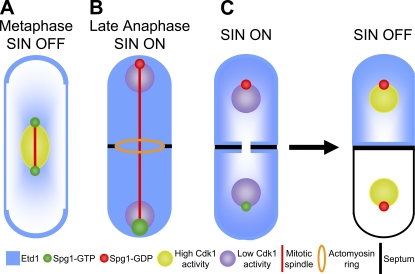
Spatial cues regulate cytokinesis: fully elongated spindles initiate cytokinesis in late anaphase, and the resulting cellular asymmetry triggers the process to end.
Abstract
Cytokinesis must be initiated only after chromosomes have been segregated in anaphase and must be terminated once cleavage is completed. We show that the fission yeast protein Etd1 plays a central role in both of these processes. Etd1 activates the guanosine triphosphatase (GTPase) Spg1 to trigger signaling through the septum initiation network (SIN) pathway and onset of cytokinesis. Spg1 is activated in late anaphase when spindle elongation brings spindle pole body (SPB)–localized Spg1 into proximity with its activator Etd1 at cell tips, ensuring that cytokinesis is only initiated when the spindle is fully elongated. Spg1 is active at just one of the two SPBs during cytokinesis. When the actomyosin ring finishes constriction, the SIN triggers disappearance of Etd1 from the half of the cell with active Spg1, which then triggers Spg1 inactivation. Asymmetric activation of Spg1 is crucial for timely inactivation of the SIN. Together, these results suggest a mechanism whereby cell asymmetry is used to monitor cytoplasmic partitioning to turn off cytokinesis signaling.
Introduction
Coordination of cytokinesis with mitosis is required to ensure proper chromosome segregation and genomic stability. It is unclear how cells determine when to initiate and when to terminate cytokinesis. In the fission yeast Schizosaccharomyces pombe, cytokinesis is regulated by the septum initiation network (SIN). Activation of the SIN in late anaphase triggers initiation of cytokinesis. In SIN mutants, the actomyosin contractile ring disassembles prematurely, causing cells to fail cytokinesis and become multinucleate. In contrast, failure to inactivate the SIN triggers repeated rounds of cytokinesis and defective entry into the next interphase (for reviews see Balasubramanian et al., 2004; Doxsey et al., 2005; Krapp and Simanis, 2008). How SIN activity is coordinated with cell cycle progression is unclear.
SIN signaling requires activation of the GTPase Spg1 (Schmidt et al., 1997). Once activated, Spg1-GTP binds the Cdc7 kinase and recruits it to the spindle pole body (SPB; Sohrmann et al., 1998). SPB-localized Cdc7 then promotes activation of the Sid2 protein kinase (Sparks et al., 1999). Active Sid2 translocates from the SPB to the cell division site to trigger cytokinesis. Most GTPases are regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). Although a GAP for Spg1 is known, no GEF has been identified. The Byr4 and Cdc16 proteins form a two-component GAP, which inactivates Spg1 (Furge et al., 1998). The Byr4–Cdc16 complex inhibits cytokinesis in interphase by binding to Spg1 at the SPB and keeping it in the inactive GDP-bound state (Cerutti and Simanis, 1999). Upon entry into mitosis, Byr4–Cdc16 leaves the SPBs, allowing Spg1 to become partially active at both SPBs (Sohrmann et al., 1998; Cerutti and Simanis, 1999). However, further activation of the SIN is blocked by Cdk1 activity (Guertin et al., 2000; Li et al., 2000; Chang et al., 2001; Krapp et al., 2008). At anaphase onset, two things happen: (1) loss of Cdk1 activity allows increased SIN activity (Guertin et al., 2000; Li et al., 2000; Chang et al., 2001; Krapp et al., 2008), and (2) Spg1 is inactivated at one of the two spindle poles by Byr4–Cdc16 (Cerutti and Simanis, 1999; Li et al., 2000). It is known that Spg1 remains active at the newer, or daughter, SPB (Sohrmann et al., 1998; Grallert et al., 2004), but the purpose of asymmetric activation of the SIN pathway is unknown. Although return of Byr4–Cdc16 to the SPB or SPBs is important for SIN inactivation after completion of cytokinesis, it has been unclear how cells sense completion of cytokinesis and turn off Spg1 and SIN signaling.
In the budding yeast Saccharomyces cerevisiae, the pathway analogous to the SIN is known as the mitotic exit network (MEN; for reviews see Seshan and Amon, 2004; Fraschini et al., 2008). Similar to the SIN, the MEN components display asymmetric localization patterns. In budding yeast, this asymmetry appears to be important for coordinating MEN activation and mitotic exit with other cell cycle events. It has been proposed that passage of the SPB into the bud brings the SPB-localized GTPase Tem1 (the Spg1 homologue) into contact with its putative GEF Lte1 that localizes exclusively in the bud. This mechanism ensures that mitotic exit only occurs after the spindle has oriented correctly, allowing the nucleus to move through the mother–bud neck into the bud (Bardin et al., 2000; Pereira et al., 2000). The molecular mechanism for the activation of Tem1 by Lte1 is still unknown. Although Lte1 has homology to the Ras exchange factor Cdc25, it has not been shown to directly activate Tem1 in vitro, and additional experiments showed that an Lte1 mutant lacking the Cdc25 homology domain (CHD) can still promote mitotic exit (Yoshida et al., 2003). Homologues of Lte1 have not been identified in S. pombe.
In this study, we examined the function of a recently identified component of the SIN called Etd1. A previous study identified Etd1 as an essential component of the SIN pathway that localizes to the cell cortex and division site but not to the SPB (Daga et al., 2005). Our work in this study suggests that Etd1 is a positive regulator of the Spg1 GTPase and a potential homologue of budding yeast Lte1. We show that proper regulation of Etd1 is crucial for both activation of Spg1 in anaphase and inactivation of Spg1 when cytokinesis is complete.
Results
etd1Δ-null mutants display a cold-sensitive phenotype similar to that of lte1 mutants in budding yeast
Activation of the Spg1 GTPase is a key step for initiation of cytokinesis in fission yeast. Budding yeast Lte1 promotes activation of the Spg1 homologue in budding yeast called Tem1. However, a fission yeast counterpart to Lte1 has not been identified. The SIN component Etd1 is the only SIN component that, like Lte1, localizes to the cell cortex and not the SPB. Although conventional blast searches using Etd1 or Lte1 did not suggest that they might be homologues, comparison of the protein sequences of Etd1 and three different budding yeast Lte1 orthologues using multiple sequence alignment (Corpet, 1988) showed that the full length of Etd1 aligns specifically with the Ras GEF CHD of the Lte1 orthologues, although the total sequence similarity is low (Fig. S1 A). This weak similarity between Etd1 and Lte1 suggested that these two proteins might be homologues. The budding yeast lte1Δ deletion mutant is viable at high but not low temperatures (Shirayama et al., 1994). Similarly, tetrad analysis of heterozygous diploids (etd1+/etd1Δ∷ura4+) showed that the etd1Δ deletion mutant is viable at 36°C but dead at lower temperatures, indicating that, like lte1Δ cells, etd1Δ cells are cold sensitive (Fig. S1 B and not depicted). A previous study citing data not depicted found that etd1+ was essential at 36°C (Daga et al., 2005); however, we repeatedly observed viability of etd1Δ spores after germination and growth at 36°C. In addition, we found that a strain in which etd1+ transcription was shut off using a regulatable promoter was also viable at 36°C (Fig. S1 B). Thus, we are unable to explain the earlier observations. Although etd1Δ cells grew at 36°C (Fig. S1 B), 21% (193/907) of cells failed cytokinesis with a SIN phenotype (Fig. S1 C, arrows). Upon shift to 25°C, etd1Δ cells died with a SIN phenotype, failing cytokinesis and becoming multinucleate (Fig. S1, C and D). Thus, like budding yeast lte1Δ mutants, etd1Δ cells are sick but viable at high temperatures.
