Abstract
It has been well documented that nordihydroguaiaretic acid (NDGA), a phenolic lignan isolated from the creosote bush, Larrea tridentate, has anti-cancer activity in vitro and in vivo. Several mechanisms have been identified that could contribute to these actions, as NDGA directly inhibits metabolic enzymes and receptor tyrosine kinases that are established anti-cancer targets. In the present study, we show that NDGA inhibits the transforming growth factor β (TGF-β) type I receptor, a serine threonine kinase receptor. In cultured cells, NDGA treatment repressed Smad2 phosphorylation induced by TGF-β treatment and by a constitutively active mutant of TGF-β type I receptor (T202D). NDGA also inhibited downstream transcriptional activation mediated by both TGF-β treatment and the constitutively active mutant receptor. In vitro, NDGA inhibited TGF-β type I receptor mediated Smad2 phosphorylation in crude cell lysates and in a purified preparation. Importantly, screening select analogs demonstrated that modification of NDGA’s structure resulted in altered potency against the receptor. These results indicated that the structure of NDGA can be modified to achieve increased potency. Together our data provide a novel mechanism for NDGA activity which could help explain its anti-cancer activity, and suggest that NDGA could serve as a structural motif for developing serine/threonine kinase inhibitors with selectivity for TGF-β type I receptor.
Keywords: TGF-β, NDGA
1. Introduction
Nordihydroguaiaretic acid (NDGA) is a phenolic lignan originally isolated from the creosote bush, Larrea tridentate, which was used by native North Americans to treat many diseases (Arteaga et al., 2005). NDGA inhibits proliferation of multiple types of cancer cells in culture, including lung (Avis et al., 1996), prostate (Huang et al., 2004), gastric (Shimakura and Boland, 1992), neuroblastoma (Meyer et al., 2007), and breast cancer (Rose and Connolly, 1990). NDGA was also observed to inhibit tumor growth in xenograft models of neuroblastoma (Meyer et al., 2007), small cell lung cancer (Avis et al., 1996), and cervical cancer (Seufferlein et al., 2002) and in a syngeneic mouse model of breast cancer (Youngren et al., 2005).
NDGA has recently been employed clinically in phase I trials with prostate cancer patients (Ryan et al., 2008). However, the mechanisms underlying the anti-cancer effects of NDGA are not fully understood. NDGA does inhibit several metabolic enzymes known to influence the growth of certain tumors (Maria et al., 2006; De et al., 2003; Li et al., 2005; Jiang et al., 2006; Van and Goossens, 1983). In addition, NDGA was recently shown to inhibit several receptor tyrosine kinases, including insulin-like growth factor (IGF-1) receptor, cerbB2/HER2/neu (HER2/neu) receptor (Meyer et al., 2007; Youngren et al., 2005), and fibroblast growth factor receptor (Meyer et al., 2008). NDGA likely exerts its anti-cancer activity at least in part through inhibiting these tumor-promoting receptor tyrosine kinases. However, NDGA also inhibits the Ap-1 transcription factor (Gonzales and Bowden, 2002; Huang et al., 2003; Kumar et al., 2007), while its effects on other transcription pathways important in tumor biology have not been explored.
Transforming growth factor β (TGF-β) belongs to a large family of proteins with pleiotropic properties. TGF-β binds to cell surface receptors, forming a bi-dimeric receptor complex including type I receptor and type II receptor. Both these two receptors are transmembrane serine/threonine kinases. Type II receptor activates the type I receptor through phosphorylation. Activated type I receptor phosphorylates Smad2, leading to activation of the downstream signaling cascade and transcriptional regulation (Massague and Gomis, 2006; Wrana et al., 1992; Wrana et al., 1994). Although Smad signaling is the main downstream pathway, there are other mediators such as the mitogen-activated protein kinases ERK and Rho family members (Derynck and Zhang, 2003). TGF-β plays a complex role in tumor biology. At the early stage of neoplasia, TGF-β functions as a tumor suppressor, while at later stage of carcinogenesis, TGF-β can function as a stimulus for tumor progression (Akhurst and Derynck, 2001; Massague and Gomis, 2006; Truty and Urrutia, 2007). In advanced tumors, the TGF-β system can promote tumor growth, invasion, metastasis, and evasion of immunological surveillance (Derynck et al., 2001; Dumont and Arteaga, 2003; Gorelik and Flavell, 2002; Siegel and Massague, 2003; Thomas and Massague, 2005). Therefore, TGF-β pathway constitutes an attractive target for anticancer agents and TGF-β inhibitors have been under investigations as the therapy for cancer and other diseases (Callahan et al., 2002; Dumont and Arteaga, 2003; Peng et al., 2005).
In the present study, we examine the effects of NDGA on TGF-β signaling and its underlying mechanisms.
2. Materials and Methods
2.1. Chemicals
NDGA, compound 1, 2, 1.1, and 1.2 were described previously (Blecha et al., 2007; Chen et al., 1998; Youngren et al., 2005). Compound 3 and 1.3 were synthesized as an expansion of the previously described library.
2.2. Plasmids and Proteins
Eukaryotic expression constructs pRK5 TGF β type I receptor (T202D) Flag was obtained from Addgene (Cambridge MA) and described previously (Feng and Derynck, 1996). Firefly luciferase reporter CAGA12-Luc was a kind gift from Dr. Rosemary Akhurst at UCSF. Firefly luciferase reporters Sp1-Luc and TATA-Luc were kind gifts from Dr. Fred Schaufele at UCSF. Renilla luciferase pRL-null was purchased from Promega. Bacterial expression constructs GST-Smad2 (L+MH2) were obtained from Addgene and described previously (He et al., 2006). Recombinant proteins were expressed in BL21 Escherichia coli (Novagen, Gibbstown NJ), GST fusion proteins were purified using BugBuster GST•Bind Purification Kit (Novagen).
