Abstract
Due to an increasing prevalence, morbidity and mortality associated with asthma, the National Heart, Lung and Blood Institute created the Asthma Clinical Research Network (ACRN) and the Childhood Asthma Research and Education (CARE) Network to improve public health. The objectives of these clinical research networks are to conduct multiple, well designed clinical trials for rapid evaluation of new and existing therapeutic approaches to asthma and to disseminate laboratory and clinical findings to the health care community. These trials comprise a large proportion of the data driving the treatment guidelines established and reviewed by the National Asthma Education and Prevention Program. This article will review the basic design and major findings of selected ACRN and CARE Network trials involving both adults and children with asthma. Collectively, these studies have helped refine the therapeutic role of existing controller medications, establish standard models for side effect evaluation and risk-benefit models, validate symptom based assessments for asthma control, and identify baseline characteristics that may predict individual patient responses. Remaining challenges include shaping the role of novel therapeutics in future guidelines, incorporating pharmacogenomic data in treatment decisions, and establishing better implementation strategies for translation to community settings, all with the goal of reducing the asthma burden on society.
Introduction
The Asthma Clinical Research Network (ACRN) and the Childhood Asthma Research and Education (CARE) Network were initiated in 1993 and 1999, respectively, from requests for proposals (RFPs) that were developed and funded by the National Heart Lung and Blood Institute. The ACRN and CARE were developed to specifically address therapeutic issues in asthma in both adults and children based on concerns related to the increase in the incidence and prevalence of worldwide asthma, and in response to clinical researchers who desired a mechanism by which dispassionate therapeutic trials in asthma could be conducted to answer important and relevant therapeutic questions that were unlikely to be addressed by industry.
The ACRN was initially funded and developed with six clinical centers (University of California, San Francisco; National Jewish Medical and Research Center, Denver; University of Wisconsin, Madison; Thomas Jefferson Medical College, Philadelphia; Harlem Lung Center, New York; Brigham and Women’s Hospital, Boston) and one data coordinating center (Pennsylvania State University, Hershey). 1 The CARE Network was next developed in 1999 and consisted of five clinical centers (University of California, San Diego; University of Arizona, Tucson; Washington University, St. Louis; University of Wisconsin, Madison; National Jewish Medical and Research Center, Denver) and one data coordinating center (Penn State University, Hershey). The research mission for both networks was twofold. First, to provide a mechanism for evaluating new and existing therapeutic approaches for asthma in order to contribute to the evidence-base of the NAEPP guidelines. Second, to provide avenues for the rapid dissemination of the clinical and laboratory findings that resulted from any protocols to the health care community. The following is a review of selected trials that have been conducted to date by these networks, also summarized in Table 1.
Table 1.
Synopsis of reviewed trials. In each case, the study acronym is followed by a brief summary of the main conclusions of the trial. The last three studies in the table have recently been submitted for publication. These are “Combination therapy with a long-acting β-agonist and a leukotriene antagonist in moderate asthma” (SLIMSIT) and “Predicting response to inhaled corticosteroid efficacy” (PRICE) through the ACRN and “Acute intermittent management strategies” (AIMS) by the CARE Network.
| Trial | Summary |
|---|---|
| BAGS | Scheduled albuterol usage is neither beneficial nor harmful |
| SOCS | Salmeterol cannot be used as monotherapy for moderate disease |
| SLIC | Control gained with salmeterol addition allows ICS dose reduction |
| BARGE | Genotype at the 16th amino acid residue of the beta2-adrenergic receptor affects the long-term response to albuterol use |
| DICE | Partial suppression of overnight plasma cortisol levels is a reliable method for comparing ICS side effect potency |
| MICE | Maximum improvement in FEV1 after a 6 wk ICS trial occurs at lower doses with less HPA-axis suppression than those required to affect airway hyperresponsiveness |
| IMPACT | Symptom-driven, intermittent use of ICS in mild disease is not associated with increased exacerbation rates |
| PEAK | ICS are effective in reducing pediatric asthma symptom burden but do not modify asthma natural history |
| CLIC | More children have an improved FEV1 on ICS than on montelukast |
| PACT | Low dose fluticasone provides children more asthma control days than does montelukast |
| SLIMSIT | Compares ICS-salmeterol versus LTRA-salmeterol as controller strategies for moderate asthma |
| PRICE | Establishes biomarker predictors of short- and long-term ICS responses |
| AIMS | Compares acute high dose ICS to montelukast or albuterol at the onset of viral upper respiratory tract infections to reduce subsequent asthma symptoms in preschool children |
Asthma Clinical Research Network
The beta agonist study (BAGS)
In the early 1990’s, due to observations made primarily with the use of fenoterol, considerable controversy existed regarding the safety of regular beta2 agonist use in asthma. Some investigators reported that the regular use of this class of compounds had the potential of increasing both morbidity and mortality from asthma2, while others were not convinced that such angst was warranted.3 To directly address this controversy, the first protocol developed by the ACRN was the Beta Agonist Study (BAGS).4 BAGS was designed to answer the following question: “Is the regular use of beta agonists in asthma safe?” BAGS enrolled 255 patients with mild asthma (age 12–55 yrs; FEV1 ≥ 70% predicted; methacholine PC20 ≤ 16 mg/ml; no inhaled corticosteroids for 6 weeks prior to enrollment; beta agonist use in a specified range). Subjects were treated for sixteen weeks with albuterol, 2 inhalations 4 times daily, or matching placebo followed by a 4-week withdrawal period. The major outcome measure evaluated was change in morning peak expiratory flow (PEF), collected and recorded daily. The trial results provided the following answer: regular beta agonist treatment was found to be neither harmful nor beneficial compared to as needed use. Based on these results, it was concluded that inhaled albuterol should be prescribed for patients with mild asthma on an as-needed basis. The NAEPP incorporated this evidence into its recommendations that as-needed short-acting beta agonist use was an essential component of both categorizing the severity of asthma and controlling it.