Etd1 interacts genetically and physically with Spg1
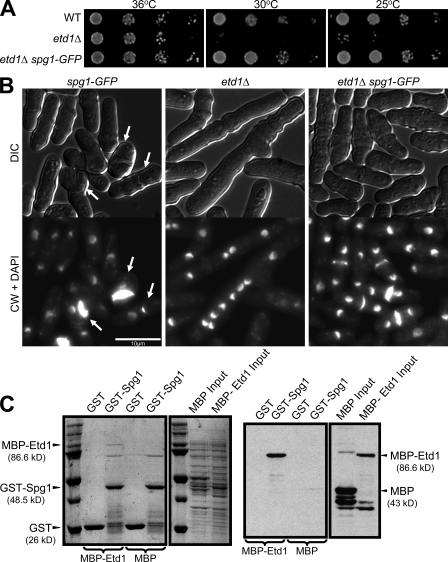
To test whether Etd1 functions like Lte1, we examined the relationship between Etd1 and the Spg1 GTPase. Several lines of evidence suggest that Etd1 functions upstream of Spg1. First, ectopic expression of spg1+ in wild-type cells induces multiple rounds of actomyosin ring assembly and septum formation without cell separation (Schmidt et al., 1997). Similarly, etd1Δ cells also showed multiple septa after moderate or strong overproduction of Spg1 (Fig. S2 A). Second, moderate overproduction of Spg1 partially rescued the growth defect of etd1Δ cells at low temperatures (Fig. S2 B). Third, we found that the etd1Δ growth defect could be suppressed by a partially activated allele of Spg1 (Fig. 1 A). In these experiments, partial activation of Spg1 was achieved by fusing GFP to its C terminus, which results in occasional interphase septa, suggesting a gain of Spg1 function (Fig. 1 B, arrows; and not depicted). In addition to the growth defect, spg1-GFP also suppressed the etd1Δ cytokinesis defect (Fig. 1 B). In contrast, the spg1-GFP allele was unable to rescue mutations in SIN pathway components downstream of Spg1 (Fig. S2 C). Together, these results suggest that Etd1 functions upstream of Spg1.
Figure 1.
Etd1 shows genetic and physical interactions with Spg1. (A) Spg1-GFP rescues the growth defect of etd1Δ cells at low temperatures. spg1-GFP (wild type [WT]), etd1Δ, and etd1Δ spg1-GFP cells were grown in YE at 36°C, and then serial dilutions were spotted on YE plates and incubated at the indicated temperatures for 3–5 d. (B) Wild-type, etd1Δ, and etd1Δ spg1-GFP cells were grown at 36°C and shifted to 25°C for 8 h, and then harvested, fixed, and stained with DAPI and CW to detect DNA and septa, respectively. Arrows indicate ectopic septa. (C) Etd1 and Spg1 bind directly in vitro. Lysates from cells expressing GST alone or GST-Spg1 were incubated for 2 h at 4°C with lysates from bacteria expressing MBP-Etd1 and MBP alone. Complexes were pulled down using glutathione beads, and the bound protein was analyzed by Western blotting using anti-MBP antibodies (right) or stained with Coomassie (left).
These observations prompted us to test whether Etd1 binds directly to Spg1. Spg1 was expressed in bacteria as a GST fusion. Lysates from cells expressing GST-Spg1 or GST alone were incubated with lysates from bacteria expressing Etd1 fused with maltose-binding protein (MBP) or MBP alone, and complexes were pulled down using glutathione beads. Analysis of the bound material showed that MBP-Etd1 (but not MBP) was specifically pulled down by GST-Spg1 (Fig. 1 C). These results demonstrate that Spg1 can interact directly with Etd1. However, we were unable to coimmunoprecipitate endogenous Spg1 with Etd1 expressed either from its own or an inducible promoter (unpublished data), suggesting that the interaction is either transitory or relatively weak.
Etd1 is essential for the activation of the SIN at the end of anaphase
To characterize the effect of Etd1 on Spg1 activity in vivo, we used an indirect reporter of Spg1 activity. Because GTP-bound Spg1 recruits its downstream effector, the Cdc7 kinase, to the SPB (Sohrmann et al., 1998), Cdc7 levels at the SPB can be used as an indirect measure of Spg1 activity in vivo. In wild-type cells, Cdc7-GFP localizes to both SPBs in early mitosis and then to just one SPB in anaphase and telophase (Fig. 2 A, left; Sohrmann et al., 1998; Cerutti and Simanis, 1999; Li et al., 2000). Interestingly, quantitative analysis of Cdc7-GFP signal showed that when Cdc7-GFP localizes to a single SPB in late anaphase the fluorescence increased ∼2.5-fold in 21 of 23 cells imaged (Fig. 2, A and B, left). In etd1Δ cells, Cdc7-GFP initially localized similarly to wild-type cells (Fig. 2 A, right), but the signal did not increase at the SPB during anaphase B (n = 13; Fig. 2 B, right). Our results suggest that the key function of Etd1 is to cause the hyperactivation of Spg1 that occurs in late anaphase. This interpretation differs from an earlier study, which suggested that Etd1 is required for maintaining Spg1 activity (Daga et al., 2005). However, our revised interpretation is consistent with their data.
Figure 2.
etd1Δ cells do not fully activate Spg1 in late anaphase. (A) Cdc7-GFP was imaged by time-lapse microscopy in wild-type (left) and etd1Δ cells (right) at 25°C. The dotted lines show the edge of each cell. Time is indicated in minutes. (B) Quantification of Cdc7-GFP fluorescence for each SPB (lSPB, left SPB; rSPB, right SPB) using arbitrary units (A.U.) is shown.
Spindle elongation allows Etd1-dependent SIN activation in late anaphase
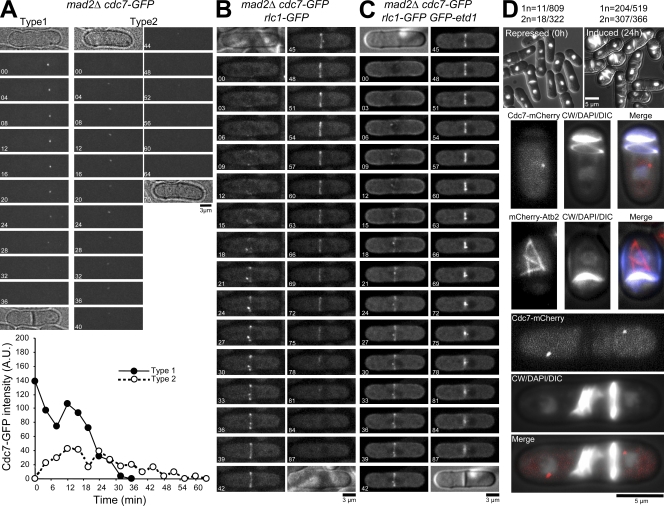
One explanation for the previous results could be that Spg1 only becomes fully activated when the elongating spindle brings the SPB into closer proximity to the cell tips, which are enriched in Etd1 (Daga et al., 2005). Therefore, we tested whether spindle elongation was required for full activation of the SIN by disrupting spindle assembly with the microtubule-depolymerizing drug methyl-2-benzimidazole-carbamate (MBC). To avoid metaphase arrest, we used mad2Δ cells, which continue to cycle without any microtubules present (He et al., 1997). In MBC-treated cultures, we observed two different patterns of Cdc7-GFP localization. Cells that had already completed spindle elongation when placed in MBC showed bright Cdc7-GFP signal at one SPB near the cell tip (n = 43; Fig. 3 A, type 1), similar to untreated cells. In cells that entered mitosis after being put in MBC, SPB-localized Cdc7-GFP appeared faintly but never increased in brightness before disappearing (n = 34; Fig. 3 A, type 2). One explanation for these results is that activation of the SIN at late anaphase requires the elongation of the spindle during anaphase B, which could bring SPB-localized Spg1 closer to cortical Etd1 at cell tips.
Figure 3.
Etd1 helps coordinate spindle elongation with SIN activation. (A) mad2Δ cdc7-GFP cells were grown in YE at 25°C and then shifted to YE containing MBC. Cdc7-GFP was imaged by time-lapse microscopy in the presence of MBC. Changes in fluorescence of Cdc7-GFP were measured in arbitrary units (A.U.) for either of the two types of cells: those that had already completed anaphase at the time of MBC addition and displayed Cdc7-GFP signal at one SPB toward the cell tip (type 1) and those that entered mitosis after the addition of MBC and displayed Cdc7-GFP signal at one or two SPBs near the cell center (type 2). A total of 43 type 1 cells and 34 type 2 cells were imaged. One representative experiment for each type is shown. (B and C) mad2Δ cdc7-GFP rlc1-GFP cells (B) or mad2Δ nmt41-GFP-etd1 cdc7-GFP rlc1-GFP cells (C), which moderately overexpressed etd1+, were grown without thiamine and then imaged by time-lapse microscopy in the presence of MBC. (A–C) Time is indicated in minutes. (D) Targeting of Etd1 to the SPB induces ectopic activation of the SIN pathway. etd1Δ cdc7-mCherry or etd1Δ mCherry-atb2 cells carrying the pREP41-EGFP-Etd1-Ppc89261–783 plasmid were cultured at 36°C in EMM liquid medium plus thiamine and shifted to media without thiamine for an additional 24 h. Samples were taken at 0 (repressed) and 24 h after thiamine removal (induced), fixed, and visualized for DIC, DAPI, CW (top panels), and Cdc7-mCherry and mCherry-Atb2 fluorescence (bottom panels). The number of mononucleate cells (1n) with a septum and binucleate cells (2n) with more than one septum versus the total number of cells is shown for each condition.
