Abstract
The comparative characterization of a series of 4-acyl-1,6-dialkylpiperazin-2-ones as potent cell entry inhibitors of the hemorrhagic fever arenavirus Lassa (LASV) is disclosed. The resolution and examination of the individual enantiomers of the prototypical LASV cell entry inhibitor 3 (16G8) is reported and the more potent (–)-enantiomer was found to be 15-fold more active than the corresponding (+)-enantiomer. The absolute configuration of (–)-3 was established by asymmetric synthesis of the active inhibitor (–)-(S)-3 (lassamycin-1). A limited deletion scan of lassamycin-1 defined key structural features required of the prototypical inhibitors.
A serious threat to public health in areas of Africa and South America is a group of arenaviruses that cause severe viral hemorrhagic fevers in humans with rates of fatality among hospitalized patients in the range of 15–35%.1 These arenaviruses, which include the Old World Lassa virus2.3 (LASV) and the New World arenaviruses Junin2 (JUNV), Guanarito4 (GTOV), and Machupo4 (MACV), have been classified as Category A pathogens by the Centers for Disease Control (CDC) due to the extremely high mortality rates and because there currently exists limited therapeutic options to combat these human pathogens.5 The need for effective treatments against these arenaviruses is substantial not only in the viral endemic regions, but also worldwide as international air traffic has facilitated the spread of arenaviral hemorrhagic fever cases into metropolitan areas.6,7 In order to facilitate the discovery of small molecule therapeutics to treat these hemorrhagic fevers, a high-throughput screen utilizing pseudotyped virion particles bearing the glycoproteins (GPs) of these arenaviruses was developed and utilized to screen a series of synthetic combinatorial libraries for inhibitors of cellular infection.8 Emerging from these screening efforts was a series of 4-acyl-1,6-dialkylpiperazin-2-ones9 that were found to be the first potent and selective inhibitors of hemorrhagic arenavirus cellular entry (Figure 1A).8 These small molecules proved to be potent viral entry inhibitors against both Old World and New World hemorrhagic arenaviruses not only using pseudotyped virion particles, but they also effectively inhibited infection of human and primate cells with live hemorrhagic fever viruses with IC50 values of 500–800 nM (conducted by CDC).8 Characterization of their mechanism of inhibition revealed that compounds 1–3 selectively block the late endosomal pH-dependent fusion mediated by the arenavirus GPs, exhibiting IC50 values of 200–350 nM in a GP-mediated cell-cell fusion assay and defining a new stage at which to inhibit viral entry (Figure 1B).8
Figure 1.
A: Lead compounds. B: Mechanism of inhibition of hemorrhagic arenaviruses by compounds 1–3. The compounds inhibit infection by blocking late endosomal pH-dependent membrane fusion. RNP = ribonucleoparticle.
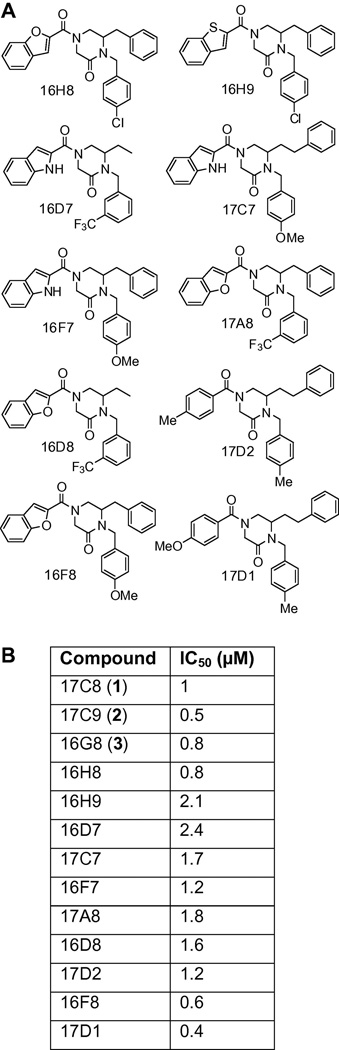
