Abstract
Background
Individuals with cocaine use disorder (CUD) chose cocaine over non-drug rewards. In two newly designed laboratory tasks with pictures, we document this modified choice outside of a cocaine administration paradigm.
Methods
Choice for viewing cocaine, pleasant, unpleasant, or neutral pictures - under explicit contingencies (where choice was made between two fully-visible side-by-side images) and under more implicit contingencies (where selections were made between pictures hidden under flipped-over cards) - was examined in 20 CUD and 20 matched healthy controls. Subjects also provided self-reported ratings of each picture’s pleasantness and arousal.
Results
Under both contingencies, CUD chose to view more cocaine pictures than control subjects, group differences that were not fully explained by the self-reported picture ratings. Further, whereas CUD’s choice for viewing cocaine pictures exceeded choice for viewing unpleasant pictures (but did not exceed choice for viewing pleasant pictures, in contrast to their self-reported ratings), healthy controls avoided viewing cocaine pictures as frequently as, or even more than, unpleasant pictures. Finally, CUD with the most cocaine viewing selections, even when directly compared to selections of the pleasant pictures, also reported the most frequent recent cocaine use.
Conclusions
Enhanced drug-related choice in cocaine addiction can be demonstrated even for non-pharmacological (pictorial) stimuli. This choice, which is modulated by alternative stimuli, partly transcends self-reports (possibly indicative of a disconnect in cocaine addiction between self-reports and objective behavior) to provide an objective marker of addiction severity. Neuroimaging studies are needed to establish the neural underpinnings of such enhanced cocaine-related choice.
Keywords: cocaine addiction, choice behavior, reward, salience, IAPS pictures, craving, unconscious motivation, neuropsychology
Introduction
Cocaine addicted individuals pursue cocaine and cocaine-related stimuli over non-drug related goals (1). The underlying mechanism may involve reduced striatal dopamine D2 receptor availability (2–5) and altered function in dopaminergically innervated corticolimbic areas that mediate processing of reward salience (6–8), such as the orbitofrontal cortex (9–11). In support of this suggested neurobiological mechanism, research in drug addicted individuals has indeed demonstrated reduced activation of corticolimbic brain areas when viewing erotic as compared to cocaine stimuli (12), as well as a unique pattern of neurocognitive changes including attentional bias toward drug-related stimuli (13–16).
This altered valuation of rewards in drug addiction is especially evident in studies that juxtapose choice for drug against choice for competing reinforcers. For example, previously drug exposed animals choose cocaine over novelty (17), adequate maternal behavior (18), and even food (19–21). Parallel human studies similarly show that drug addicted individuals routinely choose cocaine over money (22–24).
A neuropsychological task using pictures could provide an opportunity for similarly testing choice for drug-related as compared to competing stimuli outside of an acute drug administration paradigm, therefore suitable for use even when direct drug administration is not feasible or ethical (e.g., in abstaining or treatment-seeking drug addicted individuals). Following the perspective that drug-related stimuli become increasingly “wanted” in drug addiction (25), drug-related choice in drug addiction should extend to such non-pharmacological drug-related reinforcers (stimuli that increase behavior). Because drug-related choice may not be fully accessible to conscious awareness (26), as indeed supported by a disconnect between subjective and objective markers of behavior in drug addiction (11, 27, 28), such behavioral choice may not be fully captured with self-reported ratings.
In this study we report on two newly developed choice tasks that utilized four types of pictures (cocaine, pleasant, unpleasant, and neutral) to probe choice behavior for non-pharmacological stimuli in drug addiction. Subjects also provided self-reported ratings of each picture’s pleasantness and arousal. The following hypotheses guided our study: (A) overall choice for cocaine picture viewing will be higher for individuals with cocaine use disorders (CUD) than for healthy control subjects; (B) these group differences in cocaine picture choice will not be fully explained by the self-reported ratings; and (C) within the CUD group, heightened choice for drug-related stimuli, especially when compared to choice for other positively valued stimuli (i.e., pleasant pictures), will relate to indices of cocaine addiction severity.
Methods & Materials
Subjects
Subjects were recruited through advertisements in local newspapers, word-of-mouth, and local treatment facilities (see demographics in Table 1). Subjects met the following criteria, as ensured by initial screening by telephone and subsequent on-site medical and neurological evaluation: (A) absence of head trauma with loss of consciousness; (B) absence of current neurological or medical disease that required hospitalization or regular monitoring (subjects were free of any medications); and (C) except for cocaine in the CUD, negative urine screens for all other drugs or their metabolites. Of an initial pool that included 23 CUD and 22 controls, the current study used 20 CUD and 20 matched healthy control subjects (see Table 1 for matched demographic variables). Subjects were right-handed native English speakers.
Table 1.
