Abstract
Summary
In response to DNA damage, eukaryotic cells activate ATM-Chk2 and/or ATR-Chk1 to arrest the cell cycle and initiate DNA repair. We show that in the absence of p53, cells depend on a third cell cycle checkpoint pathway involving p38MAPK/MK2 for cell cycle arrest and survival after DNA damage. MK2 depletion in p53-deficient cells, but not in p53 wild-type cells, caused abrogation of the Cdc25A-mediated S-phase checkpoint after cisplatin exposure and loss of the Cdc25B-mediated G2/M checkpoint following doxorubicin treatment, resulting in mitotic catastrophe and pronounced regression of murine tumors in vivo. We show that the Chk1 inhibitor UCN-01 also potently inhibits MK2, suggesting that its clinical efficacy results from the simultaneous disruption of two critical checkpoint pathways in p53-defective cells.
Significance
Many anti-cancer agents induce DNA damage as part of their mechanism for tumor cytotoxicity. Here we show that DNA-damaging chemotherapeutic drugs directly activate the p38MAPK/MK2pathway downstream of ATM and ATR. In p53-proficient cells, signaling through this pathway is dispensable for survival after DNA damage. In marked contrast, however, activation of this pathway is essential for preventing chemotherapy-mediated cell death in p53-deficient cells. The finding that cells which lack functional p53 become dependent on recruiting a general stress kinase pathway to control the cell cycle rationalizes the therapeutic targeting of MK2as a new strategy to selectively kill p53-defective tumor cells with low dose chemotherapy.
Introduction
In response to DNA damage, eukaryotic cells activate a complex signaling network to arrest the cell cycle and facilitate DNA repair (Kastan and Bartek, 2004; Zhou and Elledge, 2000). This signaling network has traditionally been divided into two major protein kinase pathways, one mediated by Ataxia-Telangiectasia mutated (ATM) through Chk2, and the other mediated by Ataxia-Telangiectasia and Rad-3 related (ATR) through Chk1 (Bartek and Lukas, 2003; Shiloh, 2003). Some cross-talk exists between the ATM/Chk2 and ATR/Chk1 kinase pathways, particularly when signaling through one pathway is partially or totally deficient (Bartek and Lukas, 2003). Normally, however the pathways show only partial functional overlap in response to particular forms of DNA damage. The ATM/Chk2 pathway responds primarily to DNA double strand breaks (DSBs), while the ATR/Chk1 pathway is activated by bulky DNA lesions, and following replication fork collapse during S-phase (Zhou and Elledge, 2000).
The tumor suppressor protein p53 is a major downstream effector of these DNA damage kinase pathways (Harris and Levine, 2005). In normal cells, p53-dependent signaling results in G1 arrest, mainly mediated by transcriptional up-regulation of 21 (Vogelstein et al., 2000). In addition, p21 also appears to play a role in sustaining the G2 checkpoint after γ-irradiation (Bunz et al., 1998). If the DNA damage is extensive, however, then p53-dependent pathways target the damaged cell for apoptotic cell death through both the intrinsic and extrinsic pathways (Fridman and Lowe, 2003; Vousden and Lu, 2002). Most tumor cells show specific disruptions in the p53 pathway (Vogelstein et al., 2000), leading to selective loss of the G1 checkpoint. These cells are then entirely dependent on intra-S and G2/M checkpoints to maintain their genomic integrity in response to DNA damage.
In contrast to the DNA damage-specific activation of Chk1 and Chk2, the p38MAPK pathway is a general stress-activated kinase pathway that responds to various cellular stimuli, including cytokines, hyperosmolarity, and UV irradiation (Roux and Blenis, 2004). Fornace and colleagues recently reported that p38MAPK activity was important for G2/M checkpoint function in immortalized fibroblasts and HeLa cells following UV exposure (Bulavin et al., 2001). We found that MAPKAP Kinase-2 (MK2) was the critical downstream effector kinase of p38MAPK required for UV-induced cell cycle checkpoints in U2OS cells (Manke et al., 2005). Whether the observed activation of p38 MAPK/MK2 is a direct result of UV-induced DNA lesions, or results instead from other non-genotoxic effects of UV radiation is not known. Similarly, whether the p38MAPK/MK2 pathway is an important part of a general cellular response to genotoxic stress is unclear, and if so, how does this stress kinase pathway interconnect with more canonical DNA damage kinases that control cell cycle progression and DNA repair.
We now report that the p38MAPK/MK2 stress kinase pathway is activated in response to the commonly used DNA damaging agents cisplatin, doxorubicin and camptothecin, and that its cell cycle checkpoint function is essential in cells with defective p53. We go on to show that both Chk1 and MK2 are activated independently of each other, and that UCN-01, a small molecule currently in clinical trials for cancer therapy, directly inhibits both MK2 and Chk1 at similar concentrations.
Results
The p38MAPK-MK2 pathway is activated by drugs that directly damage DNA
To investigate whether the p38 MAPK/MK2 pathway was involved in the DNA damage response of cells following exposure to clinically useful chemotherapeutic agents, we treated human U2OS osteosarcoma cells with the DNA crosslinking agent cis-Platinum (cisplatin), the topoisomerase I inhibitor camptothecin, or the topoisomerase II inhibitor doxorubicin (Fig. 1). p38 MAPK activation was assessed using an antibody specific for the Thr-180/Tyr-182 doubly phosphorylated active form of the kinase. Activation of MK2 was monitored by its altered mobility on SDS-PAGE, and by immunoblotting using a phospho-specific antibody for pThr-344, a site in the auto-inhibitory domain whose phosphorylation by p38MAPK results in a dramatic elevation of MK2 activity (Ben-Levy et al., 1995; Engel et al., 1995).
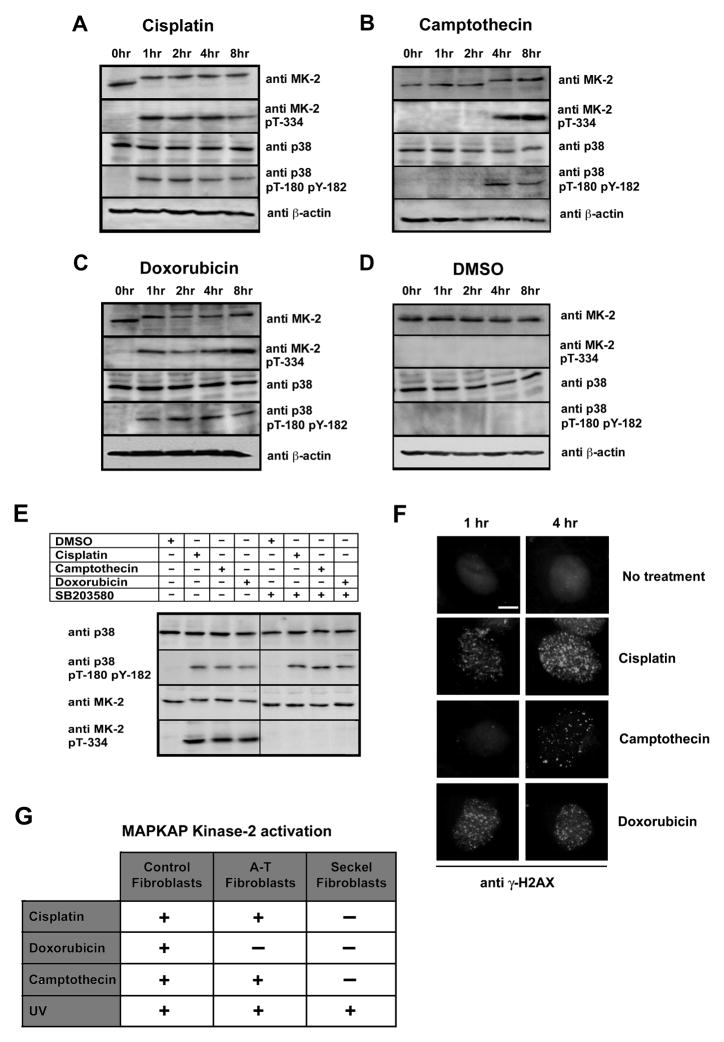
Figure 1. The p38MAPK/MK2pathway is activated by DNA- damaging drugs.
(A–D) Kinetics of p38 MAPK and MK2 activation. U2OS cells were treated with 10μM cisplatin (A), 10μM camptothecin (B), 10μM doxorubicin (C) or DMSO control (D) for the indicated times. Cell lysates were probed for total and phosphorylated/activated forms of p38MAPK and MK2(MK-2) by Western blotting. β-actin served as a loading control.
(E) MK2 activation is p38MAPK-dependent. U2OS cells were treated with the p38 MAPK specific inhibitor SB203580 (10μM) or DMSO vehicle for 30 min prior to exposure to chemotherapeutic drugs as in panels A–D. Total and phosphorylated/activated p38 was determined by immunoblotting as above.
(F) Activation of MK2 parallels the formation of γH2AX nuclear foci. U2OS cells were either mock treated or incubated with cisplatin (10μM), camptothecin (10μM) or doxorubicin (10μM). Cells were immunostained 1 and 4h later using an antibody against γ-H2AX, and counterstained with DAPI. Scale bar, 2μm.
(G) Summary of the requirement for ATM and/or ATR for the activation of MK2.
