compared, as well as baseline HPV DNA prevalence (any type, high risk, low risk, multiple types, multiple high risk types and multiple low risk types) and baseline cervical cytological abnormality prevalence.
Logistic random-effect mixed models were fitted for the binary longitudinal data on detection of DNA of HPV of any type, high risk HPV types, low risk HPV types, multiple HPV types and multiple high risk HPV types. Duration of follow-up, age, sexual activity, the presence of a cervical cytological abnormality, CD4 count and plasma HIV RNA at baseline as well as current sexual activity were explored as independent variables. The SAS GLIMMIX procedure was used. The correlation type was assumed unstructured in all models. Sensitivity analysis was conducted and similar models were fitted on a subset of data including only subjects who completed all visits.
Pooled logistic regression models were fitted on the subset of subjects without a cervical cytological abnormality at baseline and with at least one follow-up visit. In this population of HIV infected women beginning antiretroviral therapy, detection of a cervical cytological abnormality was defined if a squamous intraepithelial lesion was detected after a previous cervical cytology result was read as normal or ASCUS. The models explored the effect of HPV DNA status (any type positive, high risk type positive, low risk type positive, multiple types positive and multiple high risk types positive, one at a time) at baseline on cervical cytological abnormality detection, after adjusting for baseline CD4 count, age (above or below 35), sexual activity at baseline and log plasma HIV RNA at baseline. Adjustments were also made for the current CD4 count, current plasma HIV RNA (<50 or detectable), and current sexual activity.
Results
Demographics and baseline characteristics
A total of 147 subjects were enrolled in the study but baseline data were missing for 1 subject. Characteristics for the 146 subjects included in the analysis are shown in Table 1. The majority of study subjects were members of racial or ethnic minority groups. Slightly over half of the subjects reported that they were sexually active at the time of enrollment. The group had a median CD4 count of 238.
Table 1.
Baseline characteristics of study subjects
| Total (n=146) | ||
|---|---|---|
| Age at baseline | median (range) | 35 (16,70) |
| Race/ethnicity | Non-Hispanic White | 24 (16%) |
| Non-Hispanic Black | 64 (44%) | |
| Hispanic | 51 (35%) | |
| Asian | 5 (3%) | |
| Native American | 1 (<1%) | |
| Other/unknown | 1 (<1%) | |
| Sexually active at baseline? | Yes | 84 (56%) |
| Cigarette smoker at baseline | Ever | 70 (48%) |
| Current | 41 (28%) | |
| IDU* at baseline | Never | 131 (90%) |
| Previously | 15 (10%) | |
| Baseline CD4 count | median (Interquartile-range) | 238 cells/mm3 (121, 339) |
| Baseline plasma HIV 1 viral load | median (Interquartile-range) | 13,894 copies/mL (1,378, 59,556) |
Injecting drug use history
HPV DNA detection at baseline
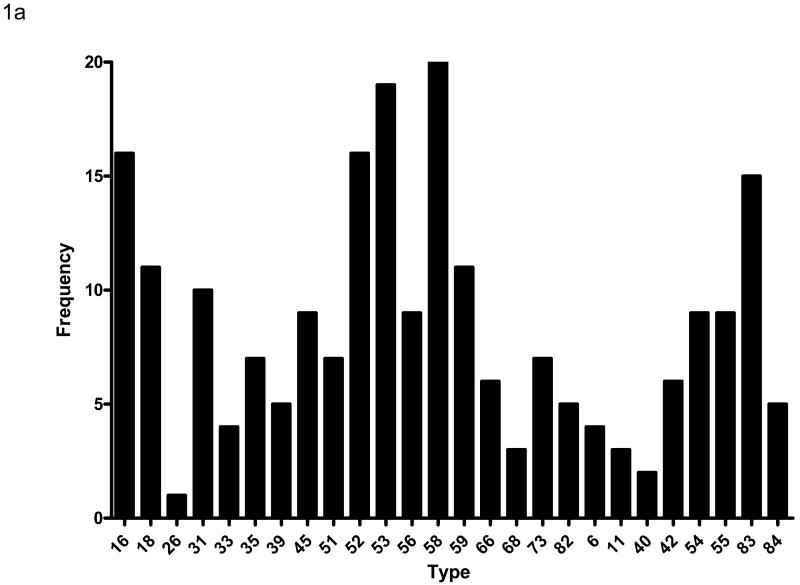
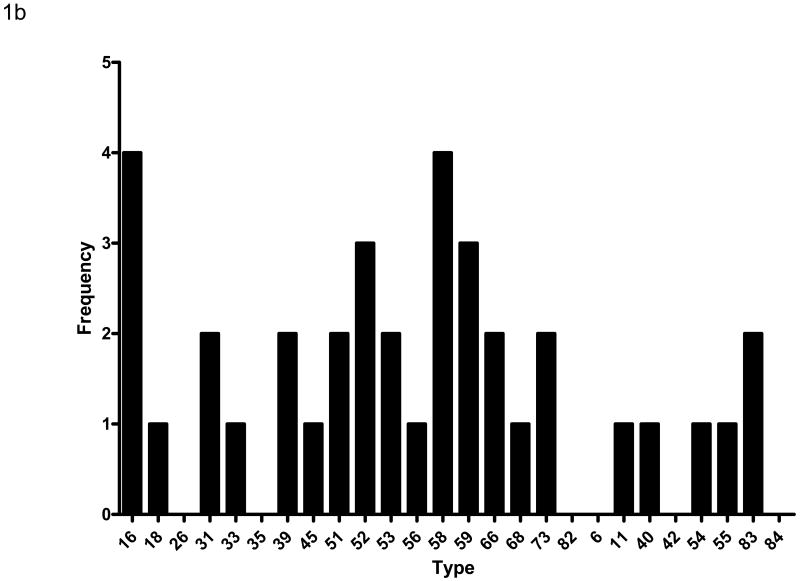