Previous studies have shown that SIN activity is required for maintenance and assembly of the actomyosin ring in late mitosis (Fankhauser et al., 1995; Balasubramanian et al., 1998; Hachet and Simanis, 2008). Because the SIN activity seems to be reduced in the absence of the anaphase spindle, we examined whether this had any effect on actomyosin ring maintenance in cells treated with MBC. Examination of mad2Δ cdc7-GFP rlc1-GFP cells by time-lapse microscopy in the presence of MBC showed that 17 out of 23 cells failed to complete cytokinesis as a result of actomyosin ring disassembly before the completion of ring constriction. Of these 17 cells, 4 showed no ring constriction, and 13 showed very slight to partial constriction and displayed incomplete septa as visualized by differential interference contrast (DIC; Fig. 3 B and Fig. S3, A and B). These results suggest that anaphase spindle elongation is essential for full activation of the SIN, which in turn is required for maintenance of the actomyosin ring and cytokinesis.
If failure to bring SPB-localized Spg1 into proximity with Etd1 at cell tips is the reason for the cytokinesis defects that occur when spindle elongation is blocked, artificially increasing the cellular concentration of Etd1 would be predicted to rescue these defects. To test this hypothesis, we examined the effect of moderate overexpression of Etd1 on actomyosin ring maintenance and cytokinesis in the presence of MBC. We observed that 92% (n = 13) of cells moderately overexpressing GFP-Etd1 in the presence of MBC maintained the actomyosin ring until it finished constriction and thus completed cytokinesis like unperturbed wild-type cells (Fig. 3 C and Fig. S3 C). These results suggest that full activation of the SIN at late anaphase requires elongation of the spindle during anaphase B, which could bring SPB-localized Spg1 closer to cortical Etd1 at cell tips.
Among known SIN components, Etd1 is the only one that does not localize to the SPB. Localization of Etd1 to cell tips might be a way to ensure that the SIN is only activated at the correct point in the cell cycle. We wondered whether selective targeting of Etd1 to the SPB might cause defects in the timing of SIN activation. To test this idea, we targeted Etd1 to the SPB by fusing it to the SPB-binding domain of Ppc89. Ppc89 is a structural component of the SPB that links the proteins of the SIN to the SPB (Rosenberg et al., 2006). GFP-etd1-ppc89 was expressed in cells using a thiamine-regulatable promoter. Induction of GFP-etd1-ppc89 expression caused the majority of the cells to arrest with an activated SIN phenotype in which repeated rounds of cytokinesis resulted in accumulation of multiple septa (Fig. 3 D, compare the top right panel with uninduced control [top left]). Interestingly, 39% of mononucleate cells (n = 519) exhibited an ectopic activation of the SIN pathway, as indicated by the presence of septa and Cdc7 at the SPB (Fig. 3 D, middle panels). The mononucleate cells that contained septa were in interphase, as judged by the presence of interphase microtubules (Fig. 3 D, middle panels). This result shows that targeting of Etd1 to the SPB triggers precocious activation of the SIN pathway. In addition, we observed that 84% of the cells with two nuclei (n = 366) displayed multiple septa and Cdc7 at both SPBs, indicating prolonged activation of the SIN pathway (Fig. 3 D, bottom panels). Curiously, the overexpression phenotype was much stronger at 36°C than at 25°C (unpublished data). This result could be explained if Spg1 is intrinsically more active at 36°C than at 25°C, which could also explain why Etd1 is not essential at 36°C (see Discussion). Overall, these results suggest that localization of Etd1 to the cells tips is important to ensure that the SIN is not fully activated until spindle elongation brings SPB-localized Spg1 close to the cell tips. Either Etd1 overexpression or forced localization of Etd1 to the SPB disrupts the dependence of SIN activation on spindle elongation.
Etd1 and SIN inactivation at the end of cytokinesis
Our aforementioned experiments show that Etd1 is required for SIN activation and may need to be inactivated to terminate SIN signaling. We wondered whether inhibition of Etd1 might be important for the eventual inactivation of Spg1 at the end of cytokinesis. First, we examined precisely when Spg1 becomes inactivated in wild-type cells using the Cdc7-GFP reporter. Observation of wild-type cells expressing Cdc7-GFP by time-lapse analysis showed that, in all cells observed (n = 28), Spg1 is completely inactivated as soon as cells complete actomyosin ring constriction and septum formation (Fig. 4 A), indicating that SIN inactivation is coincident with the partitioning of the cytoplasm between the two daughter cells. We then examined whether Etd1 underwent any changes in localization at the time of Spg1 inactivation. Because endogenous Etd1-GFP is difficult to observe (Daga et al., 2005), we examined Etd1 localization using a strain that expressed increased GFP-Etd1 levels from a heterologous promoter. As reported previously (Daga et al., 2005), we observed that GFP-Etd1 localized to the cell cortex and was enriched at cell tips during interphase (Fig. 4 B and not depicted). However, we also noticed that in late anaphase and telophase, GFP-Etd1 left the cell cortex and was present in the cytoplasm (Fig. 4 B and not depicted). In cells that had completed cytokinesis and septum formation, we found that GFP-Etd1 localized asymmetrically, with one of the resulting daughter cells having a much brighter signal in both the cell cortex and cytoplasm (Fig. 4 B and not depicted). Examination of 530 cells that had completed septum formation showed that in 86.80% of the cells, the GFP-Etd1 signal was brighter in the cell with inactive SIN, as indicated by the absence of Cdc7-GFP fluorescence at the SPB (Fig. 4 B and not depicted). Using either GFP-Etd1 to visualize the septum (Fig. 4 C) or the actomyosin ring marker Rlc1-mCherry (Fig. 4 D) to monitor progression through cytokinesis, it was clear that Etd1 localization became asymmetric at about the time that the ring finished constriction and septum formation completed. Quantification of total GFP-Etd1 fluorescence in each half of the cell showed that the asymmetry was generated by a combination of an increase in fluorescence in one half of the cell and a decrease in the other half. The fluorescence intensity only became asymmetric when cytokinesis was completed (Fig. 4 C). These results show that Etd1 is lost from the daughter cell with the SPB with active SIN. Because Etd1 is required for full Spg1 activation, loss of Etd1 at this time could promote inactivation of Spg1 and the SIN.
Figure 4.
Etd1 localizes asymmetrically at the end of cytokinesis. (A) Cdc7-GFP and Rlc1-GFP were imaged by time-lapse microscopy. The dotted lines show the edge of each cell. (B) nmt41-GFP-etd1 cdc7-GFP cells were grown in EMM liquid without thiamine. Cells representative of the indicated cell cycle stages were selected. (C) Fluorescence intensity measurements of Etd1 during cytokinesis. nmt41-GFP-etd1 cells were analyzed by time-lapse microscopy. A single medial z plane for each time point for two representative cells are shown (top). (bottom) Plots of the mean of total fluorescence for the right daughter cell and the left daughter cell. Etd1 signal at the septum was not included in the measurements because of the difficulty in determining which cell the signal was coming from. The total number of cells imaged, all of which showed similar results, is shown in parentheses. (D) Etd1 localizes asymmetrically after completion of actomyosin ring contraction. nmt41-GFP-etd1 cdc7-GFP rlc1-mCherry cells were imaged by time-lapse microscopy. (A, C, and D) Time is indicated in minutes.
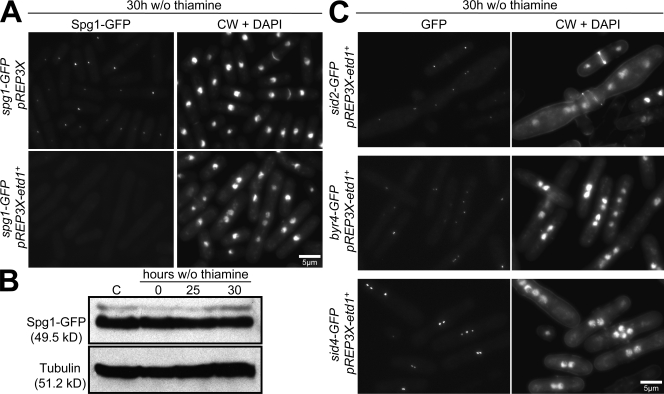
If loss of Etd1 at the end of cytokinesis is important for turning off the SIN, elevated Etd1 levels should prevent SIN inactivation. However, a previous study found that strong overproduction of Etd1 resulted in a SIN loss of function phenotype (Daga et al., 2005). When we characterized this phenotype further, we found that strong overexpression of Etd1 caused Spg1 to become displaced from SPBs in the majority of cells (Fig. 5 A). Western blot analysis showed that these phenotypes were not caused by changes in Spg1 levels (Fig. 5 B), and the SPB localization of other SIN components was unperturbed by Etd1 overproduction (Fig. 5 C), suggesting a specific effect on Spg1. These results suggest that Etd1 may bind to Spg1 in a way that blocks the ability of Spg1 to localize to the SPB, so that when Etd1 is strongly overexpressed, it titrates Spg1 away from the SPB and thus blocks SIN activity.