Demographic Characteristics and Drug Use by Study Subjects
| Cocaine subjects (N=20) | Control subjects (N=20) | |
|---|---|---|
| Gender (male/female) | 19/1 | 17/3 |
| Ethnicity (African-American/Caucasian/Other) | 11/4/5 | 9/10/1 |
| History of cigarette smoking (current or past/never)† | 18/2 | 4/16 |
| Daily frequency of smoking (for current users; N=16) | 5.6 ± 5.3 | 10.0 ± 0.0 |
| Hours since last cigarette (for current users; N=16) | 11.6 ± 15.1 | 3.0 ± 0.0 |
| Education (years) | 12.6 ± 1.4 | 13.6 ± 1.9 |
| Age (years) | 45.1 ± 9.3 | 42.4 ± 5.3 |
| Socio-economic status (51) | 29.3 ± 11.1 | 31.7 ± 10.7 |
| Non-verbal intellectual functioning: Wechsler Abbreviated Scale of Intelligence: Matrix Reasoning scaled score (52) | 10.3 ± 5.1 | 11.3 ± 2.5 |
| Self-reported state depression (53)‡ | 7.9 ± 6.9 | 1.7 ± 2.9 |
| Age at onset of cocaine use (years) | 27.7 ± 5.8 | -- |
| Duration of use (years) | 15.5 ± 7.5 | -- |
| Frequency of use (days/week): last 30 days | 3.0 ± 2.9 | -- |
| Current use in $ per use (min – max, median): last 30 days | 0–60, 25 | -- |
| Duration of current abstinence (days) (min – max, median) | 0–1825, 5 | -- |
| Total score on the Cocaine Selective Severity Assessment Scale (measure of withdrawal symptoms) (range: 0–126) (54) | 15.4 ± 7.7 | -- |
| Severity of Dependence Scale (range: 0–15) (55) | 6.3 ± 4.2 | -- |
| Cocaine Craving Questionnaire (range: 0–45) (56) | 14.5 ± 2.7 | -- |
Note: Numbers are M ± SD. Subjects were matched on sex, age, intellectual functioning, education, and socioeconomic status;
χ2=19.8, p<0.001;
Mann-Whitney U: Z=−3.4, p<0.01.
Based on a comprehensive diagnostic interview (see supplemental online material for a complete listing of interview components), all CUD met DSM-IV criteria for current cocaine dependence (N=18) or cocaine dependence in full sustained remission (N=2). Among those meeting current dependence criteria, urine screen results confirmed the presence of cocaine in nine CUD [the other CUD were in active treatment where cocaine use is prohibited (N=6) or had not used cocaine within 72 hours of the study (N=3)]. Urine screens for all other drugs were negative (the control subjects tested negative for all drugs, including cocaine; see Table 1 for drug use variables in all CUD subjects). Comorbid diagnoses within CUD included current marijuana abuse (N=1), alcohol use disorder in full sustained remission (N=7), and major depression disorder (current N=1; in full sustained remission N=1). Given the high degree of lifetime overlap between cocaine addiction and depression, especially among those seeking treatment (29), we retained subjects with comorbid major depression, which increases generalizability of findings. Nevertheless, we accounted for the possibility that comorbid depression could explain our findings as reported below (see also supplemental online material). Subjects received full information about the research and provided written consent in accordance with the local institutional review board.
Stimuli
Both tasks used 90 pictures selected from the International Affective Picture System (IAPS) (30); of these, 30 depicted pleasant scenes (e.g., smiling faces, nude images), 30 depicted neutral scenes (e.g., neutral faces, household objects), and 30 depicted unpleasant scenes (e.g., sad faces, violent images). Additionally, we created a fourth picture category that included 30 images of cocaine and individuals preparing, using, or simulating use of cocaine (e.g., snorting or smoking), collected from freely available online sources and adapted (as still images) from a cocaine video used previously in our laboratory (31). Cocaine pictures were matched to the IAPS pictures on size and ratio of human to non-human content.
Picture Ratings
Before completing the two choice tasks, subjects underwent recordings of event-related potentials while passively viewing each of these pictures for 2000 msec (these results will be reported separately). Subjects then rated each picture on pleasantness (“rate how pleasant or unpleasant you felt about this picture”) and arousal (“rate how strong of an emotional response you had to this picture”). Subjects responded using a computerized version of the Self-Assessment Manikin (SAM) (32). For these ratings subjects chose the numbers ‘1’ through ‘9’ that appeared below the SAM characters (‘1’ corresponded to unhappy/no response manikins; ‘9’ corresponded to happy/high visceral response manikins).