2.3. Cell Treatment and Immunoblot
Panc-1 cells were treated with or without 40 µM NDGA, 100 µg/ml vitamin C, 30 µM caffeic acid, 30 µM 5,8,11-eicosatriynoic acid (ETI), 4 µM AG538, or 4 µM picropodophyllin (PPP) (Biomol, Plymouth Meeting, PA) in the absence or presence of 10 ng/ml TGF-β (Invitrogen, Carlsbad CA) for one hour. Cell lysates were subjected to SDS-PAGE and analyzed by immunoblotting with anti-phospho-Smad2 (Ser465/467) (Cell Signaling, Danvers MA) and anti-Smad2 (Cell Signaling). Panc-1 cells were treated with or without 40 µM NDGA in the absence or presence of 10 ng/ml TGF-β for three days; with medium changed and NDGA replenished every two days. Cell lysates were subjected to SDS-PAGE and analyzed by immunoblotting with anti-phospho-Smad2 (Ser465/467) and anti-Smad2. 293FT cells were transfected with or without eukaryotic expression constructs pRK5 TGF β type I receptor (T202D) Flag using Fugene HD (Roche, Indianapolis IN). Culture medium was replaced 24 hours after transfection and cells were treated with or without 40 µM NDGA for overnight. Cell lysates were subjected to SDS-PAGE and analyzed by immunoblotting with anti-phospho-Smad2 (Ser465/467) and anti-Smad2. Values were determined by scanning densitometry.
2.4. Transfections and Reporter Assays
In order to study TGF-β-mediated gene transcription, 293 FT and Panc-1 cells were seeded at 10 000 cells/well in a 96-well plate and transfected with internal control Renilla luciferase pRL-null and CAGA12-Luc reporter using Fugene HD. Alternatively, cells were transfected with internal control pRL-null, and TATA-Luc reporter or Sp1-Luc reporter. Culture medium was replaced 24 hours after transfection and cells were treated with or without 10ng/ml TGF-β in the absence or presence of different concentration of NDGA or its analogs for another 6 hours. Cells were then harvested, and luciferase assays were performed using a dual-luciferase reporter assay system (Promega, Madison WI). Panc-1 cells were seeded at 10 000 cells/well in a 96-well plate and transfected with internal control Renilla luciferase pRL-null, CAGA12-Luc reporter, and pRK5 TGF β type I receptor (T202D) Flag using Fugene HD. Culture medium was replaced 24 hours after transfection and cells were treated with 10ng/ml TGF-β in the absence or presence of different concentration of NDGA or its analogs overnight. Cells were then harvested after treatment, and luciferase assays were performed. All assays were performed in triplicate.
2.5. In Vitro Pull-down Assays
Pre-cleaned cell lysates were prepared from TGF β type I receptor (T202D) transfected 293 FT cells and then incubated with purified GST-Smad2 (L+MH2) in the absence or presence of different amount of NDGA in phosphorylation buffer described previously (Lee et al., 2007) with modifications (25mM HEPES, pH 7.6, 10mM MgCl2, 2mM MnCl2, 1mM NaF, 20mM ATP, 100 nM okadaic acid, and proteinase inhibitor mixture) for 30 min at 37 °C. The reaction mixtures were then incubated with GST•Bind resin. Resins were washed extensively, and bound proteins were separated by SDS-PAGE and analyzed by immunoblotting with anti-phospho-Smad2 (Ser465/467) and anti-GST.
2.6. Immunoprecipitations
293FT cells were transfected with eukaryotic expression construct pRK5 TGF β type I receptor (T202D) Flag using Fugene HD. 24 hours after transfection, cells were harvested using lysis buffer containing 25 mM Tris-HCl, pH 8.0, 10% glycerol, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 1 mM dithiothreitol, and protease inhibitor mixture. Cleared cell lysates were incubated with anti-FLAG (Sigma, St. Louis MO) antibody bound to protein G beads (Santa Cruz, Santa Cruz CA). The beads were washed extensively, and bound proteins were used in the in-vitro kinase assay.
2.7. Phosphorylation of GST- Smad2 by eukaryotically expressed TGF β type 1 receptor (T202D)
Purified GST-Smad2 (L+MH2) was incubated with immunoprecipitated TGF β type I receptor (T202D) in the absence or presence of different amount of NDGA in phosphorylation buffer described previously for 30 min at 37 °C. The reactions were terminated by incubating the samples on ice, and centrifuged. The supernatants were separated by SDS-PAGE and analyzed by immunoblotting with anti-phospho-Smad2 (Ser465/467) and anti-GST (Bethyl, Montgomery TX). Alternatively, 293FT cells transfected with TGF β type I receptor (T202D) were harvested using phosphorylation buffer, incubated on ice for 30 minutes. Swollen cells were broken by passing through 25 G needle, and the cell lysates were cleaned by centrifugation at 2000g for 5 minutes. The supernatants were used in the phosphorylation reaction by adding Purified GSTSmad2 (L+MH2) and incubated for 30 min at 37 °C in the absence or presence of different amount of NDGA. The reactions were terminated by incubating the samples at ice, followed by in vitro pull down assays.
2.8. Statistical Analyses
All data analysis was performed employing MedCalc statistical software (Mariakerke, Belgium). Values are presented as mean ± SEM. Differences groups were analyzed by Student’s T-test.
3. Results
3.1. NDGA represses transcription and Smad2 phosphorylation induced by TGF-β treatment
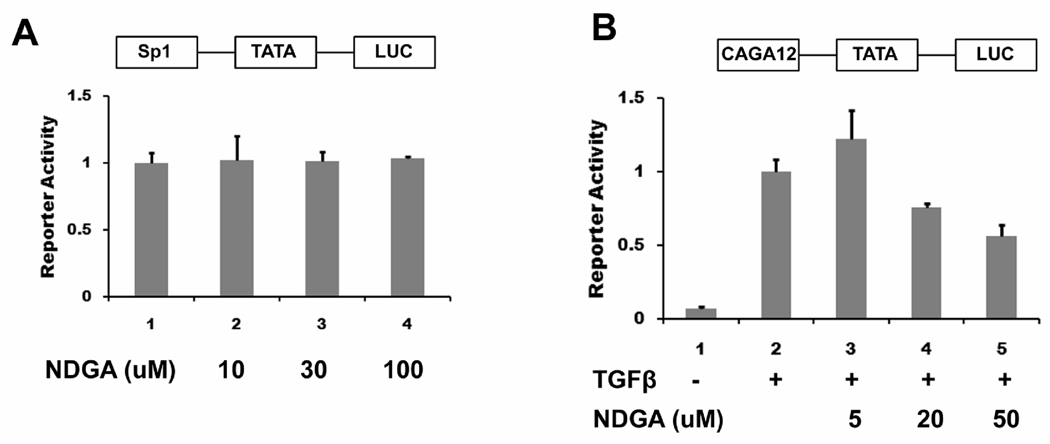
NDGA has been reported to repress the activator protein (AP1) mediated transcription. An analog of NDGA, in which all of the four hydroxyls of the two catechol rings were methylated (aka M4N), was shown to repress Sp1 mediated transcription. (Gonzales and Bowden, 2002; Huang et al., 2003; Kumar et al., 2007). Therefore, we studied the ability of NDGA to modulate the activity of two unrelated transcription pathways. We first tested the effects of NDGA on Sp1-mediated transcription by transfecting 293 FT cells with Sp1-Luc construct, a luciferase reporter driven by the transcription factor Sp1. To exclude the possibility that the transcriptional repression by 100 µM NDGA is nonspecific, the TATA-Luc reporter, which represents transcription driven by the minimal promoter region, was used as a control and luciferase activity of Sp1-Luc was normalized to basal TATA-Luc reporter activity. NDGA treatment showed no effects on the Sp1 specific reporter activity on concentrations up to 100 µM NDGA (Fig 2A).
