Abstract
Background & Aims
Osteoprotegerin (OPG), an immunoregulatory member of the TNF receptor superfamily, is expressed in inflamed intestinal mucosa. We investigated whether OPG is produced by intestinal epithelial cells and tested the hypothesis that single nucleotide polymorphisms (SNPs) in the osteoprotegerin gene are associated with travelers’ diarrhea (TD) among US travelers to Mexico.
Methods
OPG concentration was measured in the supernatants of T84 cells infected with various diarrheagenic Escherichia coli pathotypes. Genotyping was performed for four SNPs in the OPG gene from 968 US travelers with and without TD. Stools from travelers with TD were evaluated for the presence of enteric pathogens.
Results
T84 cells produced higher OPG levels in response to infection with various diarrheagenic E. coli pathotypes than E. coli controls (P < .05). A SNP in exon1 region of the OPG gene (OPG+1181 G>C) was associated with TD in Caucasian travelers who stayed in Mexico for more than one week during the summer (P = .009) and for TD due to non-secretory pathogens (P = .001).
Conclusions
Our study suggests that OPG is secreted by intestinal epithelial cells in response to enteropathogens and that a polymorphism in the OPG gene is associated with an increased susceptibility to TD.
Keywords: Travelers’ diarrhea, genetic susceptibility to infection, osteoprotegerin, OPG, single nucleotide polymorphisms
INTRODUCTION
Forty to 60% of travelers from industrialized nations suffer from travelers’ diarrhea (TD) when visiting developing countries [1]. The majority of cases are due to enteropathogens that are endemic to the countries visited [2, 3]. Attack rates vary according to the regions visited and season of travel. The main causal agents of TD are diarrheagenic Escherichia coli with enterotoxigenic E. coli (ETEC), and enteroaggregative E. coli (EAEC) accounting for approximately 50% of all cases [3, 4]. Invasive bacterial pathogens including other E. coli pathotypes, Shigella, Campylobacter and Salmonella are responsible for 10−25% of cases.
In addition to environmental risk factors, individual host genetics could influence susceptibility to TD. Recently, our group demonstrated associations between common polymorphisms the in IL-8 gene promoter and also in exon15 of the lactoferrin gene with infection due to bacterial enteropathogens in US travelers to Mexico[5, 6].
Osteoprotegerin (OPG) was identified a decade ago as a member of the tumor necrosis factor (TNF) receptor superfamily. OPG functions as soluble decoy for receptor activator of nuclear factor-Kappa B ligand (RANKL) and for TNF-related apoptosis-inducing ligand (TRAIL) [7-10]. OPG lacks [8, 9] transmembrane and cytoplasmic domains and when secreted as a mature protein of 308 amino acids, functions as a decoy receptor. OPG's ligand, TRAIL, and its receptors TRAIL-R1, -R2 and –R4 have vital roles in regulation of inflammation in the gut [11, 12] and in determining T helper cell differentiation [11-13]. OPG also serves as an important mediator in bone physiology by blocking the effect of RANKL (expressed by activated cells, osteoblasts and bone marrow cells) on osteoclasts activation [9, 14-16], RANKL/OPG also regulates dendritic cell function [14, 16], B cell maturation and the development of efficient antibody responses [17]. OPG knock-out mice developed severe osteoporosis [18, 19] while overexpression of OPG in transgenic mice results in osteopetrosis [8]. The systemic administration of OPG results in an increase in bone mineral density and prevents ovariectomy-induced bone loss in rats [8] and singe subcutaneous injection of OPG reduced bone resorption in postmenopausal women [20].
The human OPG gene (TNFRSF11B) is a single copy gene located on chromosome 8q23−24 and is composed of five exons spanning 29 kb of the human genome [21]. A number of single nucleotide polymorphisms (SNPs) in the OPG gene have been identified and found to be associated with bone mineral density [22-25], osteoporotic fractures [26], and coronary artery disease [27].
Human intestinal epithelial cell lines constitutively express OPG mRNA, secrete OPG and express mRNA for RANKL. The expression of OPG is increased in inflamed human colonic epithelium compared to healthy controls [28]. The RANKL/OPG system is activated in inflammatory bowel disease (IBD). Intestinal explant cultures obtained from patients with IBD release larger amounts of OPG when compared to tissue from healthy controls or non-inflammed segments obtained from patients with IBD [29].
OPG is up-regulated during epithelial infection and during, human experimental infection with Cryptosporidium oocysts. Furthermore, pretreatment of epithelial cells with TRAIL was shown to induce cell apoptosis and reduce parasite numbers [30].
Since OPG is expressed in multiple tissues including the gastrointestinal tract in response to Cryptosporidium and OPG production is determined by OPG polymorphisms, we sought to investigate if a) OPG was produced in vitro in response to infection with bacterial enteric pathogens, b) OPG increased in response to infection with agents of TD in humans and c) if OPG polymorphisms known to be associated with decreased production of OPG were associated with risk of acquiring TD.
METHODS AND SUBJECTS
T84 cell culture and bacterial infection
A human colonic epithelial cell line (T84) was used to study OPG production in vitro in response to enteric pathogens. T84 cells were seeded into 75-cm2 tissue culture flasks and grown in Dulbecco's modified Eagle medium-F-12 medium (Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 1% antibiotic-antimycotic solution. Flasks were incubated at 37°C under 5%CO2. When the cell monolayer reached confluence or near-confluence, cells were detached from the bottom of the flask by using trypsin-EDTA and were split into 12-well cell culture plate containing antibiotic free medium. T84 cells were then infected at bacteria-to-cell ratio of 100:1 with overnight grown enteroaggregative E. coli strain 042 (EAEC), enterohaemorrhagic E.coli strain 91−8123 (EHEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli strain 115 (ETEC) in separate wells. E. coli HS a commensal strain, and E. coli DH5α, a non-invasive and non-toxigenic strain were included as non-pathogenic controls [31, 32]. Briefly, T84 cells were infected with above mentioned E. coli strains for 3h at 37°C containing in an atmosphere 5% CO2. After 3h at 37°C, serum free-supernatants were collected from each well and human OPG was measured by ELISA as described below. Infection experiments were repeated three times with appropriate controls.