The salmeterol or corticosteroids study (SOCS)
Whereas long acting β2-agonists (LABA) were known to provide greater symptom relief compared to short acting β2-agonists and the addition of salmeterol was shown to be more effective than increasing the dose of inhaled corticosteroids (ICS),5, 6 the role of LABAs as monotherapy in asthma was undocumented. Therefore, the Salmeterol Or Corticosteroids Study (SOCS) was designed to test the question of whether salmeterol substitution could maintain asthma control first established by ICS.7 This randomized, placebo-controlled, triple-blinded trial enrolled 164 subjects with moderate persistent asthma on entry, who were able to achieve control, demonstrated by an FEV1 > 80% of predicted and peak expiratory flow variability < 20%, after a 6-week run in period on 400 µg inhaled triamcinolone twice daily. The three intervention arms were designed to continue participants on this fixed dose of triamcinolone, or switch to either inhaled salmeterol (42 µg twice daily) or placebo. The primary endpoint was a change in morning (PEF) after 28 weeks of therapy. Secondary endpoints included a change in FEV1, asthma symptom scores, rescue bronchodilator usage, treatment failures, exacerbations, bronchial methacholine responsiveness, and both sputum eosinophils and exhaled nitric oxide as markers of airway inflammation.
Although there was no significant difference in morning PEF between the active treatments, those subjects who continued triamcinolone demonstrated a lower rate of treatment failures (6, 24, and 36%) and exacerbations (7, 20, 29%) compared to participants switched to either salmeterol or placebo respectively. These results translate to a number needed to treat (NNT) of 8 favoring triamcinolone over salmeterol and an NNT of 5 versus placebo with respect to the numbers of patients needed to treat with triamcinolone to prevent one exacerbation in 28 weeks. Additionally, there was good consistency for the secondary endpoints in that all were stable on ICS, and all were worse on either salmeterol or placebo. The benefits of 28 weeks of triamcinolone were not maintained during a placebo run-out period, demonstrating the validity in the randomization scheme and suggesting the need for daily ICS therapy in this defined population. Thus, salmeterol monotherapy could not be recommended in persistent asthma, and the trial results contributed to the need for reevaluation of the value of lung function measures as the primary endpoint for future asthma studies.
The salmeterol ± inhaled corticosteroids (SLIC) trial
Adding salmeterol to medium dose ICS was emerging as a better strategy than increasing the ICS dose,8–10 but the question of reducing ICS after control was established still remained. To address this, the SLIC Trial was designed. This randomized, blinded, double-dummy controlled study, performed in parallel with SOCS and sharing the 6 week run-in period on 400 µg triamcinolone inhaled twice daily, enrolled asthmatic subjects who remained uncontrolled (FEV1 ≤ 80% or PEF variability > 20%) despite documented adherence to medium-dose ICS.11 The hypothesis tested was that subjects who attain control after the addition of salmeterol to ICS could safely reduce or eliminate the dose of triamcinolone without increased risk of treatment failure. Subjects with moderate asthma (n=175) who remained symptomatic after the 6 week ICS run-in were randomized to added inhaled salmeterol (42 µg twice daily) or placebo (designated placebo-minus) for 18 weeks with a 13:2 randomization weight scheme favoring salmeterol. After two weeks, the salmeterol group was 1:1 randomized to continue the 400 µg triamcinolone inhaled twice daily (salmeterol-plus) or to taper and eliminate the ICS dose (salmeterol-minus). The placebo-minus and the salmeterol-minus groups received 200 µg triamcinolone inhaled twice daily for 8 weeks followed by ICS-placebo for another 8 weeks. The primary endpoint was the time-to-treatment failure as defined by prospectively identified lung function measurement declines, increases in rescue albuterol use, oral or parenteral corticosteroid use, or physician clinical judgment on the basis of safety concerns.
During the ICS reduction phase, there was no difference in the rates of treatment failures between the salmeterol-plus and salmeterol-minus groups (2.8% and 8.3% with an insignificant relative risk of 2.2 (95% CI of 0.5 to 9.2)). However after the ICS-elimination phase, the salmeterol-plus group had significantly fewer treatment failures (13.7%) compared to the salmeterol-minus group (46.3%) with a relative risk of 4.3 (95% CI, 2.0–9.2) associated with the latter. Examination of sequential effects showed that the mean pre-salmeterol FEV1 was the only secondary endpoint with a between group difference favoring the salmeterol-plus group after ICS reduction, whereas after ICS elimination this group had improved peak flows, symptom and quality of life scores, and less rescue bronchodilator usage. SLIC provided crucial evidence that moderate asthmatics achieving control upon the addition of salmeterol to medium-dose ICS could safely undergo a 50% ICS dose reduction, but could not have their ICS regimen eliminated
The beta-adrenergic response by genotype (BARGE) trial
As discussed above, the conclusion of the BAGS trial was that regularly scheduled use of albuterol in patients with mild asthma is neither beneficial nor harmful when compared to as-needed usage.4 The discovery of β2-adrenergic receptor functional differences encoded by common genetic differences raised the possibility that select patients with asthma may not benefit or may even be harmed from frequent exposure to albuterol, an idea supported by retrospective studies evaluating genotypes for the 16th amino acid of the receptor.12–14 In this regard, the Beta-Adrenergic Response by Genotype (BARGE) trial was designed to prospectively answer the proof-of-principal question of whether patients with mild asthma and different β2-adrenergic receptor genotypes have altered lung function measures when treated with regularly scheduled albuterol vs. placebo.15 After a 6 week placebo run-in, 78 genotype-stratified (Arg/Arg and Gly/Gly at amino acid 16), FEV1-matched subjects with mild asthma were randomized in a double-blind, cross-over trial to receive 16 weeks of scheduled albuterol or placebo followed by an 8 week washout period on scheduled placebo and then 16 weeks on the opposite treatment arm, using ipratropium bromide as the rescue inhaler throughout. The primary outcome was the change in morning PEF at the end of the 16 week treatment periods (scheduled albuterol vs. placebo) within a given genotype; the between-genotype comparisons of PEF was a secondary outcome.