Explicit Task
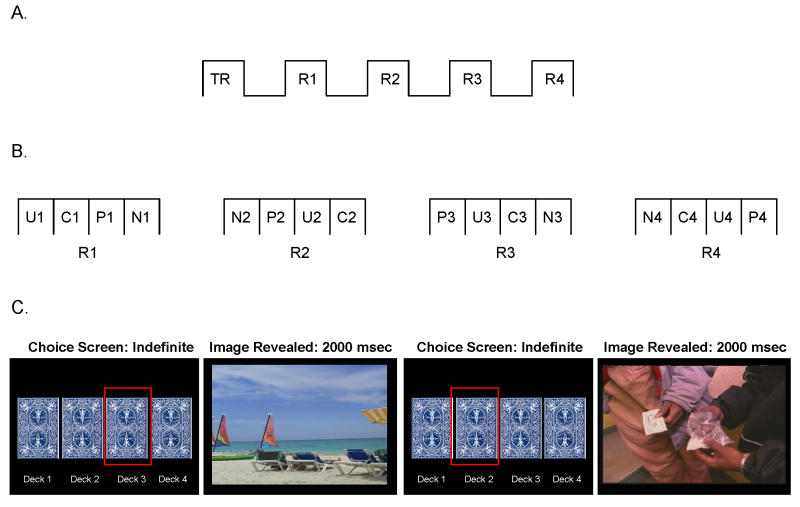
In the ‘explicit’ choice task, subjects chose via continued button pressing between two simultaneously presented (side-by-side) picture types (Figure 1). Image categories included the cocaine, pleasant, unpleasant, or neutral pictures described above or images of a blank (black) screen (inclusion of blank screens allowed for comparisons between the respective pictorial stimuli and non-stimuli). To ensure that each trial contained unique pictures, only 28/30 pictures from each category could be included; two pictures from each of the four picture categories were randomly excluded (the same blank screen was presented 28 times). On each trial, one picture was pseudorandomly paired with another picture from any of the other four picture categories/screens [28 pictures/screens × 5 categories = 140/2 (pictures per screen) = 70 unique trials]. The side (left vs. right) of presentation also varied pseudorandomly: each picture category appeared on each side of the screen 14 times to protect against perseverative responding (e.g., repeatedly choosing pictures from one side). Pressing the button corresponding to the image on the left enlarged this picture to fit the entire screen; pressing the button corresponding to the image on the right enlarged this picture instead (toggling between pictures was allowed). Continued button pressing allowed the chosen picture to remain on the screen for the entire trial duration of 5000 msec; upon non-response for 500 msec, the side-by-side images reappeared for the trial duration. We summed the number of button presses (across the 70 trials) per picture category. Scores on this task therefore reflect how much subjects worked for each picture type, modeled after previous research in which healthy control subjects button pressed/worked for viewing beautiful as compared to non-beautiful faces (33).
Figure 1.
Experimental paradigm for the ‘explicit’ task. The explicit task included training and 1 block, consisting of 70 trials. Two sample trials (A and B) are displayed. At trial onset, two side-by-side images appeared (this default screen remained for the 5000 msec duration unless subjects executed a response). Continuous button pressing enlarged the corresponding image, as shown in both examples (A: right picture; B: left picture, both indicated by red box), for the 5000 msec trial duration; no response (for 500 msec) after initial response returned the side-by-side display. Each trial onset was preceded by a 1500 msec fixation cross to separate trials (not depicted in figure).
Implicit Task
In the ‘implicit’ choice task, subjects selected via a single button press one of four flipped-over cards; upon selection, the picture was uncovered to fit the entire screen and passively viewed for 2000 msec (Figure 2). Subjects could then select again from the same deck or switch to another deck. Each deck contained a total of 30 pictures, which unbeknownst to the subjects were pseudorandomly sorted according to the following two constraints (except for these two constraints, pictures occurred in a completely random order within a deck): (A) there were no picture repetitions between the four decks; and (B) each deck contained 26 pictures (87%) of one picture category (e.g., cocaine), two pictures (7%) of another category (e.g., pleasant), and one picture (3%) of each of the remaining two categories (e.g., unpleasant, neutral; this task did not use blank screens). Following the Iowa Gambling Task (34), these percentages were selected to reduce awareness of deck identity, while still allowing for preference to be established. Similarly to the Wisconsin Card Sorting Task (WCST) (35, 36), a run terminated when subjects selected from a particular deck for a total of eight times. In contrast to the WCST, these eight selections could be nonconsecutive (because there were no “correct” or “incorrect” choices, imposing a rule of eight consecutive selections from a particular deck could have decreased interest in the task). Subjects completed four such runs. To further reduce awareness of deck identity, and to overcome the potential impact on results of perseverative responding (e.g., repeatedly choosing from the same deck across the runs), the dominant picture categories were pseudorandomized across the decks between the runs (i.e., the deck location of the four picture categories did not repeat across the runs). Because there was no significant effect of run or interaction with diagnosis (CUD, control) [Fs(3,36)<2.2, p>0.05], we summed the total number of cards selected per picture category across the four runs.
Figure 2.
Experimental paradigm for the ’implicit’ task. (A) Overall design, consisting of training (TR) and 4 runs (R). (B) Breakdown of deck identity across the runs: each run contained four decks that were comprised of mostly (87%) cocaine (C), pleasant (P), unpleasant (U), or neutral (N) pictures. Deck location did not repeat across the runs. (C) Sample trials: in each trial within a run, subjects had to choose one of four flipped-over cards; a run terminated after eight total choices from the same deck (maximum of 29 selections). Subjects pressed one of four buttons corresponding to their chosen deck. After the image appeared for 2000 msec, the choice screen reappeared, and subjects made another selection. Here in two sample trials, decks 3 and 2 are selected, respectively, indicated by the red box. The choice screen remained until a selection was made.