Figure 5.
Etd1 overexpression phenotype. (A) Cells of the indicated genotype were cultured at 25°C in the presence of thiamine and then cultured in the absence of thiamine for an additional 30 h to induce etd1+ expression. Samples were then fixed and visualized for GFP, DAPI, and CW fluorescence. (B) Strong overexpression of etd1+ does not change the levels of Spg1-GFP. spg1-GFP pREP3X (control [C]) or spg1-GFP pREP3X-etd1+ cells were grown as in A and harvested at the indicated times, and cell lysates were probed by Western blotting. (C) Cells of the indicated genotype were cultured and visualized as in A. w/o, without.
When more moderate overexpression was used via an attenuated promoter such as the one used to analyze GFP-Etd1 localization (Fig. 4), Spg1 remained active longer than in wild-type cells, as judged by Cdc7-GFP localization at the SPB. Wild type and cells moderately overexpressing GFP-Etd1 were fixed and analyzed both for the presence of Cdc7-GFP at the SPB and for whether they had completed actomyosin ring constriction using Rlc1-mCherry to mark the actomyosin ring. Examination of wild-type cells showed that, of cells displaying Cdc7-GFP signal at the SPB, only 3% (n = 116) appeared to have completed ring constriction. This is in contrast to cells moderately overexpressing GFP-Etd1, where 48% (n = 143) of cells with Cdc7-GFP at the SPB had completed ring constriction. Similar results were obtained using time-lapse analysis, which showed that 100% of wild-type cells (n = 28) inactivated Spg1 as soon as the ring finished constriction (Fig. 4 A). In contrast, 91% (n = 55) of cells moderately overexpressing GFP-Etd1 kept Spg1 active after ring constriction (Fig. 4 D) and sometimes even after cells separated (Fig. 4 B and Fig. S4 A). Although these cells were viable, they often underwent an additional round of actin ring assembly in the daughter cell with lingering SIN activity, as determined by SPB-bound Cdc7, followed by septum formation (Fig. S4, A and B). Examination of asynchronous cells overexpressing GFP-Etd1 showed that 31% of septated cells (n = 109; Fig. S4 B) contained ectopic septa. Thus, increased levels of Etd1 interfere with Spg1 inactivation at the end of cytokinesis.
The SIN antagonizes Etd1 cortical localization and promotes Etd1 asymmetry
Both because Etd1 leaves the cell cortex when the SIN becomes active in late anaphase and because Etd1 is lost from the daughter cell that has the SPB with active SIN, we wondered whether SIN signaling was responsible for these changes in Etd1 localization. To test whether SIN activity affected Etd1 localization, we reduced SIN activity using the sid2-250 mutation, which results in only partial Sid2 function even at the permissive temperature of 25°C (Mishra et al., 2005). In late anaphase and during early septum formation in telophase, Etd1 was released from the cortex and found uniformly localized in the cytosol in 95% (n = 55) of wild-type but only 2% (n = 49) of sid2-250 cells (Fig. 6 A), showing that release of Etd1 from the cortex in anaphase and telophase requires the SIN. Examination of septated cells showed that 87% of septated wild-type cells (n = 102) exhibited an asymmetric localization of Etd1 (Fig. 6 B, left, arrowheads). In contrast, only 22% of sid2-250 cells (n = 139) displayed asymmetric Etd1 localization, with most cells displaying symmetric localization of Etd1 at the cortex of both mother and daughter cells (Fig. 6 B, right, asterisks). To examine this further, we quantified the ratio of GFP-Etd1 signal between the brighter and less bright cell halves in septated wild-type and sid2-250 cells. This showed that wild-type cells (1.4 ± 0.14; n = 12) had a significantly greater asymmetry of GFP-Etd1 localization (P < 0.0001) compared with sid2-250 cells (1.1 ± 0.14; n = 14). Thus, the SIN seems to be required both to inhibit cortical localization of Etd1 and to promote the disappearance of Etd1 in the cell with active SIN. It is unclear whether loss of Etd1 from the cortex promotes Etd1 degradation or whether the SIN promotes the two events independently.
Figure 6.
SIN activity regulates both cortical and asymmetric localization of Etd1. (A) Reduced SIN activity causes prolonged localization of Etd1 to the cell cortex during anaphase/telophase. Cells of the indicated genotypes were grown at 25°C, stained, and visualized for GFP and CW fluorescence. (B) Cells with the indicated genotypes were grown as in A, and GFP-Etd1 images from a single medial focal plane are shown. Arrowheads indicate cells that display an asymmetrical localization of GFP-Etd1 in control cells, whereas asterisks mark symmetric localization in sid2-250 cells. (C) Interphase activation of the SIN causes loss of cortical and total GFP-Etd1. cdc16-116 nmt41-GFP-etd1 or cdc16-116 nmt41-GFP-etd1 cdc7-GFP cells were grown at 25°C and then shifted to 36°C for 2 h and visualized for GFP (right) and DIC images (left). GFP-Etd1 images from a single medial focal plane (top) and GFP-Etd1 and Cdc7-GFP z projection (bottom) are shown. Arrows indicate compartments that have a nucleus, as judged by nuclear exclusion of GFP-Etd1 or the presence of Cdc7-GFP. Arrowheads indicate compartments lacking a nucleus and SPB.
Given that SIN activity seems to control the movement of Etd1 from the cell cortex to the cytosol, we reasoned that constitutive SIN signaling should prevent cortical localization of Etd1. To test this possibility, we used a temperature-sensitive form of Cdc16, the GAP for Spg1 (Furge et al., 1998). Inactivation of Cdc16 causes constitutive SIN signaling and repeated rounds of cytokinesis and septum formation without cell separation (Minet et al., 1979). When Cdc16 is inactivated, two types of cells are observed (Minet et al., 1979), those that form septa in interphase (Fig. 6 C, 1) and those that form a septa at the normal time but then form additional septa adjacent to the first septum (Fig. 6 C, 2). Both types of cells contain anucleate compartments (Fig. 6 C, arrowheads) as well as compartments that have nuclei, as judged by the exclusion of cytoplasmic GFP-Etd1 (Fig. 6 C, arrows), and an SPB with active SIN (Fig. 6 C, bottom; Sohrmann et al., 1998). GFP-Etd1 was lost from the cortex and was only faintly visible in the cytoplasm of nucleate compartments containing active SIN. Interestingly, very bright GFP-Etd1 signal was observed at the cortex and cytoplasm of compartments lacking a nucleus and SPB, presumably because of the lack of SPB-localized SIN signaling (Fig. 6 C, arrowheads). Quantification of total GFP-Etd1 fluorescence from 29 cells showed a ratio of 1.4 ± 0.14 between the compartments without a nucleus and those that displayed a nucleus, indicating that the compartments without an SPB and active SIN contain more GFP-Etd1. Thus, we conclude that SIN activity is important for the translocation of Etd1 from the cell cortex to the cytoplasm during anaphase and early cytokinesis. Furthermore, SIN activity is required for the disappearance of Etd1 at the time of septum formation.