Cervical swabs were collected at baseline and tested for HPV DNA by PCR. A total of 97 (66%) subjects had HPV DNA detected in the baseline specimen. The median CD4 count of subjects with HPV DNA detected was 215 compared with a CD4 count of 311 among those who were HPV DNA negative (p=0.042). There was no association between detection of HPV DNA in the cervix and plasma HIV viral load (p=0.241). Ninety subjects (62%) were positive for one or more high risk HPV types while 36 (25%) were positive for one or more low risk HPV types. Sixty one (42%) subjects were positive for multiple HPV types including some with both high and low risk types. The distribution of HPV types detected is shown in Figure 1a. DNA from 26 of the 27 HPV types detected by the assay used for this study was identified in specimens from at least one subject. The most common type detected was HPV 58; at least 10 subjects had DNA from HPV types (in order of frequency) 53, 16, 52, 83, 18, 59, and 31 detected. The median number of HPV types detected was 2; 16 subjects had 4 or more HPV types detected. Subjects with higher baseline CD4 counts were more likely to have a single HPV type detected (as opposed to multiple types) than subjects with lower CD4 counts (p=0.049). Among those with only one HPV type detected, the distribution of types was similar to that of the overall population (Figure 1b), although HPV 16 was found as often as HPV 58. There was no association of HPV DNA detection with age at baseline (p=0.480), race.ethnicity (p=0.753), history of injecting drug use (p=0.088), current sexual activity (p=0.992), or current or previous cigarette smoking (p=0.253, p=0.383, respectively).
Figure 1.
Figure 1a – HPV DNA types detected at baseline among the 146 women. HPV types are displayed on the horizontal axis and the frequency that each type was detected on the vertical axis. The high cancer risk types (as defined by epidemiologic association 11) are displayed on the left (types 16 through 82) and the low cancer risk types on the right (types 6 through 84). Note that many subjects had multiple HPV types detected.
Figure 1b – Distribution of HPV types detected among the 36 subjects who had only one HPV type detected at baseline. The orientation of the display is the same as in Figure 1a.
Antiretroviral treatment outcomes
Most subjects responded to antiretroviral therapy with an increase in CD4 count and a decrease in HIV 1 viral load. The treatment results over the course of the study are shown in Table 2. There was a steady rise in median CD4 count and over 60% of subjects had undetectable HIV viral loads at all time points after baseline.
Table 2.
Results of antiretroviral therapy
| #Subjects with data available | Median CD4+ cells/mm3 | % HIV 1 RNA <50 copies/mL | |
|---|---|---|---|
| Baseline | 141 | 238 | 4.1 |
| Week 24 | 125 | 309 | 60.3 |
| Week 48 | 107 | 380 | 66.7 |
| Week 96 | 107 | 426 | 62.9 |
Genital tract HIV 1 viral loads also were measured in this study. At baseline, 53% of subjects had detectable genital tract HIV 1 viral loads. The proportion of subjects with detectable genital tract HIV 1 RNA dropped after initiation of HAART and was strongly correlated with plasma HIV 1 RNA (p<0.001). The proportion of subjects with a detectable genital tract HIV 1 viral load at weeks 24, 48, and 96 was 11%, 15%, and 18%, respectively. There was no significant association between cervical HPV DNA detection and genital tract HIV 1 viral load (data not shown).
Longitudinal HPV DNA detection
A summary of the HPV DNA detection results at baseline and at weeks 24, 48, and 96 is shown in Table 3a for the entire study population and in Table 3b for the subset of subjects who had specimens available from all time points. The prevalence of cervical HPV DNA detection declined from 66% at baseline to 49% at week 96 and the prevalence of the DNA of a high risk HPV type declined from 62% to 39% over the same time period.