Statistical Analyses
The explicit task used a 5 (picture type: pleasant, unpleasant, neutral, cocaine, blank) × 2 (diagnosis: CUD, control) mixed analysis of variance (ANOVA). The implicit task used a 4 (picture type: pleasant, unpleasant, neutral, cocaine) × 2 (diagnosis: CUD, control) mixed ANOVA. Both ANOVAs were followed by analyses of covariance (ANCOVA) with total button presses across trials (explicit task) or number of picture selections across runs (implicit task) as covariates to control for individual differences in response frequency. Two 4 (picture type: pleasant, unpleasant, neutral, cocaine) × 2 (diagnosis: CUD, control) mixed ANOVAs examined differences in self-reported pleasantness and arousal ratings. The Greenhouse-Geisser correction was used if the assumption of sphericity was not met. Significant interactions were followed by paired (within-group) and independent (between-group) parametric t-tests (choice task variables and self-report ratings were normally distributed). Depression and cigarette smoking status, which differed between the groups (Table 1), were covaried in subsequent ANCOVAs if these measures were significantly associated with the choice task variables or self-report ratings (37). Associations with depression (which was not normally distributed) were examined with nonparametric Spearman correlations. The dichotomous smoking status was inspected with independent t-tests. In all ANOVAs and follow-up comparisons, p<0.05 was considered significant.
To establish between-task reliability, we performed between-task partial correlations (pr); in these analyses we controlled for the total number of button presses/selections. To establish validity of our tasks, we performed partial correlations between the two cocaine choice scores and (A) self-report picture ratings and (B) drug use variables listed in Table 1. Spearman correlations were used for drug use variables (which were not normally distributed). In all correlational analyses, p<0.01 was considered significant to protect against Type I error (after satisfying this initial criterion, we retained significant correlations if they achieved a significance level of p<0.05 when accounting for depression or smoking, if necessary).
Results
Picture Ratings
Pleasantness
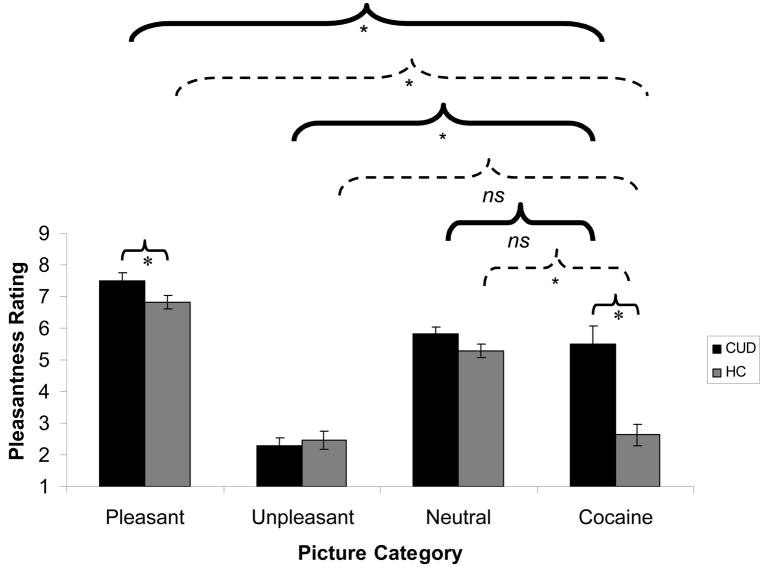
Results of the mixed ANOVA revealed main effects of picture type (pleasant>neutral>cocaine>unpleasant) [F(1.9,68.7)=83.4, p<0.001] and diagnosis (CUD>control) [F(1,37)=20.6, p<0.001]. An interaction between picture type and diagnosis showed that picture type ratings differed as a function of drug addiction [F(1.9,68.7)=8.6, p<0.01] (Figure 3A). This interaction was explained by the CUD group’s higher ratings of pleasant pictures [t(37)=2.1, p<0.05] and especially higher ratings of cocaine pictures [t(30.7)=4.2, p<0.001], but no differences between the groups in ratings of unpleasant or neutral pictures [ts(37)<1.7, p>0.09]. Interestingly, CUD provided higher ratings for pleasant pictures than cocaine pictures [t(19)=2.9, p<0.01], consistent with the picture type main effect. CUD also provided higher ratings for cocaine pictures than for unpleasant pictures [t(19)=5.1, p<0.001] but not neutral pictures [t(19)=0.5, p>0.6]. In contrast, healthy controls provided higher ratings for neutral than cocaine pictures [t(19)=7.3, p<0.001], but their ratings for cocaine and unpleasant pictures did not differ [t(18)=0.4, p>0.6]. The picture type × diagnosis interaction remained significant after accounting for depression, but not for cigarette smoking history (p>0.2); this was not unexpected based on the almost parallel distribution between the study groups with cigarette smoking history. In support of this idea, entering as covariates number of cigarettes currently smoked or time since last cigarette did not attenuate this interaction (p<0.001).
Figure 3.
Results of the picture ratings for (A) pleasantness and (B) arousal for each of the four picture types (pleasant, unpleasant, neutral, and cocaine) for individuals with cocaine use disorders (CUD; N=20), as compared to healthy comparison subjects (HC; N=19; one control subject did not complete picture ratings due to keypad malfunction). For both (A) and (B), error bars represent standard error of the mean. Between-group comparisons are indicated by light, solid lines; within-group comparisons for CUD are indicated by heavy, solid lines; and within-group comparisons for HC are indicated by broken lines; * p<0.05; all comparisons shown directly pertain to our a priori hypotheses. Supplementary Table 1 presents all significant comparisons (including comparisons not displayed in Figure 3), as well as means and standard errors for both picture ratings.