Asymmetric SIN activity is crucial for SIN inactivation at the end of cytokinesis
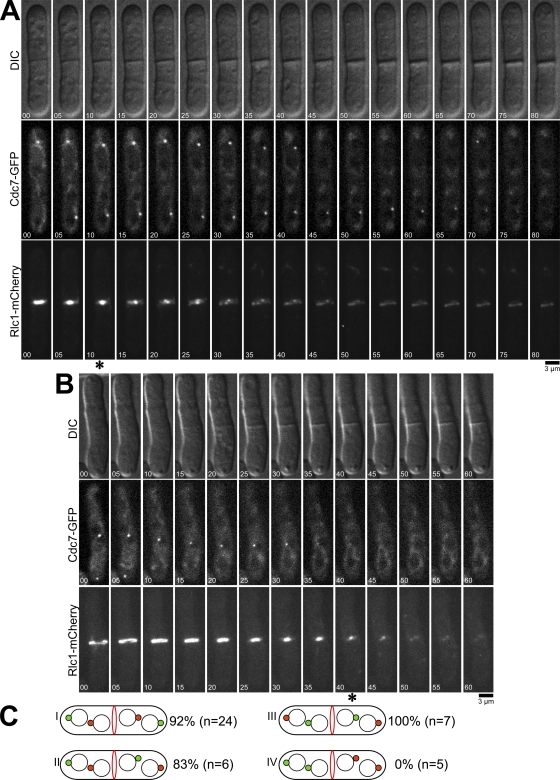
A key question is how SIN signaling is turned off once cytokinesis is complete. As we have shown, the SIN becomes inactivated precisely when the actomyosin ring finishes constriction. Furthermore, we found that the SIN triggers disappearance of Etd1 from the daughter cell with active SIN upon cytoplasmic partitioning at the end of cytokinesis. Loss of Etd1 could then cause inactivation of the SIN and termination of cytokinesis. An unanswered question is why does SIN signaling trigger the disappearance of Etd1 only when cytokinesis is complete? To further explore this issue, we wanted to test whether asymmetric SIN signaling was important for timely inactivation of the SIN. Symmetric SIN signaling can be achieved by inactivating Cdc16, the GAP for Spg1, but this leads to constitutive SIN signaling (Sohrmann et al., 1998). However, a recent study (Okazaki and Niwa, 2008) showed that by blocking one round of cytokinesis, binucleate dikaryon cells could be generated, which continue to propagate as binucleate cells for multiple generations. Interestingly, these dikaryon cells, which are about twice the size of a wild-type cell, enter mitosis and form an actomyosin ring in the cell center, and then each nucleus undergoes spindle elongation, causing each daughter cell to inherit two nuclei. During spindle elongation, Cdc7 localizes to just one SPB on each spindle, resulting mostly with each daughter cell getting one SPB with active SIN, but occasionally both Cdc7-containing SPBs go the same cell. Through this approach, we created cells that had either symmetric or asymmetric SIN signaling and then monitored whether these cells inactivated Spg1 normally when actomyosin ring constriction completed. Dikaryon cells expressing Cdc7-GFP and Rlc1-mCherry were generated by transient inactivation of the cdc11-123 mutation and then analyzed by time-lapse microscopy. As in the earlier study (Okazaki and Niwa, 2008), we observed four different types of dikaryons based on the patterns of Cdc7-GFP localization to the SPBs (Fig. 7 C). The type I, II, and III dikaryons displayed symmetric Cdc7-GFP localization, with one Cdc7-GFP–containing SPB in each future daughter cell. Interestingly, 92% (n = 37) of these cells maintained Cdc7-GFP at one or both SPBs after completion of actomyosin ring constriction and often formed additional rings and septa (Fig. 7 A). In contrast, 100% (n = 5) of the dikaryons that displayed Cdc7-GFP signal at both SPBs in one of the future daughter cells had lost Cdc7-GFP from both SPBs upon completion of actomyosin ring constriction and septum formation, as is observed in wild-type cells (Fig. 7, B and C [type IV]). Consistent with our earlier results in wild-type cells, in type IV cells, GFP-Etd1 localized asymmetrically to the daughter cell without active SIN after completion of cytokinesis (Fig. 8 A), whereas GFP-Etd1 localization remained symmetric after cell division in type I, II, and III cells, which had symmetric SIN signaling (Fig. 8 B and not depicted). Similar to normal haploid cells (Fig. 4 D), moderate overexpression of GFP-Etd1 produced a prolonged activation of the SIN after completion of cytokinesis (Fig. 8, A and B). Overall, these results show that asymmetric SIN signaling is crucial for timely inactivation of SIN signaling and suggest potential models for how termination of SIN signaling could be coupled to completion of cell division (see Discussion).
Figure 7.
Analysis of dikaryons shows that asymmetric SIN signaling is important for SIN inactivation. cdc11-123 cdc7-GFP rlc1-mCherry cells were grown in YE at 25°C, shifted to 36°C for 2.5 h to allow binucleate cells to form, and then returned to 25°C for an additional 2 h and imaged by time-lapse microscopy at 25°C. (A and B) Cells with symmetric (A) or asymmetric (B) SIN activation are shown. Asterisks indicate completion of actomyosin ring contraction. Time is indicated in minutes. (C) Distribution patterns of Cdc7-GFP (green circles) during late anaphase and telophase in cdc11-123 dikaryons. Percentages of cells with a prolonged SIN activity are shown. n indicates the total number of cells of each type imaged.
Figure 8.
Asymmetric SIN signaling is required for Etd1 asymmetry. (A and B) Localization of Etd1 in S. pombe dikaryons with asymmetrically (A) or symmetrically localized Cdc7 (B) is shown. cdc11-123 cdc7-GFP nmt41-GFP-etd1 rlc1-mCherry cells were grown at 25°C and then shifted to 36°C for 2.5 h and finally to 25°C for an additional 2 h. Cdc7-GFP and GFP-Etd1 were then imaged by time-lapse microscopy at 25°C. Time is indicated in minutes.
Discussion
Is Etd1 a functional orthologue of budding yeast Lte1?
The Spg1 and Tem1 small GTPases act at the top of the SIN and MEN pathways, respectively, to regulate cytokinesis and mitotic exit. Both GTPases are negatively regulated by a two-component GAP and formed by Byr4–Cdc16 in fission yeast and Bub2-Byr4/Bfa1 in budding yeast (for reviews see Bardin and Amon, 2001; McCollum and Gould, 2001; Krapp and Simanis, 2008). It has been unclear whether Spg1 is regulated by a GEF. In budding yeast, the putative GEF Lte1 positively regulates Tem1 at the end of mitosis (Shirayama et al., 1994; Bardin et al., 2000; Pereira et al., 2000). However, obvious homologues of Lte1 have not been apparent in the fission yeast genome, and therefore, it was thought that Spg1 might be regulated simply through its GAP. Etd1 was first described as an essential component of the SIN required to connect SIN activity with the actomyosin ring (Daga et al., 2005). Our work suggests that Etd1 is a functional homologue of Lte1. Both Etd1 and Lte1 are cortical proteins that promote activation of their respective GTPases (Shirayama et al., 1994; Bardin et al., 2000; Pereira et al., 2000; Molk et al., 2004; Daga et al., 2005; this study). Etd1 has limited sequence similarity to the Ras GEF CHD of Lte1. Additionally, Etd1 and Spg1 bind to each other in vitro. Similar to lte1Δ mutants, etd1Δ cells show cell division arrest at lower temperatures but are able to grow at 36°C. Overall, these data are consistent with Etd1 being the S. pombe homologue of Lte1.
It is intriguing that Etd1 and Lte1 are not essential at 36°C. Although etd1Δ cells are viable at 36°C, they have a high rate of cytokinesis failure, suggesting that they barely have enough SIN signaling for viability. Thus, the viability of etd1Δ cells at 36°C could be the result of a slight enhancement of the activity of other pathways that regulate the SIN at 36°C. The enhanced expression of spg1+ observed in response to heat shock and other environmental stresses could also increase SIN activity at higher temperatures (Chen et al., 2003). Alternatively, high temperatures could enhance the spontaneous nucleotide exchange rates for Spg1 and Tem1, which both have high intrinsic exchange rates in vitro (Furge et al., 1998; Geymonat et al., 2002). Our preliminary experiments indicate that both wild-type and etd1Δ cells have higher levels of Cdc7 at the SPB at 36°C than at 25°C (unpublished data), suggesting that Spg1 is more active at 36°C.
An important question is whether Lte1 and Etd1 are in fact GEFs for their respective GTPases. Lte1 was originally considered to be a GEF because of its C-terminal Ras GEF CHD, to which Etd1 displays limited similarity. Surprisingly, this domain does not appear to be essential for Lte1 function (Jensen et al., 2002; Yoshida et al., 2003), and Lte1 has not been demonstrated to possess GEF activity in vitro. Similarly, we have been unable to demonstrate an exchange activity for Etd1 in vitro, although this could be because the bacterially expressed proteins are not fully functional or require posttranslational modifications. Furthermore, Spg1 and Etd1 still bind in vitro in the presence of MgCl2 and an excess of GppNHp (a nonhydrolyzable analogue of GTP), which is a behavior not typically observed for most GEFs (unpublished data). Because in vivo experiments show that Lte1 and Etd1 promote activation of their respective GTPases, it is also possible that they promote GTPase activation through an alternative mechanism such as antagonizing a guanine nucleotide dissociation inhibitor (Dirac-Svejstrup et al., 1997).