Table 3.
| Table 3a – HPV DNA detection at baseline and follow up for all subjects | ||||||
|---|---|---|---|---|---|---|
| N | HPV DNA+ # (%) |
HR* HPV DNA+ # (%) |
LR* HPV DNA+ # (%) |
Multiple HPV Types DNA+ # (%) |
Multiple HR* HPV Types DNA+ # (%) |
|
| Baseline | 146 | 97 (66) | 90 (62) | 36 (25) | 61 (42) | 46 (32) |
| Week 24 | 119 | 73 (61) | 63 (53) | 27 (23) | 38 (32) | 31 (26) |
| Week 48 | 104 | 63 (61) | 56 (54) | 29 (28) | 33 (32) | 21 (20) |
| Week 96 | 94 | 46 (49) | 37 (39) | 19 (20) | 23 (24) | 15 (16) |
| Duration of follow-up** (every 24 weeks) |
OR(CI)** P-value |
0.87 (0.77, 1.00) 0.043 |
0.83 (0.74, 0.94) 0.002 |
0.99(0.85, 1.15) 0.907 |
0.87 (0.78, 0.97) 0.013 |
0.83 (0.72, 0.95) 0.009 |
| Significant Covariates (P<0.05) | ||||||
| Baseline CD4 count (every 50 cells) |
OR(CI)** P-value |
0.89 (0.81, 0.96) 0.005 |
0.90 (0.83, 0.98) 0.018 |
0.90(0.82,0.99) 0.039 |
0.88 (0.79, 0.97) 0.011 |
0.90 (0.81, 1.00) 0.050 |
| CCA* at Baseline | OR(CI)** P-value |
- NS |
2.26 (1.01, 5.09) 0.049 |
- NS |
2.42 (1.09, 5.41) 0.031 |
2.70 (1.20, 6.06) 0.017 |
| Table 3b – HPV DNA detection at baseline and follow up for subjects with data at all time points | ||||||
| N | HPV DNA+ # (%) |
HR* HPV DNA+ # (%) |
LR* HPV DNA+ # (%) |
Multiple HPV Types DNA+ # (%) |
Multiple HR* HPV Types DNA+ # (%) |
|
| Baseline | 72 | 43 (60) | 40 (56) | 12 (17) | 26 (36) | 20 (28) |
| Week 24 | 72 | 39 (54) | 35 (49) | 11 (15) | 18 (25) | 14 (19) |
| Week 48 | 72 | 40 (56) | 36 (50) | 15(21) | 19 (26) | 11 (15) |
| Week 96 | 72 | 36 (50) | 30 (42) | 14 (19) | 16 (22) | 11 (15) |
| Duration of follow-up** (every 24 weeks) |
OR(CI)** P-value |
0.86 (0.71, 1.04) 0.113 |
0.83 (0.71, 0.97) 0.017 |
1.04(0.85, 1.27) 0.695 |
0.84 (0.71, 1.00) 0.047 |
0.77 (0.61, 0.98) 0.037 |
| Significant Covariates (P<0.05) | ||||||
| Baseline CD4 count (every 50 cells) |
OR(CI)** P-value |
0.76 (0.67, 0.87) <0.001 |
0.79 (0.69, 0.91) 0.002 |
- NS |
0.80 (0.68, 0.94) 0.008 |
0.80 (0.65, 0.99) 0.043 |
| CCA* at Baseline | OR(CI)** P-value |
- NS |
- NS |
- NS |
4.51(1.36,14.99) 0.015 |
7.67(1.69,34.73) 0.009 |
| Age >35 at Baseline | OR(CI)** P-value |
0.22 (0.09, 0.55) 0.001 |
0.23 (0.08, 0.62) 0.004 |
- NS |
- NS |
- NS |
HR – high cancer risk HPV type; LR – low cancer risk HPV type; CCA – cervical cytological abnormality (see methods section)
Based on the logistic random-effect mixed models; OR(CI)- Odds Ratio (95% Confidence Interval)
Comparing subjects with or without HPV data at all visits, Hispanic subjects were much more likely (p=0.004) to have all HPV data. There was no significant difference in other baseline demographic and health characteristics. A higher rate of high risk HPV DNA detection at baseline was marginally associated (p=0.089) with missing HPV data for at least one follow up visit, whereas a higher rate of low risk HPV DNA detection at baseline was significantly associated (p=0.030) with missing follow up data. Overall, it appeared that HPV-infected women had less complete HPV data. This indicated that we could not assume the missing data pattern as missing completely at random when assessing the trend of HPV prevalence over time. Likelihood-based logistic random-effect mixed models for longitudinal exploration were therefore chosen to take into account the correlation between the repeated measures over time within one subject with appropriate assumptions.
