Abstract
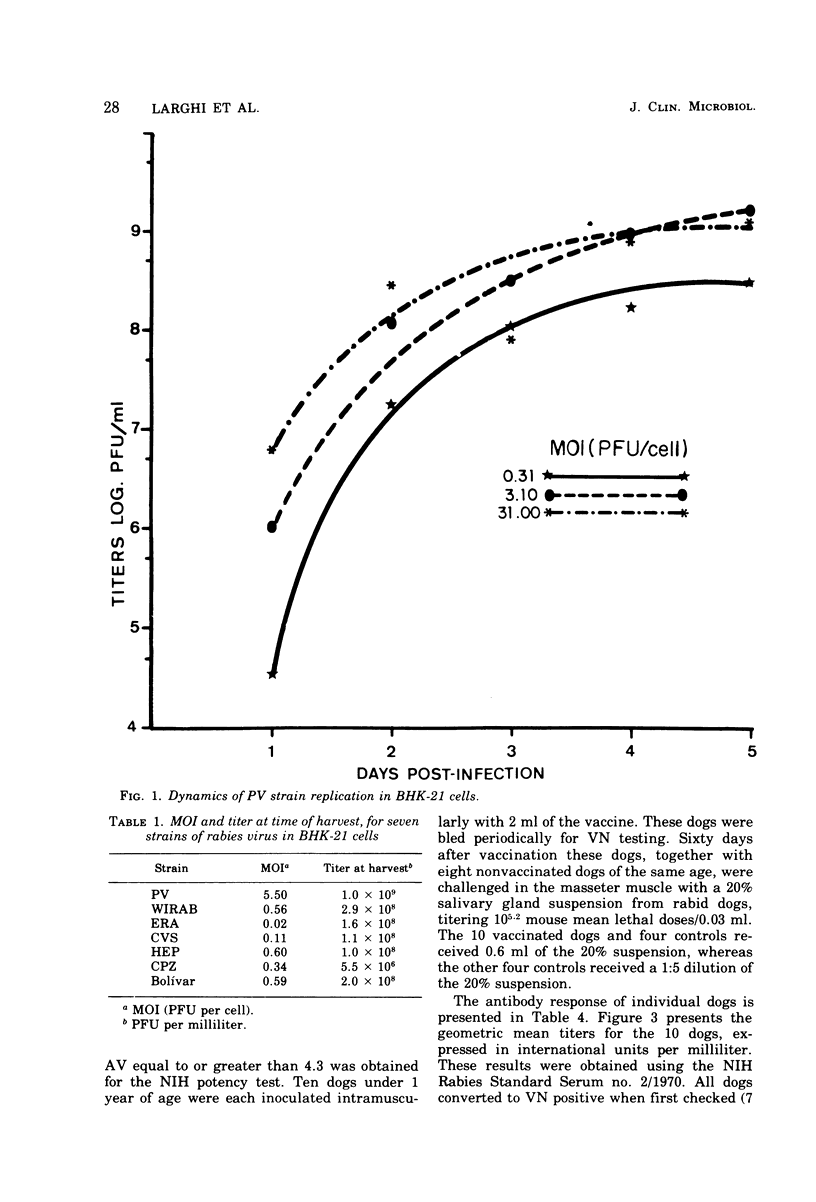
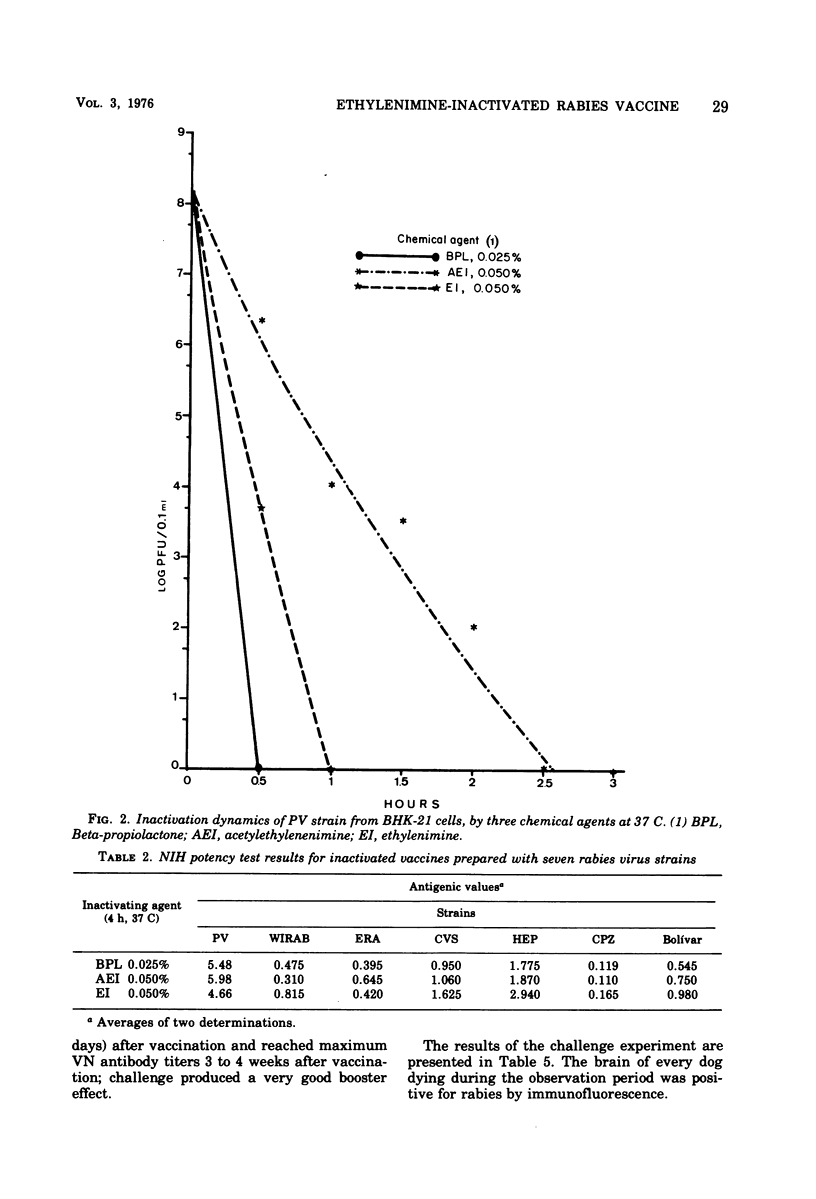
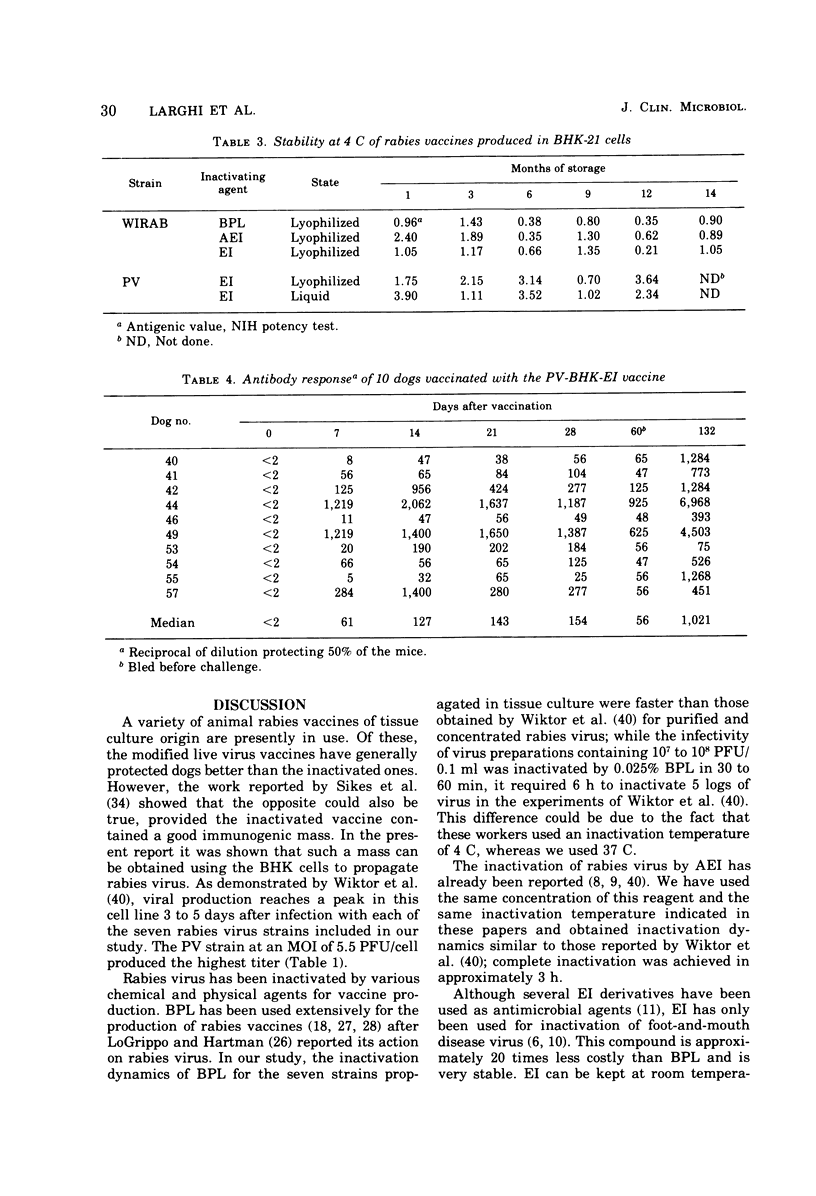
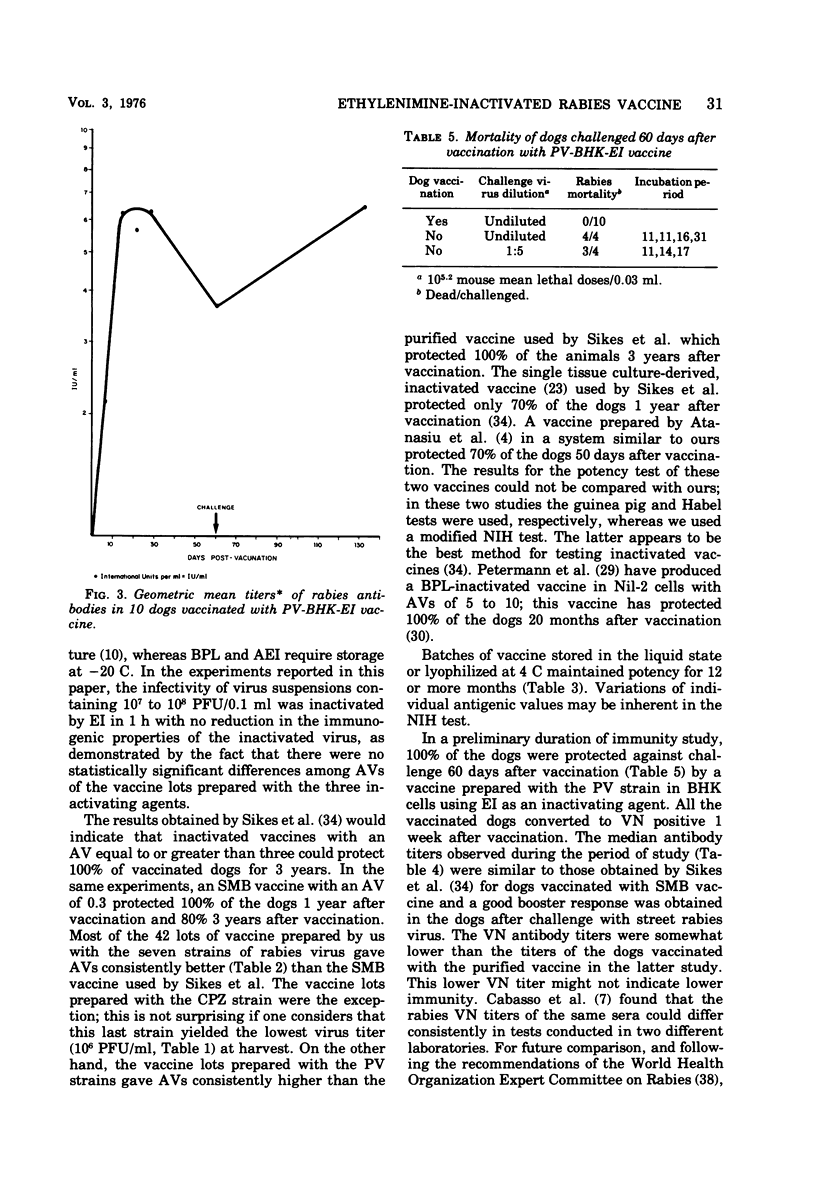
The replication of seven rabies virus strains (CVS, HEP, PV, ERA, WIRAB, CPZ and BOLIVAR) in BHK cells and the inactivation dynamics of these strains by beta-propiolactone, acetylethylenimine, and ethylenimine were studied to find the most immunogenic strain and the most economic and stable inactivating agent for the production of an inactivated tissue culture rabies vaccine for animal use. The seven strains reached the peak of virus production 3 to 5 days after inoculation of the cell culture; PV yielded the highest virus titer (10(9) plaque-forming units/ml). The infectivity of virus suspensions containing 10(7) to 10(8) plaque-forming units/0.1 ml was inactivated by beta-propiolactone in 0.5 h, acetylethylenimine in 3.0 h, and ethylenimine in 1.0 h. Most of the vaccine lots prepared with the different strains and inactivating agents passed a modified National Institutes of Health potency test. The vaccines prepared with the PV strain had consistently higher antigenic values (equal or better than four) than the other six strains. This difference was highly significant (F6,12=59.8), whereas there were no statistically significant differences among the antigenic values of the vaccine lots prepared with the three inactivating agents. Batches of lyophilized and liquid vaccine stored at 4 C maintained potency for over 1 year. Ten dogs vaccinated with a vaccine prepared with the PV strain and