Activation of the SIN in anaphase
Cytokinesis should not be initiated until anaphase spindle elongation has moved the chromosomes away from the cell division site. Anaphase SIN activation appears to be governed at least in part by loss of Cdk1 activity, which occurs when cyclin B is degraded in anaphase (Sparks et al., 1999; Guertin et al., 2000; Chang et al., 2001; Krapp et al., 2008). In this study, we show that Etd1 also acts in anaphase to promote SIN signaling by increasing Spg1-GTP levels. One explanation for how Etd1 promotes anaphase SIN activation could be that spindle elongation brings SPB-localized Spg1 closer to cortical Etd1 at cell tips. In this way, cells would ensure that the SIN does not become fully activated until chromosomes have been segregated away from the midzone. Consistent with this, we showed that a block in spindle elongation caused a defect in anaphase Spg1 activation similar to that in etd1Δ mutant cells, producing cells in which the contractile actomyosin ring is prematurely disassembled, leading to defective cytokinesis. Importantly, moderate overexpression of Etd1 rescues the defects observed when spindle elongation is blocked. These results can be collectively encapsulated in a model shown in Fig. 9. It is interesting that SIN signaling causes the release of Etd1 from the cell cortex in late anaphase when the SIN becomes active. It is unclear how the release of Etd1 from the cortex affects its function, but if Etd1 interacts with Spg1 in the cytoplasm, release of Etd1 from the cortex could potentially be a mechanism for the SIN to promote its own activation through positive feedback.
Figure 9.
Model for the role of Etd1 in the initiation and termination of SIN signaling. (A) During early anaphase, Spg1-GTP localizes weakly to both SPBs (small green circles), whereas Etd1 is localized to the cell cortex (blue). The cell displays low SIN activity because of inhibition by high Cdk1 activity (nucleus in yellow). (B) Loss of Cdk1 activity (nucleus in purple) in anaphase allows some increase in SIN activity. When the mitotic spindle displays maximal elongation, SPB-localized Spg1 comes into proximity of Etd1 at cell tips and is fully activated (big green circle), and Etd1 is released from the cell cortex to the cytoplasm, which might further enhance Spg1 activation. The return of the GAP complex to the old SPB promotes Spg1 inactivation (red circle). (C) At the end of cytokinesis, the SIN pathway is inactivated. Coincident with the partition of the cytoplasm by the septum (black line), Etd1 disappears from the cell that had the active SIN, which triggers inactivation of Spg1 and the SIN.
Asymmetry and SIN inactivation
The cell must ensure that cytokinesis occurs only once each cell cycle. We showed that SIN signaling is normally inactivated as soon as cells complete actomyosin ring constriction and septum formation, which partitions the cytoplasm between the two daughter cells. This suggests that cells can somehow sense that cytokinesis is complete and inactivate Spg1 to turn off the SIN. Inhibition of Etd1 function may be one mechanism for turning off SIN signaling. Models to explain the timely termination of SIN signaling must explain/incorporate three types of observations revealed in this study: (1) Etd1 stimulates SIN signaling, and yet, paradoxically, SIN signaling in turn appears to inactivate Etd1, (2) SIN activity and the consequent disappearance of Etd1 are asymmetric in the cell, and (3) inactivation of SIN signaling is coincident with the partitioning of cellular cytoplasm into two compartments.
Etd1 activates SIN signaling, which then promotes asymmetric disappearance of Etd1 after cytokinesis. This situation, in which the activity of one cell cycle component triggers its own eventual inactivation, is analogous to that of Cdk1–cyclin B and the anaphase-promoting complex (APC), where Cdk1 activity is required for the APC to become active (Félix et al., 1990; Hershko et al., 1994; Minshull et al., 1994; Shteinberg and Hershko, 1999) and then the APC inactivates Cdk1 by degrading cyclin B. In both cases, there are additional events that must take place before the second step inactivates the first. For example, the chromosomes must be properly attached to the mitotic spindle before the APC can become active, and for the SIN pathway, Etd1 is only lost from the cell compartment with the active SIN after septum formation is complete.
It is unclear how completion of septum formation is monitored and promotes the disappearance of Etd1. Our results show that asymmetric SIN signaling is important for timely inactivation of the SIN. Therefore, it seems likely that completion of cytokinesis is monitored indirectly by a mechanism that uses the asymmetry in SIN signaling to sense partitioning of the cytoplasm. One explanation for these results could be that during cytokinesis, SIN signaling promotes degradation of Etd1, but this degradation is limited because too much Etd1 degradation would reduce SIN activity, resulting in an equilibrium state during cytokinesis. At this stage, Etd1 is present throughout the cell, and the SIN is active at just one SPB. When cytokinesis partitions the cell, the ratio of SIN signaling to Etd1 increases twofold in the cell with active SIN, which could conceivably tip the balance toward Etd1 degradation and eventual loss of SIN signaling. Although the relative change in Etd1 and SIN activities is only twofold, there are a wide variety of ways that the system could work, including positive feedback (Ferrell, 2008), which would make it sensitive to twofold changes. Our data using dikaryon cells are consistent with this type of model. It is important to note that the dikaryon cells were generated by blocking cytokinesis and then allowing them to proceed through another growth cycle as binucleates before they entered the next round of mitosis. This results in the cells being twice the size of a normal wild-type cell when they attempt cytokinesis (unpublished data). Thus, during cytokinesis, these cells should have the same ratio of SIN activity to Etd1 as in wild-type cells because although SIN signaling comes from two SPBs, these cells have twice as much Etd1 as the result of being twice the size of normal wild-type cells. In the situation in which each cell inherits an SPB with active SIN, upon completion of cytokinesis, nothing changes about the ratio of SIN activity to Etd1 in each daughter cell because although each cell has half as much Etd1, they have half as much SIN signaling. Therefore, SIN signaling is maintained after cell division because cytoplasmic partitioning does not alter the SIN to Etd1 ratio. In contrast, when one daughter cell inherits both SPBs with active SIN, the ratio of SIN to Etd1 doubles as in wild-type cells, which could then tip the balance toward Etd1 degradation and SIN inactivation. In the future, a combination of molecular modeling and quantitative in vivo measurements will be necessary to work out the exact mechanism for terminating SIN signaling. In conclusion, this work provides an example of how asymmetric localization of cellular factors may be important for cell division even in symmetrically dividing cells, and it raises the possibility that mechanisms for asymmetric cell division in stem cells in metazoans may have their origins in systems for regulating cell division in simple unicellular ancestors.
Materials and methods
Strains and culture conditions
Fission yeast strains used in this study are listed in Table S1. Strains not created in this study were provided by J. Jimenez (Centro Andaluz de Biología del Desarrollo, Sevilla, Spain) and M. Balasubramanian (The National University of Singapore, Singapore, Republic of Singapore). Genetic crosses and general yeast techniques were performed as described previously (Moreno et al., 1991). S. pombe strains were grown in rich medium (yeast extract [YE]) or Edinburgh minimal medium (EMM) with appropriate supplements (Moreno et al., 1991). EMM with 5 µg/ml thiamine or YE was used to repress expression from the nmt1 promoter. For expression of GFP-Etd1, cells were grown for 2 d in EMM solid without thiamine at 25°C and then grown in EMM liquid without thiamine for an additional 20 h. YE containing 100 mg Geneticin (G-418; Sigma-Aldrich) per liter was used for selecting KanR cells. For serial dilution drop tests for growth, four serial 10-fold dilutions were made, and 5 µl of each dilution was spotted on plates with the starting cell number of 104. Cells were grown in liquid YE or EMM at 25°C and then spotted onto YE or EMM plates at the indicated temperatures and incubated for 3–5 d before photography. All strains with an etd1Δ genotype were built by genetic crosses with the strain DM3547. After tetrad dissection, the YE plates containing single spores were incubated at 36°C, and the final ura+ clones were screened for the etd1Δ phenotype at 25°C and for the absence or presence of the plasmid-containing etd1+ gene through the leucine marker. GFP fluorescence or G-418 resistance was used to screen for other markers in the strains of interest. For treatment with MBC (Sigma-Aldrich), log-phase cells were treated for 10 min with MBC used at the final concentration of 25 µg/ml from a 10 mg/ml stock and then centrifuged in a microfuge (model 5415C; Eppendorf) to concentrate the cells and imaged in the presence of 25 µg/ml MBC.
Preparation of dikaryons using the cdc11-123 mutant
S. pombe dikaryon cells were obtained as previously described (Okazaki and Niwa, 2008). In brief, early log-phase cdc11-123 cells were shifted to 36°C for 2.5 h. The formation of binucleate cells was checked before returning to permissive temperature at 25°C. The cells were returned to 25°C for 2 h. The binucleate cells were then imaged by time-lapse microscopy at 25°C (see Microscopy).