Arousal
Results of the mixed ANOVA revealed main effects of picture type (pleasant>all other categories) [F(2.0,74.9)=7.3, p<0.01] and diagnosis (CUD>control) [F(1,37)=8.3, p<0.01]. The interaction was also significant [F(2.0, 74.9)=4.0, p<0.05] (Figure 3B), driven by the CUD group’s higher arousal ratings only for the cocaine pictures [t(37)=3.4, p<0.01]; other group differences were not significant [ts(37)<2.0, p>0.06]. Further, CUD provided arousal ratings that were higher for cocaine than unpleasant pictures [t(19)=2.1, p<0.05], whereas this pattern was reversed in healthy controls who rated cocaine pictures as less arousing than unpleasant pictures [t(18)=3.7, p<0.01]. The interaction again remained significant when controlling for depression, but not cigarette smoking history. However, the interaction remained significant when entering as covariates number of cigarettes currently smoked or time since last cigarette (p<0.05).
Explicit Task
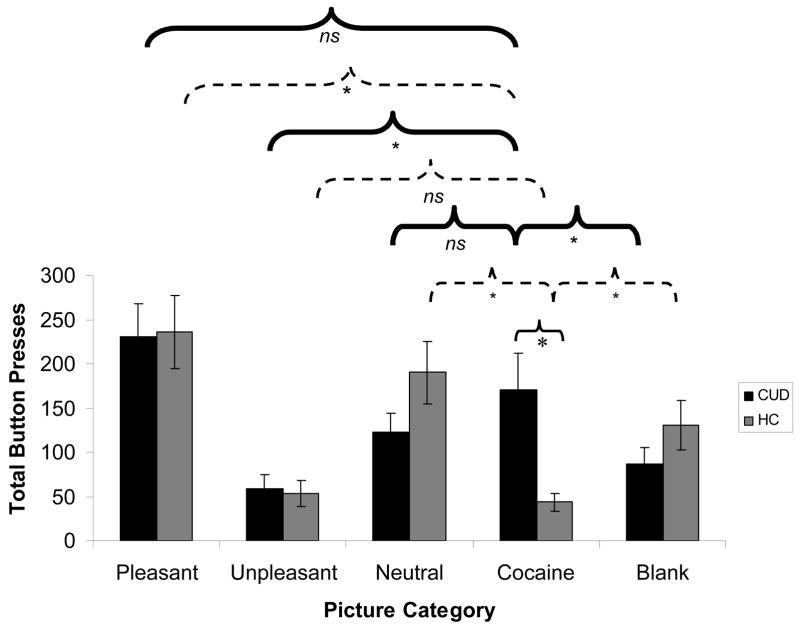
Results of the mixed 2-way ANOVA revealed a main effect of picture type (pleasant>all other picture categories), indicating more button presses for pleasant pictures in all subjects [F(2.9,111.0)=20.1, p<0.001]. There was no main effect of diagnosis [F(1,38)=0.0, p>0.9]. Importantly, the picture type × diagnosis interaction reached significance [F(2.9,111.0)=6.4, p<0.01] (Figure 4A), driven by group differences in button pressing for the cocaine pictures [CUD>control; t(21.1)=2.9, p<0.01]. Further, CUD button pressed more for cocaine pictures than for unpleasant and blank pictures [ts(19)>2.2, p<0.05; a similar trend was observed for neutral pictures, t(19)=1.4, p>0.1], but not for pleasant pictures [t(19)=−1.8, p>0.08]. This pattern was reversed in the healthy controls, who button pressed for cocaine pictures significantly less than for all other picture categories [ts(19)>3.2, p<0.01] except for unpleasant pictures [t(19)=0.7, p>0.4), similarly to their pleasantness ratings. This interaction remained significant after accounting for depression and total button presses (p<0.01), but became attenuated with smoking history as a covariate (p>0.1). Nevertheless, covarying for cigarettes smoked per day and time since last cigarette did not attenuate this interaction (p<0.01), again indicating that the effect of cigarette smoking history may be attributable to its correspondence with diagnosis.
Figure 4.
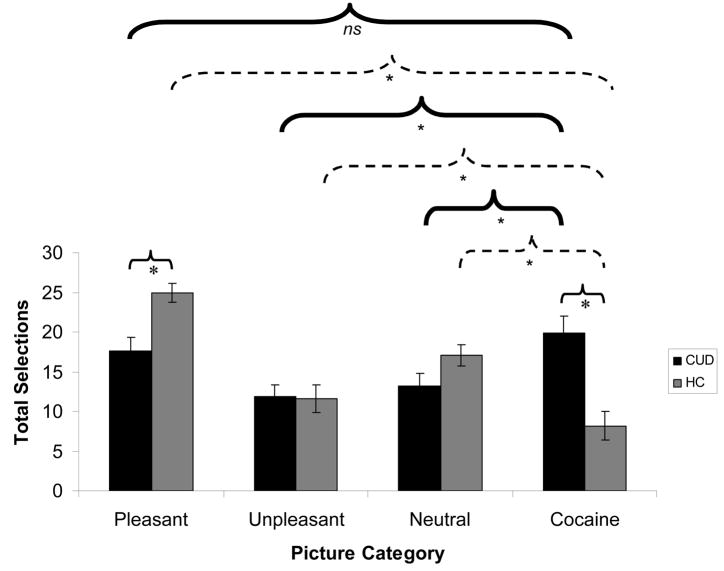
(A) Results of the ‘explicit’ task, showing total button presses for each of the five picture categories (pleasant, unpleasant, neutral, cocaine, and blank) for individuals with cocaine use disorders (CUD; N=20), as compared to healthy comparison subjects (HC; N=20). (B) Results of the ‘implicit’ task, showing total picture selections for each of the four picture categories for the same two subject groups. For both (A) and (B), error bars represent standard error of the mean. Between-group comparisons are indicated by light, solid lines; within-group comparisons for CUD are indicated by heavy, solid lines; and within-group comparisons for HC are indicated by broken lines; * p<0.05; all comparisons shown directly pertain to our a priori hypotheses. Supplementary Table 1 presents all significant comparisons (including comparisons not displayed in Figure 4), as well as means and standard errors for both choice tasks.