After adjusting for age, sexual activity at baseline, cervical cytological abnormality at baseline, CD4 count, and plasma HIV 1 viral load at baseline as well as current sexual activity, the duration of follow-up was found to be significantly associated with decreased detection of HPV DNA of any type (OR=0.87, 95%CI (0.77, 1.00), p=0.043); high risk HPV type (OR=0.83, 95%CI (0.74, 0.94), p=0.002); multiple HPV types (OR=0.87, 95%CI (0.78, 0.97), p=0.013)); and multiple high risk HPV types (OR=0.83, 95%CI (0.72, 0.95), p=0.009) (see Table 3a). The odds of detecting HPV DNA of any type were estimated to decrease by 13% after every 24 weeks of follow up. The same model was applied to the subset of subjects who had specimens available at each time point (n=72) and it yielded similar results except that the decrease for HPV DNA of any type was no longer significant (p=0.113. Table 3b).
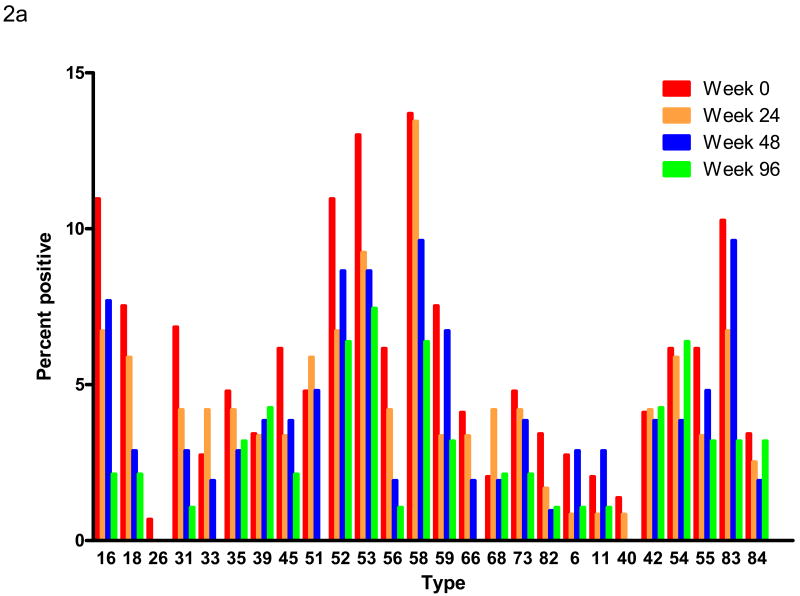
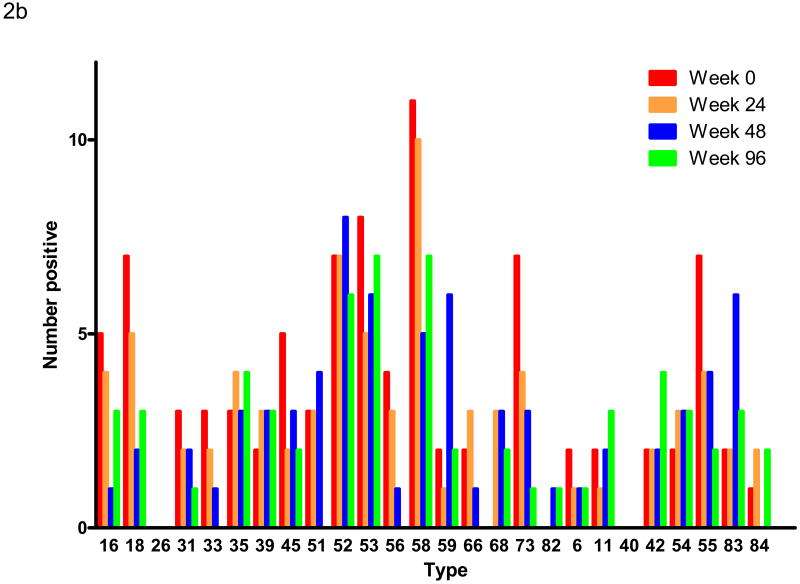
The frequency of detecting DNA of nearly all individual HPV types also decreased with time of follow up. The longitudinal detection of DNA of each HPV type is shown in Figure 2a. Because of the loss of subjects over time, these results are presented as the percentage of available specimens that were positive for DNA of each HPV type. All types showed a decrease in prevalence except for the high risk types HPV 39 and 68 and the low risk types HPV 42 and 54 that showed no change or a marginal increase in prevalence. Figure 2b shows similar data for the subset of subjects with specimens available at each time point. In this figure, the absolute number of each type is shown because the number of available specimens is the same at each time point. The result is similar, but the smaller numbers make detailed comparisons difficult.
Figure 2.