The Gly/Gly genotype subjects experienced an increase in PEF while on scheduled albuterol that did not occur during the placebo treatment. By contrast, the Arg/Arg genotype subjects had no change in PEF on albuterol, but improved while on placebo, with a genotype-attributable difference of 24 L/min (p=0.0003). Secondary outcome variables were consistent in that the Arg/Arg subjects had a lower FEV1, higher symptom scores, and more rescue bronchodilator use while on scheduled albuterol, compared to those subjects with the Gly/Gly genotype. An effect on exacerbations was not observed given the relatively short treatment periods in subjects with mild disease. Whereas the mechanism(s) at the genetic, cellular and physiological levels remain to be determined, this study was the first prospectively designed, genotype-stratified trial to suggest that patients with the Arg/Arg genotype might benefit from avoiding short-acting β2-adrenergic receptor agonists.
The SOCS/SLIC salmeterol response by genotype analysis
The higher adverse-event rates in patients with moderate or severe asthma suggests that there may be a select group of patients in whom long-acting β2-adrenergic receptor agonist usage promotes risk of exacerbation or even asthma-related death.6, 16, 17 The ACRN was in a unique position to begin to address the question of whether Arg/Arg genotype individuals also respond poorly to salmeterol, as SOCS and SLIC prospectively collected DNA samples on consenting participants. Genotyping was performed on 53 subjects on salmeterol and 43 on placebo in the SOCS trial, as well as on 74 subjects on salmeterol and a fixed dose of triamcinolone in the SLIC study.
In the two trials combined, there were 25 subjects who were Arg/Arg homozygotes and 48 who were Gly/Gly homozygotes.18 Baseline characteristics of these two subject groups were comparable with the exception that the Arg/Arg subjects in SOCS were slightly older than those with the Gly/Gly genotype (36.99 ± 12.64 vs 29.14 ± 10.59, p = 0.03). At the end of the treatment periods in both studies, subjects with the Arg/Arg genotype had worse morning PEF compared to the Gly/Gly subjects (−51.4 and −36.8 L/min in the SOCS and SLIC trials respectively), despite a transient improvement in SLIC subjects with the Arg/Arg genotype on both salmeterol and triamcinolone. A genotype-attributable difference in exacerbation rates was not observed in either trial, however, Arg/Arg subjects in the SLIC study had lower FEV1, higher morning symptom scores, and more rescue bronchodilator usage compared to those with the Gly/Gly genotype. In sum, this retrospective study suggests B16 Arg/Arg homozygotes have a reduced therapeutic response to salmeterol, providing foundation for the ongoing prospective trial named LARGE (Long Acting Bronchodilator Response by Genotype).
The dose of inhaled corticosteroids with equisystemic effects (DICE) study
Numerous factors influence the specific choice of ICS in asthma management. As little comparative data existed with respect to the incidence of side effects, the goal of the DICE study was to establish the dose of six different ICS products that rendered similar degrees of hypothalamic-pituitary-adrenal axis suppression.19 ICS-naive subjects (n=156) with mild to moderate asthma were randomized to a six week-escalating dose regimen of one of six ICS or matching placebo. The study medications included beclomethasone dipropionate-chlorofluorocarbon (BDP-CFC), flunisolide (FLU-CFC), fluticasone propionate (FP-CFC), and triamcinolone actonide (TAA-CFC) were all delivered by metered dose inhalers (MDIs). Additionally, budesonide (BUD-DPI) and fluticasone proprionate (FP-DPI) were delivered by dry powder inhalers (DPIs). Several methods were used for assessing HPA axis suppression including serial urine and plasma cortisol concentrations; serum osteocalcin measurements were also made. Using the dose causing 10% HPA axis suppression assessed by overnight plasma cortisol levels, a rank order of side effect potency was established according to labeled doses: FLU-CFC (1), TAA-CFC (1.19:1), BDP-CFC (1.69:1), FP-DPI (2.08:1), BUD-DPI (3.45:1), and FP-CFC (8.33:1). Thus, the DICE results support ICS dose equivalence estimates in the NAEPP guidelines, although HPA-axis suppression from FP-DPI was less than expected.
