Abstract
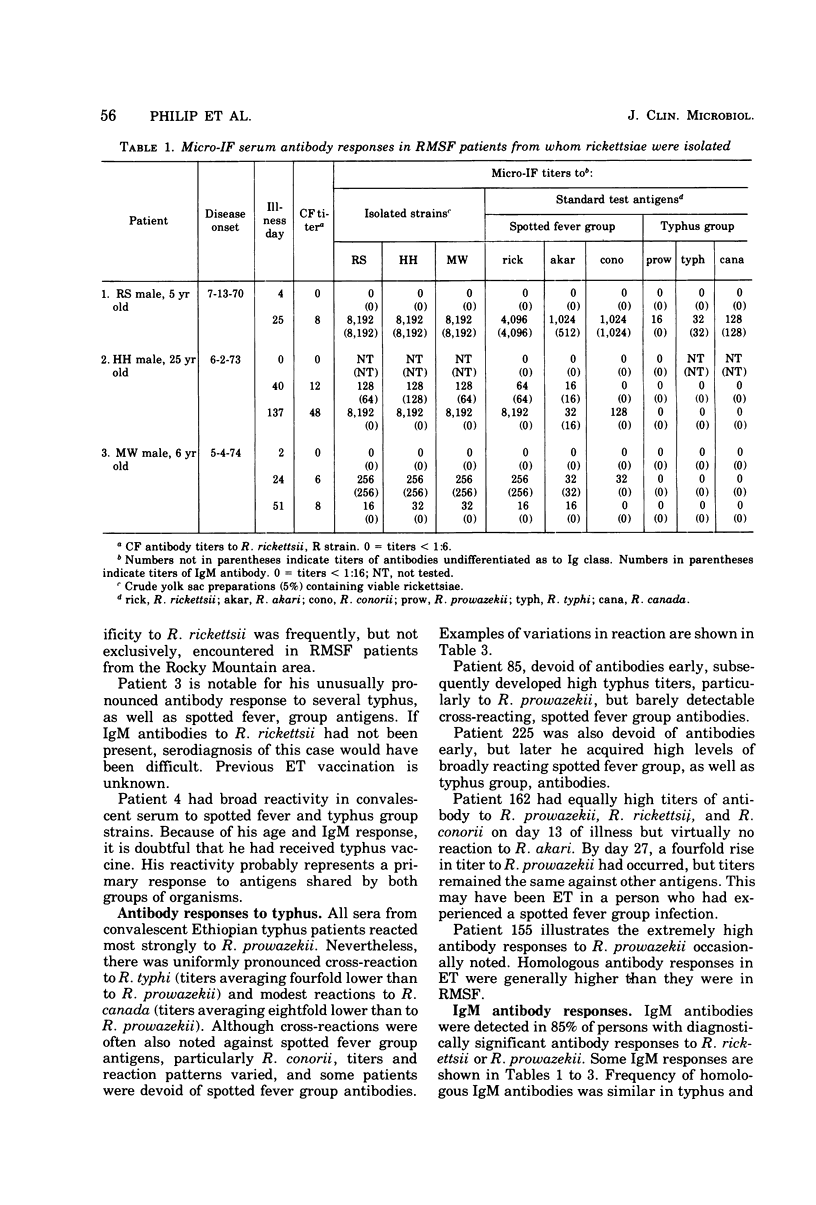
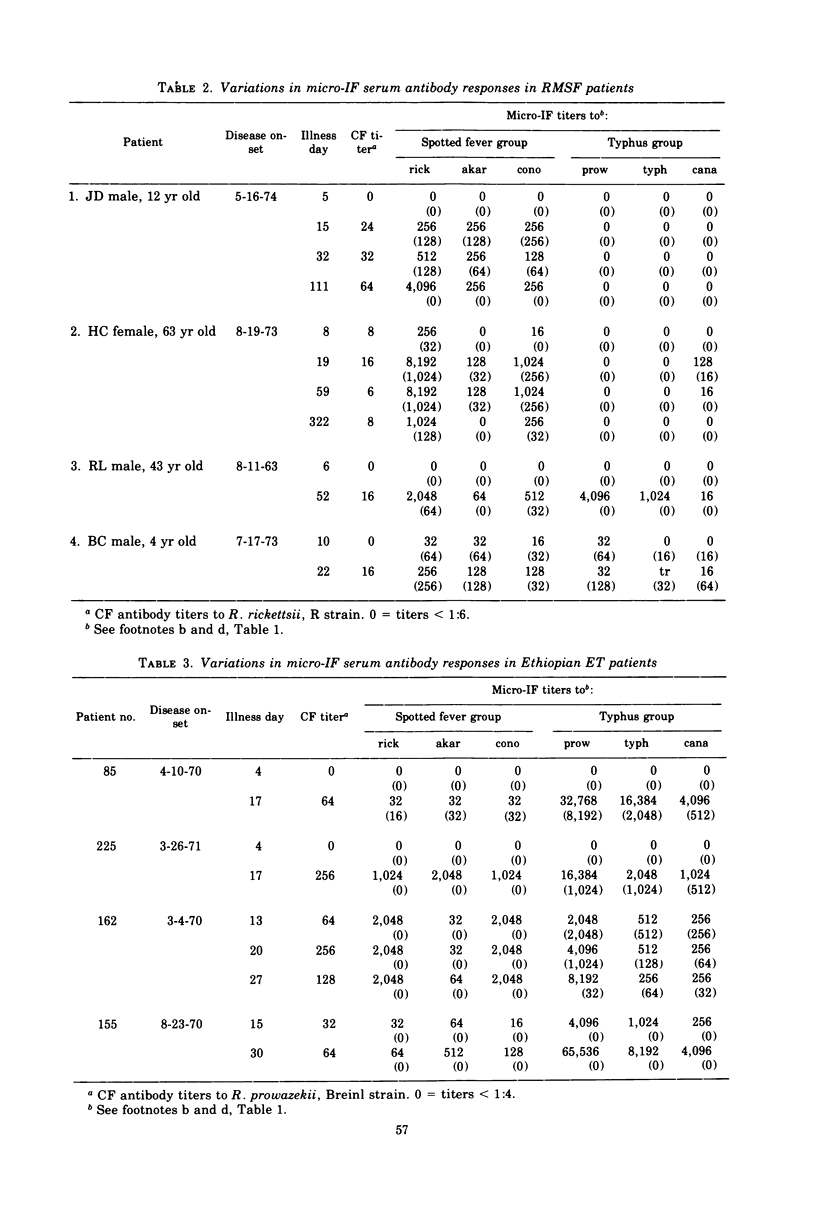
A microimmunofluorescence test was used to study antibody responses to various spotted fever group and typhus group rickettsiae during Rocky Mountain spotted fever (RMSF) and epidemic typhus (ET). Patients with RMSF reacted most strongly to Rickettsia rickettsii; those with ET reacted predominantly to R. prowazekii. The degree of cross-reaction to other rickettsial strains varied from patient to patient, but a particular pattern of cross-reaction was consistently observed in serial sera from the same patient. Fresh isolates from three Montana RMSF cases were indistinguishable from each other and from strain R of R. rickettsii used as a standard antigen in all tests. Immunoglobulin M (IgM) antibodies were usually present in high titer in early-convalescent-phase sera from RMSF, as well as ET, patients. After RMSF, IgM antibodies persisted for a few months and, in one instance, for as long as 10 months. IgM responses to laboratory-acquired infections were infrequent in persons previously vaccinated with antigens related to the infecting strain. Previous antigenic conditioning from infection or vaccination may have accounted partly for the apparent lack of IgM response in a few study participants.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL E. J., KOHLS G. M., STOENNER H. G., LACKMAN D. B. NONPATHOGENIC RICKETTSIAS RELATED TO THE SPOTTED FEVER GROUP ISOLATED FROM TICKS, DERMACENTOR VARIABILIS AND DERMACENTOR ANDERSONI FROM EASTERN MONTANA. J Immunol. 1963 May;90:770–781. [PubMed] [Google Scholar]
- BELL E. J., PICKENS E. G. A toxic substance associated with the rickettsias of the spotted fever group. J Immunol. 1953 May;70(5):461–472. [PubMed] [Google Scholar]