Fig. 2.
NDGA has minimal effects on Sp1 mediated transcription but represses TGF-β activated transcription. A. 293 FT cells were transiently transfected with luciferase reporters pRL-null and Sp1-Luc or TATA-Luc. Cells were treated with different amounts of NDGA for 6 hours before being harvested for the dual luciferase assay. Luciferase activity of Sp1-Luc was normalized to TATA-Luc reporter activity. There was no effect on transcription by NDGA treatment at 10 and 30 µM, and 100 µM. B. Panc-1 cells were transiently transfected with luciferase reporters pRL-null and CAGA12-Luc or TATA-Luc. Cells were treated with 10 ng/ml TGF-β and different amounts of NDGA for 6 hours before being harvested for the dual luciferase assay. Luciferase activity of CAGA12-Luc was normalized to TATA-Luc reporter activity. TGF-β treatment induced activation of the reporter, and NDGA repressed this activation in a dose-dependent manner.
To test the effects of NDGA on Smad2 mediated transcription, we then transfected Panc-1 cells with the CAGA12-Luc construct, a promoter activated by Smads with a luciferase reporter. As above, luciferase activity of CAGA12-Luc was normalized to basal TATA-Luc reporter activity. TGF-β treatment of these cells induced transcriptional activation of the reporter as reported previously, and NDGA significantly inhibited reporter activation in a dose dependent manner with 46 ± 7% inhibition observed at the highest concentration employed (50 µM) (Fig 2B). These data suggest that NDGA inhibits Smad2 mediated transcription activated by TGF-β treatment.
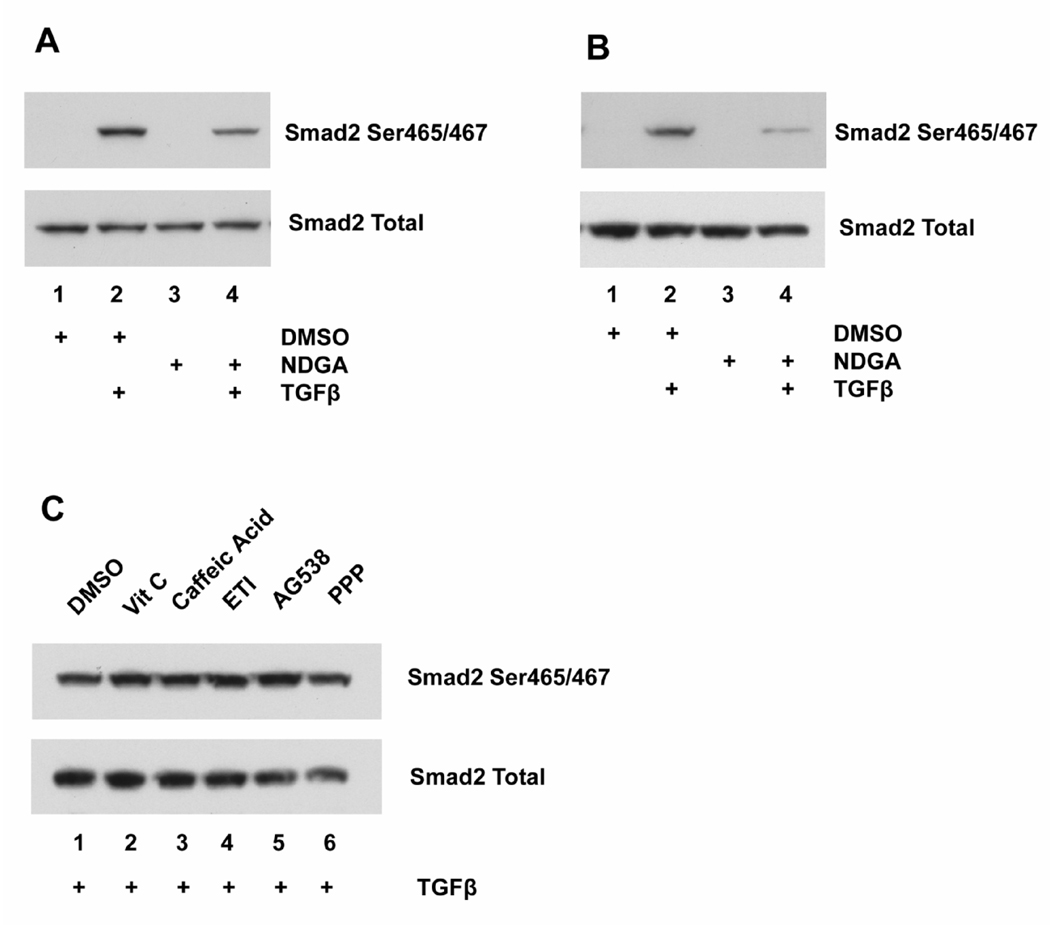
The repression of TGF-β activated transcription by NDGA could be due to many mechanisms. Since Smad2 phosphorylation is a critical step in this event, we tested the hypothesis that NDGA repress transcription through inhibiting Samd2 phosphorylation. As expected, short-term NDGA treatment inhibited Smad2 phosphorylation induced by TGF-β treatment in Panc-1 cells by 50 ± 3% with no effect on the total Smad2 protein level (Fig 3A). It is possible that long-term NDGA treatment might have different effects on TGF- β signaling than short-term treatment. Therefore, we treated Panc-1 cells with NDGA and TGF-β for three days, and determined Smad2 phosphorylation by immunoblotting. As shown in Fig 3B, long-term NDGA treatment also inhibited TGF-β induced Smad2 phosphorylation to similar extent (56 ± 13%), while the total Smad2 level remained unchanged. As an initial test to ensure that these effects on Smad2 phosphorylation related specifically to actions on the TGF-β signaling pathway, we assessed the effects of several small molecules that reproduce several other known cellular actions of NDGA on Smad2 phosphorylation in Panc-1 cells. No effect on Smad2 phosphorylation was observed by incubation with the anti-oxidant vitamin C, the lipoxygenase inhibitors caffeic acid or 5,8,11-eicosatriynoic acid (ETI), or the IGF-1 receptor inhibitors AG538 or picropodophyllin (PPP) (Fig 3C). These results suggest that in Panc-1 cells, NDGA inhibits Smad2 phosphorylation via a specific effect on TGF-β signaling.