Prior to exposure of cells to DNA damaging compounds (Fig. 1A–C, 0 hr lanes), or in cells treated with DMSO (vehicle) alone (Fig. 1D), MK2 ran as a single band that did not cross-react with the anti-pThr-344 antibody. Within 1 hr after exposure of the cells to cisplatin and doxorubicin, or within 4 hr following treatment with camptothecin, MK2 displayed a significant reduction in its electrophoretic mobility. The upshifted MK2 band appeared with the same kinetics as both the MK2 pThr-344- and the p38MAPK pThr-180/pTyr-182 immunoreactive bands. Activation of MK2 was entirely dependent on p38MAPK, since addition of the p38MAPK selective inhibitor SB203580 to the growth media 30 minutes prior to application of the DNA damaging agents completely abolished MK2 activation, while preserving activation of p38MAPK (Fig. 1E). Similar results for MK2 activation in response to cisplatin, camptothecin and doxorubicin were also observed in HeLa cervical carcinoma cells, U87MG human glioblastoma cells, and primary MEFs (Fig. 2A and data not shown). The time course of MK2 activation upon treatment with each of these drugs (Fig. 1A–D) matched the rate of appearance of γ-H2AX nuclear foci (Fig. 1F). These data indicate that treatment of cells with these genotoxic agents results in MK2 activation, likely as a direct result of chemotherapy-induced DNA damage.
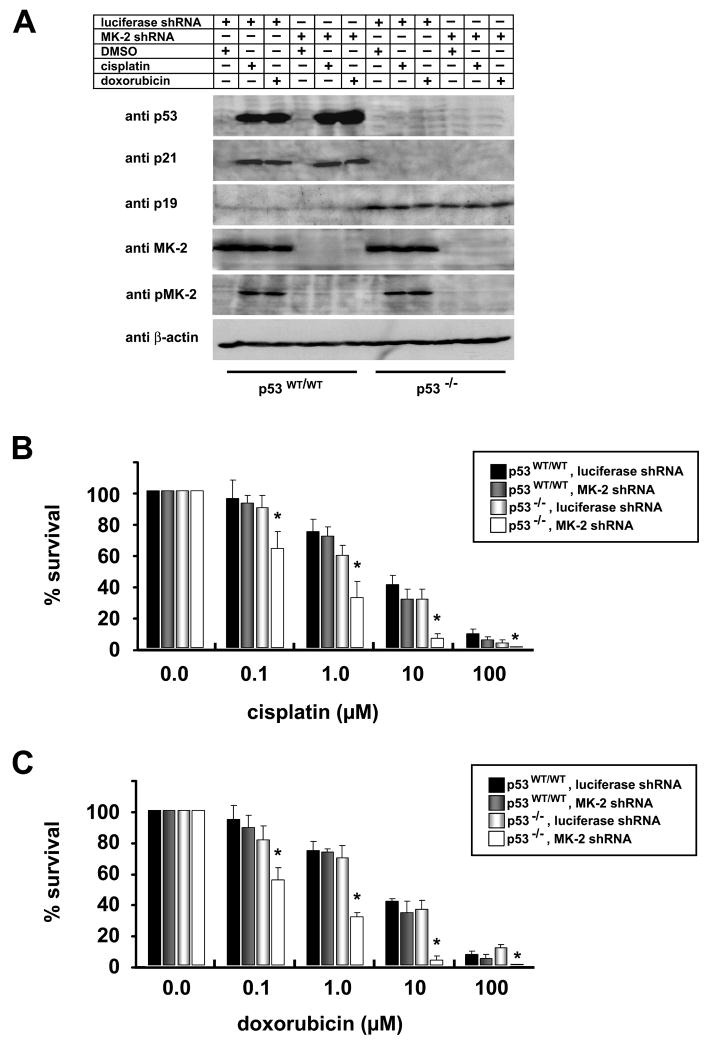
Figure 2. p53 deficient MEFs require MK2 for survival after DNA damage.
(A) p21 induction is independent of MK2 activation. p53WT/WT and p53−/− MEFs stably expressing luciferase shRNA or MK2 shRNA were treated with 10μM cisplatin or 10μM doxorubicin. MK2activation and components of the p53 pathway were monitored by SDS-PAGE and Western blot analysis.
(B, C) Clonogenic survival assay. p53WT/WT and p53−/− MEFs stably expressing luciferase shRNA or MK2 shRNA were mock treated or treated with increasing doses of cisplatin (B) or doxorubicin (C) for 4 hours, washed in PBS, trypsinized and replated at 5000 cells/10cm dish. 14 days later, surviving colonies were fixed, stained with crystal violet, and counted. Assays were performed in triplicate for each condition and normalized to mock treated cells. Asterisks denote statistically significant differences (2-tailed Student’s t-test, p<0.04).
ATM and ATR are required for p38MAPK/MK2 activation following genotoxin-induced DNA damage but not in response to UV irradiation
We analyzed the p38MAPK/MK2activation profile in ATM-deficient and ATR-defective fibroblasts (O’Driscoll et al., 2003) (Supp. Figs. 1A–D and 2, Fig. 1G). We also studied the effect of pharmacological inhibition of these kinases by addition of caffeine (Supp. Fig 1E,F). Activation of the p38MAPK/MK2 complex in response to cisplatin, camptothecin or UV exposure occurred normally in ATM deficient fibroblasts, while doxorubicin treatment failed to activate either p38MAPK or MK2 in these cells. ATR-defective fibroblasts, on the other hand, failed to activate p38MAPK or MK2 following either cisplatin, doxorubicin, or camptothecin exposure. However, UV-induced p38MAPK/MK2 activation in these cells was unaffected. Similarly, treatment of U2OS cells with 20mM caffeine, a concentration sufficient to inhibit ATM, ATR and DNA-PK (Block et al., 2004; Sarkaria et al., 1999), for 30 minutes prior to exposure to cisplatin and doxorubicin completely abrogated the p38MAPK/MK2 response, while the activation of these kinases by UV occurred normally under these conditions. Taken together, these data indicate that cisplatin, camptothecin, and doxorubicin require ATR for p38MAPK/MK2 activation, that doxorubicin also requires ATM activity, and that UV irradiation is capable of activating the p38MAPK/MK2 in a manner that is independent of ATM, ATR, or DNA-PK function.
Loss of p53 renders cells dependent on MK2 signaling for survival after chemically-induced DNA damage
The p53 tumor suppressor protein plays an important role in the cellular response to DNA damage by transcriptionally upregulating the Cdk inhibitor p21 to induce a G1 and G2 arrest (Bartek and Lukas, 2001; Bunz et al., 1998; Vogelstein et al., 2000). Cancer cells frequently show disruptions in the p53 pathway, eliminating this component of the DNA damage response, and leaving the cells entirely dependent on remaining checkpoint signaling pathways (Dixon and Norbury, 2002; Kawabe, 2004). To examine whether the ATR/ATM-p38MAPK-MK2 pathway was required for cell survival after genotoxin-induced DNA damage in p53 wild-type and p53−/− MEFs, we used RNAi to deplete MK2, and examined the response of these otherwise genetically identical cells to cisplatin and doxorubicin using a colony survival assay (Fig. 2).
Cells were infected with lentiviruses delivering shRNAs against luciferase (control) or MK2 and analyzed 6 days later (Fig. 2A). Luciferase and MK2 knockdown MEFs were then exposed to increasing doses of cisplatin or doxorubicin. As seen in Figs. 2B and C, there was no difference in the number of surviving colonies in the p53wt/wt MEFs, regardless of the presence or absence of MK2, at any dose of cisplatin or doxorubicin examined. In contrast, downregulation of MK2 in p53−/− cells dramatically reduced the number of surviving colonies. These results demonstrate that depletion of MK2 specifically sensitizes p53-deficient cells to the anti-proliferative effects of chemotherapy-induced DNA damage.
We used Western blot analysis to profile activation of the MK2 pathway and the p53 network following cisplatin and doxorubicin treatment in these four cell lines (Fig. 2A). The presence or absence of MK2 had no effect on the strong induction of p53 and p21 following exposure to cisplatin or doxorubicin in the p53 WT/WT cells. Only minimal amounts of the p53 inducer protein p19ARF were detected. Neither p53 or p21 induction was detectable in p53−/− MEFs in the presence or absence of MK2. However, the tumor suppressor p19ARF was strongly induced in these cells even in the absence of DNA damaging chemotherapy, likely reflecting a feedback response due to the inability of these cells to induce p53. Thus, we concluded that MK2 is not required for the normal p53/p21 induction or stabilization in response to DNA damage in wild-type primary cells, and that MK2 is unable to induce p21 expression after DNA damage in the absence of functional p53.