ELISA for OPG
Concentration of human OPG in stool samples and cell culture supernatant was determined by a commercial sandwich ELISA kit (RayBiotech Inc., Norcross, GA) according to the manufacturer's instructions. Briefly, ∼50mg or 50μl of stool samples were diluted five times in dilution buffer and 100μl of samples was added to individual wells in duplicate. A standard concentration curve was constructed using purified human OPG with negative control. After overnight incubation at 4°C, plates were washed with washing buffer and fecal OPG concentration was determined using the standard curve were included in each run.
ELISA for RANKL
Concentration of human RANKL in fecal samples was determined by using a commercial RANKL ELISA kit (BioVendor Laboratory Medicine Inc., Modrice, Czech) according to the manufacturer's instruction. Approximately 50mg or 50μl of stool was diluted five times with dilution buffer and 100μl of the sample was loaded in duplicate onto each well. Plates were processed for ELISA after incubation at 4°C overnight. Recombinant human RANKL was used as standard along with appropriate positive and negative controls. A standard normal curve was generated and used to relate optical density to concentration.
Human Subjects
Adult North American travelers 18 years or older or children > 16 years of age with parental consent, who attended summer language educational programs in Guadalajara and Cuernavaca, Mexico during the summers of 2002−2006 were invited to participate in the study. Visitors were eligible to participate if they were otherwise in good health, and had not traveled to an area of risk for travelers’ diarrhea within the preceding 6 months. Visitors who had pre-existing irritable bowel syndrome, known lactose intolerance, or were taking any medications to prevent travelers’ diarrhea or history of significant underlying enteric, pulmonary, cardiac, or renal disease or used antacids, H2 blockers, or proton pump inhibitors routinely were excluded from this study. Travelers completed a daily diary indicating the number and consistency of bowel movements, and the presence of gastrointestinal symptoms, including abdominal pain, excess gas/bloating, nausea, vomiting, urgency, tenesmus, and fever. Participants were asked to report to clinic on a weekly basis to exchange diaries if they remained healthy. Participants were asked to provide a stool sample for microbiological analysis if they became ill with travelers’ diarrhea. Appropriate treatment was given for diarrheal illness after stools were obtained for studies. Subjects were followed prospectively in Mexico for up to 6 weeks. The presence of acute diarrhea was defined as the passage of 3 or more unformed stools within a 24 hour period plus 1 or more supportive symptom of enteric infection including the following abdominal pain, cramping, excessive gas, nausea, vomiting, fever, urgency, bloody or mucus stool, and tenesmus. Written and informed consent to participate in this study was obtained from all subjects. This study was approved by the University of Texas Health Science Center Committee (Houston) for the Protection of Human Subjects.
Microbiology Examination
Stool samples from subjects with diarrhea were collected and transported at 4°C directly or in Cary-Blair transport media to the laboratory. An aliquot of stool was frozen for analyzing various markers. Fecal specimens were subjected to microbiologic analysis in our field laboratory in Mexico. Five individual E. coli like colonies were immediately stored at −20°C until further processed. Stools were also examined for enteric protozoal parasites, including Giardia lamblia, Entamoeba histolytica and Cryptosporidium species, by use of EIAs (Alexon, Indianapolis, IN). Cultures for enteric bacteria were performed by using 6 standard media: MacConkey, Tergitol, Hektoen enteric, Yersinia, TCBS, and Campylobacter agar plates. The presence of diarrheagenic E. coli (EAEC, ETEC, EPEC, STEC, EIEC) was detected by colony hybridization as previously described [33], by colony PCR, or by fecal PCR using specific primers for respective virulence markers (Table 1) [34-36]. Stools were evaluated for the presence of mucus, fecal leukocytes, and occult blood by conventional methods.
Table 1.