Implicit Task
Results of the mixed 2-way ANOVA similarly revealed a main effect of picture type (pleasant>all other categories), indicating increased selection of pleasant pictures in all subjects [F(2.3,85.9)=15.8, p<0.001], and no main effect of diagnosis [F(1,38)=0.0, p>0.9]. The picture type × diagnosis interaction reached significance [F(2.3,85.9)=16.5, p<0.001] (Figure 4B). Follow-up tests indicated that this interaction was again driven by differences between the groups in selection of cocaine pictures [CUD>control; t(38)=4.1, p<0.001], and (unique to this implicit task) by differences between the groups in selection of positive pictures [CUD<control; t(33.5)=3.5, p<0.01]. This time, the enhanced selection of cocaine pictures in CUD was greater than selection of all other picture categories, reaching significance for the unpleasant [t(19)=3.0, p<0.01] and neutral pictures [t(19)=2.2, p<0.05]. Conversely, in healthy controls, cocaine picture selection was significantly lower than all other picture categories including unpleasant pictures [ts(19)>3.0, p<0.01]. This interaction remained significant after controlling for all covariates (p<0.05).
Task Intercorrelations and Correlations with Self-Reported Ratings
Correlation analyses indicated good between-task agreement for cocaine choice in CUD (pr=0.71, p<0.01). Further, in the CUD, both total cocaine button presses and total cocaine selections correlated with both cocaine picture ratings, yielding four significant correlations (cocaine button presses: pr=0.61 and pr=0.68, p<0.01, for pleasantness and arousal ratings, respectively; cocaine selections: pr=0.74 and pr=0.80, p<0.001, for pleasantness and arousal ratings, respectively). Together, these correlations highlight our tasks’ reliability and validity in probing choice for cocaine pictures in CUD.
Given these correlations and following our second a priori hypothesis, we repeated the two task ANOVAs using each of the self-reported ratings as a separate covariate; we also controlled for total button presses/selections as appropriate. The picture type × diagnosis interaction was still detected when controlling for pleasantness (implicit task only: p<0.05) and arousal (implicit task: p<0.01; and a similar trend for the explicit task: p<0.06), indicating that enhanced cocaine-related choice in the CUD as compared to controls was not fully explained by these self-reports, as best demonstrated with the implicit task.
Spearman Correlations with Drug Use Variables
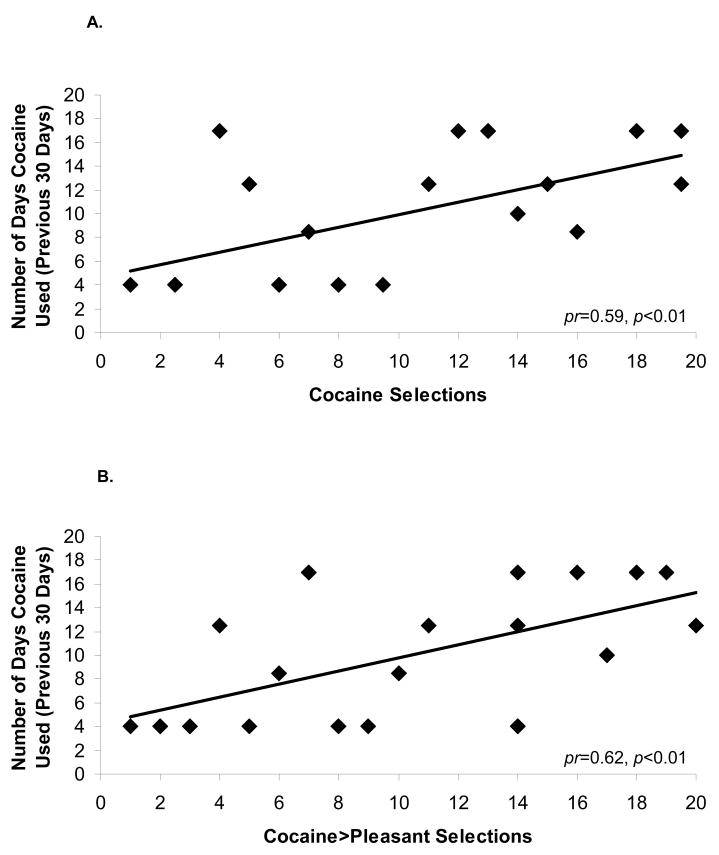
Total cocaine selections (implicit task) positively correlated with frequency of cocaine use in the month preceding this study (pr=0.59, p<0.01; Figure 5A). Following our third a priori hypothesis, we calculated a change score that subtracted pleasant selections from cocaine selections. This change score also correlated with frequency of cocaine use in the previous month (pr=0.62, p<0.01; Figure 5B). This latter correlation indicates that the higher the choice to view cocaine pictures over hedonically positive pictures, the more severe the current drug use. Other correlations with the drug use variables listed in Table 1 were not significant.