Down-regulation of MK2 leads to mitotic catastrophe after DNA damage in p53−/− cells
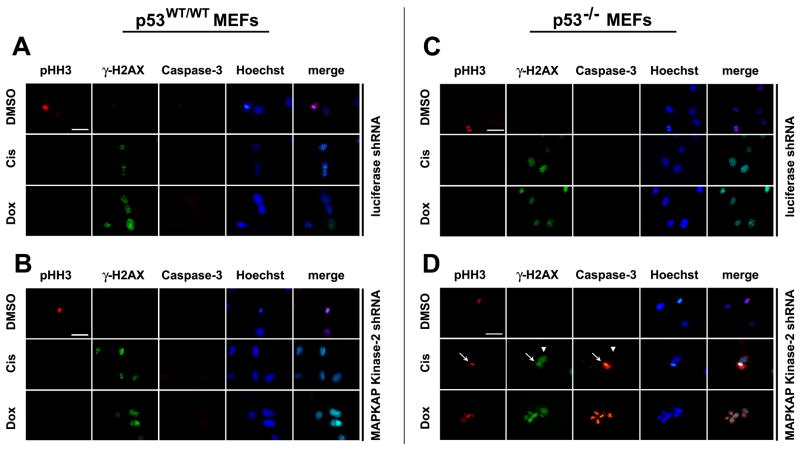
We speculated that the reduced colony formation observed in MK2-depleted p53−/− MEFs after DNA damaging chemotherapy might be due to mitotic catastrophe resulting from defective cell cycle checkpoints. A hallmark of mitotic catastrophe is entry of cells into mitosis despite the presence of damaged DNA, resulting in activation of the apoptotic cell death pathway (Castedo et al., 2004). To investigate this, luciferase shRNA control and MK2 depleted p53 wild-type or null MEFs were treated with low doses of doxorubicin or cisplatin for 30 h and immunostained with antibodies against histone H3 pSer-10 as a marker for mitotic entry, histone γ-H2AX as marker for persistent DNA damage, and cleaved caspase-3 as a marker for apoptosis (Fig. 3). Luciferase shRNA-treated p53wt/wt and p53−/− cells showed robust γ-H2AX foci after exposure to DNA damaging chemotherapy. No phospho-histone H3 or cleaved caspase-3 staining was observed in MK2-containing cells, consistent with an intact DNA damage response regardless of the presence (Fig. 3A) or absence (Fig. 3C) of p53. Similarly, an intact DNA damage checkpoint response was also observed in MK2 depleted cells that contained wild-type p53 (Fig. 3B). In sharp contrast, however, in the MK2-deficient p53−/− cells, a substantial fraction of the γ-H2AX positive cells also stained positively for both phospho-histone H3 and cleaved caspase 3 (Fig. 3D). Interestingly, no caspase 3 staining was observed in γ-H2AX-positive cells that did not also contain phospho-histone H3 (Fig. 3D, arrowhead). Thus, in the absence of MK2, p53 null primary cells treated with cisplatin and doxorubicin lose one or more critical cell cycle checkpoints and undergo mitotic catastrophe.
Figure 3. Depletion of MK2 sensitizes p53-deficient MEFs to the anti-proliferative effects of cisplatin and doxorubicin by inducing mitotic catastrophe.
p53WT/WT and p53−/− MEFs stably expressing RNAi hairpins against luciferase (A, C) or MK2(B, D) were treated with low dose cisplatin (1.0μM) or doxorubicin (0.1μM) for 30hr, fixed and stained with antibodies against phospho-histone H3, γ-H2AX and cleaved caspase-3. Positive staining for γ-H2AX in combination with phospho-histone H3 and cleaved caspase-3 labeling is indicative of mitotic catastrophe, was only observed in MK2 depleted p53−/− cells (panel D, arrows). Arrowhead in that panel shows a γ-H2AX-positive cell that does not stain for either phospho-histone H3 or cleaved caspase-3. Scale bar, 5μm.
Down-regulation of MK2 causes regression of established p53−/− tumors in vivo after low dose treatment with DNA damaging agents
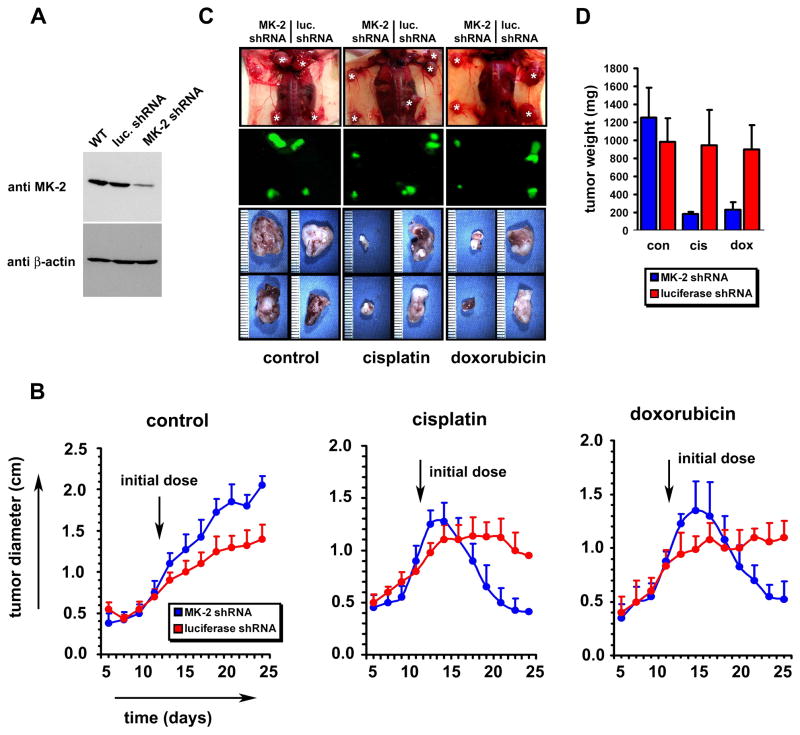
We investigated whether the chemo-sensitizing effect of MK2 depletion in p53 null cells in culture could also be observed when pre-existing p53 deficient tumors were treated with DNA damaging drugs in vivo. In these experiments H-Ras-V12 transformed p53−/− MEFs were stably transfected with control shRNA or MK2 shRNA expressed from a murine U6 promoter, using a lentiviral delivering system (Fig. 4A, Supp. Fig. 3). The lentiviral transfer vector also encoded GFP under the control of a CMV promoter, allowing for fluorescent detection of tumors in situ. Tumors were induced by injection of 106 cells into the flanks of nude mice. Twelve days later ~ 1 cm diameter tumors had formed at all injection sites, and treatment with cisplatin, doxorubicin, or vehicle was begun (Fig. 4B). In the absence of treatment with DNA damaging drugs, the tumors arising from the MK2 depleted cells in the right flanks of these animals grew slightly larger than those of the luciferase shRNA control cells in the left flanks (Fig. 4B–D). Following treatment with cisplatin or doxorubicin, the control tumors showed either minimal reduction in size, or slow continued growth (Fig. 4B, D, red symbols). In contrast, the MK2 depleted tumors showed a dramatic reduction in weight and diameter (Fig. 4B, D, blue symbols). Tumors depleted of MK2 shrank from 1.3 cm to 0.4 cm over14 days when treated with cisplatin, and from 1.4 to 0.5 cm when treated with doxorubicin. Thus, the sensitizing effect of MK2 depletion on DNA damage-induced cell death in p53-deficient primary cells observed in cell culture was also maintained in vivo. These results strongly suggest that MK2 may be a useful target for the design of new cancer treatment agents.
Figure 4. MK2 depletion enhances regression of established tumors after DNA damaging chemotherapy in a murine model.
(A) H-Ras-V12 transformed p53 −/− MEFs (Ferbeyre et al., 2002) were infected with lentiviruses encoding U6 promoter-driven luciferase shRNA or MK2shRNA, and CMV promoter-driven GFP. 3d post-infection, GFP expressing cells were selected by FACS and cultured for an additional 3d. Efficiency of MK2knock-down in the entire GFP-positive population was then assessed by immunoblotting of total cell lysates.
(B) Following subcutaneous injection of 106 shRNA-containing cells into the flanks of NCR nude outbred mice, tumor growth was measured every 2 days, starting at day 6 post injection. Arrow indicates the start of intraperitoneal administration of DMSO, 2 mg/kg cisplatin, or 4 mg/kg doxorubicin on day 12, given 3 times/week. In the absence of DNA-damaging chemotherapy, the MK2depleted tumors were statistically significantly larger than the control tumors at each time point beginning on day 13 (Student’s t-test, 2-tailed, p<0.02). In contrast, after cisplatin or doxorubicin treatment the MK2depleted tumors were statistically smaller than the control tumors beginning on days 21 and 23, respectively (p<0.02).
(C) Upper panels: dorsal view of the tumors in situ 14 days after initiation of the indicated treatments, corresponding to 26 days after tumor cell implantation. Middle panels: corresponding fluorescence images. Lower panels: close-up view of the excised tumors.
(D) Tumor weight was analyzed at the 26 day endpoint.