Primers used in this study for the identification of enteropathogens
| E. coli pathotype | Gene | Primer sequence | Amplicon (bp in size) |
|---|---|---|---|
| EAEC | aggR | 5-CTAATTGTACAATCGATGTA-3' | 308 |
| 5'-CTGAAGTAATTCTTGAAT-3' | |||
| aatA | 5'-CTGGCGAAAGACTGTATCAT-3' | 630 | |
| 5'-CAATGTATAGAAATCCGCTGTT-3' | |||
| STEC | stx1 | 5'-CTGGATTTAATGTCGCATAGTG-3' | 150 |
| 5'-AGAACGCCCACTGAGATCATC-3' | |||
| stx2 | 5'-GGCACTGTCTGAAACTGCTCC-3' | 255 | |
| 5'-TCGCCAGTTATCTGACATTCTG-3' | |||
| EIEC | ial | 5'-GGTATGATGATGATGAGTCCA-3' | 650 |
| 5'-GGAGGCCAACAATTATTTCC-3' | |||
| ipaH | 5'-GTTCCTTGACCGCCTTTCCGATACCGTC-3' | 620 | |
| 5'-GCCGGTCAGCCACCCTCTGAGAGTAC-3' | |||
| EPEC | bfpA | 5'-AATGGTGCTTGCGCTTGCTGC-3' | 324 |
| 5'-GCCGCTTTATCCAACCTGGTA-3' | |||
| eaeA | 5'-GACCCGGCACAAGCATAAGC-3' | 384 | |
| 5'-CCACCTGCAGCAACAAGAGG-3' | |||
| ETEC | est | 5'-ATTTTTCTTTCTGTATTGTCTT-3' | 190 |
| 5'-CACCCGGTACAAGCAGGATT-3' | |||
| elt | 5'-GGCGACAGATTATACCGTGC-3' | 450 | |
| 5'-CGGTCTCTATATTCCCTGTT-3' |
EAEC, enteroaggregative Escherichia coli; STEC, Shiga toxin producing Escherichia coli; EIEC, enteroinvasive Escherichia coli; EPEC, enteropathogenic Escherichia coli; ETEC, enterotoxigenic Escherichia coli
Genotyping
Human genomic DNA was extracted from peripheral blood leukocytes using a genomic DNA purification kit (Gentra System Inc., Minneapolis, MN) or from human saliva using Oragene DNA purification kit (DNA Genoteck Inc., Ottawa, Ontario, Canada). The SNPs in the OPG gene were determined by the SNPlex Genotyping System (Applied Biosystems Inc., Foster City. CA), using approximately 80ng of DNA as per manufacturers’ instruction. Four OPG SNPs were studied and they are described in table 2. These SNPs include a SNP in position −950 of the promoter region, a SNP in position +1181 in exon1, and SNPs in introns 2 and 4. GeneMapper software (Applied Biosystems Inc., Foster City, CA) was used for analyzing the sequencing data and calling SNP genotypes, which assigns individual genotypes based on the intensity and location of peaks.
Table 2.
Demographic characteristics of study participants and risk factors associated with travelers’ diarrhea
| Characteristic | All subjects n=968 | Healthy n=494 (51%) | Travelers’ Diarrhea n=474 (49%) | RR (95% CI) | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 308 (31.8) | 152 (30.8) | 156 (32.9) | 1.051 (0.915−1.2) | .491 |
| Female | 660 (68.2) | 342 (69.2) | 318 (67.1) | ||
| Race | |||||
| Caucasian | 872 (90.1) | 438 (88.7) | 434 (91.6) | ||
| African-American | 54 (5.6) | 32 (6.5) | 22 (4.6) | 1.222 (0.93−1.745) | .197 |
| Asian | 23 (2.4) | 16 (3.2) | 7 (1.5) | ||
| Indian American | 3 (0.3) | 2 (0.4) | 1 (0.2) | ||
| Pacific Islander | 3 (0.3) | 2 (0.4) | 1 (0.2) | ||
| Other | 13 (1.3) | 5 (1.0) | 8 (1.7) | 1.225 (0.972−1.599) | .106 |
| Ethnicity | |||||
| Non-Hispanic | 856 (88.4) | 438 (88.7) | 418 (88.2) | ||
| Hispanic | 112 (11.6) | 56 (11.3) | 56 (11.8) | 0.977 (0.814−1.23) | .841 |
| Age, mean years ± SD | 30.3±13.4 | 31.8±14.1 | 28.7±12.4 | < .0001 | |
| Length of stay, mean days ± SD | 25.2±10.8 | 23.2±10.2 | 27.3±11.1 | < .0001 | |
| Season of travel | |||||
| Summer | 773 (80.0) | 377 (76.3) | 396 (83.5) | 1.291 (1.08−1.566) | |
| Other | 194 (20.0) | 117 (23.7) | 77 (16.2) | .005 |
RR, relative risk; CI, confidence interval
Statistical Analysis
Univariate and multivariate analyses were performed using SPSS version 15.0 software package (SPSS Company, Chicago, IL) for Windows. The association between OPG genotypes and outcome phenotypes such as microbiological, clinical data was assessed by Chi-square test. Fisher's exact test was used to calculate the association between OPG genotypes and clinical outcomes. In each case, the relative risks (RR) and 95% confidence Intervals (CIs) were calculated. Continuous variables were compared between cases and healthy controls by ANOVA.
RESULTS
OPG production after in vitro infection
The OPG concentrations found in culture supernatants of T84 cell line with and without infection by the various E. coli strains are presented in figure 1. T84 cells infected with EAEC, EHEC, EPEC and ETEC induced the production of significantly higher levels of OPG when compared to infection-free T84 cells (P < .05) (Fig.1). OPG production did not increase in the supernatant of cells exposed to non-pathogenic E. coli strains HS and DH5α.
Figure 1.
Osteoprotegerin levels (pg/ml) measured by ELISA in supernatants of human intestinal cell cultures infected with various E. coli strains. T84 cells were infected with enteroaggregative E. coli 042 (EAEC) enterohaemorrhagic E.coli 91−8123 (EHEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli 115 (ETEC), E. coli, HS and E. coli DH5α at bacteria-to-cell ratio of 100:1 for 3h at 37°C. ELISA was performed from culture supernatants and results were expressed in pg/ml. The experiment was performed three times in duplicate.
Study population and risk factors for travelers’ diarrhea
Nine hundred and sixty eight travelers were included in this study. As depicted in table 2, 68.2% of participants were female, 872 (90.1%) were white, 54 (5.6%) were black, 23 (2.4%) were Asian, and 19 (2%) were of other races. The hispanic ethnic group was composed of 112 (11.6%) subjects. Univariate analysis revealed that younger age, longer duration of stay and summer time travel [37] had significant influences on rates of diarrhea (Table 2).