Compounds 1–3 emerged from the initial screen of our library10–13 (ca. 95,000 compounds) as remarkably potent and robust inhibitors already possessing drug-like characteristics. This trisubstituted piperazinone scaffold is clearly well-suited in the spatial and configurational display of the aryl substituents necessary for interaction with the protein target which mediates the inhibitory activity of the compounds. Evidence for this can be found in the well-defined structure-activity relationship (SAR) exhibited by the 150-membered piperazinone library from which the lead compounds emerged. Analysis of the trends indicated that key elements important for activity included the closely related 2-indole, 2-benzofuran, or 2-benzothiophene aryl substituent attached to N4 of the piperazinone core. These bicyclic aryl substituents proved more potent than the corresponding 2-pyrrole, 2-furan, 2-thiophene, or phenyl aryl substituents defining a clear pattern of activity. Similarly, although not as widely explored, the activity was observed most often or significantly with a phenethyl C6 substituent (PhCH2CH2 > PhCH2 > Et) and the nature of the aryl substitution at N1 significantly modulated this activity. Important in this work was the number of active inhibitors that arose from the piperazinone sublibrary during the primary screening. Only compounds 1–3 were further characterized in the initial studies that subsequently focused on defining the scope and selectivity of their activity and the underlying mechanism.8 However, ten additional compounds were identified that displayed significant activity comparable to compounds 1–3 in the LASV pseudotype assay and they have now been characterized (IC50 determination, Figure 2). The evaluation of these additional structures reveals that a consistently potent combination of aryl substituents is C6 benzyl combined with the benzofuran at N4 and a para-substituted (Me = Cl ≈ OMe) benzyl group on N1 (see 16G8 (3), 16H8, and 16F8). 3-Trifluoromethyl substitution on the N1 benzyl ring or replacement of the benzofuran with a benzothiophene (16H8 vs. 16H9) or indole (16F7 vs. 16F8) all proved slightly less active. Another potent combination contains the C6 phenethyl substituent and a para-substituted N1 benzyl substituent. Unlike the C6 benzyl series, however, there is substantial flexibility at the N4 position for this series, with the benzothiophene, benzofuran, indole, p-methoxyphenyl and tolyl groups all leading to active compounds. Direct comparison of these N4 groups reveals that benzothiophene, benzofuran, and indole exhibit very comparable activity (compare 17C9 (2), 17C8 (1), and 17C7) and p-methoxyphenyl > tolyl (compare 17D1 and 17D2). Several of these additionally characterized compounds proved to be equally (16H8) or even more potent (16F8, 17D1) than the original lead compounds in Figure 1A. These additional inhibitors of LASV GP-mediated cellular infection represent exciting additional lead structures available to pursue as we move forward in optimization of these compounds for in vivo examination.
Figure 2.
A: Additional LASV entry inhibitors. B: Inhibition of LASV pseudotype infection of permissive human A549 lung epithelial cells, see ref. 8.