MK2is required for the G2/M checkpoint following doxorubicin treatment in p53-deficient cells
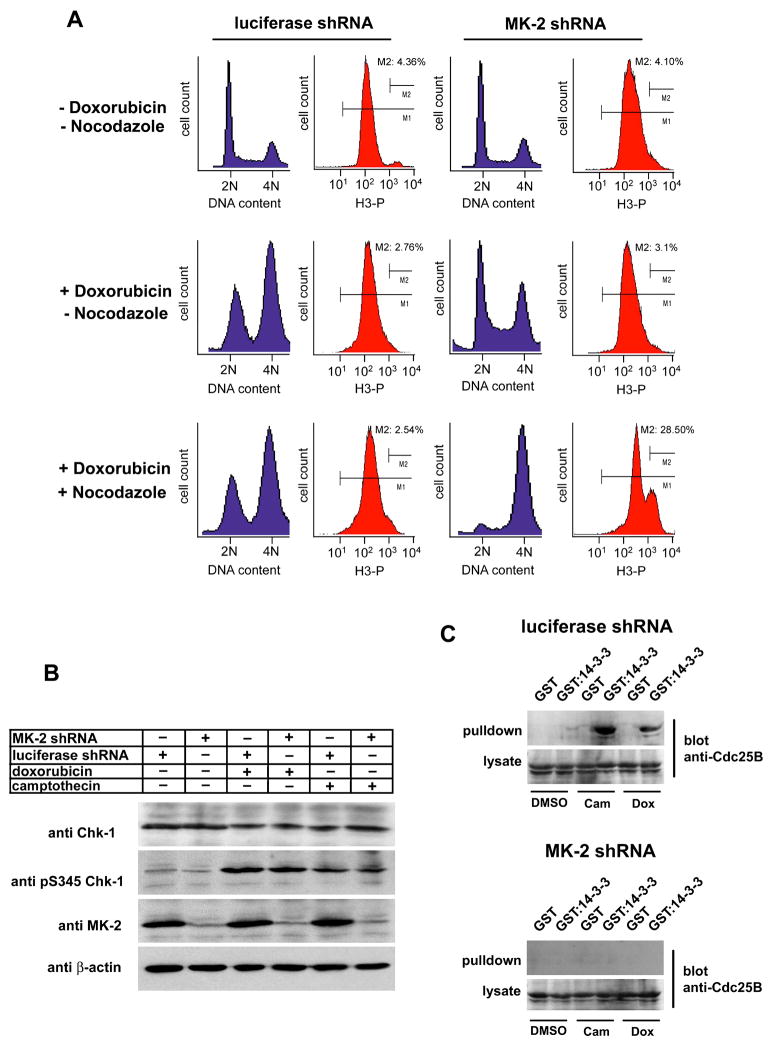
To investigate the molecular mechanisms involved in MK2 dependent responses to DNA lesions, we examined cell cycle profiles of control and MK2 depleted p53−/− MEFs. Asynchronous MK2- or control knock-down p53−/− MEFs were mock treated or exposed to doxorubicin for 30 hr, and cell cycle distribution monitored by FACS. In one set of experiments the spindle poison nocodazole was added to the media 3 hr after addition of doxorubicin, to cause any cells progressing through the cell cycle to arrest in mitosis. DNA content was monitored by PI staining; phospho-histone-H3 staining was used as an indicator of mitotic entry. As shown in the left panels of Fig. 5A, treatment of control knock down p53−/− cells with doxorubicin led to the accumulation of cells with 4N DNA content, and a lack of phospho-histone H3 staining in either the absence or presence of nocodazole, indicative of an intact G2/M checkpoint. These cells expressing control shRNAs behaved identically to an untransfected control p53−/− cell population (Supp. Fig. 4A,B). In marked contrast, MK2 depleted p53−/− cells treated with doxorubicin displayed a cell cycle profile similar to that of untreated cells (Fig. 5A, right upper and middle panels), with only a small increase in the 4N peak compared to the doxorubicin-treated luciferase shRNA controls, a slightly increased S-phase population, and the appearance of a sub-G1 population indicative of apoptosis. Addition of nocodazole following doxorubicin treatment to the MK2 depleted cells caused them to accumulate in a 4N DNA containing peak, with 28.5% of the cells staining positively for phospho-histone H3 (Fig. 5A, right lower panels), a value similar to that of untreated p53−/− cells blocked in mitosis with nocodazole (29.5%, Supp. Fig. 4D). Intriguingly, MK2 depletion did not alter total Chk1 levels or reduce Chk1 activation following DNA damage (Fig. 5B). These findings demonstrate that loss of MK2 prevents p53-deficient cells from establishing a functional G2/M checkpoint following doxorubicin-induced DNA damage, despite the presence of activated Chk1. Identical results were obtained using a second unrelated shRNA against MK2 (data not shown). Importantly, the checkpoint defect could be fully rescued in the MK2 depleted cells by expressing an shRNA-resistant form of MK2 at comparable levels to the endogenous protein (Supp. Fig. 5).
Figure 5. MAPKAP Kinase 2 mediates a G2/M arrest following doxorubicin treatment.
(A) RNAi down-regulation of MK2 ablates the doxorubicin-induced G2/M checkpoint. p53−/− MEFs stably expressing control luciferase shRNA (left) or MK2 shRNA (right) were cultured in the absence or presence of 10 μM doxorubicin and cell cycle profiles analyzed 30h later by FACS using PI for DNA content (blue) and phospho-histone H3 staining as an indicator of mitosis (red). In the lower set of panels, nocodazole (100 ng/ml) was added 3h following doxorubicin addition. Note that in addition to loss of the prominent G2/M checkpoint, the G1 and S phase components are also eliminated in MK2-depleted cells following doxorubicin + nocodazole treatment.
(B) Down-regulation of MK2 does not impair Chk1 activation. Luciferase shRNA-or MK2 shRNA expressing p53−/− MEFs were mock treated or exposed to 10μM doxorubicin or 10μM camptothecin for 30h. Total cell lysates were immunoblotted for the presence of MK2and total and activated forms of Chk1.
(C) Doxorubicin and camptothecin-induced binding of Cdc25B to 14-3-3 is lost in MK2 depleted cells. p53−/− MEFs cells either expressing a luciferase hairpin (upper panel) or a MK2 specific hairpin (lower panel) were mock treated or treated with 10μM camptothecin (cam) or 10μM doxorubicin (dox) for 8 hr. The presence of 14-3-3-binding sites on Cdc25B was monitored by incubating the lysates with bead-bound GST-14-3-3β/ζ followed by immunoblotting of the pulled-down material.
Two Cdc25 family members, Cdc25B and C, play important roles in initiating and maintaining mitotic entry in normal cells (Nilsson and Hoffmann, 2000), and are prominent targets of the G2/M checkpoint (Donzelli and Draetta, 2003). Cdc25B is believed to function by activating Cdk1/Cyclin B at the centrosome in late G2 as an initiator of early mitotic events (Jackman et al., 2003), while Cdc25C functions to further amplify Cdk1/CyclinB activity within a nuclear auto-amplification loop once mitosis has begun (Hoffmann et al., 1993). In response to γ- or UV-irradiation-induced DNA damage, checkpoint kinases phosphorylate Cdc25B and C on Ser-323 and 216, respectively, to induce their binding to 14-3-3 proteins, sequestering them in the cytoplasm away from their cyclin/Cdk substrates (Forrest and Gabrielli, 2001; Graves et al., 2001; Kumagai and Dunphy, 1999; Peng et al., 1997). Recent studies by van Vugt et al. suggest that Cdc25B plays a particularly crucial role in initiating and maintaining normal cell cycle G2/M checkpoint responses, since reactivation of Cdc25B is critical for DNA-damaged cells to re-enter the cell cycle (van Vugt et al., 2004). We therefore investigated whether MK2 signaling was required for association of Cdc25B with 14-3-3 in response to DNA damage by chemotherapeutic drugs. As shown in Fig. 5C, both doxorubicin and camptothecin treatment, resulted in the generation of stable 14-3-3-binding sites on Cdc25B in the luciferase shRNA control cells. No 14-3-3 binding of Cdc25B, however, was detected in lysates from the MK2 depleted cells (Fig. 5C, lower panel). This result is in good agreement with the cell cycle studies in panel A, which showed loss of the G2/M checkpoint in MK2 depleted cells after treatment with the topoisomerase inhibitor doxorubicin. These data indicate that loss of the G2/M checkpoint after DNA lesions in MAPKAP Kinase-2-depleted p53-defective cells likely arises, at least in part, from loss of Cdc25B binding to 14-3-3 proteins.
MK2 is required for S-phase checkpoint arrest following cisplatin treatment in p53-deficient cells
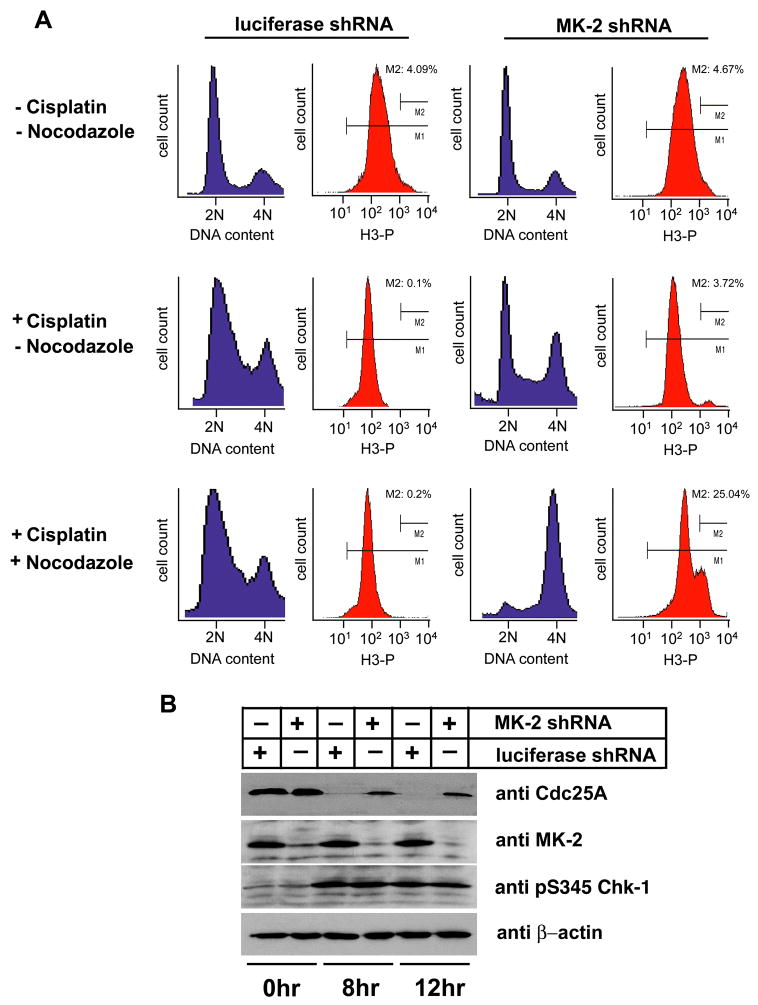
Treatment with the DNA intra-strand cross-linker cisplatin caused p53−/− cells to predominantly accumulate in S phase of the cell cycle (Supp. Fig. 4C). RNA interference was used to investigate the role of MK2 in this process. p53−/− control knock-down cells showed an identical accumulation in S phase after cisplatin exposure (Fig. 6A, left middle panels) as that seen in untransfected p53−/− cells. Addition of nocodazole to the luciferase knock-down cells 3 hr following cisplatin treatment did not reveal the appearance of any mitotic cells over the ensuing 27 hr, as monitored by phospho-histone H3 staining (Fig. 6A, lower left panels), indicating a functionally intact S-phase checkpoint. Depletion of MK2 prior to cisplatin exposure resulted in a dramatically different result. As seen in the right panels of Fig. 6A, MK2-depleted p53−/− cells showed a cell cycle profile after cisplatin treatment that was similar to that of untreated cells, other than a slight increase in the total number of cells in S-phase and the appearance of a sub-G1 population consistent with apoptosis. Strikingly, when nocodazole was added 3 hrs following cisplatin addition, the MK2 depleted p53−/− cells accumulated in a 4N DNA containing peak with ~25 % of the cells staining positive for phospho-histone H3. The same cell cycle defects after cisplatin exposure were observed using a second unrelated shRNA sequence against MK2 (data not shown), and the MK2 shRNA phenotype was completely reversed by expression of an RNAi-resistant form of MK2 at physiological levels (Supp. Fig. 5). Similar to what was observed following doxorubicin treatment (Fig. 5B), MK2 depletion did not impair activation of Chk1 after cisplatin exposure (Fig. 6B). These data imply that MK2 is essential for the cisplatin induced S-phase arrest in p53-deficient cells, and that loss of MK2 enables these cells to override the cisplatin-induced S-phase checkpoint, despite the presence of activated Chk1, and proceed into mitosis.