OPG SNPs and travelers’ diarrhea
Among the 4 SNPs examined, the SNP at position +1181 (rs#2073618) in the exon 1 region, demonstrated a significant association with TD among North American travelers to Mexico (RR, 1.26; 95% CI, 1.09−1.43). This +1181G>C, polymorphism, resulted in a substitution that causes corresponding amino acid change at codon3 from lysine (AAG) to asparagine (AAC). In contrast, a SNP in promoter region, OPG-950T>C and two SNPs in intronic regions were not associated with TD (Table 3). The genotype distribution for the SNPs examined and their outcome is shown in table 3. A multivariate logistic regression analysis of risk factors for TD in which the OPG+1181G>C genotype was included demonstrated that length of stay (OR, 1.03; 95% CI, 1.01−1.04; P < .0001), summer season travel (OR, 2.93; 95% CI, 0.79−10.84; P = .01), younger age at travel (OR, 0.99; 95% CI, 0.97−1.0; P = .06) and presence of the OPG+1181 CC genotype (OR, 1.6; 95% CI, 1.2−2.2; P = .001) as independently significant risk factors for development of diarrhea.
Table 3.
Human osteoprotegerin gene single nucleotide polymorphisms studied and their association with diarrhea in North American travelers to Mexico
| SNP name | rs # | SNP | Location | Genotype | All subjects | Healthy | Travelers’ Diarrhea | Pa |
|---|---|---|---|---|---|---|---|---|
| OPG-950T>C (n=960) | 2073617 | T/C | Promoter | T/T | 224 (23.3) | 122 (24.8) | 102 (21.7) | |
| T/C | 496 (51.7) | 255 (51.9) | 241 (51.3) | |||||
| C/C | 240 (25.0) | 114 (23.2) | 126 (26.8) | .31 | ||||
| OPG+1181G>C (n=968) | 2073618 | G/C | Exon1 | G/G | 265 (27.3) | 134 (27.1) | 131 (27.6) | |
| G/C | 448 (46.3) | 252 (51) | 196 (41.3) | |||||
| CC | 255 (26.3) | 108 (21.8) | 147 (31.0) | .002 | ||||
| OPG2 (n=831) | 1564858 | G/A | Intron2 | G/G | 639 (76.8) | 333 (76.4) | 306 (77.5) | |
| G/A | 118 (14.2) | 66 (15.1) | 52 (13.2) | |||||
| A/A | 74 (8.9) | 37 (8.5) | 37 (9.4) | .67 | ||||
| OPG4 (n=930) | 7844539 | T/G | Intron4 | T/T | 732 (78.7) | 368 (78.3) | 364 (79.1) | |
| T/G | 183 (19.7) | 95 (20.2) | 88 (19.1) | |||||
| G/G | 15 (1.6) | 7 (1.5) | 8 (1.7) | .88 |
rs#, reference SNP number; TD, traveler's diarrhea
P value based on 3×2 chi-square test
We then compared the distribution of genotypes for the SNP in position +1181 among the various racial groups. Among Caucasians travelers, 25.1% were of the GG, 47.2% were of the GC and 27.6% were of the CC genotypes. In contrast, 53.7% of African-American visitors were of the GG, 40.7% of the GC and 5.5% of the CC genotypes (P < .0001 for Caucasians compared to African-American and all other racial groups).
Since the majority of our study participants (90%) were Caucasians, and the genotype distribution was influenced by race, we stratified our analysis and focused on the Caucasian participants to decrease genetic variability. Given the influence of length of stay and season of travel we conducted a subgroup analysis that included Caucasian participants traveling for 7 days or more during summer. In this subgroup analysis composed of 688 travelers, the distribution of the OPG+1181 genotypes in TD cases was compared to the genotypes in healthy controls. As shown in table 4 the genotype distribution was significantly different. Further analysis demonstrated that travelers with the CC genotype were more likely to experience TD (23 vs.32%; RR, 1.23; 95% CI, 1.06−1.42). This suggests that the influence of the OPG+1181 G>C genotype follows a recessive model of inheritance. The genotype frequencies were in agreement with Hardy-Weinberg equilibrium (P > .05).
Table 4.
Distribution of OPG+1181G>C SNP genotypes and alleles and their association with travelers’ diarrhea and markers of inflammation
| Clinical Outcome | Stools with | |||||
|---|---|---|---|---|---|---|
| All subjects n=688 | Healthy n=331 (48) | Diarrhea n=357 (52) | WBC n=32 | RBC n=9 | Mucus n=60 | Any Inflammatory Marker n=75 |
| GG n=183 | 86 (26) | 97 (27) | 10 (31) | 3 (33) | 11 (18) | 15 (20) |
| GC n=312 | 168 (51) | 144 (40) | 13 (41) | 5 (56) | 23 (38) | 29 (39) |
| CC n= 193 | 77 (23) | 116 (32) | 9 (28) | 1 (11) | 26 (43) | 31 (41) |
| P value a | .009 | 0.54 | 0.67 | .005 | .006 | |
| Non-CC n=495 | 254 (77) | 241 (68) | 23 (72) | 8 (89) | 34 (57) | 44 (59) |
| CC n=193 | 77 (23) | 116 (32) | 9 (28) | 1 (11) | 26 (43) | 31 (41) |
| P value b | .009 | .68 | .65 | .002 | .002 | |
| Alleles | ||||||
| G | 340 (51) | 338 (47) | 33 (52) | 11 (61) | 45 (37) | 59 (39) |
| C | 322 (49) | 376 (53) | 31 (48) | 7 (39) | 75 (63) | 91 (61) |
| P value b | .13 | .92 | .56 | .007 | .01 | |
Travelers were stratified in a subgroup of Caucasian travelers who traveled for 1 or more weeks during the summer season.
3×2 chi-square test comparing with healthy travelers.
2×2 chi-square test comparing with healthy travelers.