Fig. 3.
NDGA inhibits Smad2 phosphorylation induced by TGF-β treatment in Panc-1 cells. A. Panc-1 cells were pretreated with 40 µM NDGA or DMSO for one hour, followed by treatment with 10 ng/ml TGF-β for another hour. Phospho-Smad2 and Smad2 were tested by immunoblotting. TGF-β treatment induced increased Smad2 phosphorylation, and NDGA treatment inhibited this induction. There was no effect on total Smad2 level by any treatment. B. Panc-1 cells were treated with 40 µM NDGA or DMSO in the presence or absence of 10 ng/ml TGF-β for 3 days, with medium changed every two days. Phospho-Smad2 and Smad2 were tested by immunoblotting. TGF-β treatment increased Smad2 phosphorylation, and NDGA treatment inhibited this induction. There was no effect on total Smad2 level by any treatment. C. Panc-1 cells were pretreated with 100 µg/ml vitamin C, 30 µM caffeic acid, 30 µM 5,8,11-eicosatriynoic acid (ETI), 4 µM AG538, 4 µM picropodophyllin (PPP), or DMSO for one hour, followed by treatment with 10 ng/ml TGF-β for another hour. Phospho-Smad2 and Smad2 were tested by immunoblotting. There was no effect on Smad2 phosphorylation and total Smad2 level by any treatment.
3.2 NDGA inhibits TGF β type I receptor in vivo and in vitro
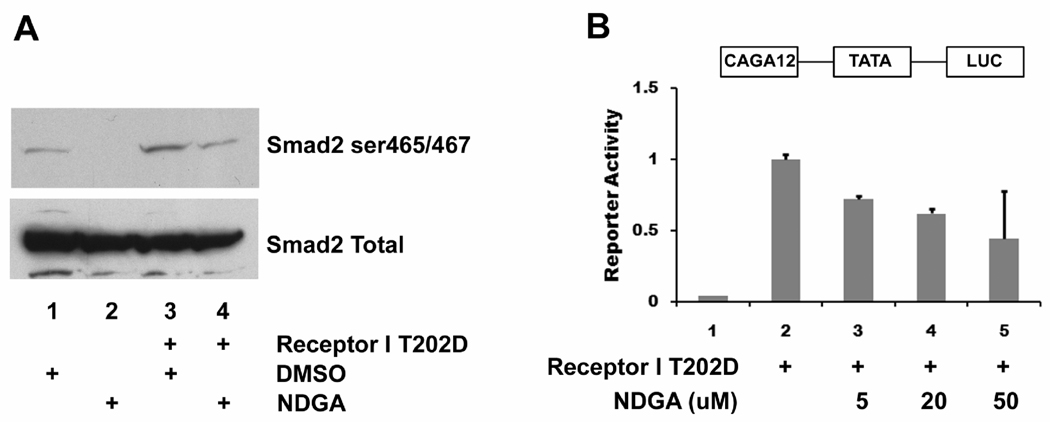
TGF-β signaling activation involves three receptors, TGF β type I receptor, type II receptor, and type III receptor. NDGA has been shown to directly inhibit multiple receptor tyrosine kinases (Domin et al., 1994; Meyer et al., 2008; Youngren et al., 2005). In order to determine whether NDGA inhibition of TGF-β induced Smad2 phosphorylation resulted from specific inhibition of the serine/threonine kinase activity of the TGF β type I receptor, we employed a constitutively active mutant of the TGF β type I receptor (T202D). Transfection of the mutant receptor into 293 FT cells resulted in an approximately 2.5 fold increase in Smad2 phosphorylation level as reported previously, while the total Smad2 level remained unchanged (Fig. 4A). NDGA treatment inhibited basal Smad2 phosphorylation by 67% as well as the mutant TGF β type I receptor-mediated increase in Smad2 phosphorylation by 63% (Fig. 4A). To confirm this inhibitory effect of NDGA on the TGF β type I receptor, we then performed the luciferase reporter assay using CAGA12-Luc as described previously. Co-transfection of the constitutively active TGF β type I receptor mutant activated the reporter as described previously, and NDGA inhibited this transcriptional activation in a dose-dependent manner (Fig. 4B).
Fig. 4.
NDGA inhibits Smad2 phosphorylation and transactivation mediated by TGF β type I receptor in cells. A. 293 FT cells were transfected with TGF β type I receptor T202D (receptor I T202D), a constitutively active mutant of TGF β type I receptor. Cells were treated with 40 µM NDGA or DMSO overnight. Phospho-Smad2 and Smad2 were by immunoblotting. Transfection of mutant receptor significantly increased Smad2 phosphorylation, and NDGA treatment inhibited this induction as well as the basal phosphorylation level of Smad2. There was no effect on total Smad2 level by any treatment. B. Panc-1 cells were transiently transfected with luciferase reporters CAGA12-Luc and pRL-null in the presence or absence of TGF β type I receptor T202D (receptor I T202D). Cells were treated with different amounts of NDGA overnight before being harvested for a luciferase assay. Transfection of mutant the receptor induced activation of the reporter, and NDGA repressed this activation in a dose-dependent manner.