inactivated with ethylenimine developed a good antibody response and resisted challenge 60 days after vaccination, while seven of eight nonvaccinated controls died of rabies. This information indicates that an inactivated, stable, economic, and easy-to-prepare rabies vaccine can be produced in BHK cells by using the PV strain and ethylenimine as an inactivating agent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATANASIU P., BAHMANYAR M., BALTAZARD M., FOX J. P., HABEL K., KAPLAN M. M., KISSLING R. E., KOMAROV A., KOPROWSKI H., LEPINE P. Rabies neutralizing antibody response to different schedules of serum and vaccine inoculations in non-exposed persons. Bull World Health Organ. 1956;14(4):593–611. [PMC free article] [PubMed] [Google Scholar]
- Abelseth M. K. An Attenuated Rabies Vaccine for Domestic Animals Produced in Tissue Culture. Can Vet J. 1964 Nov;5(11):279–286. [PMC free article] [PubMed] [Google Scholar]
- Aoki F. Y., Tyrrell D. A., Hill L. E. Immunogenicity and acceptability of a human diploid-cell culture rabies vaccine in volunteers. Lancet. 1975 Mar 22;1(7908):660–662. doi: 10.1016/s0140-6736(75)91761-4. [DOI] [PubMed] [Google Scholar]
- Atanasiu P., Ribeiro M., Tsiang H. Vaccins antirabiques de culture cellulaire obtenus avec la souche Pasteur. Résultats de vaccination. Ann Inst Pasteur (Paris) 1972 Sep;123(3):427–441. [PubMed] [Google Scholar]
- Atanasiu P., Tsiang H., Gamet A. Nouveau vaccin antirabique humain de culture cellulaire primaire. Ann Microbiol (Paris) 1974 Oct-Nov;125B(3):419–432. [PubMed] [Google Scholar]