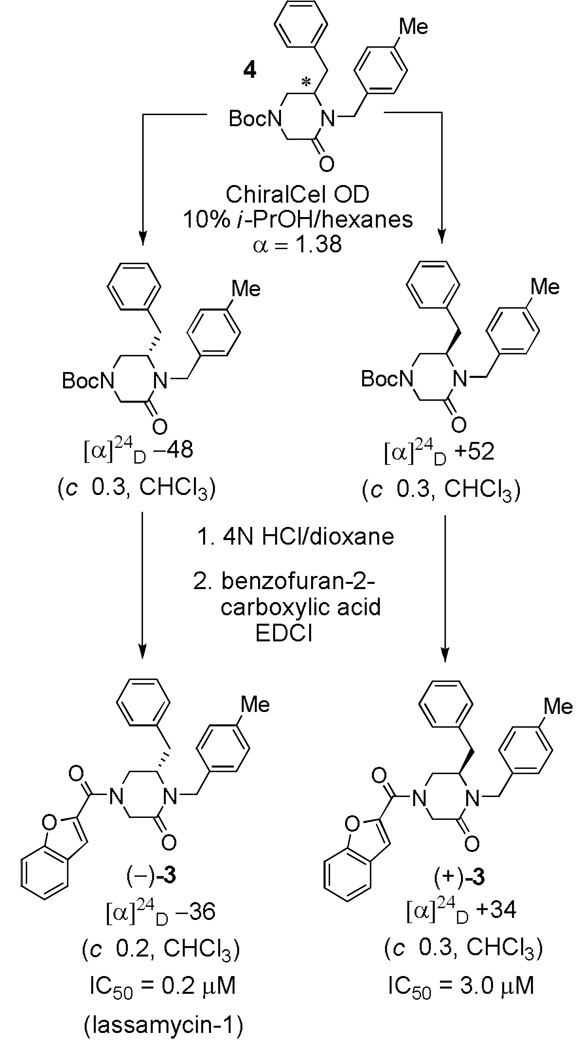
Among the first and most important questions yet to be addressed in the examination of this initial set of hemorrhagic arenavirus entry inhibitors was the activities of the individual enantiomers of the compounds. To address this question and after unsuccessful efforts to resolve 1–3 directly, we found that the penultimate Boc-protected precursor to 3, but not those leading to 1–2, could be resolved into its enantiomers using chiral HPLC (Scheme 1). N-Boc deprotection of the individual enantiomers of 4 (4N HCl/dioxane) and subsequent acylation of the free amine with benzofuran-2-carboxylic acid (EDCI, HOAt, 2,6-lutidine, DMF) gave (+)-3 and (–)-3 (Scheme 1). Examination of the two enantiomers of 3 in the LASV pseudotype assay revealed a considerable difference (15-fold) in their inhibitory activity, with (–)-3 being slightly more potent than the racemic mixture (±)-3.
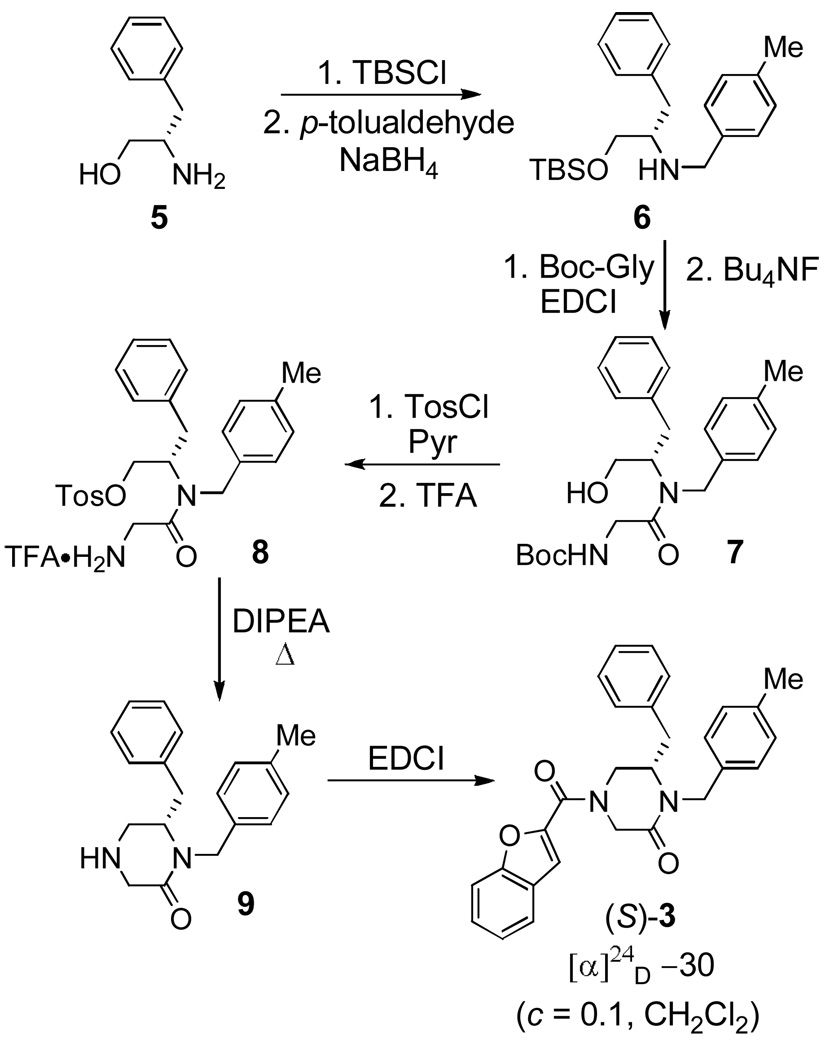
Scheme 1.