Figure 2a – Longitudinal HPV DNA detection. The HPV types are displayed on the horizontal axis as in Figure 1. Each bar within a type represents a different time point after initiation of HAART as indicated. The vertical axis shows the percentage of specimens collected at each time point that were positive for that type. The number of specimens tested at each time point was: week 0, n=146; week 24, n=119; week 48, n=104; week 96, n=94.
Figure 2b – Longitudinal HPV DNA detection in the subset of 72 subjects who had specimens available at each follow up visit. The orientation of the display is the same as in Figure 2a except that the vertical axis shows the number of specimens positive for each type (all 72 specimens were tested at each time point).
Association of cervical cytological abnormalities with HPV and HAART response
Among the 106 subjects who did not have a cervical cytological abnormality at baseline and who had at least one follow-up visit, 21 had a cervical cytological abnormality subsequently detected. The pooled logistic models showed that the presence of HPV DNA of any type (OR=6.74, 95%CI (2.09, 21.81), p=0.001) and HPV DNA from a high risk type (OR=10.69, 95% CI (3.40, 33.62), p<0.001) at baseline were positively associated with detection of a cervical cytological abnormality, after controlling for CD4 count, age, sexual activity and plasma HIV 1 viral load at baseline, as well as the current CD4 count, plasma HIV 1 viral load and sexual activity. The presence of HPV DNA from a low risk type at baseline was found to be inversely associated with the detection of a cervical cytological abnormality (OR=0.23, 95%CI (0.08, 0.69), p=0.008). The presence of multiple HPV DNA types or multiple high risk types at baseline was not found to be significantly associated with the detection of a cervical cytological abnormality. After adjustment for sexual activity, HPV DNA status (any type), CD4 count and plasma HIV 1 viral load at baseline, as well as the current plasma HIV RNA and sexual activity, the model showed that the current CD4 count (OR 0.77 for every 50 cell increase, 95% CI (0.65, 0.91) p=0.002) and age >35 (OR 0.35 95% CI (0.14, 0.85) p=0.020) were inversely associated with the detection of a cervical cytological abnormality.
Discussion
Retrospective studies and small prospective studies have shown varying effects of HAART on HPV infection and on HPV-associated dysplasia and cancer. This study was designed to prospectively evaluate the impact of HAART on HPV infection and disease in previously untreated women.
The 66% prevalence of HPV DNA detection at baseline was similar to that reported in previous studies that used HPV DNA detection methods of similar sensitivity 1, 2, 33. The distribution of HPV types detected also was similar to other reports in comparable populations 33, 34, 35, although there were small differences of uncertain significance. Although HPV 16 and 18 cause the majority of cervical cancers 11 and are targeted by current HPV vaccines 36, 37, there were other HPV types that were detected more commonly than HPV 16 and 18. This could have implications if HPV immunization is offered to HIV-infected women in the future or if women who previously received an HPV vaccine are subsequently infected with HIV 38. However, the purpose of the vaccine is to prevent high-grade dysplasia and cancer; the relative importance of these additional HPV types compared to HPV 16 and 18 in advanced disease (especially cancer) in this patient population has not been conclusively established. One study suggested that even though types other than HPV 16 and 18 were commonly detected in HIV-infected women, HPV 16 and 18 were associated with about 65% of invasive cervical cancer, a prevalence similar to that of matched control subjects 39.
As with most persons initiating antiretroviral therapy, the subjects in this study responded well to treatment with improvements in CD4 counts and declines in HIV 1 viral load. A variety of different antiretroviral regimens were used but there was no adjustment for regimen. The proportion of subjects with undetectable plasma HIV 1 viral loads at 48 and 96 weeks (67% and 63%, respectively) was somewhat lower than expected. For example, some subjects in this study were co-enrolled in ACTG protocol 5095 that had rates of undetectable viral loads of approximately 80% at 48 and 96 weeks 40. However, many of the subjects in the current study were on treatment by prescription and women have been found to have somewhat higher failure rates in non-clinical trial settings 41.
Any prospective study relies on longitudinal data that are as complete as possible, so we were concerned about subject loss. Our analysis suggested that subjects were not lost at random. A logistic random effect mixed model accounts for the association of loss with covariates. Although this is a valid approach, there is still some uncertainty because the exact nature of the missing data is unknown. Using this model, we demonstrated a decline of detection of any HPV type of 13% for every 24 weeks on study. The decline was most evident among the high risk HPV types and was seen for nearly every individual HPV type detected. An analysis of two large cohorts of HIV-infected women (WIHS and HERS) suggested that detection of HPV 16 was less closely related to immunosuppression as measured by CD4 count than was detection of other HPV types 33. Based on that observation, the authors predicted that HAART would have relatively less impact on HPV 16 detection than on detection of other HPV types. Although the numbers in the current study are relatively small, there was no suggestion that there was a differential effect of HAART on HPV 16 compared to other high risk HPV types. There was no significant effect of HAART on the detection of low risk HPV types. The reasons for this are not certain. It may be that our relatively small sample size and lower prevalence of low risk HPV types were insufficient to detect a difference.