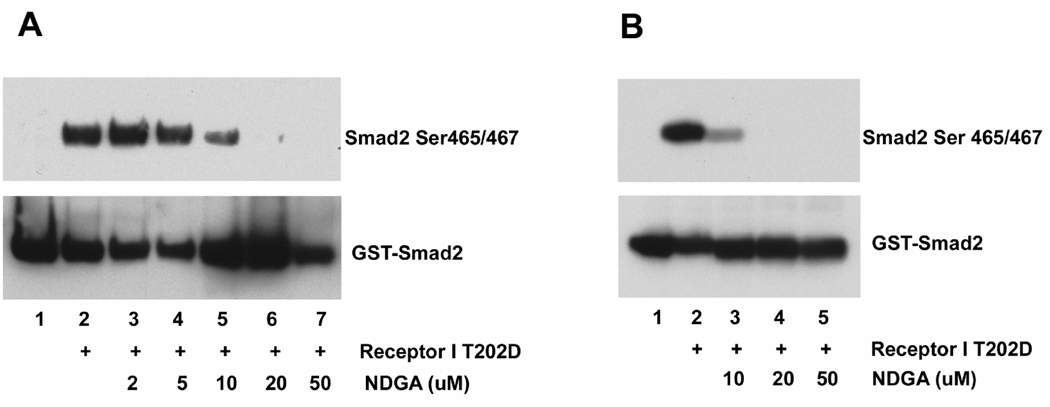
To further explore whether this effect of NDGA on TGF β type I receptor signaling is due to direct or indirect inhibition, we performed in vitro kinase assays in cell free systems. Crude cells lysates from T202D transfected cells were incubated with purified GST-Smad2 fusion protein, and the phosphorylation of Smad2 was analyzed by in vitro pull-down followed by immunoblotting. As shown on Fig. 5A, Smad2 phosphorylation was observed in reactions using lysates from transfected cells but not in reactions using lysates from control cells, suggesting that the observed Smad2 phosphorylation is due to the activity of the transfected mutant receptor. Addition of NDGA to this reaction mixture inhibited Smad2 phosphorylation in a dose-dependent manner. To further confirm that this in vitro inhibition of TGF β type I receptor by NDGA was the result of a direct and then specific interaction, we performed an in vitro kinase assay using immunoprecipitated TGF β type I receptor (T202D) and purified GST-Smad2. When incubated with ATP, the immunoprecipitated receptor directly phosphorylated purified Smad2 while the control immunoprecipitation showed no kinase activity. NDGA effectively inhibited Smad2 phosphorylation in a dose-dependent manner (Fig. 5B), indicating that NDGA directly inhibits the kinase activity of TGF β type I receptor.
Fig. 5.
NDGA inhibits Smad2 phosphorylation mediated by TGF β type I receptor in vitro. A. Cell lysates from TGF β type I receptor T202D (receptor I T202D) transfected cells were incubated with purified GST-Smad2 fusion protein, and the phosphorylated Smad2 was analyzed by in vitro pull-down followed by immunoblotting. Immunoblotting with anti-GST were used as a pull-down and loading control. Smad2 phosphorylation was observed in reactions using lysates from transfected cells but not in reactions using lysates from control cells. NDGA inhibited Smad2 phosphorylation in a dose-dependent manner. B. Immunoprecipitated TGF β type I receptor T202D (receptor I T202D) were incubated with purified GST-Smad2, and the phosphorylated Smad2 was analyzed by immunoblotting. Immunoblotting with anti-GST was used as a loading control. Immunoprecipitated receptor showed kinase activity against Smad2 while the control immunoprecipitation showed no activity. NDGA inhibited Smad2 phosphorylation in a dose-dependent manner.
3.3. Effects of the NDGA analogs on TGF-β signaling
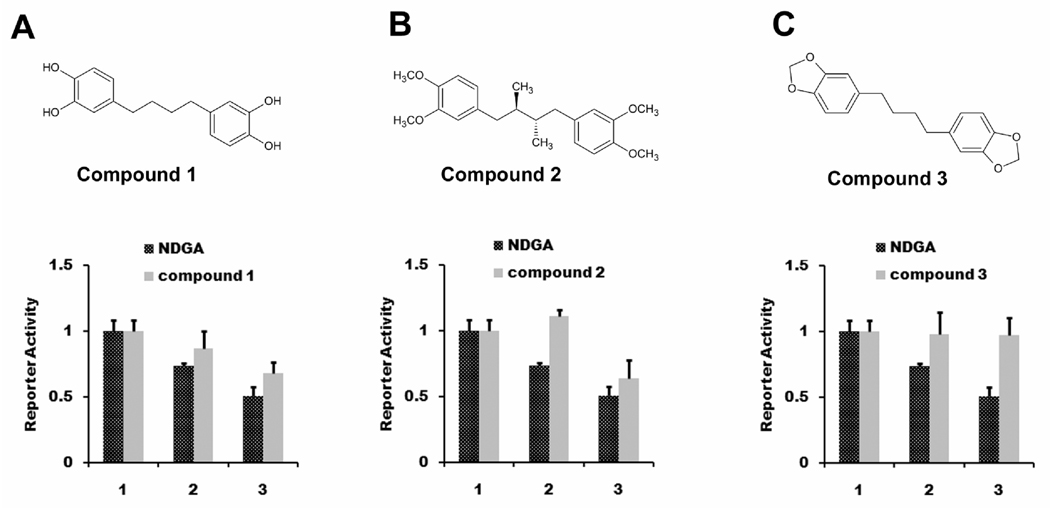
In order to determine whether the inhibition of the TGF β type I receptor was a specific effect that was uniquely related to the structural properties of NDGA, we employed a small chemical library of NDGA analogs to examine the importance of different structural groups of NDGA to its inhibitory effects on TGF-β signaling. In order to assess the contribution of the basic bridge structure, we employed the CAGA12-Luc reporter in TGF-β treated Panc-1 cells. We first tested compound 1, an analog of NDGA in which the methyl substituents on the bridging butyl chain are absent, in this assay. The transcriptional repression activity of compound 1 was decreased compared with NDGA (Fig. 6A), suggesting that the methyl substituent on the bridging butyl chain contributes in part to this activity. In order to determine the importance of the bicatechol structure of NDGA on inhibition of TGF-β signaling, we employed compound 2, an analog of NDGA in which all of the four hydroxyls of the two catechol rings were methylated (aka M4N) (Chen et al., 1998). The tetramethyl analog of NDGA showed diminished inhibition of TGF- β induced transcriptional activity compared to NDGA (Fig. 6B), indicating that these hydroxyl groups also contribute in part to this function. To confirm these observations, we tested compound 3, an analog of NDGA in which the methyl substituent on the bridging butyl chain and all of the four hydroxyls of the two catechol rings were replaced. The transcriptional repression activity of NDGA was abolished completely (Fig. 6C), indicating that the transcriptional repression is dependent on both of these two structural groups.
Fig. 6.
NDGA analogs displayed differential effects on TGF-β mediated transcription. Panc-1 cells were transiently transfected with luciferase reporters CAGA12-Luc and pRL-null. Cells were treated with 10 ng/ml TGF-β and different amounts of NDGA analogs (1, DMSO control; 2, NDGA or NDGA analogs 20µM; 3, NDGA or NDGA analogs 50µM.) for 6 hours before harvested for dual luciferase assay. A) Compound 1 was less potent than NDGA. B) Compound 2 was less potent than NDGA. C) Compound 3 lost the inhibitory effects of NDGA.