The measuring inhaled corticosteroid efficacy (MICE) study
The objective of the MICE study was to establish a standard model for making benefit-to-risk assessments for controller therapies.20 This protocol used a feasibility design enrolling 30 subjects with mild-to-moderate asthma who were then randomized to 18 weeks of escalating doses of either BDP-CFC or FP-CFC MDIs followed by a three-week administration of high FP-DPI to assess maximum effects. Major endpoints for efficacy included changes in FEV1 and methacholine PC20 as well as prevention of exercise-induced bronchospasm. Changes in exhaled nitric oxide (eNO) and measures of sputum eosinophil were also evaluated. ICS risk was assessed by suppression of overnight plasma cortisol concentrations. Despite equivalent dosing strata, low dose FP-CFC was associated with the maximum improvement in FEV1 while medium dose BDP-CFC was required to achieve a similar effect size. For both agents, HPA axis suppression was observed at doses higher than that needed to achieve the maximum FEV1 improvement. The optimum improvement in airway reactivity occurred at one dose step higher than that needed to achieve the FEV1 benefit (medium and high respectively). Significant variability was noted among subjects and a secondary cohort analysis suggested that subjects with a good FEV1 response (defined as ≥15% improvement) to either ICS could be predicted by baseline characteristics including elevated eNO, higher bronchodilator reversibility, and lower FEV1/FVC ratios. Finally, about a third of the subjects had poor responses (<5% improvement in FEV1) to ICS, consistent with the notion that more severe patients may require higher ICS doses to prevent exacerbations. The MICE findings also substantiate the variability in ICS responses, in this defined asthma population.
The improving asthma control trial (IMPACT)
National and international guidelines have recommended daily anti-inflammatory “controller” therapy even for mild persistent asthma, a classification characterized by consistent but low morbidity.21, 22 This recommendation was prompted by studies reporting that such treatment improves PEF, FEV1, severity of symptoms, exacerbation frequency, and quality of life,23–25 which was reinforced by reports that ICS therapy may prevent progressive loss of lung function.26–28 These latter findings, noted in studies conducted in both adults and children, strongly influenced guideline recommendations for early recognition and continuous treatment of patients with mild persistent asthma.21, 22 However, these studies involved small numbers of participants and their design was not ideal to provide solid evidence that would convincingly validate these recommendations.
Moreover, despite this emphasis on the prescribing of daily controller therapy, anecdotal reports and analysis of pharmacy records indicated that many patients were not diligent about renewing their prescriptions for controller medications (ICS and leukotriene-receptor antagonists).29 Thus, patients with mild persistent asthma appeared to be using their controller medications intermittently because they did not perceive the need to use therapy daily. To more firmly establish if daily controller therapy was essential in mild persistent asthma, not only to control symptoms and reduce exacerbations but also to prevent any future loss of lung function, the ACRN formulated the following research question and designed and conducted a year long clinical study, IMPACT, to answer it. In patients with NAEPP Guideline definition of mild persistent asthma, is it essential to administer controller therapy on a daily basis in order to establish and maintain adequate asthma control and to prevent loss of lung function?
The inclusion criteria for IMPACT included the following: physician-diagnosed asthma, age 18–65 years, and FEV1 ≥ 70% of predicted, with either ≥ 12% (and ≥ 200 ml improvement) following albuterol inhalation or bronchial hyperreactivity (methacholine PC20 < 16 mg/ml). Patients were randomized to one of three treatment arms: (1) budesonide (200 µg bid); (2) oral zafirlukast (20 mg bid); (3) placebo (“intermittent-only” treatment). All were instructed to take open-label budesonide or prednisone as guided by a symptom-based action plan. The run-in and treatment phases both ended with a 14-day period of intense combined therapy, called PICT (prednisone 0.5 mg/kg/d, budesonide 800 µg 2x/d, and zafirlukast 20 mg 2x/d) and acute treatment with albuterol (540–720 µg). The primary outcome was morning PEF and secondary outcomes included those directly perceived by patients, namely, asthma exacerbations, days lost from work or school, symptom-free days, and asthma-related quality of life. Other secondary outcomes included a panel of physiological and biological measures of asthma activity.
There were 225 randomized patients (73 on budesonide, 76 on zafirlukast, and 76 on placebo) in IMPACT. The three groups yielded similar increases in morning PEF (7–9%, ~32 L/min; p = 0.90) and similar rates of asthma exacerbation (14/73 on budesonide, 6/76 on zafirlukast, and 10/76 on placebo, p = 0.238), even though the intermittent-only group took budesonide for an average of only 0.5 weeks of the year. The budesonide group displayed greater improvement in pre-bronchodilator FEV1 (+4.02% ± 1.20%; zafirlukast, −1.06% ± 1.00%; “intermittent only,” +0.66% ± 1.09%; p = 0.005) but not in post-bronchodilator FEV1 (p > 0.25). Budesonide treatment was associated with improvements in questionnaire scores for asthma control and symptom-free days but not for quality of life.
The results of IMPACT suggested that guideline criteria for mild persistent asthma may define a condition so mild that patients instructed in an action plan may decide whether to take daily anti-inflammatory therapy on the basis of their assessment of the importance of their symptomatic improvements.30 These findings clearly were unexpected and have lead many asthma researchers to compare and contrast these results with other studies to determine how treatment recommendations for mild persistent asthma may need to be modified.31–33 Alternatively, it is conceivable that the criteria used to define mild persistent asthma may need to be redefined as well. Nonetheless, the findings from IMPACT are indeed provocative and will no doubt influence the design of future clinical trials focused on providing additional evidence that would solidify the definition of mild persistent asthma and its appropriate treatment both short and long term.