There are inherent methodologic limitations in this study design. Detection of HPV DNA at two time points could reflect persistence of HPV or clearance and reinfection. Detection of DNA of a new HPV type could reflect a new infection or reactivation of a previously acquired HPV. Similarly, loss of detection could represent clearance, sampling variability, or other technical problems. Despite these limitations, the steady decline of HPV detection after initiation of HAART is consistent with a treatment effect. The impact of HAART on HPV infection appears to be limited mostly to the high risk HPV types. In addition, the observation that the detection of cervical cytological abnormalities was inversely associated with current CD4 count at follow up visits also suggests a treatment effect.
Further studies will be needed to confirm and extend these observations. Few women in this study developed high-grade dysplasia and there were no cases of cervical cancer. It will be important to measure the impact of HAART on key endpoints of HPV infection, high-grade dysplasia and cervical cancer. Some such studies have been initiated in developing countries where cervical cancer is more prevalent 42.
Acknowledgments
The authors would like to thank Linda Naini for her assistance in the preparation of this manuscript and Brahim Qadadri for technical assistance with the HPV assays. We would also like to thank Dr. Anna-Barbara Moscicki for helpful comments on the manuscript.
A5029 sites and contributing personnel include: Hannah Edmondson-Melancon, RN, MPH and Lorraine Sanchez-Keeland, PA-C- University of Southern California (A1201) CTU Grant # AI27673
Elizabeth Livingston, MD and Joan Riddle, RN- Duke University Medical Center (A1601) CTU Grant # AI69684
Mary Albrecht, MD- Beth Israel Deaconess Med Ctr (A0103) and Sigal Yawetz, MD- Brigham and Women's Hospital (A0107) CTU Grant # AI069472; AI060354
Margie Vasquez, RN and Judith A. Aberg, MD- New York University/NYC HHC at Bellevue (A0401) CTU Grant # AI069532, GCRC Grant # RR00096
Donna McGregor, NP and Oluwatoyin Adeyemi, MD- Northwestern University (A2701) and Cook County CORECenter (A2705) CTU Grant # AI 69471
Laura Bachmann, MD, MPH and Angela Rivers, RN- University of Alabama at Birmingham (A5801) CTU Grant # AI069452-01, GCRC Grant # RR-00032, CFAR Grant # AI027767
Helen Grubbs, RN,MSN- Indiana University (A2601) CTU Grant # AI25859, GCRC Grant # RR000750
Jane Reid, RNc, MS, ANP and Carol Greisberger, RN- University of Rochester (A1101) CTU Grant # AI69411, GCRC Grant # RR00044
Michelle V. Lisgaris, MD and Barbara Philpotts, RN- Case Western Reserve University (A2501) CTU Grant # AI069501
Anneris Delgado, PA and Margaret A. Fischl, MD- University of Miami AIDS Clinical Research Unit (A0901) CTU Grant # AI069477
Sharon Riddler, MD, MPH and Barbara Rutecki, MSN, MPH, CRNP- University of Pittsburgh (A1001) CTU Grant # AI 69494
Karen T. Tashima, MD and Deborah K. Perez, RN-The Miriam Hospital (A2951) CTU Grant AI46381
Charles Davis and Barbara Glick- University of Maryland, Inst. of Human Virology (A4651)
Rodrigo Díaz-Velasco, MD, BC, Antonio Rodríguez- Mimoso, MD, Elvia Pérez-Hernández, BS, MEd., MA, MPH and Lourdes Angeli-Nieves, RN, BSN, MPH, San Juan City Hospital (Westat/NICHD 5031) Contract # HD33345
Sharon Nachman, MD, Paul Ogburn, MD and Jennifer Griffin, NP- SUNY at Stony Brook School of Medicine, Division of Ped. Infect. Dis. (Westat/NICHD 5040) Contract # HD33345
Irma L. Febo, MD and Hazel Ayala, RN- University of Puerto Rico, Childrens Hospital (P6601)
Mykyelle Crawford, RN BSN, and Madeline Torres, RN BSN- Columbia Collaborative HIV/AIDS Clinical Trials Unit (A7802) CTU Grant # AI069470
Esmine Leonard, RN and Ana M. Puga, MD- North Broward Hosp. District, Children's Diagnostic & Treatment Center, Inc. (Westat/NICHD 5055) Contract # HD33345
Kristin Mondy, MD and Michael K. Klebert, RN-CS, MSN, ANP- Washington University in St. Louis (A2101) CTU Grant # AI069495
Donna Mildvan, MD and Gwendolyn Costantini, FNP- Beth Israel Medical Center (A2851) CTU Grant # AI46370
Vicki Bailey, RN; Beverly Byram, MSN, FNP- Vanderbilt AIDS Clinical Trials Center (A3652) CTU Grant # AI069439
Cris Milne, RN, CNP and Marilou Marcelo Gonzalez, BS- University of Hawaii at Manoa (A5201) CTU Grant# AI34853
Douglas Watson and Sally Snader- University of Maryland Med Ctr, Div. of Ped Immunology & Rheumatology (P3702)
Daniel Johnson and Dominika Kowalski- Mt. Sinai Hospital Med Ctr, Womens & Childrens HIV Program (P4005)