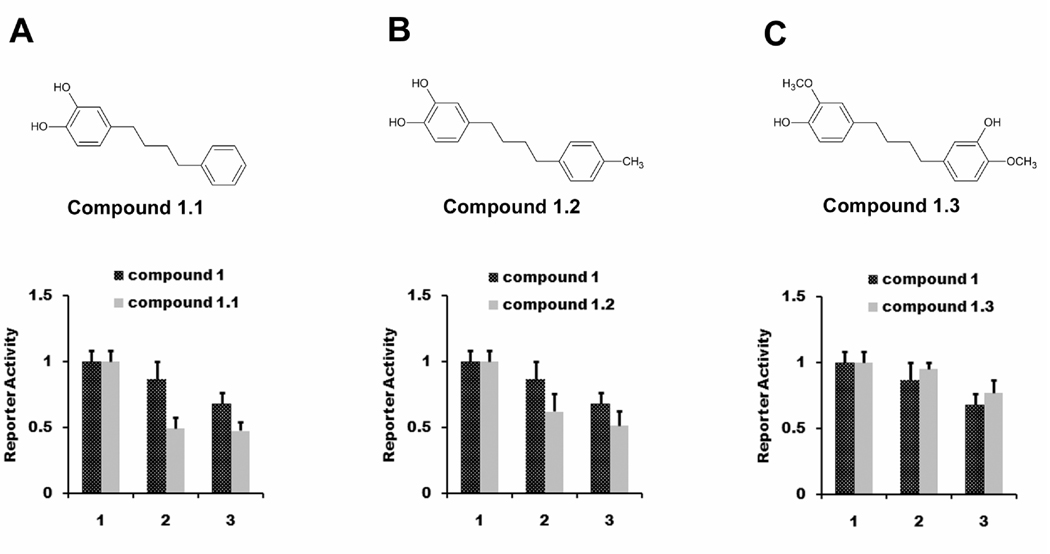
Based on these limited studies, changing the four hydroxyls groups appeared to have a stronger effect on TGF-β inhibitory activity than changing the butane-chain methyl groups. Therefore, we performed experiments to test the relative importance of the second symmetrical dicatechol ring. We obtained three analogs based on structure of compound 1, compounds 1.1, 1.2, and 1.3. In compounds 1.1 and 1.2, the two catechols on the second ring were removed, and were replaced by a 3’ methyl group in compound 1.2. These compounds were studied for their ability to inhibit TGF-β mediated transcriptional activity. Interestingly, with compounds 1.1 and 1.2, in which two hydroxyls from one catechol rings were replaced, we observed significantly improved inhibition of transcription activity, compared with compound 1 (Fig. 7A, 7B). With compound 1.3, in which one hydroxyl from each of the two catechol rings were replaced, this improvement was lost (Fig. 7C). These data indicate that the structure of NDGA is not optimal for inhibiting TGF-β signaling and that its structure can be modified to improve its potency against this target.
Fig. 7.
NDGA analogs can be structurally refined to achieve higher potency. Panc-1 cells were transiently transfected with luciferase reporters CAGA12-Luc and pRL-null. Cells were treated with 10 ng/ml TGF-β and different amounts of NDGA analogs (1 DMSO; 2, NDGA analogs 20µM; 3, NDGA analogs 50µM.) for 6 hours before harvested for dual luciferase assay. A) Compound 1.1 was more potent than compound 1. B) Compound 1.2 was more potent than compound 1. C) Compound 1.3 had similar potency as compound 1.
4. Discussion
NDGA directly inhibits several established anti-cancer targets, including metabolic enzymes and receptor tyrosine kinases. Our study suggests that NDGA also functions as a direct inhibitor of the serine/threonine kinase receptor, TGF β type I receptor. This conclusion is based on the following observations: a) NDGA inhibits TGF-β-induced Smad2 phosphorylation and its downstream transcription in cells; b) NDGA inhibits Smad2 phosphorylation and transcription induced by a constitutively active mutant of TGF β type I receptor in cells; c) NDGA inhibits Smad2 phosphorylation by purified TGF β type I receptor in vitro. This new activity of NDGA allows us to better understand the multiplicity of NDGA’s anti-cancer effects and enable us to further explore its clinical implications.
TGF-β signaling was well established to play a key role in carcinogenesis, and TGF-β inhibitors have been investigated as cancer treatment (Dumont and Arteaga, 2003; Peng et al., 2005). TGF-β neutralizing antibodies have been shown to inhibit growth and metastasis of breast tumors in mice. However, TGF-β targeting therapies have also been shown to increase proliferation and aggressiveness of other cancer models. While there are no clinically approved small molecule TGF-β inhibitors, several are currently under development despite the complicated role for this pathway in tumor biology. Considering the dichotomous role of TGF-β in cancer, NDGA and structurally related analogs represent ideal compounds for inhibition of TGF-β signaling in cancer due to their ability to inhibit additional anti-cancer targets and their consistent growth inhibitory actions across a wide range of cancer cell lines and tumor types. TGF-β signaling is also involved in several additional disorders, and TGF-β inhibitors are under investigation as therapeutics for these disorders including diffuse systemic sclerosis (Dumont and Arteaga, 2003), glaucoma (Siriwardena et al., 2002), and progressive fibrosis (Callahan et al., 2002). The possibility that NDGA or related analogs could also be useful candidates for treating these disorders deserves additional study.
NDGA inhibits TGF-β signaling at micro molar concentration, which is similar to its potency for other targets. It is not clear, however, which cellular actions attributed to NDGA might take precedence in vivo, or how these different effects might interact to produce the observed anti-cancer actions. NDGA has consistently demonstrated anti-cancer actions in a variety of animal models, with a wide range of dosing protocols, although information on target interactions in vivo is limited. Only the IGF-1 receptor and HER2 tyrosine kinase receptors have been shown to be specifically inhibited in tumors of NDGA treated mice (Youngren et al., 2005).