The potential association between genotypes and agents of diarrhea is shown in Table 5. The frequencies of the genotypes seen in subjects in whom a pathogen was indentified were compared to the frequencies seen in the travelers that remained healthy. Of the 357 subjects that experienced TD, 255 (71%) visited the clinic and provided a stool samples for diagnostic testing. We identified a potential bacterial pathogen in 145 (57%) visitors that submitted a diarrheal stool sample. Among them, an inflammatory pathogen (non-secretory pathogens) was indentified in 106 (42%) visitors (Table 5). At least one of the diarrheagenic E. coli pathotypes tested was detected in the stools of 128 (50%) subjects; while EAEC was identified alone or with other agents in 93 (36%) of subjects; ETEC was identified in 82 (32%) of the cases and included heat-labile, heat stable and heat-labile/heat stable toxin producing strains. EPEC were found in the stools of 62 (24%) subjects whereas STEC were found in the stools of 18 (7%) subjects. In the case of EIEC, ial and ipaH genes were found by PCR in 13 (5%) of stools (Table 5). Salmonella, Shigella and Campylobacter were found in 8 (3%), 3 (1%), and 2 (<1%) subjects respectively. Overall, subjects with the OPG+1181 CC genotype were more likely to have inflammatory pathogens indentified in their stools (RR, 1.85; 95% CI, 1.34−2.54) while no association was seen in the cases due to ETEC (Table 5).
Table 5.
Fecal microbiology, genotype and allele distribution of the single nucleotide polymorphisms OPG+1181 G>C in US visitors to Mexico
| Pathogensa | |||||||
|---|---|---|---|---|---|---|---|
| All subjects n=688 | Healthy n=331 | EAEC n=93 | ETEC n=82 | EPEC n=62 | STEC n=18 | EIEC n=13 | Inflammatory Pathogenb n=106 |
| GG n=183 | 86 (26) | 22 (24) | 24 (29) | 13 (21) | 6 (33) | 4 (23) | 26 (24) |
| GC n=312 | 168 (51) | 39 (42) | 32 (39) | 23 (37) | 3 (17) | 2 (15) | 36 (34) |
| CC n= 193 | 77 (23) | 32 (34) | 26 (32) | 26 (42) | 9 (50) | 7 (54) | 44 (42) |
| P valuec | .09 | .134 | .008 | .009 | .017 | .001 | |
| Non-CC n=495 | 254 (77) | 61 (66) | 56 (68) | 36 (42) | 9 (50) | 6 (38) | 62 (58) |
| CC n=193 | 77 (23) | 32 (34) | 26 (32) | 26 (58) | 9 (50) | 7 (54) | 44 (42) |
| P valued | .03 | .11 | .004 | .02 | .01 | .0004 | |
| Alleles | |||||||
| G | 340 (51) | 83 (45) | 80 (49) | 49 (40) | 15 (42) | 10 (38) | 88 (42) |
| C | 322 (49) | 103 (55) | 84 (51) | 75 (60) | 21 (58) | 16 (62) | 124 (58) |
| P valued | .11 | .6 | .02 | .3 | .2 | .01 | |
Travelers were stratified in a subgroup of Caucasian travelers who traveled for 1 or more weeks during the summer season.
EAEC, enteroaggregative Escherichia coli; ETEC, enterotoxigenic Escherichia coli; EPEC, enteropathogenic Esch erichia coli; STEC, Shiga toxin producing Escherichia coli; EIEC, enteroinvasive Escherichia coli
The total number of pathogens exceeds the number of specimens since mixed infections. In this analysis, each pathogen was considered independently.
Inflammatory pathogens = Salmonella, Shigella, Campylobacter, STEC, EIEC, EPEC.
3 ×2 chi-square test comparing with healthy travelers.
2×2 chi-square test comparing with healthy travelers.
OPG and intestinal inflammation
To study the impact of the OPG+1181 SNP on intestinal inflammation, we compared frequencies of inflammatory markers in stools. We noted a significant difference in the distribution of the CC genotype among subjects with mucus identified in their stools compared to healthy controls [26 (43%) of 60 versus 77 (23%) of 331 respectively; P = .005)]. When we used the presence at least one of the markers for intestinal inflammation, including of blood, mucus or fecal leukocytes as an indicator inflammation we noted that 31 (41%) of 75 subjects belonged to the CC genotype in contrast to 77 (23%) of 331 healthy individuals (P = .006) (Table 4).
OPG in fecal samples
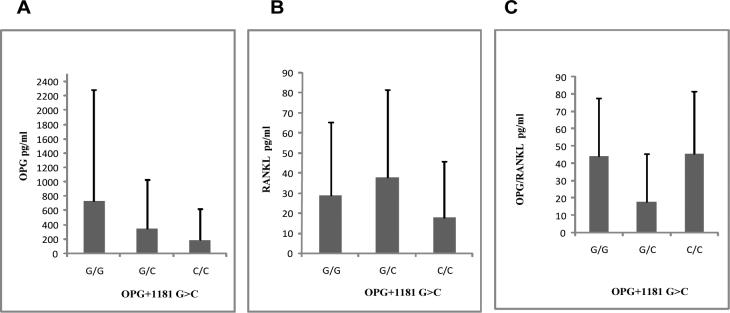
We then measured the concentrations of OPG in stool specimens from healthy subjects and patients with diarrhea. Fecal OPG levels ranged from ≥8 to 1567 pg/ml for healthy visitors and ≥8 to 4536 pg/ml in the case of visitors with diarrhea. The mean OPG level from TD cases tended to be slightly higher, although not significantly so, than that OPG levels in healthy subjects (mean OPG level, 186.3±504.5pg/ml for TD cases vs. 121.1±351.1pg/ml for healthy controls). Since we noticed a significant association between this SNP and clinical outcomes of TD, we sought to investigate the association of genotypes with the level of fecal OPG among travelers with diarrhea. The concentration of fecal OPG was lower in subjects with the CC genotype than visitors with GG and GC genotypes, but the differences did not reach statistically significance (Fig 2A). Similarly, no significant difference between the 3 genotypes was noted in the level of RANKL or in the ratio of OPG/RANKL (Fig. 2 B & C).