Alice Stek, MD and Ana Melendrez, RN- # 5048 Los Angeles County/University of Southern California PACTU/Maternal-Child-Adolescent HIV Center (Westat/NICHD 5048) NICHD Contract # HD33345, Westat Subcontract Grant #7735-S042, GCRC Grant # RR000043
N. Jeanne Conley, RN and Ann C. Collier, MD- University of Washington (A1401) CTU Grant #AI27664 and AI 069434
Susan Pedersen, RN and Kristine Patterson, MD- University of North Carolina at Chapel Hill (A3201) CTU Grant # AI69423-01, CFAR Grant # AI50410, GCRC Grant #RR00046
Mobeen H. Rathore, MD and Ana Alvarez, MD- University of Florida/Jacksonville (Westat/NICHD 5051) Contract # HD33345
Supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases including grants AI68636, AI68634, AI38558, AI25859, AI38855, AI68634, RR00750, and NIH AIDS Clinical Trials Group awards to other participating institutions (see acknowledgements).
Footnotes
Conflicts of interest: All authors: no relevant conflicts.
Presented in part at the XV International AIDS Conference, Bangkok, 11-16 July 2004, abstract #ThPeA6952 and at the 17th International Society for Sexually Transmitted Diseases Research Meeting, Seattle, WA
References
- 1.Sun XW, Kuhn L, Ellerbrock TV, et al. Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med. 1997;337(19):1343–1349. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 2.Cu-Uvin S, Hogan JW, Warren D, et al. Prevalence of lower genital tract infections among human immunodeficiency virus (HIV)-seropositive and high-risk HIV-seronegative women. Clin Infect Dis. 1999;29(5):1145–1150. doi: 10.1086/313434. [DOI] [PubMed] [Google Scholar]
- 3.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91(3):226–236. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson DJ, Duerr A, Burk R, et al. Characterization of genital human papillomavirus infection in women who have or who are at risk of having HIV infection. Am J Obstet Gynecol. 2002;186(1):21–27. doi: 10.1067/mob.2002.119776. [DOI] [PubMed] [Google Scholar]
- 5.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)- positive and HIV-negative women. J Infect Dis. 2001;184(6):682–690. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 6.Moscicki AB, Ellenberg JH, Farhat S, et al. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004;190(1):37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 7.Rowhani-Rahbar A, Hawes SE, Sow PS, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis. 2007;196(6):887–894. doi: 10.1086/520883. [DOI] [PubMed] [Google Scholar]
- 8.Massad LS, Riester KA, Anastos KM, et al. Prevalence and predictors of squamous cell abnormalities in Papanicolaou smears from women infected with HIV-1. J Acquir Immune Defic Syndr. 1999;21(1):33–41. doi: 10.1097/00126334-199905010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283(8):1031–1037. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 10.Duerr A, Paramsothy P, Jamieson DJ, et al. Effect of HIV infection on atypical squamous cells of undetermined significance. Clin Infect Dis. 2006;42(6):855–861. doi: 10.1086/500404. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 12.Ho GYF, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338(7):423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 13.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337(11):734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 14.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337(11):725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 15.Hankins C, Coutlee F, Lapointe N, et al. Prevalence of risk factors associated with human papillomavirus infection in women living with HIV. Can Med Assoc J. 1999;160(2):185–191. [PMC free article] [PubMed] [Google Scholar]
- 16.Delmas MC, Larsen C, van Benthem B, et al. Cervical squamous intraepithelial lesions in HIV-infected women: prevalence, incidence and regression. AIDS. 2000;14(12):1775–1784. doi: 10.1097/00002030-200008180-00013. [DOI] [PubMed] [Google Scholar]
- 17.Levi JE, Fernandes S, Tateno AF, et al. Presence of multiple human papillomavirus types in cervical samples from HIV-infected women. Gynecol Oncol. 2004;92(1):225–231. doi: 10.1016/j.ygyno.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92(22):1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 19.Heard I, Schmitz V, Costagliola D, et al. Early regression of cervical lesions in HIV-seropositive women receiving highly active antiretroviral therapy. AIDS. 1998;12(12):1459–1464. doi: 10.1097/00002030-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lillo FB, Ferrari D, Veglia F, et al. Human papillomavirus infection and associated cervical disease in human immunodeficiency virus-infected women: Effect of highly active antiretroviral therapy. J Infect Dis. 2001;184(5):547–551. doi: 10.1086/322856. [DOI] [PubMed] [Google Scholar]