Relatively high doses of NDGA have been administered and well tolerated in both animal studies and clinical trials with minimal toxicity. Anticancer activities were observed in a xenograft model of pancreatic cancer when NDGA was administered at 250 mg/kg/day by gavage, although significant tumor inhibition has been observed with much lower doses (Tong et al., 2002). Interestingly, daily consumption of NDGA estimated at over 400 mg/kg/day resulted in an increased life span of male mice, demonstrating the safety of long-term NDGA ingestion (Strong et al., 2008). Clinically, a phase I study of prostate cancer patients indicated that up to 2500 mg per day of NDGA was well tolerated. (Ryan et al., 2008).
Because of the ability of NDGA to inhibit multiple targets, we performed a preliminary investigation on the structure-potency relationship of NDGA on TGF-β inhibition to determine if this activity was specifically related to any of the structural characteristics of NDGA. We found that modification of the methyl substituents on the bridging butyl chain and the four hydroxyls of the two catechol rings dramatically change the potency of NDGA for TGF-β inhibition. More importantly, we also showed that the potency of two NDGA analogs improved after the two hydroxyl groups from one of the two catechol rings were replaced with a simple phenyl ring or alkyl-substituted phenyl. These findings indicate that the structure of NDGA can be refined to achieve higher potency against TGF-β, and that a compound with a single catechol ring is likely to show improved performance against this target. Interestingly, the asymmetrical compounds tested in the present study that showed increased potency against TGF-β signaling were reported in our previous publication to show improved growth inhibition of breast cancer cells compared to NDGA (Blecha et al., 2007).
In sum, our data demonstrate a new activity of NDGA as an inhibitor of the serine/threonine kinase receptor, TGF β type I receptor. Our study also highlights the possibility that increased potency against this kinase can be achieved through modifying NDGA structure, suggesting NDGA may provide a novel structural motif to generate serine /threonine kinase inhibitors with selectivity for TGF β type I receptor. This present study suggests that NDGA and it analogs hold a great potential to be developed as effective therapeutics against cancer and other illnesses based on this novel mechanism.
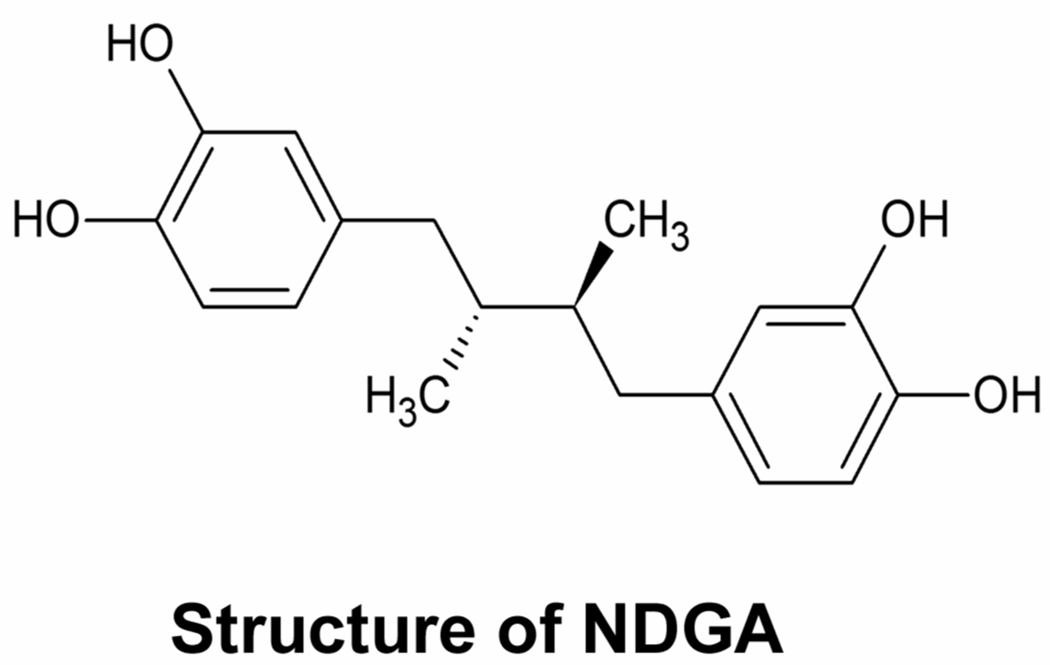
Fig. 1.
Structure of NDGA.
Acknowledgements
We greatly appreciate the gifts of CAGA12-Luc plasmid from Rosemary Akhurst and Sp-Luc, TATA-Luc plasmids from Fred Schaufele. We would also like to give our thanks to Ira Goldfine, Betty Maddux, Lance Stapleton, Michael Campbell, Dhemy Padilla, Markus Lacher, Matthew Lee, and Susan Smith for technical assistance and many helpful suggestions.
This work was supported in part by the National Institutes of Health [Grant U-56 CA92616-04].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- Arteaga S, ndrade-Cetto A, Cardenas R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J Ethnopharmacol. 2005;98:231–239. doi: 10.1016/j.jep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Avis IM, Jett M, Boyle T, Vos MD, Moody T, Treston AM, Martinez A, Mulshine JL. Growth control of lung cancer by interruption of 5-lipoxygenase-mediated growth factor signaling. J Clin Invest. 1996;97:806–813. doi: 10.1172/JCI118480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecha JE, Anderson MO, Chow JM, Guevarra CC, Pender C, Penaranda C, Zavodovskaya M, Youngren JF, Berkman CE. Inhibition of IGF-1R and lipoxygenase by nordihydroguaiaretic acid (NDGA) analogs. Bioorg.Med Chem.Lett. 2007;17:4026–4029. doi: 10.1016/j.bmcl.2007.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R, Mathur A, Olson BA, Weinstock J, Laping NJ. Identification of novel inhibitors of the transforming growth factor beta1 (TGF-beta1) type 1 receptor (ALK5) J Med Chem. 2002;45:999–1001. doi: 10.1021/jm010493y. [DOI] [PubMed] [Google Scholar]
- Chen H, Teng L, Li JN, Park R, Mold DE, Gnabre J, Hwu JR, Tseng WN, Huang RC. Antiviral activities of methylated nordihydroguaiaretic acids. 2. Targeting herpes simplex virus replication by the mutation insensitive transcription inhibitor tetra-O-methyl-NDGA. J Med Chem. 1998;41:3001–3007. doi: 10.1021/jm980182w. [DOI] [PubMed] [Google Scholar]
- De SE, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–3804. [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Domin J, Higgins T, Rozengurt E. Preferential inhibition of platelet-derived growth factor-stimulated DNA synthesis and protein tyrosine phosphorylation by nordihydroguaiaretic acid. J Biol.Chem. 1994;269:8260–8267. [PubMed] [Google Scholar]
- Dumont N, Arteaga CL. Targeting the TGF beta signaling network in human neoplasia. Cancer Cell. 2003;3:531–536. doi: 10.1016/s1535-6108(03)00135-1. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Ligand-independent activation of transforming growth factor (TGF) beta signaling pathways by heteromeric cytoplasmic domains of TGF-beta receptors. J Biol.Chem. 1996;271:13123–13129. doi: 10.1074/jbc.271.22.13123. [DOI] [PubMed] [Google Scholar]