Figure 5.
Scatterplots in the CUD (N=20) showing associations between cocaine selection and drug use variables on the ‘implicit’ task: (A) correlations between cocaine selection and number of cocaine use days in the past 30 days; and (B) cocaine>pleasant selection and number of cocaine use days in the past 30 days. Because these analyses used Spearman correlations, all scores and drug use scores are presented as ranks.
Discussion
In the current study we compared cocaine addicted individuals and healthy controls on choice for viewing pictures of cocaine and standardized pleasant, unpleasant, and neutral pictures, exploring objective cocaine-related choice as a potential marker of addiction severity. We also tested whether choice for viewing cocaine pictures could be fully explained by self-reported ratings of pleasantness and arousal of these stimuli, exploring the novel hypothesis of compromised insight into behavior in drug addiction.
Consistent with our first a priori hypothesis, CUD chose cocaine pictures more than healthy controls. This finding demonstrates drug-related choice in cocaine addiction even for non-pharmacological (pictorial) stimuli, and is consistent with research showing that cocaine-related stimuli have attention-biasing properties in CUD (13–16). Other contributing factors to this choice in CUD may have included enhanced interest, affinity, motivation, salience, or familiarity with these particular drug stimuli. Nevertheless, in the present study drug-related choice in CUD cannot be attributed to motor perseveration (in both tasks location of the cocaine pictures was pseudorandomly varied) or overall increased button pressing/selection (no group main effect emerged in either task, and results remained significant in subsequent ANCOVAs that controlled for total button press/selection).
Consistent with our second a priori hypothesis, this enhanced drug-related choice in the CUD was not completely explained by their self-reported ratings. Moreover, CUD’s self-reported ratings were incongruent with their choice behavior, as indicated by discrepancies between picture ratings (cocaine<pleasant) and objective choice as assessed in both tasks (cocaine=pleasant). Together, such results suggest a disconnect in drug addiction between self-reports (as measures of conscious awareness) and objective markers of behavior, as possibly indicative of impaired awareness of internal drives (11, 27, 28) or of cognitive-behavioral performance (38, 39). Such impaired awareness may potentially underlie the evasive nature of using self-reported craving to predict relapse in drug addiction (40), and highlights the potential utility of our choice tasks as objective markers of individualized clinical outcomes.
Finally, results of both tasks showed that cocaine-related choice in CUD surpassed unpleasant, but not pleasant, picture choice. These findings suggest that drug seeking in CUD (as approximated here with cocaine picture choice) may be higher in the presence of aversive stimuli, but not in the presence of alternative pleasant stimuli, consistent with both human and animal studies (41–48). This interpretation is also consistent with the significant correlations in the current study between choice for viewing cocaine pictures, even when directly compared to selections of reportedly more pleasant pictures, and frequency of actual cocaine use. Therefore, behavior on this task may be an indirect marker of actual drug-choice behavior, as remains to be tested in future studies.
Limitations of this study include: (A) previous viewing and rating of the same cocaine pictures may have precipitated cue-induced craving in CUD (49). Counterbalancing picture ratings and picture choice tasks should be implemented in future studies; (B) unexpectedly, healthy control subjects selected fewer cocaine than unpleasant pictures in the implicit task. A completely masked task would eliminate the possible confounding influence of socially desirable responding and other demand characteristics that may have partially driven this finding. Similarly, for CUD, the discrepancy between pleasantness ratings and task performance could have also reflected socially desirable self-reporting; (C) habituation could have resulted from viewing the same blank screen throughout. However, control subjects pressed for these blank screens over the unpleasant or cocaine pictures, suggesting habituation did not significantly impact our results and further highlights control subjects’ aversion to such pictures; (D) our CUD group was heterogeneous, as it included both active users and treatment-seekers. Larger CUD samples can ascertain whether study results differ as a function of active cocaine use (e.g., 50).