Childhood Asthma Research and Education (CARE) Network
The prevention of early asthma in kids (PEAK) trial
Based on data generated in both pediatric28 and adult26, 34 patients with asthma, current asthma guidelines recommend that daily controller therapy should be initiated in individuals whose symptoms place them in the mild persistent asthma category. However, in preschool aged children, wheezing is a common manifestation of viral respiratory tract infections,35 and properly diagnosing asthma in this age group so that appropriate treatment can be initiated has posed a challenge for many clinicians. On the one hand, early recognition and treatment may be important in altering the natural history of the disease in terms of both symptom and exacerbation control as well as loss of lung function. On the other hand, treating children who only have a transient wheezing phenotype may be putting them at unnecessary potential risk from medication side effects (e.g. reduced growth velocity from ICS).35–37 To address these critical questions in childhood asthma, the first trial conducted by the CARE network was Prevention of Early Asthma in Kids (PEAK) study.38 The main research question posed by PEAK was as follows: can early recognition and treatment of children at increased risk of developing asthma prevent its clinical expression or any alterations in lung function measures that occur as a result of it?
PEAK was a three year long, double blind, randomized, parallel group design trial in which children ages 2–3 years with a positive modified asthma predictive index (API) were treated for two years with either fluticasone 44 mcg/puff MDI, 2 puffs twice daily via a spacer and face mask, or matching placebo. At the end of this treatment period, all children were taken off active medications for a one-year observation period. Treatment algorithms (step up and step down controller regimens) were utilized throughout the trial to maximize participant safety while minimizing prolonged interventions that would dampen the ability to document that this type of intervention was indeed therapeutic both short and long term.
The primary outcome was the difference between the study groups in the proportion of episode-free days during the year-long observation period. Episode-free days were defined as those during which there were no asthma-like symptoms, no unscheduled medical visits for respiratory symptoms, and no use of any supplementary asthma medications including pre-exercise albuterol. Secondary outcomes included the proportion of episode-free days during the treatment period. Other outcomes during both the treatment and observation periods included the number and time to systemic corticosteroid courses and controller medications, and blood eosinophilia. Impulse oscillometry was performed using established procedures to assess the contribution of resistance and reactance to the total impedance of the respiratory system; reactance at low frequencies reflects the elastic properties of the respiratory system and will be more negative in patients with airway dysfunction.39–41 An exacerbation was defined as the need for a prednisolone course to control asthma-like symptoms. In addition, potential effects of long-term use of the ICS on growth velocity were assessed.
During the observation-year, no significant differences in the proportion of episode-free days, number of exacerbations, or lung function were observed between the two groups. During the two-year treatment period, ICS use was associated with a 4.8% greater proportion of episode-free days (p=0.006), a 32.0% lower rate of exacerbations (p<0.001), and a 53.0% reduction in supplementary ICS use (p<0.001). Although symptom control during the treatment period was clearly improved with daily ICS therapy, some side effects from the treatment on linear growth were observed. The mean increase in height in the ICS-group was 1.1 cm less than the placebo-group at 24 months (p<0.001). However, following cessation of daily therapy, the ICS-group had a similar growth velocity compared to the placebo group during the last 12 months of treatment, and a higher velocity during the observation-year, resulting in a mean increase in height that was 0.7 cm less in the ICS-group at the end of the trial (p=0.008). The participants in PEAK are currently being followed more long term to determine the overall effects that ICS treatment could have on growth velocity.
The results of PEAK have clearly provided new insights into the success of potential interventions that may alter the natural history of asthma disease expression in early childhood. It is clear from the PEAK data that, during treatment, ICS reduced symptom burden, exacerbations, and respiratory system reactance. Thus, treatment was very effective in controlling symptom burden. Although growth velocity diminished early in treatment, it subsequently accelerated even in spite of continued therapy. The long-term consequences of this therapeutic intervention on growth are currently under study. Despite these positive benefits in terms of the control of symptom burden during therapy, once it was stopped, symptoms returned in approximately two to three months. Thus, the natural history of asthma in high-risk preschool aged children was not altered by two years of ICS therapy. PEAKs supports the disease controlling, but not modifying effects, of ICS therapy.
Characterizing the response to a leukotriene receptor antagonist and an inhaled corticosteriod (CLIC)
Evidence from the ACRN DICE and MICE trials suggested that considerable interindividual variability in response to ICS exists in adults with persistent asthma, and that phenotypic attributes might predict such response. Therefore, the CARE Network designed the CLIC trial42 to examine the variability of responses to both ICSs and LTRAs in children with asthma to identify patient features that would serve as indicators for selection of the controller medications most likely to achieve a favorable response in individual patients. Children, 6 to 17 years of age, with mild-to-moderate persistent asthma were enrolled. Children were excluded if they had FEV1<70% predicted, or had evidence of severe disease. All participants demonstrated improvement in FEV1 of 12% or greater after maximal bronchodilator or methacholine PC20≤12.5 mg/ml. Eligible children were randomized to one of two crossover sequences, including 8 weeks of FP-DPI 100 mcg twice daily and 8 weeks of montelukast 5–10 mg nightly (depending on age), in a double-masked design.
Response was assessed on the basis of improvement in FEV1, from baseline to the end of each treatment period, and defined as an improvement in FEV1 of ≥7.5%. Based on this definition, 17% of 126 participants responded to both study medications, 23% responded to FP alone, 5% to montelukast alone, and 55% responded to neither medication. Similar to the adult studies, responses to FP and montelukast varied considerably.