- Bahnemann H. G. The inactivation of foot-and-mouth disease virus by ethylenimine and propylenimine. Zentralbl Veterinarmed B. 1972 Jul;20(5):356–360. doi: 10.1111/j.1439-0450.1973.tb01136.x. [DOI] [PubMed] [Google Scholar]
- Cabasso V. J., Dobkin M. B., Roby R. E., Hammar A. H. Antibody response to a human diploid cell rabies vaccine. Appl Microbiol. 1974 Mar;27(3):553–561. doi: 10.1128/am.27.3.553-561.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman W. G., Ramshaw I. A., Crick J. Inactivated rabies vaccine produced from the Flury LEP strain of virus grown in BHK-21 suspension cells. Appl Microbiol. 1973 Dec;26(6):858–862. doi: 10.1128/am.26.6.858-862.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick J., Brown F. An inactivated baby hamster kidney cell rabies vaccine for use in dogs and cattle. Res Vet Sci. 1971 Mar;12(2):156–161. [PubMed] [Google Scholar]
- Cunliffe H. R. Inactivation of foot-and-mouth disease virus with ethylenimine. Appl Microbiol. 1973 Nov;26(5):747–750. doi: 10.1128/am.26.5.747-750.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENJE P. A rabies vaccine from hamster kidney tissue cultures: preparation and evaluation in animals. Can J Microbiol. 1960 Dec;6:605–609. doi: 10.1139/m60-072. [DOI] [PubMed] [Google Scholar]
- FENJE P. Propagation of rabies virus in cultures of hamster kidney cells. Can J Microbiol. 1960 Oct;6:479–484. doi: 10.1139/m60-055. [DOI] [PubMed] [Google Scholar]
- KISSLING R. E. Growth of rabies virus in non-nervous tissue culture. Proc Soc Exp Biol Med. 1958 Jun;98(2):223–225. doi: 10.3181/00379727-98-23997. [DOI] [PubMed] [Google Scholar]