- 21.Minkoff H, Ahdieh L, Massad LS, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS. 2001;15(16):2157–2164. doi: 10.1097/00002030-200111090-00011. [DOI] [PubMed] [Google Scholar]
- 22.Branca M, Garbuglia AR, Benedetto A, et al. Factors predicting the persistence of genital human papillomavirus infections and PAP smear abnormality in HIV-positive and HIV-negative women during prospective follow-up. Int J STD AIDS. 2003;14(6):417–425. doi: 10.1258/095646203765371321. [DOI] [PubMed] [Google Scholar]
- 23.Uberti-Foppa C, Ferrari D, Lodini S, et al. Long-term effect of highly active antiretroviral therapy on cervical lesions in HIV-positive women. AIDS. 2003;17(14):2136–2138. doi: 10.1097/00002030-200309260-00021. [DOI] [PubMed] [Google Scholar]
- 24.Ahdieh-Grant L, Li R, Levine AM, et al. Highly active antiretroviral therapy and cervical squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2004;96(14):1070–1076. doi: 10.1093/jnci/djh192. [DOI] [PubMed] [Google Scholar]
- 25.Del Mistro A, Bertorelle R, Franzetti M, et al. Antiretroviral therapy and the clinical evolution of human papillomavirus-associated genital lesions in HIV-positive women. Clin Infect Dis. 2004;38(5):737–742. doi: 10.1086/381681. [DOI] [PubMed] [Google Scholar]
- 26.Paramsothy P, Jamieson DJ, Heilig CM, et al. The effect of highly active antiretroviral therapy on human papillomavirus clearance and cervical cytology. Obstet Gynecol. 2009;113(1):26–31. doi: 10.1097/AOG.0b013e31819225cb. [DOI] [PubMed] [Google Scholar]
- 27.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 28.Coombs RW, Wright DJ, Reichelderfer PS, et al. Variation of human immunodeficiency virus type 1 viral RNA levels in the female genital tract: implications for applying measurements to individual women. J Infect Dis. 2001;184(9):1187–1191. doi: 10.1086/323660. [DOI] [PubMed] [Google Scholar]
- 29.Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191(2):182–192. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38(1):357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravitt PE, Peyton CL, Apple RJ, et al. Genotyping of 27 HPV types from L1 consensus PCR products using a single hybridization, reverse line-blot detection method. J Clin Microbiol. 1998;36(10):3020–3027. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown DR, Schroeder JM, Bryan JT, et al. Detection of multiple human papillomavirus types in condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37(10):3316–3322. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strickler HD, Palefsky JM, Shah KV, et al. Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst. 2003;95(14):1062–1071. doi: 10.1093/jnci/95.14.1062. [DOI] [PubMed] [Google Scholar]
- 34.Luque AE, Jabeen M, Messing S, et al. Prevalence of human papillomavirus genotypes and related abnormalities of cervical cytological results among HIV-1-infected women in Rochester, New York. J Infect Dis. 2006;194(4):428–434. doi: 10.1086/505876. [DOI] [PubMed] [Google Scholar]
- 35.Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007 doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 37.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncology. 2005;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 38.Bollen LJ, Chuachoowong R, Kilmarx PH, et al. Human papillomavirus (HPV) detection among human immunodeficiency virus-infected pregnant Thai women: implications for future HPV immunization. Sex Transm Dis. 2006;33(4):259–264. doi: 10.1097/01.olq.0000187208.94655.34. [DOI] [PubMed] [Google Scholar]
- 39.De Vuyst H, Gichangi P, Estambale B, et al. Human papillomavirus types in women with invasive cervical carcinoma by HIV status in Kenya. Int J Cancer. 2008;122(1):244–246. doi: 10.1002/ijc.23045. [DOI] [PubMed] [Google Scholar]
- 40.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296(7):769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 41.Kuyper LM, Wood E, Montaner JS, et al. Gender differences in HIV-1 RNA rebound attributed to incomplete antiretroviral adherence among HIV-Infected patients in a population-based cohort. J Acquir Immune Defic Syndr. 2004;37(4):1470–1476. doi: 10.1097/01.qai.0000138379.39317.62. [DOI] [PubMed] [Google Scholar]
- 42.Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, et al. Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecol Oncol. 2006;103(3):1017–1022. doi: 10.1016/j.ygyno.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]