CLIC also provided the opportunity to assess medication responses for relationships to baseline asthma phenotype-associated biomarkers. Greater differential responses to FP over montelukast (defined on the basis of FEV1 improvement) was associated with higher bronchodilator use, bronchodilator response, eNO levels, and eosinophil cationic protein levels and lower methacholine PC20 and pulmonary function values.
Due to the poor correlation of pulmonary function responses to controller therapy with many nonpulmonary clinical control outcomes43, the CARE Network also reported additional CLIC asthma clinical control outcomes in a subsequent manuscript.44 As expected, improvements in most clinical asthma control measures occurred with both FP and montelukast. However, clinical outcomes (asthma control days, the Asthma Control Questionnaire, and albuterol use), pulmonary responses (FEV1/forced vital capacity, PEF variabilities, morning PEF, and measures of impedance), and inflammatory biomarkers (eNO) improved significantly more with FP than with montelukast treatment. eNO served as both a predictor of asthma control days (ACD) and a response indicator in documenting the difference in ACD response between FP and montelukast. The short-term results of the CLIC trial both supported the guidelines’ preference of ICS as first-line therapy for mild-to-moderate persistent asthma in children and the potential role of eNO to individualize choice of controller therapy.
Pediatric asthma controller trial (PACT)
PACT was designed to compare three contemporary asthma controller regimens as first-line therapy for the management of mild-to-moderate persistent asthma in school-aged children.45 The guideline recommendations to date had largely relied on trials in adults with more moderate diseases, resulting in extrapolations to the pediatric population. Whereas, important data to manage childhood asthma was provided in CLIC, the trial was too short-term to adequately assess the important outcome of asthma exacerbations. Therefore, PACT enrolled 285 children (ages 6–14 years) with mild-to-moderate persistent asthma based on symptoms, but who all purposefully had baseline FEV1≥80% predicted. Bronchodilatior reversibility was collected, but was not an entry criterion. All children demonstrated methacholine FEV1 PC20≤12.5 mg/ml. During the baseline placebo run-in, PACT participants averaged only 27% ACDs, documenting their need for controller therapy. Children were randomized to one of three double-blind 48 week treatments: FP DPI 100 mcg twice daily (FP monotherapy); FP 100 mcg/salmeterol 50 mcg in the morning and salmeterol 50 mcg in the evening (PACT combination); montelukast 5 mg in the evening. The primary outcome was ACDs; a multitude of secondary outcomes were determined including asthma exacerbation, humanistic measurements, and pulmonary function measurements.
All three PACT controller therapies resulted in an improvement of ACDs during the 48 weeks, as compared to that documented at baseline. However, the FP monotherapy group gained an average of 42 asthma control days per year, compared to the montelukast monotherapy group (p=0.004). FP monotherapy and PACT combination were comparable in many patient-measured outcomes, including percent of ACDs and prevention of asthma exacerbations, but FP monotherapy was superior for clinic-measured FEV1/FVC maximum bronchodilator response, and PC20. FP was superior to montelukast for ACDs and for all other PACT control outcomes. The NNT for both FP monotherapy and PACT combination as compared to montelukast is approximately 6.5, meaning that 7 children would need to be treated with FP monotherapy or PACT combination instead of montelukast in order to achieve one additional treatment response (a priori defined as a 20% increase in asthma control days). Importantly, growth over 48 weeks was not statistically different in the three treatment groups (FP 5.3 cm, PACT combination 5.3 cm, montelukast 5.7 cm).
Of the regimens tested, the PACT study findings favor FP monotherapy due to its overall success in improving asthma control outcomes. Therefore, this study provides the definitive evidence in support of guideline recommendations of low dose ICS in treating children with mild-to-moderate persistent asthma with FEV1 ≥ 80% predicted.
Summary
This review has highlighted the major contributions of the ACRN and CARE Network trials to the current NAEPP asthma treatment guidelines, depicted in Figure 1. First, the stepwise positioning (level of severity between intermittent and persistent) of as-needed short acting β2-agonists was established (BAGS) and later, whether or not a subgroup of patients may be at risk if these agents were used on a regular basis (BARGE). Second, the use of the long acting beta agonist, salmeterol, was evaluated to determine if it could be used as monotherapy in mild persistent asthma (SOCS), and if it would enable steroid reduction and/or elimination in more moderate disease (SLIC). Both studies clearly demonstrated that long acting beta agonists should not be used as monotherapy, and later retrospective analyses based on beta adrenergic receptor genotypes indicated that the results seen in the BARGE trial with short acting beta agonists were applicable to long acting beta agonists as well. Third, models for standardized assessment of controller therapy side-effect comparisons and risk-benefit calculations were established (MICE, DICE). Fourth, for individuals with mild persistent asthma (using strict NAEPP2 guideline criteria), intermittent ICS use directed by symptoms was found not to be associated with higher rates of exacerbations compared to daily use with ICS (IMPACT). Fifth, despite concerns of the transient effects on growth, ICS usage in school-aged children and adolescence was found to be safe and preferable to montelukast for improving pulmonary function and establishing symptom control (CLIC, PACT). Finally, in preschool-aged children at high risk of developing asthma, daily treatment with an ICS was able to control symptom burden and exacerbations but was unable to alter the natural history of asthma once the therapy was discontinued (PEAK). Both the ACRN and CARE network continue to develop protocols that will provide answers to questions regarding both the short and long term management of asthma in both adults and children that will assist clinicians in providing optimal care for their patients.