- KISSLING R. E., REESE D. R. ANTI-RABIES VACCINE OF TISSUE CULTURE ORIGIN. J Immunol. 1963 Sep;91:362–368. [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick embryo adapted rabies virus. III. Duration of immunity in vaccinated dogs. Proc Soc Exp Biol Med. 1952 Jul;80(3):410–415. doi: 10.3181/00379727-80-19640. [DOI] [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick-embryo-adapted rabies virus. VII. Immunological responses of animals to vaccination with high egg passage Flury strain. J Immunol. 1954 Jun;72(6):503–510. [PubMed] [Google Scholar]
- LOGRIPPO G. A., HARTMAN F. W. Antigenicity of beta-propiolactone-inactivated virus vaccines. J Immunol. 1955 Aug;75(2):123–128. [PubMed] [Google Scholar]
- Larghi O. P., Jimenez E. Methods for accelerating the fluorescent-antibody test for rabies diagnosis. Appl Microbiol. 1971 Apr;21(4):611–613. doi: 10.1128/am.21.4.611-613.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larghi O. P., Nebel A. E., Lazaro L., Savy V. L. Sensitivity of bhk-21 cells supplemented with diethylaminoethyl-dextran for detection of street rabies virus in saliva samples. J Clin Microbiol. 1975 Mar;1(3):243–245. doi: 10.1128/jcm.1.3.243-245.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H. L., Jobim G. O., Moura M. do C. Vacina anti-rábica tipo Fuenzalida modificada (cinco anos de produço observaçes. Rev Inst Med Trop Sao Paulo. 1971 Mar-Apr;13(2):114–120. [PubMed] [Google Scholar]