Figure 2.
Human osteoprotegerin and RANKL concentrations in fecal samples collected from travelers with OPG+1181 G>C SNP. Data are presented in pg/ml as mean ± SD for G/G (n=35), G/C (n=46) and C/C (n=27) genotype groups.
(A) Association of human fecal osteoprotegerin levels and OPG+1181 G>C SNP (rs#2073618) genotypes. Fecal osteoprotegerin was determined by a sandwich ELISA.
(B) Association of human fecal RANKL levels and OPG+1181 G>C SNP genotypes. Fecal RANKL was measured by a by specific ELISA.
(C) Osteoprotegerin and RANKL ratios corresponding study population genotypes.
DISCUSSION
In this study, we found that a SNP located in exon1 at position +1181 of the OPG gene is associated with diarrhea due to non-secretory enteropathogens in US travelers to Mexico. This is the first report of the association between OPG production in response to intestinal bacterial infection in humans as well as between OPG SNPs and diarrheal disease. Of interest, the +1181 SNP CC was associated with E. coli pathotypes that have traditionally have been associated with inflammatory diarrhea (EPEC, EHEC, and EIEC) rather than with pathotypes associated with toxin production such as ETEC. We noted little correlation with diarrhea due to EAEC, an organism that has the ability to cause non-inflammatory and inflammatory response [4, 31].
The OPG+1181 SNP results in a transition from lysine, a charged polar amino acid to asparagine, an uncharged amino acid. This transition in the OPG signal peptide is thought to lead to decreased OPG secretion. Of interest, postmenopausal women with the OPG+1181 CC genotype have lower serum levels of OPG and increased bone mineral density [38]. Similarly, we found that travelers with diarrhea with the CC genotype (Asn-Asn) had a trend towards lower levels of fecal OPG than visitors with the GG and GC genotypes. This could indicate that a change of amino acid from lysine to asparagine may affect the kinetics of OPG secretion. Our study did not show an association between three OPG genotypes and concentrations of RANKL or with the OPG/RANKL ratio as has been shown in other study [38]. The variability in the range of fecal OPG levels seen in this study could be due to a dilution effect from diarrhea or degradation of OPG while in transit through the gastrointestinal tract, or sample freezing and processing. It is also possible that polymorphic OPG was converted into monomeric OPG [39] and not equally measured by the ELISA.
OPG acts as a decoy receptor for RANKL, inhibiting its binding to RANK, and of TRAIL to its receptors [15]. OPG is widely expressed in tissues, including human intestinal epithelial cells. Several intestinal epithelial cell lines constitutively express OPG mRNA and secrete OPG [28] suggesting that OPG plays an important role in gut homeostasis and in response to inflammation. Of interest, human milk contains OPG in concentrations that are 1000-fold higher than found in normal human serum and could play a potential role in neonatal intestine and development physiology [40].
Serum OPG levels are increased in patients with Crohn's disease and ulcerative colitis [29, 41, 42], The high levels of serum OPG are thought to be in response to IBS induced osteoporosis. In the IL-2 knockout mouse colitis model, recombinant OPG modulates intestinal dendritic cells activity and reverses inflammation [43]. Similarly, we have now demonstrated increased OPG secretion by T84 cells in response to enteric pathogenic but not to non-pathogenic E. coli.
OPG is regulated by inflammatory signals, including NFκB [44]. NFκB is activated in intestinal epithelial cells in response to a number of inflammatory responses and it up regulates OPG expression. OPG may, in turn, blunt the inflammatory effects of RANKL and TRAIL. It is plausible that low OPG levels might worsen the clinical presentation in patients exposed to invasive pathogens. Based on these observations, we hypothesize that OPG functions as an anti-inflammatory mediator in the gut that increases in response to intestinal infection. SNPs in the OPG gene that result in low production of OPG may lead to more severe clinical manifestations, in subjects with infection due to non-secretory organisms.
There are several limitations to this study. First, all travelers with diarrhea did not provide stool samples for microbiology, OPG, and RANKL assays. Secondly, we did not include a control group to evaluate the role of OPG/RANKL in response to asymptomatic colonization with pathogens to further confirm the association of this OPG+1181G>C SNP with symptomatic disease. Thirdly, the exposure to diarrheal causing agents may have not been uniform among participants, as travelers report variable degrees of adherence to avoid risk food items. There is also the potential for the false associations due to multiple comparisons. Controversy exists on the appropriateness of statistical adjustments such as the Bonferroni correction in this setting, which some find is too conservative for an exploratory association study. Additional studies will be needed to confirm this observation in other study populations.
In conclusion, our in vitro cell culture infection model suggests that OPG is secreted by intestinal epithelial cells in response to invasive enteropathogens and that a SNP in exon1 (OPG+1181G>C), of the OPG gene is associated with susceptibility to inflammatory diarrhea in North American travelers to Mexico. The study of OPG and its SNPs may be of use in further understanding host susceptibility and the intestinal immune response to enteropathogens.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health to PCO (R01 AI54948-01), to the Center for Clinical and Translational Sciences (UL1RR024148) of the University of Texas Medical School at Houston, and DK56338, which funds the Texas Gulf Coast Digestive Diseases Center. We are indebted to Dorothy Ruelas, RN, Judy Guillen, RN, Margaret DuPont, MS, David Huang, MD, Lisa Armitige, MD, Jackie Vaca, RN, Stephanie A. Lee, MD, Parvathy Nair, and the administration and staff of Universidad Internacional in Cuernavaca, Morelos, Mexico for their assistance with this project.