In summary, two newly-developed tasks examined choice for viewing cocaine pictures in comparison to pleasant, unpleasant, and neutral pictures. CUD selected more, and worked more for, cocaine pictures than healthy control subjects, results that were not fully driven by (or subject to the pitfalls of) self-report. Results also revealed that drug picture choice did not differ from pleasant picture choice but was enhanced when compared to unpleasant picture choice, possibly indicative of modulation of actual drug choice by other pleasant or aversive stimuli in drug addicted individuals. Further studies are needed to uncover the neural substrates that underlie this drug-biased choice in CUD, and whether cue-reactive states enhance such choice over pleasant stimuli (1), especially in individuals with more severe drug use. Overall, such disadvantageously enhanced drug choice could provide a marker of the neurocognitive dysfunction that characterizes drug addiction.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (to RZG: 1R01DA023579; R21DA02062) and General Clinical Research Center (5-MO1-RR-10710). Notice: This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
Footnotes
Disclosure/Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nader MA, Czoty PW. PET Imaging of Dopamine D2 Receptors in Monkey Models of Cocaine Abuse: Genetic Predisposition Versus Environmental Modulation. American Journal of Psychiatry. 2005;162:1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- 3.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature Neuroscience. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. American Journal of Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 6.Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 7.McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 9.Stalnaker TA, Roesch MR, Franz TM, Burke KA, Schoenbaum G. Abnormal associative encoding in orbitofrontal neurons in cocaine-experienced rats during decision-making. European Journal of Neuroscience. 2006;24:2643–2653. doi: 10.1111/j.1460-9568.2006.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovanovski D, Erb S, Zakzanis KK. Neurocognitive Deficits in Cocaine Users: A Quantitative Review of the Evidence. Journal of Clinical and Experimental Neuropsychology. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 13.Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug and Alcohol Dependence. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176:353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- 15.Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. Journal of Psychopharmacology. 2002;16:385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- 16.Franken IHA, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroin dependence. Journal of Psychopharmacology. 2000;14:395–400. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- 17.Reichel CM, Bevins RA. Competition between the conditioned rewarding effects of cocaine and novelty. Behavioral Neuroscience. 2008;122:140–150. doi: 10.1037/0735-7044.122.1.140. [DOI] [PubMed] [Google Scholar]
- 18.Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: Pups and cocaine throughout the postpartum period. Behavioral Neuroscience. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- 19.Zombeck JA, Chen G-T, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiology & Behavior. 2008;93:637–650. doi: 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Aigner TG, Balster RL. Choice behavior in rhesus monkeys: Cocaine versus food. Science. 1978;201:534–535. doi: 10.1126/science.96531. [DOI] [PubMed] [Google Scholar]
- 21.Woolverton WL, Anderson KG. Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2006;186:99–106. doi: 10.1007/s00213-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 22.Donny EC, Bigelow GE, Walsh SL. Choosing to take cocaine in the human laboratory: Effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug and Alcohol Dependence. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- 23.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. American Journal of Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 24.Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behavioural Pharmacology. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 26.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein RZ, Parvaz MA, Maloney T, Alia-Klein N, Woicik PA, Telang F, et al. Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology. doi: 10.1111/j.1469-8986.2008.00670.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hester R, Simoes-Franklin C, Garavan H. Post-error behavior in active cocaine users: Poor awareness of errors in the presence of intact performance adjustments. Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- 29.Rounsaville BJ, Anton SF, Carroll K, Budde D. Psychiatric diagnoses of treatment-seeking cocaine abusers. Archives of General Psychiatry. 1991;48:43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- 30.Lang PJ, Bradley MM, Cuthbert BN. The International Affective Picture System (IAPS): Photographic Slides. University of Florida; Gainsville: 1998. [Google Scholar]
- 31.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 33.Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 34.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 35.Berg EA. A simple objective treatment for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 36.Heaton RK. Wisconsin Card Sorting Test: Computer Version 3 for Windows. Odessa, FL: Research Edition Psychological Assessment Resource; 1999. [Google Scholar]
- 37.Stevens J. Applied multivariate statistics for the social sciences. 2. New Jersey: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 38.Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate Hypoactivity in Cocaine Users During a GO-NOGO Task as Revealed by Event-Related Functional Magnetic Resonance Imaging. Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, et al. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Miller NS, Gold MS. Dissociation of ‘conscious desire’ (craving) from and relapse in alcohol and cocaine dependence. Annals of Clinical Psychiatry. 1994;6:99–106. doi: 10.3109/10401239409148988. [DOI] [PubMed] [Google Scholar]
- 41.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-Induced Cocaine Craving and Hypothalamic-Pituitary-Adrenal Responses Are Predictive of Cocaine Relapse Outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 42.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- 44.Brown ZJ, Erb S. Footshock stress reinstates cocaine seeking in rats after extended post-stress delays. Psychopharmacology. 2007;195:61–70. doi: 10.1007/s00213-007-0846-4. [DOI] [PubMed] [Google Scholar]
- 45.Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annual Review of Psychology. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- 46.Christensen CJ, Silberberg A, Hursh SR, Huntsberry ME, Riley AL. Essential value of cocaine and food in rats: Tests of the exponential model of demand. Psychopharmacology. 2008;198:221–229. doi: 10.1007/s00213-008-1120-0. [DOI] [PubMed] [Google Scholar]
- 47.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007 Aug 1;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behavioural Pharmacology. 2006;17:597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- 49.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 50.Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, et al. The neuropsychology of cocaine addiction: Recent cocaine use masks impairment. Neuropsychopharmacology. doi: 10.1038/npp.2008.60. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollingshead AB. Four-factor index of social status. 1975 Unpublished paper. [Google Scholar]
- 52.Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 53.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 54.Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addictive Behaviors. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 55.Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. British Journal of Addiction. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 56.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug and Alcohol Dependence. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.