Figure 6. MK2 controls the S-phase checkpoint in response to cisplatin treatment.
(A) RNAi down-regulation of MK2 ablates the cisplatin-induced S-phase checkpoint. p53−/− MEFs stably expressing control luciferase shRNA (left) or MK2shRNA (right) were cultured in the absence or presence of 10 μM cisplatin and cell cycle profiles analyzed 30 hr later by FACS using PI for DNA content (blue) and phospho-histone H3 staining as an indicator of mitosis (red). In the lower set of panels, nocodazole (100 ng/ml) was added 3 hrs following cisplatin addition.
(B) Cisplatin-induced degradation of Cdc25A is impaired in MK2 depleted cells despite activation of Chk1. Luciferase shRNA- or MK2 shRNA expressing p53−/−MEFs were mock treated or exposed to 10μM cisplatin for 8 and 12 hr. Total cell lysates were immunoblotted for Cdc25A, total MK2 and activated Chk1. β-actin was used as a loading control.
In contrast to the 14-3-3-mediated sequestration of Cdc25B and C involved in the G2/M checkpoint response, the G1 and S phase checkpoints are largely controlled by the phosphorylation-dependent degradation of another Cdc25 isoform, Cdc25A (Bartek and Lukas, 2001; Busino et al., 2004). We therefore investigated whether MK2 was required for the degradation of Cdc25A following cisplatin-induced DNA damage (Fig. 6B). Cdc25A levels were undetectable in the control luciferase knock-down cells after treatment with cisplatin. In contrast, in the MK2 depleted cells, substantial amounts of Cdc25A remain present in the lysates after cisplatin exposure, indicating that in the absence of MAPKAP Kinase-2, p53−/− MEFs cells are defective in targeting Cdc25A for degradation in response to cisplatin induced DNA damage. This impaired ability of MK2 depleted cells to degrade Cdc25A likely explains their failure to establish a sustained G1/S checkpoint following cisplatin exposure. Interestingly, Cdc25A may be a direct target of MK2 in vivo, since both MK2 and Chk1 phosphorylate Cdc25A equivalently in vitro (Supp. Fig. 6), Chk1 phosphorylation of Cdc25A in vivo has been shown to facilitate its ubiquitin-mediated proteolysis in a complex and incompletely understood manner (Jin et al., 2003; Mailand et al., 2000), and MK2 and Chk1 phosphorylate the identical optimal sequence motifs when analyzed by oriented peptide library screening (Manke et al., 2005).
MK2 and Chk1 are activated independently by DNA damage, and are both potently inhibited by UCN-01
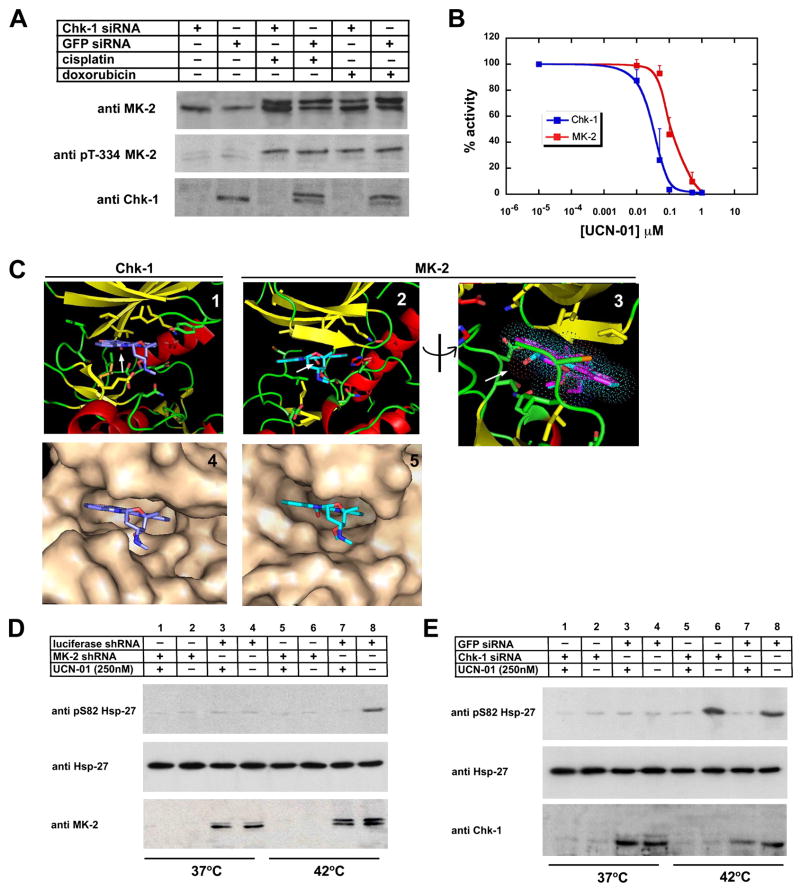
Activation of MK2 by cisplatin, camptothecin, and doxorubicin is strikingly similar to the activation profile reported for Chk1 (Agner et al., 2005; Ho et al., 2005; Mailand et al., 2000; Zhao and Piwnica-Worms, 2001). Similarly, the impaired S-phase and G2/M checkpoints seen after these DNA damaging stimuli in MK2 knock-down cells bears some resemblance to what has been previously reported for Chk1-deficient p53-defective cells (Xiao et al., 2003). These phenotypic similarities prompted us to further investigate whether the activation of Chk1 and MK2 was interdependent. As shown in Figs. 5B and 6B, activation of Chk1 in response to cisplatin and doxorubicin was unimpaired in MK2 depleted cells. We therefore investigated the converse - whether the activation of MK2 after DNA damage was dependent on Chk1. U2OS cells depleted of Chk1 using siRNA were exposed to cisplatin and doxorubicin, and analyzed for activation of MK2. As shown in Fig. 7A, phosphorylation/activation of MK2 occurred normally after treatment with these DNA damaging agents, regardless of the presence or absence of Chk1. Thus, activation of MK2 and Chk1 after drug-induced DNA damage appears to occur independently of each other, and both kinases appear to participate in parallel DNA damage checkpoint signaling pathways that are necessary for cell survival in the absence of a strong p53 response.
Figure 7. UCN-01 potently inhibits MK2 in vitro and in vivo.
(A) MK2 is activated independently of Chk1. U2OS cells were either transfected with Chk1-specific siRNA oligonucleotides or GFP control oligonucleotides and MK2activation was monitored after treatment with cisplatin (10μM) or doxorubicin (10μM). Cell lysates were probed for total and phosphorylated/activated forms of MK2with an antibody detecting total Chk1, to monitor the knock down efficiency.
(B) In vitro kinase assays were performed with Chk1 and MK2in the presence of increasing doses of UCN-01 using MK-2tide as a substrate.
(C) Structural basis for UCN-01 inhibition of MK2. Ribbon diagrams (β-strands shaded yellow, α-helices shaded red, loops shaded green) and molecular surfaces of the Chk1:UCN-01 complex (panels 1, 4) and the MK2:staurosporine complex with UCN-01 modeled onto staurosporine (panels 2,3, 5). Panel 3 is rotated 90° from the view in panel 2; the staurosporine molecule is colored purple, UCN-01 is shown with carbon atoms colored cyan, oxygens red and nitrogens blue. The arrow points to the unique 7-hydroxy group of UCN-01 with Van der Waals radii indicated by dots.
(D) UCN-01 inhibits MK2 in U2OS cells. Luciferase shRNA- or MAPKAP Kinase 2 shRNA expressing cells were incubated at 37°C or 42°C for 2 hr in the absence or presence of 250nM UCN-01. Cells were lysed and probed for total hsp-27, hsp-27 pS82, and MK2by immunoblotting.
(E) UCN-01 inhibition of hsp-27 is independent of Chk1. GFP or Chk1 siRNA transfected U2OS cells were incubated at 42°C or 37°C for 2h in the absence or presence of 250nM UCN-01. Cells were then lysed and probed for total hsp-27, hsp-27 pS82, and Chk1 by immunoblotting.