Epitope tagging and plasmids
For fission yeast expression, the etd1+ gene was amplified by PCR from wild-type fission yeast genomic DNA and cloned between the Xho1 and Sma1 sites of the pREP3X and pREP41X vectors (Forsburg, 1993). The pREP41-EGFP-Etd1-Ppc89261-–783 plasmid was created by a three-piece ligation. An Nde1–Xho1 fragment containing the etd1+ gene and an Xho1–Sma1 fragment containing the last 1,566 nucleotides of ppc89+ were generated by PCR and cloned between the Nde1 and Sma1 sites of the pREP41-EGFP vector (Craven et al., 1998). For bacterial expression, the etd1+ gene was amplified by PCR from a cDNA library (Two-hybrid; Clontech Laboratories, Inc.) and cloned between the EcoR1 and Pst1 sites of the MBP tag vector pMAL-c2X (New England Biolabs, Inc.). All resulting plasmids were sequenced to confirm the absence of nucleotide changes. K. Gould (Vanderbilt University, Nashville, TN) provided plasmids for overexpression of Spg1.
Immunoblot analysis
Cell pellets were obtained and processed as described previously (Matsuo et al., 2006). In brief, 108 (5 OD units at 595 nm) exponentially growing cells were collected and lysed by incubation with 0.6 M NaOH for 10 min. Next, the cells were collected, and the supernatant was removed. 70 µl of SDS sample buffer (60 mM Tris-HCl, pH 6.8, 4% β-mercaptoethanol, 4% SDS, 0.01% bromophenol blue, and 5% glycerol) was added to the pellet, and samples were boiled for 3 min. Proteins were subjected to 10% SDS-PAGE, blotted onto Immobilon-P (Millipore), and probed with monoclonal anti-GFP (1:250 dilution; Invitrogen) or anti–α-tubulin antibody TAT1 (1:500 dilution; a gift from K. Gull, University of Oxford, Oxford, England, UK).
Binding assay
Escherichia coli containing expression plasmids for MBP, MBP-Etd1, GST, and GST-Spg1 was induced and lysed in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 100 µg/ml PMSF, 1% NP-40, and 20% glycerol). For binding assays, the lysates were mixed and incubated at 4°C for 2 h, followed by the addition of glutathione beads and a further 1 h incubation at 4°C. Next, glutathione beads with immobilized GST or GST-Spg1 were washed three times with lysis buffer and analyzed by Western blotting with anti-MBP antibodies (New England Biolabs, Inc.).
Microscopy
GFP fusion proteins were observed in cells after fixation with −20°C methanol or in live cells. DNA and cell wall material were visualized with DAPI (Sigma-Aldrich) at 2 µg/ml and calcofluor white (CW; Sigma-Aldrich) at 50 µg/ml, respectively. Images were captured using a microscope (Eclipse E600; Nikon) with a digital camera (ORCA-ER; Hamamatsu Photonics) and IPLab Spectrum software (Signal Analytics). For time-lapse experiments, exponentially growing cells were concentrated, and 1.8 µl of the cell suspension was placed on a microscope slide with a 2% YE agar slab. Alternatively, 1.5 ml of log-phase cells was concentrated by centrifugation and suspended in 100 µl of medium (YE or EMM without thiamine for cells expressing GFP-Etd1), and 30 µl of the cell suspension was mixed with 30 µl of 1 mg/ml soybean lectin (L1395; Sigma-Aldrich), placed in 35-mm glass-bottomed culture dishes (P35-1.5-10-C; MatTek), and immersed in 3 ml of medium (YE or EMM without thiamine for cells expressing GFP-Etd1) for time-lapse imaging. Time-lapse experiments were made at 25°C by acquiring epifluorescence images in z planes and 2 × 2 binning. Cells were then viewed on a microscope (Axiovert 200; Carl Zeiss, Inc.) with an argon ion laser system (Melles Griot). Images were captured using an IEEE 1394 digital charge-coupled device camera (C4742-80-12AG; Hamamatsu Photonics) and UltraVIEW RS confocal imaging system software (PerkinElmer). Fluorescence intensity measurements for SPB-localized Cdc7-GFP were made using IPLab software (BD) by placing a circle around SPB-localized Cdc7-GFP and measuring the maximal fluorescence. A nearby cytosolic region was used as background. GFP-Etd1 fluorescence was measured using ImageJ software (National Institutes of Health) by drawing the outline of the two daughter cells, excluding the medial region, and measuring the mean of total fluorescence. A nearby region outside of the cell was used as background.
Online supplemental material
Fig. S1 shows a sequence alignment comparing Etd1 with Lte1 family members and the phenotype of etd1Δ cells. Fig. S2 shows epistasis analysis supporting the conclusion that Etd1 functions upstream of Spg1. Fig. S3 shows additional cells from time-lapse experiments of actomyosin ring dynamics in the absence of microtubules. Fig. S4 shows that moderate overexpression of Etd1 causes prolonged SIN activation and repeated rounds of cytokinesis. Table S1 lists strains used in this study. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200902116/DC1.
Acknowledgments
We are grateful to Drs. Kathy Gould, Mohan Balasubramanian, Juan Jimenez, and Rafael R. Daga for providing strains and plasmids, to Kirsten Hagstrom for use of her microscope, and Peter Pryciak for comments on the manuscript.
This work was supported by National Institutes of Health grant GM058406 to D. McCollum, and J.C. García-Cortés was supported by a postdoctoral fellowship from the Ministerio de Educación y Ciencia, Spain.
Footnotes
Abbreviations used in this paper:
- APC
- anaphase-promoting complex
- CHD
- Cdc25 homology domain
- CW
- calcofluor white
- DIC
- differential interference contrast
- EMM
- Edinburgh minimal medium
- GAP
- GTPase-activating protein
- GEF
- guanine nucleotide exchange factor
- MBC
- methyl-2-benzimidazole-carbamate
- MBP
- maltose-binding protein
- MEN
- mitotic exit network
- SIN
- septum initiation network
- SPB
- spindle pole body
- YE
- yeast extract
References
- Balasubramanian M.K., McCollum D., Chang L., Wong K.C., Naqvi N.I., He X., Sazer S., Gould K.L. 1998. Isolation and characterization of new fission yeast cytokinesis mutants.Genetics. 149:1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M.K., Bi E., Glotzer M. 2004. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells.Curr. Biol. 14:R806–R818 doi:10.1016/j.cub.2004.09.022 [DOI] [PubMed] [Google Scholar]
- Bardin A.J., Amon A. 2001. Men and sin: what’s the difference? Nat. Rev. Mol. Cell Biol. 2:815–826 doi:10.1038/35099020 [DOI] [PubMed] [Google Scholar]
- Bardin A.J., Visintin R., Amon A. 2000. A mechanism for coupling exit from mitosis to partitioning of the nucleus.Cell. 102:21–31 doi:10.1016/S0092-8674(00)00007-6 [DOI] [PubMed] [Google Scholar]
- Cerutti L., Simanis V. 1999. Asymmetry of the spindle pole bodies and spg1p GAP segregation during mitosis in fission yeast.J. Cell Sci. 112:2313–2321 [DOI] [PubMed] [Google Scholar]
- Chang L., Morrell J.L., Feoktistova A., Gould K.L. 2001. Study of cyclin proteolysis in anaphase-promoting complex (APC) mutant cells reveals the requirement for APC function in the final steps of the fission yeast septation initiation network.Mol. Cell. Biol. 21:6681–6694 doi:10.1128/MCB.21.19.6681-6694.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Toone W.M., Mata J., Lyne R., Burns G., Kivinen K., Brazma A., Jones N., Bähler J. 2003. Global transcriptional responses of fission yeast to environmental stress.Mol. Biol. Cell. 14:214–229 doi:10.1091/mbc.E02-08-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. 1988. Multiple sequence alignment with hierarchical clustering.Nucleic Acids Res. 16:10881–10890 doi:10.1093/nar/16.22.10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R.A., Griffiths D.J., Sheldrick K.S., Randall R.E., Hagan I.M., Carr A.M. 1998. Vectors for the expression of tagged proteins in Schizosaccharomyces pombe.Gene. 221:59–68 doi:10.1016/S0378-1119(98)00434-X [DOI] [PubMed] [Google Scholar]