Figure 1.
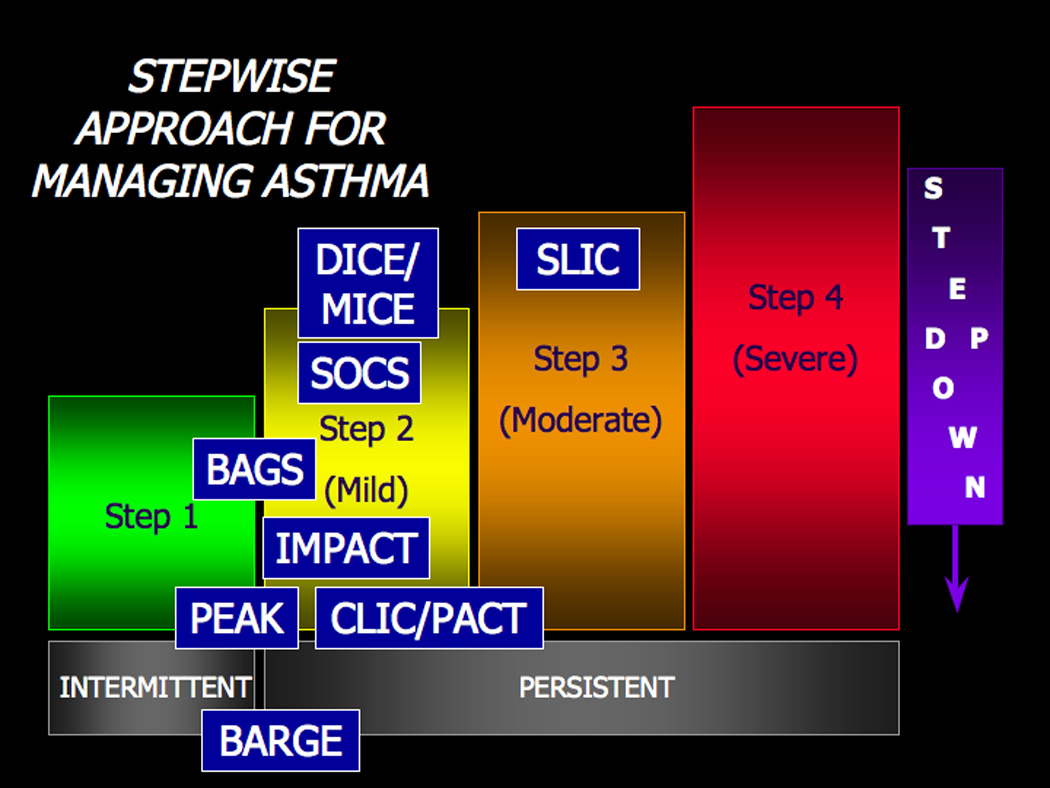
Past and present ACRN and CARE Network clinical trials separated by NAEPP severity steps. The acronyms refer to trials reviewed herein. Escalation of controller therapy selection and dose is appropriate for the moderate and severe strata. Patients who remain symptomatic despite oral corticosteroids and other agents in the severe class should be reevaluated for medication compliance issues and/or aggressive treatment of other coexisting conditions such as vocal cord dysfunction, gastroesophageal reflux, or chronic sinusitis.
Acknowledgements
We thank the clinical coordinators at all the centers and the staff at the Data Coordinating Center, without whom this work would be impossible. The ACRN (I) is supported by the National Institutes of Health, National Heart, Lung and Blood Institute grants 5U10HL051845, 5U10HL051831, 5U10HL051834, 5U10HL051843, 5U10HL056443, 5U10HL051810, and 5U10HL051823. The CARE Network is also funded by NIH/NHLBI via 5U10HL064313, 5U10HL064288, 5U10HL064305, 5U10HL064295, 5U10HL064287 and 5U10HL064307.