The staurosporine derivative 7-hydroxystaurosporin/UCN-01 has been shown to increase the cytotoxicity of chemotherapy and radiation (Mack et al., 2004; Shao et al., 2004; Tse and Schwartz, 2004) and is currently in clinical trials. It has been demonstrated that a major target of UCN-01 is the checkpoint kinase Chk1 (Busby et al., 2000; Graves et al., 2000), leading to speculation that that the increased chemo- and radiation sensitivity of cells treated with UCN-01 is a direct result of Chk1-mediated checkpoint abrogation (Kawabe, 2004). UCN-01 inhibits Chk1 with an IC50 that is ~1000 fold lower than that for Chk2, and hence has been used experimentally as a Chk1 specific inhibitor (Graves et al., 2000; Syljuasen et al., 2005). Strong circumstantial evidence, however, suggests that UCN-01 must be inhibiting other kinases involved in cell cycle control at similar concentrations as those used for Chk1 inhibition studies. For example, Chk1-depleted cells maintain phosphorylation of Ser-216, a well characterized Chk1 target site on Cdc25C, both during asynchronous growth and following γ-irradiation (Graves et al., 2000; Zhao et al., 2002b). Phosphorylation at this site is lost, however, when cells are treated with low doses of UCN-01 (~300 nM) (Busby et al., 2000; Graves et al., 2001), indicating that UCN-01 inhibitable kinase(s) other than Chk1 participate in Cdc25C Ser-216 phosphorylation. Based on our finding that MK2 depletion results in a dramatically increased chemosensitivity of malignant cells, we asked whether MK2 might be a UCN-01 target, similar to Chk1. In vitro kinase assays were performed under identical reaction conditions with Chk1 and MK2 using the same optimal peptide substrate for both kinases with the core consensus sequence L-Q-R-Q-L-S-I (Manke et al., 2005), in the presence of various concentrations of UCN-01. As shown in Fig. 7B, UCN-01 potently inhibited both kinases, with an IC50 value of ~35nM for Chk1 and ~95nM for MAPKAP Kinase-2. The IC50 value we measured for Chk1 is in good agreement with previously published data (Graves et al., 2000; Jackson et al., 2000). Importantly, the IC50 value we measured for MK2 is significantly below the concentrations of UCN-01 that are used in “Chk1-specific” checkpoint abrogation assays, suggesting that under the conditions used in those studies, both Chk1 and MK2 were being inhibited.
To examine the structural basis for UCN-01 inhibition of MK2, the structure of the MK2:UCN-01 complex was modeled using coordinates from the published MK2:staurosporine structure (Underwood et al., 2003), and compared the results with the co-crystal structure of Chk1:UCN-01 (Zhao et al., 2002a) (Fig. 7C). As seen in panels 2, 3 and 5, the 7-hydroxy moiety of UCN-01 can be easily accommodated into the MK2:staurosporine structure, where its closest neighboring residues would be Val-118 (2.8Å to Cγ2), Leu-141 (3.2 Å to Cδ1), and Thr-206 (3.6 Å to Cγ2). Lack of steric hindrance, and the overall similarity of the modeled MK2:UCN-01 structure to the Chk1:UCN-01 structure (panels 1, 4), provides a structural rationale for the tight binding observed biochemically.
To verify that MK2 is a direct target of UCN-01 within cells, we measured the phosphorylation of the MK2 substrate hsp-27 (Landry et al., 1992) after heat shock, a stimulus that activates the p38MAPK/MK2 pathway (Rouse et al., 1994). Control or MK2 shRNA expressing U2OS cells were incubated at 42°C or 37°C for 2 hr in the presence or absence of 250 nM UCN-01, and phosphorylation of hsp-27 monitored by immunoblotting. Fig. 7D shows that hsp27 is phosphorylated on Ser-82 when the control luciferase shRNA cells were placed at 42°C (lane 1). This phosphorylation was completely abrogated by treatment with UCN-01 (lane 2). No phosphorylation was observed in MK2 knock-down cells placed at 42°C regardless of the presence or absence of UCN-01 (lanes 3, 4), and no signal was observed in both the control and MK2 knock-down cells that were maintained at 37°C, with or without UCN-01 treatment (lane 5–8). Heat shock was equally effective in promoting the phosphorylation of hsp-27 on Ser-82, and UCN-01 was equally effective in blocking Ser-82 phosphorylation in cells that were depleted of Chk1 (Fig. 7E, lanes 1–4). Thus, UCN-01 inhibits MK2 in vivo, and this effect is independent of Chk1 function. This data demonstrates that UCN-01 is a direct inhibitor of MK2 within cells, and indicates that the clinical efficacy of UCN-01 in cancer treatment, particularly in p53-defective tumors, likely arises from the simultaneous inhibition of both the Chk1 and MK2 signaling pathways.
Discussion
Activation of cell cycle checkpoints in response to various types of DNA damage is essential for the maintenance of genomic stability in eukaryotic cells. Disruption of normal checkpoint function from inherited and acquired genetic mutations is increasingly recognized as a pathophysiological mechanism responsible for tumor-prone human disease syndromes (Bartek and Lukas, 2003; Kastan and Bartek, 2004; O’Driscoll et al., 2004; Shiloh, 2003). Alternatively, the loss of normal DNA damage checkpoint function in tumor cells is thought to underlie their increased susceptibility to genotoxic agents used in cancer chemotherapy. This has led to the suggestion of therapeutically targeting the remaining checkpoint pathways in tumors to increase the tumor-specific toxicity of DNA-damaging chemotherapy, particularly in cells that lack a functional p53 response (Dixon and Norbury, 2002; Kawabe, 2004; Zhou and Bartek, 2004).
The DNA damage response has largely been defined in the context of the ATM-Chk2 pathway and the ATR-Chk1 pathway. In contrast to Chk1 and Chk2, which are dedicated to DNA damage-response signaling, the p38MAPK pathway is a general stress response pathway that senses a wide variety of aberrant cell states. We found that both topoisomerase inhibitors, and intra-strand crosslinking agents activated MK2 in a p38 MAPK-dependent manner downstream of ATM and ATR. The kinetics of MK2 activation was observed to parallel the appearance of γ-H2AX foci. Rapid foci formation and MK2 activation was seen after cisplatin and doxorubicin exposure. In contrast, both foci formation and MK2 activation were delayed by ~4 hrs after camptothecin treatment, a finding that is in good agreement with previous data showing that DNA replication/S-phase progression is required for generation of a DNA lesion by this topoisomerase I inhibitor (Pommier, 1996; Tsao et al., 1992). Thus, in response to these genotoxic agents, the p38MAPK-MK2 signaling axis appears to be activated through the direct sensing of DNA damage. This mechanism of stress kinase pathway activation differs significantly from that observed following exposure to UV light, which did not require ATM or ATR, since it was activated equally well in cells that were genetically deficient for these DNA damage kinases. In agreement with our observations, Bulavin et al. reported that activation of p38MAPK was unaffected by treatment of HeLa cells with caffeine, a non-specific inhibitor of several PI 3-Kinase-like Kinases (PIKKs), prior to UV irradiation (Bulavin et al., 2001).
The requirement for particular upstream PIKKs to activate MK2 was different for different DNA damaging agents. In response to lesions created by cisplatin and camptothecin, activation of p38MAPK and MK2 required ATR, while the double strand break-inducing topoisomerase II inhibitor required both ATM and ATR. This latter finding is consistent with a recent study by Jazayeri et al. (Jazayeri et al., 2005), which showed that both ATM and Mre11 are recruited to DNA double-strand breaks, converting them into RPA-coated single-strand DNA, which then recruits ATR. In mammalian cells, p38MAPK consists of 4 isoforms -α, β, γ and δ, only two of which,α and β, are inhibited by SB203580 (Lee et al., 1999). Our finding that SB203580 inhibited the activation of MK2 in response to all of the DNA damaging drugs investigated implicates either or both of these isoforms as the relevant upstream activating kinase. The manner by which ATR, or possibly ATM, activates p38MAPK is unclear but may involve activation of any of a variety of MAPKK and MAPKKKs that are known to participate in p38MAPK activation in other contexts (Brancho et al., 2003; Lee et al., 1999).