Synthesis and characterization of both enantiomers of 16G8, (–)-3 (lassamycin-1) and (+)-3.
Having established that there is a substantial difference in the activity of the enantiomers of 3, we undertook the assignment of the absolute configuration of the active (–)-3 by synthesis of (S)-3 and subsequent comparison. An attractive approach to (S)-3 that could be generalized to the preparation of subsequent lead optimization libraries is based on the use of L-phenylalaninol as the starting material for 3 (Scheme 2). The synthesis was accomplished starting with silylation of L-phenylalaninol (5) using TBSCl (imidazole, CH2Cl2) to give the O-silyl ether followed by a stepwise reductive amination14 with p-tolualdehyde entailing pre-formation of the imine and subsequent NaBH4 reduction (MeOH) to give intermediate 6. Compound 6 was then coupled to Boc-glycine to generate the tertiary amide (EDCI, HOAt, 2,6-lutidine, DMF) and was followed by TBS removal effected by treatment with Bu4NF (THF) to give 7. Formation of the tosylate of 7 was accomplished upon treatment of 7 with tosyl chloride (TosCl) and pyridine (CH2Cl2) and subsequent Boc deprotection with TFA (1:1 TFA/CH2Cl2) gave the cyclization precursor 8. Warming 8 at 50 °C in DMF in the presence of diisopropylethylamine (DIPEA) resulted in cyclization to the piperazinone 9, which was then coupled to the benzofuran-2-carboxylic acid (EDCI, HOAt, 2,6-lutidine, DMF) to furnish (S)-3. The optical rotation of (S)-3 was measured ([α]24D –30 (c 0.1, CH2Cl2), >98% ee) and found to match that of the active inhibitor (–)-3 to which we can now assign its absolute configuration as (S).
Scheme 2.
Synthesis of (S)-3 (lassamycin-1).
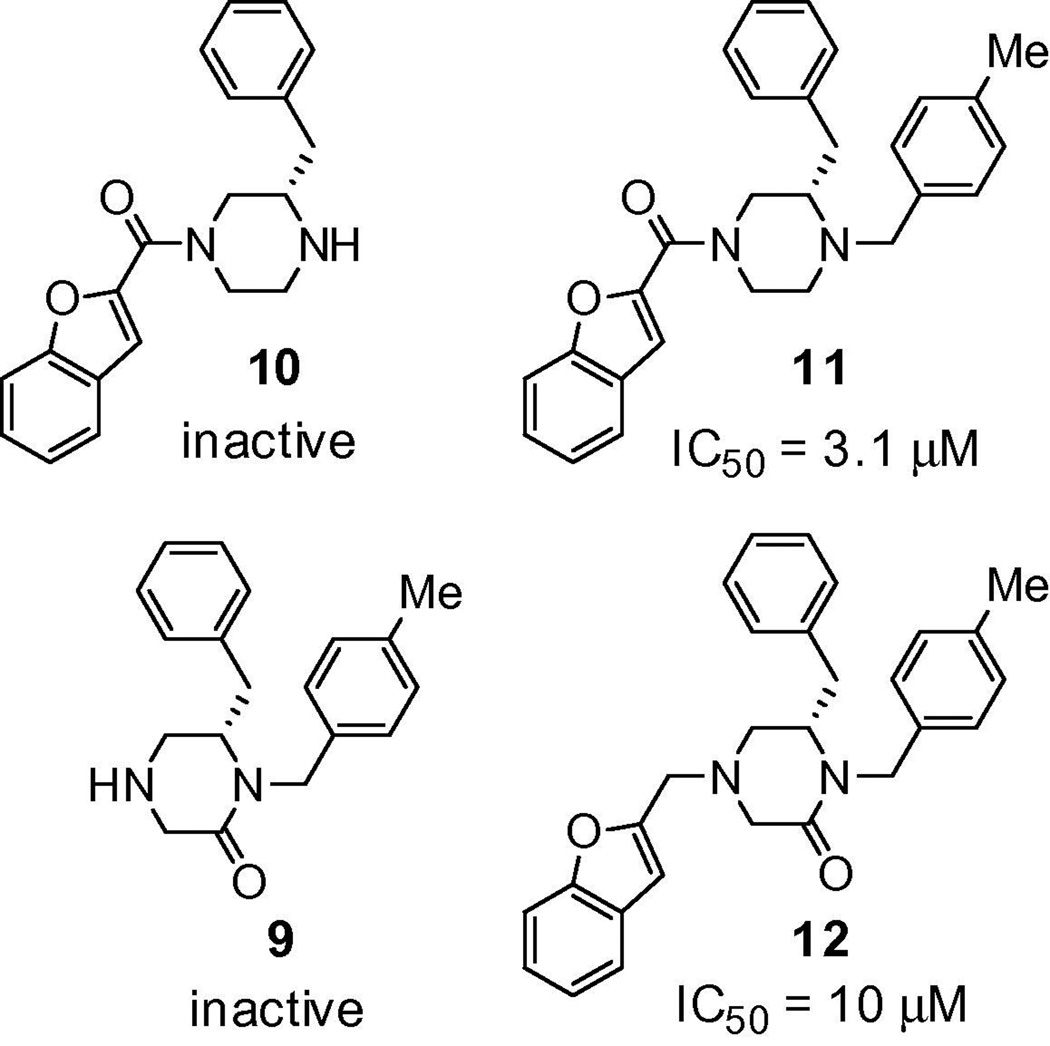
We also conducted a modest deletion scan of (S)-3 that would enable us to confirm the importance of the aryl substituents and define that of the carbonyl groups attached to the N1 and N4 centers (Figure 3). We synthesized two partial structures in which the C2 carbonyl oxygen (11) and N1 aryl substituent (10) were successively removed and the two partial structures in which the N4-substituent carbonyl oxygen (12) and the N4 substituent (9) were successively removed.15 