Financial Funding: National Institutes of Health to PCO (R01 AI54948−01), to the Center for Clinical and Translational Sciences (UL1RR024148) of the University of Texas Medical School at Houston, and DK56338, which funds the Texas Gulf Coast Digestive Diseases Center.
Footnotes
Potential conflicts of interest: All authors; no conflicts.
Presented in part: 45th Annual Meeting of Infectious Diseases Society of America, San Diego, CA, 4−7 October, 2007 (abstract 458).
References
- 1.Steffen R, Tornieporth N, Clemens SA, et al. Epidemiology of travelers’ diarrhea: details of a global survey. J Travel Med. 2004;11:231–7. doi: 10.2310/7060.2004.19007. [DOI] [PubMed] [Google Scholar]
- 2.Adachi JA, Jiang ZD, Mathewson JJ, et al. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clin Infect Dis. 2001;32:1706–9. doi: 10.1086/320756. [DOI] [PubMed] [Google Scholar]
- 3.Jiang ZD, Lowe B, Verenkar MP, et al. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J Infect Dis. 2002;185:497–502. doi: 10.1086/338834. [DOI] [PubMed] [Google Scholar]
- 4.Jiang ZD, Greenberg D, Nataro JP, Steffen R, DuPont HL. Rate of occurrence and pathogenic effect of enteroaggregative Escherichia coli virulence factors in international travelers. J Clin Microbiol. 2002;40:4185–90. doi: 10.1128/JCM.40.11.4185-4190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang ZD, Okhuysen PC, Guo DC, et al. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promotor region. J Infect Dis. 2003;188:506–11. doi: 10.1086/377102. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed JA, DuPont HL, Jiang ZD, et al. A novel single-nucleotide polymorphism in the lactoferrin gene is associated with susceptibility to diarrhea in North American travelers to Mexico. Clin Infect Dis. 2007;44:945–52. doi: 10.1086/512199. [DOI] [PubMed] [Google Scholar]
- 7.Kwon BS, Wang S, Udagawa N, et al. TR1, a new member of the tumor necrosis factor receptor superfamily, induces fibroblast proliferation and inhibits osteoclastogenesis and bone resorption. Faseb J. 1998;12:845–54. doi: 10.1096/fasebj.12.10.845. [DOI] [PubMed] [Google Scholar]
- 8.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda H, Shima N, Nakagawa N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–37. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 10.Yun TJ, Chaudhary PM, Shu GL, et al. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J Immunol. 1998;161:6113–21. [PubMed] [Google Scholar]
- 11.Strater J, Moller P. Expression and function of death receptors and their natural ligands in the intestine. Ann N Y Acad Sci. 2000;915:162–70. doi: 10.1111/j.1749-6632.2000.tb05239.x. [DOI] [PubMed] [Google Scholar]
- 12.Strater J, Walczak H, Pukrop T, et al. TRAIL and its receptors in the colonic epithelium: a putative role in the defense of viral infections. Gastroenterology. 2002;122:659–66. doi: 10.1053/gast.2002.31889. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XR, Zhang LY, Devadas S, Li L, Keegan AD, Shi YF. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death Differ. 2003;10:203–10. doi: 10.1038/sj.cdd.4401138. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 15.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 16.Wong BR, Josien R, Lee SY, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–80. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun TJ, Tallquist MD, Aicher A, et al. Osteoprotegerin, a crucial regulator of bone metabolism, also regulates B cell development and function. J Immunol. 2001;166:1482–91. doi: 10.4049/jimmunol.166.3.1482. [DOI] [PubMed] [Google Scholar]
- 18.Bucay N, Sarosi I, Dunstan CR, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno A, Amizuka N, Irie K, et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–5. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 20.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2005;20:2275–82. doi: 10.1359/jbmr.2005.20.12.2274. 2004. [DOI] [PubMed] [Google Scholar]
- 21.Morinaga T, Nakagawa N, Yasuda H, Tsuda E, Higashio K. Cloning and characterization of the gene encoding human osteoprotegerin/osteoclastogenesis-inhibitory factor. Eur J Biochem. 1998;254:685–91. doi: 10.1046/j.1432-1327.1998.2540685.x. [DOI] [PubMed] [Google Scholar]
- 22.Arko B, Prezelj J, Komel R, Kocijancic A, Hudler P, Marc J. Sequence variations in the osteoprotegerin gene promoter in patients with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2002;87:4080–4. doi: 10.1210/jc.2002-020124. [DOI] [PubMed] [Google Scholar]
- 23.Richards JB, Rivadeneira F, Inouye M, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–12. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, et al. Multiple genetic loci for bone mineral density and fractures. N Engl J Med. 2008;358:2355–65. doi: 10.1056/NEJMoa0801197. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Ando F, Niino N, Shimokata H. Association of polymorphisms of the osteoprotegerin gene with bone mineral density in Japanese women but not men. Mol Genet Metab. 2003;80:344–9. doi: 10.1016/S1096-7192(03)00125-2. [DOI] [PubMed] [Google Scholar]