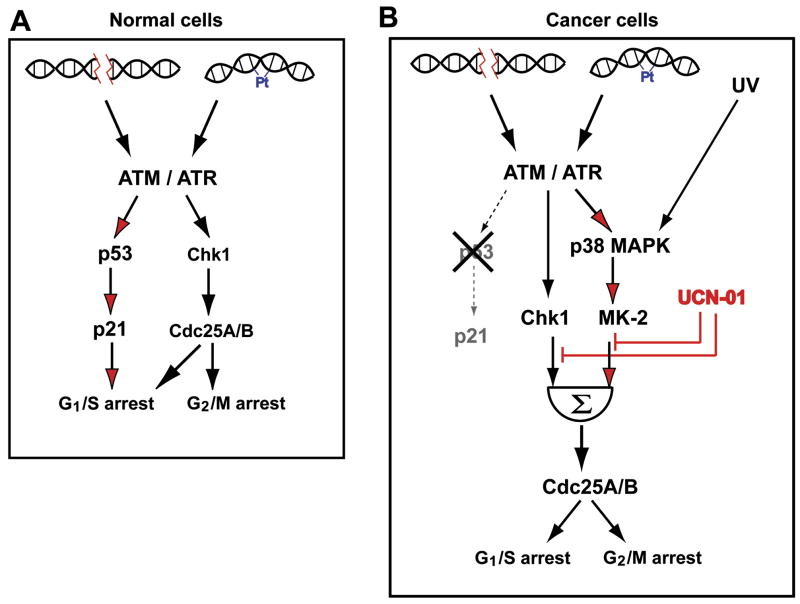
A particularly striking observation was the synthetic lethal interaction between p53 and MK2. On a systems level, this indicates a rewiring of DNA damage checkpoint signaling from a predominately p53 and Chk1-mediated arrest in p53 proficient cells (Lam et al., 2004) towards a Chk1 and p38MAPK/MK2 driven response in p53 deficient cells (Fig. 8). We found that MK2 was critical for both Cdc25B binding to 14-3-3 proteins after topoisomerase inhibition, and for establishment of a stable G2/M checkpoint in p53−/− cells exposed to doxorubicin. The 14-3-3 -binding site of Cdc25B is a direct substrate for MK2 (Lemaire et al., 2006; Manke et al., 2005), and Cdc25B function, which is inhibited by 14-3-3 binding (Forrest and Gabrielli, 2001), is required for cell cycle escape from the checkpoint after genotoxic stress (Bugler et al., 2006; Forrest and Gabrielli, 2001; van Vugt et al., 2004). Although Cdc25B is critical for mitotic control in normal cells (Dutertre et al., 2004; Lindqvist et al., 2005), Piwnica-Worms and colleagues have recently shown that Cdc25A, or some other as-yet-unknown phosphatase, appears to be able to compensate for the cell cycle and checkpoint functions of Cdc25B and C in mice genetically deficient for both of these phosphatases (Ferguson et al., 2005). We also observed that MK2 was critical for S phase arrest after cisplatin treatment. In the absence of MK2, Cdc25A, a critical effector of these cell cycle transitions, failed to undergo complete damage-induced destruction. The loss of these MK2-dependent cell cycle checkpoints permits cells with broken or damaged DNA strands to enter mitosis, resulting in mitotic catastrophe. Consequently, depletion of MK2 using RNA interference resulted in a profound increase in the sensitivity of p53−/− cells to the anti-proliferative effects of both cisplatin and doxorubicin. These effects were observed in clonogenic survival assays in culture, and in a xenograft tumor model in vivo.
Figure 8. A simplified model for re-wiring of cell cycle checkpoint pathways in p53-proficient and deficient cells.
(A) Checkpoint function in p53-proficient cells is mediated primarily through a robust, sustained p53 response downstream of ATM, together with Chk1. Although not shown explicitly in the diagram, Chk1 also directly phosphorylates p53 (Shieh et al., 2000). Under these conditions the presence of MK2 is not required for cell survival after exposure to DNA damaging agents.
(B) In p53-deficient cancer cells, checkpoint signaling following exposure to DNA damaging agents is mediated through the combined action of both the Chk1 and the p38 MAPK/MK2pathways. In this situation the p38MAPK/MK2 branch of checkpoint signaling becomes essential for cell survival after DNA damage. Both pathways are simultaneously inhibited by the indolocarbazole drug UCN-01.
Intriguingly, there appears to be an additional feedback connection between the MK2and p53 pathways, since Weber et al reported that MK2 could directly phosphorylate HDM2, the E3 ubiquitin ligase responsible for p53 destruction, to impair its function (Weber et al., 2005). In that study p53-competent MK2-deficient cells showed elevated levels of p53 and increased apoptosis after UV irradiation. we similarly observed a modest increase in p53 levels in MAPKAP Kinase-2-depleted p53+/+ MEFs, compared to control shRNA-treated p53+/+ MEFs after treatment with doxorubicin or cisplatin, along with a slight decrease in survival, but this failed to reach statistical significance. The difference between our findings and those of Weber et al. could be due to residual MK2activity using our RNAi approach compared to their gene disruption approach, or to the use of different genotoxic agents, since our data indicates that UV-induced activation of MK2 occurs independently of the ATR/ATM-mediated DNA damage response that is activated by doxorubicin and cisplatin.
Recruitment of MK2 as a critical DNA damage checkpoint kinase pathway in p53-deficient cells, but not in p53-proficient cells, suggest that inhibition of MK2 will likely function synergistically with conventional chemotherapeutic agents in human cancer treatment, similar to what has been proposed for inhibitors of Chk1 (Kawabe, 2004). UCN-01 is an indolocarbazole ATP analogue that inhibits Chk1 and increases the sensitivity of tumor cells to the anti-proliferative action of cisplatin, camptothecin and doxorubicin (Bunch and Eastman, 1996; Shao et al., 1997; Wang et al., 1996). We found that UCN-01 is a potent inhibitor of MK2 with an IC50 similar to that required for Chk1 inhibition. Typical concentrations of UCN-01 used in many cell cycle checkpoint abrogation experiments would have inhibited both Chk1 and MK2. This dual inhibition mechanism may provide a molecular rationale for the efficacy of UCN-01 in cancer therapy, particularly underlying its effectiveness in tumor cells that are defective in p53 function (Redkar et al., 2004; Wang et al., 1996), and suggests that similar agents that simultaneously target two distinct DNA damage signaling pathways will prove to be particularly efficacious. However, since disruption of the MK2 signaling pathway enhances chemotherapeutic responses in p53-deficient cells, but not in p53-wild type cells, even in the presence of a functional Chk1 response, and since MK2 knock-out mice are viable (Kotlyarov et al., 1999), in contrast to Chk1 knock-out mice (Liu et al., 2000; Takai et al., 2000), our results suggest that a MK2-specific inhibitor might provide significant clinical benefit with fewer undesirable side-effects, although we cannot dismiss the possibility that the apparently normal survival of the MK2 knock-out mice results from compensation by other genes with redundant function. The results of the current study strongly support the development of clinical MK2inhibitors as viable anti-cancer agents, and suggest that such drugs are likely to be particularly efficacious for the treatment of human p53-defective human cancers.
Experimental Procedures
RNAi
Sequences used for shRNA and siRNA were: luciferase (shRNA), 5′pTGA CCA GGC ATT CAC AGA AAT TCA AGA GAT TTC TGT GAA TGC CTG GTC TTT TTT C-3′; mMK2 (shRNA#1), 5′-pTCG ATG CGT GTT GAC TAT GAT TCA AGA GAT CAT AGT CAA CAC GCA TCG TTT TTT C-3′; mMK2 (shRNA#2) 5′-pTGG AGA GCT CTT TAG TCG AAT TCA AGA GAT TCG ACT AAA GAG CTC TCC TTT TTT C-3′; hMK2 (shRNA#1), 5′-pTTG ACC ATC ACC GAG TTT ATT TCA AGA GAA TAA ACT CGG TGA TGG TCA TTT TTT C-3′; hMK2 (shRNA#2), 5′-pTCA ATG CGC GTT GAC TAC GAT TCA AGA GAT CGT AGT CAA CGC GCA TTG TTT TTT C-3′. The murine shRNAs were used in MEFs while the human shRNAs were used in U2OS cells. GFP (siRNA) sense 5′-UCC CGG CUA UGU GCA GGA GdTdT-3′ and antisense strand 5′-CUCCUG CAC AUA GCC GGG AdTdT-3′; Chk1 (siRNA), 5′-UGG CAA CAG UAU UUC GGU AdTdT-3′ and antisense strand 5′-UAC CGA AAU ACU GUU GCC AdTdT-3′. RNAi resistant mMK2 was constructed by changing the sequence starting at nucleotide 1041 from 5′-ACG ATG CGT GTT GAC TAT GA-3′ to 5′-ACA ATG ACC ATT GAT TAC GA-3′ as described in Supplemental Methods.
Murine tumor model
For tumor regression assays, H-Ras-V12 transformed p53 −/− MEFs were stably transfected with a lentiviral transfer vector encoding for shRNA targeting either MK2or luciferase. 106 cells (107 cells/ml in PBS) were injected subcutaneously into the flanks of nude mice (Ncr nu/nu, Taconic), and tumors were allowed to form for 12 days. Mice were then treated with either cisplatin (2mg/kg, intraperitoneal administration 3x per week) or doxorubicin (4mg/kg, intraperitoneal administration 3x per week), monitored for a total of 26 days and then sacrificed. Tumor diameter was measured periodically during growth and tumors were weighed at the endpoint. Experiments were performed in quadruplicate, and data plotted as sample means with error bars showing standard deviation. These experiments were approved by the Massachusetts Institute of Technology Committee on Animal Care (CAC). A more detailed description of the experimental procedures is provided in the Supplemental Data.
Kinase assays
In vitro kinase assays for UCN-01 IC50 determination for both Chk1 and MK2were performed in identical 30μl reactions containing 20 mM HEPES (pH 7.5), 10 mM MgCl2, 3 mM 2-mercaptoethanol, 100 μg/ml BSA, 50 μM ATP, 10 μCi 32P-γ-ATP, and 50μM MK2-tide substrate (Manke et al., 2005) for 20 min at 30°C. Chk1 was used at a concentration of 0.3μM, MK2was used at a concentration of 0.1μM. Reactions were terminated by adding an equal volume of 0.5% phosphoric acid to the reaction and 5 μl was spotted onto P81 paper. After washing 5x in 0.5% phosphoric acid, sample were subjected to scintillation counting.
Molecular Modeling
The structure of MK2 bound to UCN-01 was modeled using PyMOL with the structure of MK2 bound to staurosporine (PDB ID 1NXK, (Underwood et al., 2003)) as a base model.