Evaluation of these partial structures in the LASV pseudotype assay revealed that both carbonyl to methylene modifications resulted in a substantial loss of activity, with 11 showing a 15-fold loss of activity compared to (S)-3 (IC50 = 200 nM) while compound 12 showed a 50-fold loss, demonstrating that the carbonyl oxygens are key elements in the potent inhibitory activity of (S)-3. The removal of the aryl substituents at either N1 (10) or N4 (9) gave completely inactive compounds and further underscored the critical nature of each of the aryl subunits towards activity.
Figure 3.
Partial structures of (S)-3 (lassamycin-1).
Ten additional trisubstituted piperazinones were characterized as inhibitors of LASV GP-mediated cellular infection representing the first set of small, drug-like molecules with potent and robust activity that are available for further investigation and optimization against hemorrhagic arenavirus infections. Resolution of the enantiomers of the initially reported inhibitor 3 revealed that (–)-3 (IC50 = 200 nM) is 15-fold more potent than (+)-3 and slightly more potent than (±)-3. Development of an enantioselective route to (S)-3 and comparison of its optical rotation with that of the active enantiomer allowed the assignment of the absolute configuration of (–)-3 as (S). In the process, an effective synthetic route was developed to obtain the optically active trisubstituted piperazinones starting from an enantiopure amino alcohol (such as phenylalaninol), which can be obtained from readily available optically active α-amino acids. This route can now be utilized to access a variety of optically active 4-acyl-1,6-dialkylpiperazin-2-ones bearing a natural or unnatural amino acid side chain as the substituent on the C6 chiral center. We also conducted a limited deletion scan of (S)-3 probing the importance of the two carbonyl groups in the molecule as well as the N1 and N4 aryl substituents. Results of this scan revealed that replacement of either carbonyl in the molecule with a methylene group resulted in a significant loss in activity (15-fold and 50-fold) and that further removal of either the N1 or N4 aryl substituents completely abolished activity.