- Gonzales M, Bowden GT. Nordihydroguaiaretic acid-mediated inhibition of ultraviolet B-induced activator protein-1 activation in human keratinocytes. Mol.Carcinog. 2002;34:102–111. doi: 10.1002/mc.10052. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev.Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125:929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Huang JK, Chen WC, Huang CJ, Hsu SS, Chen JS, Cheng HH, Chang HT, Jiann BP, Jan CR. Nordihydroguaiaretic acid-induced Ca2+ handling and cytotoxicity in human prostate cancer cells. Life Sci. 2004;75:2341–2351. doi: 10.1016/j.lfs.2004.04.043. [DOI] [PubMed] [Google Scholar]
- Huang RC, Li Y, Giza PE, Gnabre JN, bd-Elazem IS, King KY, Hwu JR. Novel antiviral agent tetraglycylated nordihydroguaiaretic acid hydrochloride as a host-dependent viral inhibitor. Antiviral Res. 2003;58:57–64. doi: 10.1016/s0166-3542(02)00189-4. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Watkins G, Douglas-Jones A, Mansel RE. Reduction of isoforms of 15-lipoxygenase (15-LOX)-1 and 15-LOX-2 in human breast cancer. Prostaglandins Leukot.Essent.Fatty Acids. 2006;74:235–245. doi: 10.1016/j.plefa.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Kumar S, Wedgwood S, Black SM. Nordihydroguaiaretic acid increases endothelial nitric oxide synthase expression via the transcription factor AP-1. DNA Cell Biol. 2007;26:853–862. doi: 10.1089/dna.2007.0614. [DOI] [PubMed] [Google Scholar]
- Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BH, Ma XF, Wang Y, Tian WX. Structure-activity relationship of polyphenols that inhibit Fatty Acid synthase. J Biochem. 2005;138:679–685. doi: 10.1093/jb/mvi171. [DOI] [PubMed] [Google Scholar]
- Maria MA, Morrissey C, O'Keane C, Mulligan N, Watson C, Smith J, Fitzpatrick JM, Watson RW. Effects of the dual 5 alpha-reductase inhibitor dutasteride on apoptosis in primary cultures of prostate cancer epithelial cells and cell lines. Cancer. 2006;106:2743–2752. doi: 10.1002/cncr.21938. [DOI] [PubMed] [Google Scholar]
- Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Meyer AN, McAndrew CW, Donoghue DJ. Nordihydroguaiaretic acid inhibits an activated fibroblast growth factor receptor 3 mutant and blocks downstream signaling in multiple myeloma cells. Cancer Res. 2008;68:7362–7370. doi: 10.1158/0008-5472.CAN-08-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer GE, Chesler L, Liu D, Gable K, Maddux BA, Goldenberg DD, Youngren JF, Goldfine ID, Weiss WA, Matthay KK, Rosenthal SM. Nordihydroguaiaretic acid inhibits insulin-like growth factor signaling, growth, and survival in human neuroblastoma cells. J Cell Biochem. 2007;102:1529–1541. doi: 10.1002/jcb.21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SB, Yan L, Xia X, Watkins SA, Brooks HB, Beight D, Herron DK, Jones ML, Lampe JW, McMillen WT, Mort N, Sawyer JS, Yingling JM. Kinetic characterization of novel pyrazole TGF-beta receptor I kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochemistry. 2005;44:2293–2304. doi: 10.1021/bi048851x. [DOI] [PubMed] [Google Scholar]
- Rose DP, Connolly JM. Effects of fatty acids and inhibitors of eicosanoid synthesis on the growth of a human breast cancer cell line in culture. Cancer Res. 1990;50:7139–7144. [PubMed] [Google Scholar]
- Ryan CJ, Harzstark AH, Rosenberg J, Lin A, Claros C, Goldfine ID, Kerner JF, Small EJ, Youngren JF. A pilot dose-escalation study of the effects of nordihydroguaiaretic acid on hormone and prostate specific antigen levels in patients with relapsed prostate cancer. BJU.Int. 2008;101:436–439. doi: 10.1111/j.1464-410X.2007.07330.x. [DOI] [PubMed] [Google Scholar]
- Seufferlein T, Seckl MJ, Schwarz E, Beil M, Wichert G, Baust H, Luhrs H, Schmid RM, Adler G. Mechanisms of nordihydroguaiaretic acid-induced growth inhibition and apoptosis in human cancer cells. Br.J Cancer. 2002;86:1188–1196. doi: 10.1038/sj.bjc.6600186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakura S, Boland CR. Eicosanoid production by the human gastric cancer cell line AGS and its relation to cell growth. Cancer Res. 1992;52:1744–1749. [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev.Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Siriwardena D, Khaw PT, King AJ, Donaldson ML, Overton BM, Migdal C, Cordeiro MF. Human antitransforming growth factor beta(2) monoclonal antibody--a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology. 2002;109:427–431. doi: 10.1016/s0161-6420(01)00997-6. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol.Cancer Ther. 2002;1:929–935. [PubMed] [Google Scholar]
- Truty MJ, Urrutia R. Basics of TGF-beta and pancreatic cancer. Pancreatology. 2007;7:423–435. doi: 10.1159/000108959. [DOI] [PubMed] [Google Scholar]
- Van WJ, Goossens J. Effects of antioxidants on cyclooxygenase and lipoxygenase activities in intact human platelets: comparison with indomethacin and ETYA. Prostaglandins. 1983;26:725–730. doi: 10.1016/0090-6980(83)90057-6. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Youngren JF, Gable K, Penaranda C, Maddux BA, Zavodovskaya M, Lobo M, Campbell M, Kerner J, Goldfine ID. Nordihydroguaiaretic acid (NDGA) inhibits the IGF-1 and c-erbB2/HER2/neu receptors and suppresses growth in breast cancer cells. Breast Cancer Res.Treat. 2005;94:37–46. doi: 10.1007/s10549-005-6939-z. [DOI] [PubMed] [Google Scholar]