Statistical analysis
All measurements are presented as mean values with error bars denoting standard deviations.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Drew Lowery, Isaac A. Manke and Duaa Mohammad. This work was supported by NIH grants GM60594 and CA112967, and ES015339 from the National Institute of Environmental Health Sciences (NIEHS). It’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. HCR was supported by a post-doctoral fellowship from the Deutsche Forschungsgemeinschaft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agner J, Falck J, Lukas J, Bartek J. Differential impact of diverse anticancer chemotherapeutics on the Cdc25A-degradation checkpoint pathway. Exp Cell Res. 2005;302:162–169. doi: 10.1016/j.yexcr.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 2001;490:117–122. doi: 10.1016/s0014-5793(01)02114-7. [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Ben-Levy R, Leighton IA, Doza YN, Attwood P, Morrice N, Marshall CJ, Cohen P. Identification of novel phosphorylation sites required for activation of MAPKAP kinase-2. Embo J. 1995;14:5920–5930. doi: 10.1002/j.1460-2075.1995.tb00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block WD, Merkle D, Meek K, Lees-Miller SP. Selective inhibition of the DNA-dependent protein kinase (DNA-PK) by the radiosensitizing agent caffeine. Nucleic Acids Res. 2004;32:1967–1972. doi: 10.1093/nar/gkh508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugler B, Quaranta M, Aressy B, Brezak MC, Prevost G, Ducommun B. Genotoxic-activated G2-M checkpoint exit is dependent on CDC25B phosphatase expression. Mol Cancer Ther. 2006;5:1446–1451. doi: 10.1158/1535-7163.MCT-06-0099. [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ., Jr Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature. 2001;411:102–107. doi: 10.1038/35075107. [DOI] [PubMed] [Google Scholar]
- Bunch RT, Eastman A. Enhancement of cisplatin-induced cytotoxicity by 7-hydroxystaurosporine (UCN-01), a new G2-checkpoint inhibitor. Clin Cancer Res. 1996;2:791–797. [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Busby EC, Leistritz DF, Abraham RT, Karnitz LM, Sarkaria JN. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 2000;60:2108–2112. [PubMed] [Google Scholar]
- Busino L, Chiesa M, Draetta GF, Donzelli M. Cdc25A phosphatase: combinatorial phosphorylation, ubiquitylation and proteolysis. Oncogene. 2004;23:2050–2056. doi: 10.1038/sj.onc.1207394. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- Dixon H, Norbury CJ. Therapeutic exploitation of checkpoint defects in cancer cells lacking p53 function. Cell Cycle. 2002;1:362–368. doi: 10.4161/cc.1.6.257. [DOI] [PubMed] [Google Scholar]
- Donzelli M, Draetta GF. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 2003;4:671–677. doi: 10.1038/sj.embor.embor887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C, Mirey G, Bouche JP, Theis-Febvre N, Schmitt E, et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci. 2004;117:2523–2531. doi: 10.1242/jcs.01108. [DOI] [PubMed] [Google Scholar]
- Engel K, Schultz H, Martin F, Kotlyarov A, Plath K, Hahn M, Heinemann U, Gaestel M. Constitutive activation of mitogen-activated protein kinase-activated protein kinase 2 by mutation of phosphorylation sites and an A-helix motif. J Biol Chem. 1995;270:27213–27221. doi: 10.1074/jbc.270.45.27213. [DOI] [PubMed] [Google Scholar]
- Ferbeyre G, de Stanchina E, Lin AW, Querido E, McCurrach ME, Hannon GJ, Lowe SW. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol Cell Biol. 2002;22:3497–3508. doi: 10.1128/MCB.22.10.3497-3508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AM, White LS, Donovan PJ, Piwnica-Worms H. Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases. Mol Cell Biol. 2005;25:2853–2860. doi: 10.1128/MCB.25.7.2853-2860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest A, Gabrielli B. Cdc25B activity is regulated by 14-3-3. Oncogene. 2001;20:4393–4401. doi: 10.1038/sj.onc.1204574. [DOI] [PubMed] [Google Scholar]
- Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- Graves PR, Lovly CM, Uy GL, Piwnica-Worms H. Localization of human Cdc25C is regulated both by nuclear export and 14-3-3 protein binding. Oncogene. 2001;20:1839–1851. doi: 10.1038/sj.onc.1204259. [DOI] [PubMed] [Google Scholar]
- Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O’Connor PM, Piwnica-Worms H. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- Ho CC, Siu WY, Chow JP, Lau A, Arooz T, Tong HY, Ng IO, Poon RY. The relative contribution of CHK1 and CHK2 to Adriamycin-induced checkpoint. Exp Cell Res. 2005;304:1–15. doi: 10.1016/j.yexcr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2--cyclin B and its involvement in the self-amplification of MPF at mitosis. Embo J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60:566–572. [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2005 doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Jin J, Shirogane T, Xu L, Nalepa G, Qin J, Elledge SJ, Harper JW. SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 2003;17:3062–3074. doi: 10.1101/gad.1157503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kawabe T. G2 checkpoint abrogators as anticancer drugs. Mol Cancer Ther. 2004;3:513–519. [PubMed] [Google Scholar]
- Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev. 1999;13:1067–1072. doi: 10.1101/gad.13.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MH, Liu Q, Elledge SJ, Rosen JM. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell. 2004;6:45–59. doi: 10.1016/j.ccr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- Lee JC, Kassis S, Kumar S, Badger A, Adams JL. p38 mitogen-activated protein kinase inhibitors--mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82:389–397. doi: 10.1016/s0163-7258(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Lemaire M, Froment C, Boutros R, Mondesert O, Nebreda AR, Monsarrat B, Ducommun B. CDC25B phosphorylation by p38 and MK-2. Cell Cycle. 2006;5:1649–1653. doi: 10.4161/cc.5.15.3006. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Kallstrom H, Lundgren A, Barsoum E, Rosenthal CK. Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome. J Cell Biol. 2005;171:35–45. doi: 10.1083/jcb.200503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Mack PC, Jones AA, Gustafsson MH, Gandara DR, Gumerlock PH, Goldberg Z. Enhancement of radiation cytotoxicity by UCN-01 in non-small cell lung carcinoma cells. Radiat Res. 2004;162:623–634. doi: 10.1667/rr3253. [DOI] [PubMed] [Google Scholar]
- Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, Yaffe MB. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell. 2005;17:37–48. doi: 10.1016/j.molcel.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Hoffmann I. Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res. 2000;4:107–114. doi: 10.1007/978-1-4615-4253-7_10. [DOI] [PubMed] [Google Scholar]
- O’Driscoll M, Gennery AR, Seidel J, Concannon P, Jeggo PA. An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair (Amst) 2004;3:1227–1235. doi: 10.1016/j.dnarep.2004.03.025. [DOI] [PubMed] [Google Scholar]
- O’Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Pommier Y. Eukaryotic DNA topoisomerase I: genome gatekeeper and its intruders, camptothecins. Semin Oncol. 1996;23:3–10. [PubMed] [Google Scholar]
- Redkar A, Mixter P, Daoud SS. Implications of p53 in growth arrest and apoptosis on combined treatment of human Mammary epithelial cells with topotecan and UCN-01. J Exp Ther Oncol. 2004;4:213–222. [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- Shao RG, Cao CX, Pommier Y. Abrogation of Chk1-mediated S/G2 checkpoint by UCN-01 enhances ara-C-induced cytotoxicity in human colon cancer cells. Acta Pharmacol Sin. 2004;25:756–762. [PubMed] [Google Scholar]
- Shao RG, Cao CX, Shimizu T, O’Connor PM, Kohn KW, Pommier Y. Abrogation of an S-phase checkpoint and potentiation of camptothecin cytotoxicity by 7-hydroxystaurosporine (UCN-01) in human cancer cell lines, possibly influenced by p53 function. Cancer Res. 1997;57:4029–4035. [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- Tsao YP, D’Arpa P, Liu LF. The involvement of active DNA synthesis in camptothecin-induced G2 arrest: altered regulation of p34cdc2/cyclin B. Cancer Res. 1992;52:1823–1829. [PubMed] [Google Scholar]
- Tse AN, Schwartz GK. Potentiation of cytotoxicity of topoisomerase i poison by concurrent and sequential treatment with the checkpoint inhibitor UCN-01 involves disparate mechanisms resulting in either p53-independent clonogenic suppression or p53-dependent mitotic catastrophe. Cancer Res. 2004;64:6635–6644. doi: 10.1158/0008-5472.CAN-04-0841. [DOI] [PubMed] [Google Scholar]
- Underwood KW, Parris KD, Federico E, Mosyak L, Czerwinski RM, Shane T, Taylor M, Svenson K, Liu Y, Hsiao CL, et al. Catalytically active MAP KAP kinase 2 structures in complex with staurosporine and ADP reveal differences with the autoinhibited enzyme. Structure (Camb) 2003;11:627–636. doi: 10.1016/s0969-2126(03)00092-3. [DOI] [PubMed] [Google Scholar]
- van Vugt MA, Bras A, Medema RH. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O’Connor PM. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88:956–965. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- Weber HO, Ludwig RL, Morrison D, Kotlyarov A, Gaestel M, Vousden KH. HDM2 phosphorylation by MAPKAP kinase 2. Oncogene. 2005;24:1965–1972. doi: 10.1038/sj.onc.1208389. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, Zhang H. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem. 2003;278:21767–21773. doi: 10.1074/jbc.M300229200. [DOI] [PubMed] [Google Scholar]
- Zhao B, Bower MJ, McDevitt PJ, Zhao H, Davis ST, Johanson KO, Green SM, Concha NO, Zhou BB. Structural basis for Chk1 inhibition by UCN-01. J Biol Chem. 2002a;277:46609–46615. doi: 10.1074/jbc.M201233200. [DOI] [PubMed] [Google Scholar]
- Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A. 2002b;99:14795–14800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004;4:216–225. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.