- BOZEMAN F. M., ELISBERG B. L. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc Exp Biol Med. 1963 Mar;112:568–573. doi: 10.3181/00379727-112-28107. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Sexton D. J., Gerloff R. K., Anacker R. L., Philip R. N., Thomas L. A. Rhipicephalus sanguineus: vector of a new spotted fever group rickettsia in the United States. Infect Immun. 1975 Jul;12(1):205–210. doi: 10.1128/iai.12.1.205-210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer R. S., Treharne J. D., Jones B. R., Herring J. Chlamydial infection. Results of micro-immunofluorescence tests for the detection of type-specific antibody in certain chlamydial infections. Br J Vener Dis. 1972 Dec;48(6):452–459. doi: 10.1136/sti.48.6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- GOLDWASSER R. A., SHEPARD C. C. Fluorescent antibody methods in the differentiation of murine and epidemic typhus sera; specificity changes resulting from previous immunization. J Immunol. 1959 Apr;82(4):373–380. [PubMed] [Google Scholar]
- Gutman A., Schreiber H., Taragan R. An outbreak of tick typhus in the coastal plain of Israel. 13 cases from the Sharon area. Trans R Soc Trop Med Hyg. 1973;67(1):112–121. doi: 10.1016/0035-9203(73)90328-3. [DOI] [PubMed] [Google Scholar]
- Hanna L., Jawetz E., Nabli B., Hoshiwara I., Ostler B., Dawson C. Titration and typing of serum antibodies in TRIC infections by immunofluorescence. J Immunol. 1972 Jan;108(1):102–107. [PubMed] [Google Scholar]
- Hazard G. W., Ganz R. N., Nevin R. W., Nauss A. H., Curtis E., Bell D. W., Murray E. S. Rocky mountain spotted fever in the eastern United States. N Engl J Med. 1969 Jan 9;280(2):57–62. doi: 10.1056/NEJM196901092800201. [DOI] [PubMed] [Google Scholar]