Acknowledgements
We gratefully acknowledge the financial support of the National Institutes of Health (CA78045 to D.L.B.; AI55540 to M.B.A.O.). Landon R. Whitby is a Skaggs Fellow.
References and Notes
- 1.Geisbert TW, Jahrling PB. Nat. Med. 2004;10:S110. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 2.McCormick JB, Fisher-Hoch SP. Curr. Top. Microbiol. Immunol. 2002;262:75. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 3.Weissenbacher MC, Laguens RP, Coto CE. Curr. Top. Microbiol. Immunol. 1987;134:79. doi: 10.1007/978-3-642-71726-0_4. [DOI] [PubMed] [Google Scholar]
- 4.Peters CJ. Curr. Top. Microbiol. Immunol. 2002;262:65. doi: 10.1007/978-3-642-56029-3_3. [DOI] [PubMed] [Google Scholar]
- 5.Borio L, Inglesby T, Peters CJ, Schmaljon AI, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O’Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM, Jr, Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K. JAMA. 2002;287:2391. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz H, Kohler B, Laue T, Drosten C, Veldkamp PJ, Gunther S, Emmerich P, Geisen HP, Fleischer K, Beersma MF, Hoerauf A. Microbes Infect. 2002;4:43. doi: 10.1016/s1286-4579(01)01508-8. [DOI] [PubMed] [Google Scholar]
- 7.Isaacson M. Clin. Infect. Dis. 2001;33:1707. doi: 10.1086/322620. [DOI] [PubMed] [Google Scholar]
- 8.Lee AM, Rojek JM, Spiropoulou CF, Gunderson AT, Jin W, Shaginian A, York J, Nunberg JH, Boger DL, Oldstone MBA, Kunz S. J. Biol. Chem. 2008;283:18734. doi: 10.1074/jbc.M802089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boger DL, Goldberg J, Satoh S, Ambroise Y, Cohen SB, Vogt PK. Helv. Chim. Acta. 2000;83:1825. [Google Scholar]
- 10.Boger DL, Desharnais J, Capps K. Angew. Chem. Int. Ed. Engl. 2003;42:4138. doi: 10.1002/anie.200300574. [DOI] [PubMed] [Google Scholar]
- 11.Boger DL, Tarby CM, Caporale LH. J. Am. Chem. Soc. 1996;118:2109. [Google Scholar]
- 12.Cheng S, Comer DD, Williams JP, Boger DL. J. Am. Chem. Soc. 1996;118:2567. [Google Scholar]
- 13.Cheng S, Tarby CM, Comer DD, Williams JP, Caporale LH, Boger DL. Bioorg. Med. Chem. 1996;4:727. doi: 10.1016/0968-0896(96)00069-7. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. J. Org. Chem. 1996;61:3849. doi: 10.1021/jo960057x. [DOI] [PubMed] [Google Scholar]
- 15.The synthesis of 9 is shown in Scheme 2 and 12 was synthesized from 9 by reductive amination with benzofuran-2-carboxaldehyde (NaBH(OAc)3, ClCH2CH2Cl). The synthesis of 10 and 11 started from commercially available (S)-N1-Boc-2-benzylpiperazine (Anaspec). Coupling at N4 with benzofuran-2-carboxylic acid (EDCI, HOAt, 2,6-lutidine, DMF) followed by Boc deprotection (HCl, dioxane) gave 10. Compound 11 was prepared from 10 by reductive amination with p-tolualdehyde (NaBH(OAc)3, ClCH2CH2Cl).