- PECK F. B., Jr, POWELL H. M., CULBERTSON C. G. A new antirabies vaccine for human use; clinical and laboratory results using rabies vaccine made from embryonated duck eggs. J Lab Clin Med. 1955 May;45(5):679–683. [PubMed] [Google Scholar]
- Petermann H. G., Lang R., Branche R., Soulebot J. P., Mackowiak C. Réalisation d'un nouveau vaccin rabique à partir de culture cellulaire. C R Acad Sci Hebd Seances Acad Sci D. 1967 Dec 18;265(25):2143–2144. [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann E. B., Jr Laboratory techniques in rabies. Potency-test requirements of the United States National Institutes of Health (NIH). Monogr Ser World Health Organ. 1966;23:145–151. [PubMed] [Google Scholar]
- Sikes R. K., Larghi O. P. Purified rabies vaccine: development and comparison of potency and safety with two human rabies vaccines. J Immunol. 1967 Sep;99(3):545–553. [PubMed] [Google Scholar]
- Sikes R. K., Peacock G. V., Acha P., Arko R. J., Dierks R. Rabies vaccines: duration-of-immunity study in dogs. J Am Vet Med Assoc. 1971 Dec 1;159(11):1491–1499. [PubMed] [Google Scholar]
- WIKTOR T. J., FERNANDES M. V., KOPROWSKI H. CULTIVATION OF RABIES VIRUS IN HUMAN DIPLOID CELL STRAIN WI-38. J Immunol. 1964 Sep;93:353–366. [PubMed] [Google Scholar]
- Wiktor T. J., Aaslestad H. G., Kaplan M. M. Immunogenicity of rabies virus inactivated by -propiolactone, acetylethyleneimine, and ionizing irradiation. Appl Microbiol. 1972 May;23(5):914–918. doi: 10.1128/am.23.5.914-918.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Plotkin S. A., Grella D. W. Human cell culture rabies vaccine. Antibody response in man. JAMA. 1973 May 21;224(8):1170–1171. [PubMed] [Google Scholar]