- Juchau S. V., Linscott W. D., Schachter J., Jawetz E. Inhibition of antichlamydial IgM antibody by IgG antibody in immunofluorescence tests. J Immunol. 1972 Jun;108(6):1563–1569. [PubMed] [Google Scholar]
- MURRAY E. S., GAON J. A., O'CONNOR J. M., MULAHASANOVIC M. SEROLOGIC STUDIES OF PRIMARY EPIDEMIC TYPHUS AND RECRUDESCENT TYPHUS (BRILL-ZINSSER DISEASE). I. DIFFERENCES IN COMPLEMENT-FIXING ANTIBODIES: HIGH ANTIGEN REQUIREMENT AND HEAT LABILITY. J Immunol. 1965 May;94:723–733. [PubMed] [Google Scholar]
- McKiel J. A., Bell E. J., Lackman D. B. Rickettsia canada: a new member of the typhus group of rickettsiae isolated from Haemaphysalis leporispalustris ticks in Canada. Can J Microbiol. 1967 May;13(5):503–510. doi: 10.1139/m67-065. [DOI] [PubMed] [Google Scholar]
- PARKER R. R., PICKENS E. G., LACKMAN D. B., BELLE E. J., THRAIKILL F. B. Isolation and characterization of Rocky Mountain Spotted Fever Rickettsiae from the rabbit tick Haemaphysalis leporis-palustris Packard. Public Health Rep. 1951 Apr 13;66(15):455–463. [PMC free article] [PubMed] [Google Scholar]
- PICKENS E. G., BELL E. J., LACKMAN D. B., BURGDORFER W. USE OF MOUSE SERUM IN IDENTIFICATION AND SEROLOGIC CLASSIFICATION OF RICKETTSIA AKARI AND RICKETTSIA AUSTRALIS. J Immunol. 1965 Jun;94:883–889. [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Gordon F. B., Quan A. L. Fluorescent antibody responses to chlamydial infection in patients with lymphogranuloma venereum and urethritis. J Immunol. 1974 Jun;112(6):2126–2134. [PubMed] [Google Scholar]
- WELSH H. H., JENSEN F. W., LENNETTE E. H. Q fever studies. XX. Comparison of four serologic techniques for the detection and measurement of antibody to Coxiella burnetii in naturally exposed sheep. Am J Hyg. 1959 Jul;70(1):1–13. [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis. 1974 Oct;130(4):388–397. doi: 10.1093/infdis/130.4.388. [DOI] [PubMed] [Google Scholar]