Footnotes
This article was originally published in The Journal of Allergy and Clinical Immunology. http://www.sciencedirect.com/science/journal/00916749
References
- 1.Chinchilli VM, Drazen JM, Fish JE, Lemanske RF, Jr, Lazarus SC, Martin RJ. The clinical trials in the initial five-year award period of the Asthma Clinical Research Network. Control Clin Trials. 2001;22:126S–134S. doi: 10.1016/s0197-2456(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 2.Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, Cockcroft D, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326:501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 3.McFadden ER., Jr Perspectives in beta 2-agonist therapy: vox clamantis in deserto vel lux in tenebris? J Allergy Clin Immunol. 1995;95:641–651. doi: 10.1016/s0091-6749(95)70166-4. [DOI] [PubMed] [Google Scholar]
- 4.Drazen JM, Israel E, Boushey HA, Chinchilli VM, Fahy JV, Fish JE, et al. Comparison of regularly scheduled with as-needed use of albuterol in mild asthma. N Engl J Med. 1996;335:841–847. doi: 10.1056/NEJM199609193351202. [DOI] [PubMed] [Google Scholar]
- 5.Pearlman DS, Chervinsky P, LaForce C, Seltzer JM, Southern DL, Kemp JP, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med. 1992;327:1420–1425. doi: 10.1056/NEJM199211123272004. [DOI] [PubMed] [Google Scholar]
- 6.D'Alonzo GE, Nathan RA, Henochowicz S, Morris RJ, Ratner P, Rennard SI. Salmeterol xinafoate as maintenance therapy compared with albuterol in patients with asthma. JAMA. 1994;271:1412–1416. [PubMed] [Google Scholar]
- 7.Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF, Jr, Sorkness CA, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285:2583–2593. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 8.Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med. 1996;153:1481–1488. doi: 10.1164/ajrccm.153.5.8630590. [DOI] [PubMed] [Google Scholar]
- 9.Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet. 1994;344:219–224. doi: 10.1016/s0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- 10.van Noord JA, Schreurs AJ, Mol SJ, Mulder PG. Addition of salmeterol versus doubling the dose of fluticasone propionate in patients with mild to moderate asthma. Thorax. 1999;54:207–212. doi: 10.1136/thx.54.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemanske RF, Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–2603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 12.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochem. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 13.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 14.Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax. 2000;55:762–767. doi: 10.1136/thorax.55.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- 16.Bisgaard H. Effect of long-acting beta2 agonists on exacerbation rates of asthma in children. Pediatr Pulmonol. 2003;36:391–398. doi: 10.1002/ppul.10381. [DOI] [PubMed] [Google Scholar]
- 17.Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ. 1993;306:1034–1037. doi: 10.1136/bmj.306.6884.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wechsler ME, Lehman E, Lazarus SC, Lemanske RF, Jr, Boushey HA, Deykin A, et al. beta-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med. 2006;173:519–526. doi: 10.1164/rccm.200509-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin RJ, Szefler SJ, Chinchilli VM, Kraft M, Dolovich M, Boushey HA, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med. 2002;165:1377–1383. doi: 10.1164/rccm.2105013. [DOI] [PubMed] [Google Scholar]
- 20.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 21.NAEPP. National Asthma Education and Prevention Program: Guidelines for the Diagnosis and Management of Asthma. Bethesda: National Institutes of Health; National Institutes of Health/National Heart L, and Blood Institute. 1997 ed:
- 22.GINA. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention. Bethesda: National Institutes of Health; National Institutes of Health/National Heart L, and Blood Institute. 1998 ed:
- 23.Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325:388–392. doi: 10.1056/NEJM199108083250603. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–1076. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 25.O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164:1392–1397. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 26.Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. N Engl J Med. 1994;331:700–705. doi: 10.1056/NEJM199409153311103. [DOI] [PubMed] [Google Scholar]
- 27.Selroos O, Pietinalho A, Lofroos AB, Riska H. Effect of early vs late intervention with inhaled corticosteroids in asthma. Chest. 1995;108:1228–1234. doi: 10.1378/chest.108.5.1228. [DOI] [PubMed] [Google Scholar]
- 28.Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med. 1994;88:373–381. doi: 10.1016/0954-6111(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 29.Stoloff SW, Stempel DA, Meyer J, Stanford RH, Carranza Rosenzweig JR. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol. 2004;113:245–251. doi: 10.1016/j.jaci.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352:1519–1528. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 31.Boushey HA. Daily inhaled corticosteroid treatment should not be prescribed for mild persistent asthma. Con. Am J Respir Crit Care Med. 2005;172:412–414. doi: 10.1164/rccm.2505003. [DOI] [PubMed] [Google Scholar]
- 32.Fabbri LM. Does mild persistent asthma require regular treatment? N Engl J Med. 2005;352:1589–1591. doi: 10.1056/NEJMe058020. [DOI] [PubMed] [Google Scholar]
- 33.O'Byrne PM. Daily inhaled corticosteroid treatment should be prescribed for mild persistent asthma. Am J Respir Crit Care Med. 2005;172:410–412. doi: 10.1164/rccm.2505001. [DOI] [PubMed] [Google Scholar]
- 34.Laitinen LA, Laitinen A, Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a beta 2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double-blind, parallel-group controlled trial. J Allergy Clin Immunol. 1992;90:32–42. doi: 10.1016/s0091-6749(06)80008-4. [DOI] [PubMed] [Google Scholar]
- 35.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–675. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 36.Allen DB. Influence of inhaled corticosteroids on growth: a pediatric endocrinologist's perspective. Acta Paediatr. 1998;87:123–129. doi: 10.1080/08035259850157516. [DOI] [PubMed] [Google Scholar]
- 37.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 38.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 39.Goldman MD, Carter R, Klein R, Fritz G, Carter B, Pachucki P. Within-and between-day variability of respiratory impedance, using impulse oscillometry in adolescent asthmatics. Pediatr Pulmonol. 2002;34:312–319. doi: 10.1002/ppul.10168. [DOI] [PubMed] [Google Scholar]
- 40.Klug B, Bisgaard H. Measurement of lung function in awake 2–4-year-old asthmatic children during methacholine challenge and acute asthma: a comparison of the impulse oscillation technique, the interrupter technique, and transcutaneous measurement of oxygen versus whole-body plethysmography. Pediatr Pulmonol. 1996;21:290–300. doi: 10.1002/(SICI)1099-0496(199605)21:5<290::AID-PPUL4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 41.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:317–322. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 42.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Yu C, Holgate ST, Reiss TF. Variability and lack of predictive ability of asthma end-points in clinical trials. Eur Respir J. 2002;20:1102–1109. doi: 10.1183/09031936.02.02402001. [DOI] [PubMed] [Google Scholar]
- 44.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Sorkness CA, Lemanske RF, Mauger DT, Bochemer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of three controller regimens for mild-moderate persistent asthma: The Pediatric Asthma Controller Trial (PACT) 2006 doi: 10.1016/j.jaci.2006.09.042. In press. [DOI] [PubMed] [Google Scholar]