- 26.Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF. Polymorphisms in the osteoprotegerin gene are associated with osteoporotic fractures. J Bone Miner Res. 2002;17:1245–55. doi: 10.1359/jbmr.2002.17.7.1245. [DOI] [PubMed] [Google Scholar]
- 27.Soufi M, Schoppet M, Sattler AM, et al. Osteoprotegerin gene polymorphisms in men with coronary artery disease. J Clin Endocrinol Metab. 2004;89:3764–8. doi: 10.1210/jc.2003-032054. [DOI] [PubMed] [Google Scholar]
- 28.Vidal K, Serrant P, Schlosser B, van den Broek P, Lorget F, Donnet-Hughes A. Osteoprotegerin production by human intestinal epithelial cells: a potential regulator of mucosal immune responses. Am J Physiol Gastrointest Liver Physiol. 2004;287:G836–44. doi: 10.1152/ajpgi.00428.2003. [DOI] [PubMed] [Google Scholar]
- 29.Moschen AR, Kaser A, Stadlmann S, et al. The RANKL/OPG system and bone mineral density in patients with chronic liver disease. J Hepatol. 2005;43:973–83. doi: 10.1016/j.jhep.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Castellanos-Gonzalez A, Yancey LS, Wang HC, et al. Cryptosporidium Infection of Human Intestinal Epithelial Cells Increases Expression of Osteoprotegerin: A Novel Mechanism for Evasion of Host Defenses. J Infect Dis. 2008 doi: 10.1086/528374. [DOI] [PubMed] [Google Scholar]
- 31.Huang DB, DuPont HL, Jiang ZD, Carlin L, Okhuysen PC. Interleukin-8 response in an intestinal HCT-8 cell line infected with enteroaggregative and enterotoxigenic Escherichia coli. Clin Diagn Lab Immunol. 2004;11:548–51. doi: 10.1128/CDLI.11.3.548-551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meraz IM, Arikawa K, Ogasawara J, Hase A, Nishikawa Y. Epithelial cells secrete interleukin-8 in response to adhesion and invasion of diffusely adhering Escherichia coli lacking Afa/Dr genes. Microbiol Immunol. 2006;50:159–69. doi: 10.1111/j.1348-0421.2006.tb03781.x. [DOI] [PubMed] [Google Scholar]
- 33.Murray BE, Mathewson JJ, DuPont HL, Hill WE. Utility of oligodeoxyribonucleotide probes for detecting enterotoxigenic Escherichia coli. J Infect Dis. 1987;155:809–11. doi: 10.1093/infdis/155.4.809. [DOI] [PubMed] [Google Scholar]
- 34.Huang DB, Mohamed JA, Nataro JP, DuPont HL, Jiang ZD, Okhuysen PC. Virulence characteristics and the molecular epidemiology of enteroaggregative Escherichia coli isolates from travellers to developing countries. J Med Microbiol. 2007;56:1386–92. doi: 10.1099/jmm.0.47161-0. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Saucedo C, Cerna JF, Villegas-Sepulveda N, et al. Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli. Emerg Infect Dis. 2003;9:127–31. doi: 10.3201/eid0901.01-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohamed JA, Huang DB, Jiang ZD, et al. Association of putative enteroaggregative Escherichia coli virulence genes and biofilm production in isolates from travelers to developing countries. J Clin Microbiol. 2007;45:121–6. doi: 10.1128/JCM.01128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ericsson CD, DuPont HL, Mathewson IJ. Epidemiologic Observations on Diarrhea Developing in U.S. and Mexican Students Living in Guadalajara, Mexico. J Travel Med. 1995;2:6–10. doi: 10.1111/j.1708-8305.1995.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhao HY, Liu JM, Ning G, et al. The influence of Lys3Asn polymorphism in the osteoprotegerin gene on bone mineral density in Chinese postmenopausal women. Osteoporos Int. 2005;16:1519–24. doi: 10.1007/s00198-005-1865-9. [DOI] [PubMed] [Google Scholar]
- 39.Yano K, Tsuda E, Washida N, et al. Immunological characterization of circulating osteoprotegerin/osteoclastogenesis inhibitory factor: increased serum concentrations in postmenopausal women with osteoporosis. J Bone Miner Res. 1999;14:518–27. doi: 10.1359/jbmr.1999.14.4.518. [DOI] [PubMed] [Google Scholar]
- 40.Vidal K, van den Broek P, Lorget F, Donnet-Hughes A. Osteoprotegerin in human milk: a potential role in the regulation of bone metabolism and immune development. Pediatr Res. 2004;55:1001–8. doi: 10.1203/01.pdr.0000127014.22068.15. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein CN, Sargent M, Leslie WD. Serum osteoprotegerin is increased in Crohn's disease: a population-based case control study. Inflamm Bowel Dis. 2005;11:325–30. doi: 10.1097/01.mib.0000164015.60795.ca. [DOI] [PubMed] [Google Scholar]
- 42.Franchimont N, Reenaers C, Lambert C, et al. Increased expression of receptor activator of NF-kappaB ligand (RANKL), its receptor RANK and its decoy receptor osteoprotegerin in the colon of Crohn's disease patients. Clin Exp Immunol. 2004;138:491–8. doi: 10.1111/j.1365-2249.2004.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashcroft AJ, Cruickshank SM, Croucher PI, et al. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity. 2003;19:849–61. doi: 10.1016/s1074-7613(03)00326-1. [DOI] [PubMed] [Google Scholar]
- 44.Toruner M, Fernandez-Zapico M, Sha JJ, Pham L, Urrutia R, Egan LJ. Antianoikis effect of nuclear factor-kappaB through up-regulated expression of osteoprotegerin, BCL-2, and IAP-1. J Biol Chem. 2006;281:8686–96. doi: 10.1074/jbc.M512178200. [DOI] [PubMed] [Google Scholar]