- Daga R.R., Lahoz A., Muñoz M.J., Moreno S., Jiménez J. 2005. Etd1p is a novel protein that links the SIN cascade with cytokinesis.EMBO J. 24:2436–2446 doi:10.1038/sj.emboj.7600705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirac-Svejstrup A.B., Sumizawa T., Pfeffer S.R. 1997. Identification of a GDI displacement factor that releases endosomal Rab GTPases from Rab-GDI.EMBO J. 16:465–472 doi:10.1093/emboj/16.3.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S., McCollum D., Theurkauf W. 2005. Centrosomes in cellular regulation.Annu. Rev. Cell Dev. Biol. 21:411–434 doi:10.1146/annurev.cellbio.21.122303.120418 [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Reymond A., Cerutti L., Utzig S., Hofmann K., Simanis V. 1995. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis.Cell. 82:435–444 doi:10.1016/0092-8674(95)90432-8 [DOI] [PubMed] [Google Scholar]
- Félix M.A., Labbé J.C., Dorée M., Hunt T., Karsenti E. 1990. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase.Nature. 346:379–382 doi:10.1038/346379a0 [DOI] [PubMed] [Google Scholar]
- Ferrell J.E., Jr. 2008. Feedback regulation of opposing enzymes generates robust, all-or-none bistable responses.Curr. Biol. 18:R244–R245 doi:10.1016/j.cub.2008.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S.L. 1993. Comparison of Schizosaccharomyces pombe expression systems.Nucleic Acids Res. 21:2955–2956 doi:10.1093/nar/21.12.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini R., Venturetti M., Chiroli E., Piatti S. 2008. The spindle position checkpoint: how to deal with spindle misalignment during asymmetric cell division in budding yeast.Biochem. Soc. Trans. 36:416–420 doi:10.1042/BST0360416 [DOI] [PubMed] [Google Scholar]
- Furge K.A., Wong K., Armstrong J., Balasubramanian M., Albright C.F. 1998. Byr4 and Cdc16 form a two-component GTPase-activating protein for the Spg1 GTPase that controls septation in fission yeast.Curr. Biol. 8:947–954 doi:10.1016/S0960-9822(98)70394-X [DOI] [PubMed] [Google Scholar]
- Geymonat M., Spanos A., Smith S.J., Wheatley E., Rittinger K., Johnston L.H., Sedgwick S.G. 2002. Control of mitotic exit in budding yeast. In vitro regulation of Tem1 GTPase by Bub2 and Bfa1.J. Biol. Chem. 277:28439–28445 doi:10.1074/jbc.M202540200 [DOI] [PubMed] [Google Scholar]
- Grallert A., Krapp A., Bagley S., Simanis V., Hagan I.M. 2004. Recruitment of NIMA kinase shows that maturation of the S. pombe spindle-pole body occurs over consecutive cell cycles and reveals a role for NIMA in modulating SIN activity.Genes Dev. 18:1007–1021 doi:10.1101/gad.296204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin D.A., Chang L., Irshad F., Gould K.L., McCollum D. 2000. The role of the sid1p kinase and cdc14p in regulating the onset of cytokinesis in fission yeast.EMBO J. 19:1803–1815 doi:10.1093/emboj/19.8.1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachet O., Simanis V. 2008. Mid1p/anillin and the septation initiation network orchestrate contractile ring assembly for cytokinesis.Genes Dev. 22:3205–3216 doi:10.1101/gad.1697208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Patterson T.E., Sazer S. 1997. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex.Proc. Natl. Acad. Sci. USA. 94:7965–7970 doi:10.1073/pnas.94.15.7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ganoth D., Sudakin V., Dahan A., Cohen L.H., Luca F.C., Ruderman J.V., Eytan E. 1994. Components of a system that ligates cyclin to ubiquitin and their regulation by the protein kinase cdc2.J. Biol. Chem. 269:4940–4946 [PubMed] [Google Scholar]
- Jensen S., Geymonat M., Johnson A.L., Segal M., Johnston L.H. 2002. Spatial regulation of the guanine nucleotide exchange factor Lte1 in Saccharomyces cerevisiae.J. Cell Sci. 115:4977–4991 doi:10.1242/jcs.00189 [DOI] [PubMed] [Google Scholar]
- Krapp A., Simanis V. 2008. An overview of the fission yeast septation initiation network (SIN).Biochem. Soc. Trans. 36:411–415 doi:10.1042/BST0360411 [DOI] [PubMed] [Google Scholar]
- Krapp A., Collin P., Cano Del Rosario E., Simanis V. 2008. Homoeostasis between the GTPase Spg1p and its GAP in the regulation of cytokinesis in S. pombe.J. Cell Sci. 121:601–608 doi:10.1242/jcs.022772 [DOI] [PubMed] [Google Scholar]
- Li C., Furge K.A., Cheng Q.C., Albright C.F. 2000. Byr4 localizes to spindle-pole bodies in a cell cycle-regulated manner to control Cdc7 localization and septation in fission yeast.J. Biol. Chem. 275:14381–14387 doi:10.1074/jbc.275.19.14381 [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Asakawa K., Toda T., Katayama S. 2006. A rapid method for protein extraction from fission yeast.Biosci. Biotechnol. Biochem. 70:1992–1994 doi:10.1271/bbb.60087 [DOI] [PubMed] [Google Scholar]
- McCollum D., Gould K.L. 2001. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN.Trends Cell Biol. 11:89–95 doi:10.1016/S0962-8924(00)01901-2 [DOI] [PubMed] [Google Scholar]
- Minet M., Nurse P., Thuriaux P., Mitchison J.M. 1979. Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe.J. Bacteriol. 137:440–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Sun H., Tonks N.K., Murray A.W. 1994. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts.Cell. 79:475–486 doi:10.1016/0092-8674(94)90256-9 [DOI] [PubMed] [Google Scholar]
- Mishra M., Karagiannis J., Sevugan M., Singh P., Balasubramanian M.K. 2005. The 14-3-3 protein rad24p modulates function of the cdc14p family phosphatase clp1p/flp1p in fission yeast.Curr. Biol. 15:1376–1383 doi:10.1016/j.cub.2005.06.070 [DOI] [PubMed] [Google Scholar]
- Molk J.N., Schuyler S.C., Liu J.Y., Evans J.G., Salmon E.D., Pellman D., Bloom K. 2004. The differential roles of budding yeast Tem1p, Cdc15p, and Bub2p protein dynamics in mitotic exit.Mol. Biol. Cell. 15:1519–1532 doi:10.1091/mbc.E03-09-0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe.Methods Enzymol. 194:795–823 doi:10.1016/0076-6879(91)94059-L [DOI] [PubMed] [Google Scholar]
- Okazaki K., Niwa O. 2008. Dikaryotic cell division of the fission yeast Schizosaccharomyces pombe.Biosci. Biotechnol. Biochem. 72:1531–1538 doi:10.1271/bbb.80035 [DOI] [PubMed] [Google Scholar]
- Pereira G., Höfken T., Grindlay J., Manson C., Schiebel E. 2000. The Bub2p spindle checkpoint links nuclear migration with mitotic exit.Mol. Cell. 6:1–10 doi:10.1016/S1097-2765(00)00002-2 [PubMed] [Google Scholar]
- Rosenberg J.A., Tomlin G.C., McDonald W.H., Snydsman B.E., Muller E.G., Yates J.R., III, Gould K.L. 2006. Ppc89 links multiple proteins, including the septation initiation network, to the core of the fission yeast spindle-pole body.Mol. Biol. Cell. 17:3793–3805 doi:10.1091/mbc.E06-01-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Sohrmann M., Hofmann K., Woollard A., Simanis V. 1997. The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe.Genes Dev. 11:1519–1534 doi:10.1101/gad.11.12.1519 [DOI] [PubMed] [Google Scholar]
- Seshan A., Amon A. 2004. Linked for life: temporal and spatial coordination of late mitotic events.Curr. Opin. Cell Biol. 16:41–48 doi:10.1016/j.ceb.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Shirayama M., Matsui Y., Tanaka K., Toh-e A. 1994. Isolation of a CDC25 family gene, MSI2/LTE1, as a multicopy suppressor of ira1.Yeast. 10:451–461 doi:10.1002/yea.320100404 [DOI] [PubMed] [Google Scholar]
- Shteinberg M., Hershko A. 1999. Role of Suc1 in the activation of the cyclosome by protein kinase Cdk1/cyclin B.Biochem. Biophys. Res. Commun. 257:12–18 doi:10.1006/bbrc.1999.0409 [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Schmidt S., Hagan I., Simanis V. 1998. Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p.Genes Dev. 12:84–94 doi:10.1101/gad.12.1.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks C.A., Morphew M., McCollum D. 1999. Sid2p, a spindle pole body kinase that regulates the onset of cytokinesis.J. Cell Biol. 146:777–790 doi:10.1083/jcb.146.4.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Ichihashi R., Toh-e A. 2003. Ras recruits mitotic exit regulator Lte1 to the bud cortex in budding yeast.J. Cell Biol. 161:889–897 doi:10.1083/jcb.200301128 [DOI] [PMC free article] [PubMed] [Google Scholar]