In a recent issue of The Journal of Physiology,Hajiha et al. (2009) reported a dose-related suppression of genioglossal muscle activity in anaesthetized rats in response to the direct application of opioid compounds (primarily fentanyl) onto the hypoglossal motor nucleus. The decrement in muscle activity was substantial, particularly at higher opioid doses. There is a considerable literature in man indicating that the genioglossus muscle is an important pharyngeal dilator and that decrements in the activity of this and other upper airway dilator muscles decreases pharyngeal patency and probably play an important role in the pathophysiology of obstructive sleep apnoea (White, 2005)). There is, to the best of my knowledge, no previous study in man or animals assessing the influence of opioid compounds on the motoneurons innervating the pharyngeal muscles. This raises the obvious question, are these observations clinically relevant in humans? There are two settings in which this question needs to be considered: in patients in the post-operative time period and in patients receiving chronic opioid medication. Both have systemic opiates on-board at a potentially vulnerable time: during sedation or sleep post-operatively and during sleep in patients on chronic opiates. This assumes that additional mechanisms are available and active during wakefulness to maintain airway patency and this is supported by the literature. The post-op and chronic opioid use situations will be considered below.
There is an evolving literature which suggests that patients with obstructive sleep apnoea (OSA) are at increased risk of adverse outcomes (even death) in the post-operative period (Chung et al. 2008). However, most of the actual papers are either case reports or small case series and the explanation for the increased risk is speculative at best. As the predominant abnormality in most OSA patients is an anatomically small airway, it would seem logical to assume that this anatomy deficiency plays a role in this elevated post-op risk. If we assume that opiates (based on Hajiha et al.) and possibly other sedating medication reduce pharyngeal dilator muscle activity in the post-operative period, then individuals with an anatomically small airway (OSA patients) will be at increased risk of partial or complete pharyngeal collapse during sleep or sedation. However, this reduced pharyngeal patency occurs every night in these patients (assuming their OSA is not treated) and few if any seem to suffer serious acute adverse consequences (sudden myocardial infarction, stroke or death). Thus, why are these individuals uniquely vulnerable in the post-operative period?
I suspect this relates to the recovery mechanism which normally occurs at the end of an apnoea during sleep. Generally an apnoea (or hypopnoea) is terminated by one of two mechanisms. The most common is that the individual briefly wakes up, activates dilator muscles thereby opening the airway, and takes several large breaths to correct the hypoxia and hypercapnia. Less commonly there is a gradual recruitment of pharyngeal dilator muscle activity during sleep in response to rising partial pressure of CO2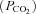 and increasing airway negative pressure such that the airway opens without arousal. In either case, the event is terminated. However, it is quite likely that opiates blunt responsiveness of the upper airway muscles to rising
and increasing airway negative pressure such that the airway opens without arousal. In either case, the event is terminated. However, it is quite likely that opiates blunt responsiveness of the upper airway muscles to rising 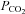 as the ventilatory response to CO2 has been shown to be substantially attenuated by the acute administration of these agents during wakefulness. This may apply to negative pressure stimulation of muscle activity as well. As a result, muscle recruitment to open the airway during stable sleep/sedation may not be possible. Thus, arousal becomes the only avenue to resume breathing. Most evidence suggests that arousal from sleep in response to hypoxia or hypercapnia is the result of increasing respiratory drive and not secondary to direct effects of either hypoxia or hypercapnia (Gleason et al. 1990). Opiates are well known to diminish respiratory drive (Lalley, 2003) thereby potentially reducing or eliminating this mechanism of event termination. Thus, opiates may yield reduced basal pharyngeal muscle activity (as demonstrated by Hajiha et al.), diminished respiratory drive (eliminating subsequent arousal), and possibly poor muscle responsiveness (yet untested), which in combination may contribute in some patients to catastrophic outcomes in the post-operative time frame.
as the ventilatory response to CO2 has been shown to be substantially attenuated by the acute administration of these agents during wakefulness. This may apply to negative pressure stimulation of muscle activity as well. As a result, muscle recruitment to open the airway during stable sleep/sedation may not be possible. Thus, arousal becomes the only avenue to resume breathing. Most evidence suggests that arousal from sleep in response to hypoxia or hypercapnia is the result of increasing respiratory drive and not secondary to direct effects of either hypoxia or hypercapnia (Gleason et al. 1990). Opiates are well known to diminish respiratory drive (Lalley, 2003) thereby potentially reducing or eliminating this mechanism of event termination. Thus, opiates may yield reduced basal pharyngeal muscle activity (as demonstrated by Hajiha et al.), diminished respiratory drive (eliminating subsequent arousal), and possibly poor muscle responsiveness (yet untested), which in combination may contribute in some patients to catastrophic outcomes in the post-operative time frame.
The picture is quite different in patients receiving chronic opioid medication for pain syndromes. In these patients, the predominant abnormality during sleep is central apnoea which may be either ataxic in nature or quite periodic, with a relatively high percentage of these patients demonstrating this respiratory pattern (30% in the largest series (Wang & Teichtahl, 2007)). Obstructive apnoea seems to be uncommon although there are case reports of apparent obstructive hypoventilation with sustained oxygen desaturation during NREM sleep. Why the two groups (post-operative patients receiving acute opioid medications versus chronic opiate users) appear to behave so differently during sleep is unclear although neither group has been well characterized clinically or physiologically. One could speculate that chronic opiate consumption has a very different effect on pharyngeal dilator muscle activity than occurs with short-term use of these agents. However, even acute administration of opiates to normal volunteers did not lead to sleep-disordered breathing of either type. Thus, reduced pharyngeal patency resulting from opiate effects on genioglossal muscle activity does not appear to be an important clinical problem in chronic users of opioid medication. However, considerable further investigation is certainly needed in this area.
References
- Chung SA, Yuan H, Chung F. Anesth Analg. 2008;107:1543–1563. doi: 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- Gleason K, Zwillich CW, White DP. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- Hajiha M, DuBord M-A, Liu H, Horner RL. J Physiol. 2009;587:2677–2692. doi: 10.1113/jphysiol.2009.171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1287–R1304. doi: 10.1152/ajpregu.00199.2003. [DOI] [PubMed] [Google Scholar]
- Wang D, Teichtahl H. Sleep Med Rev. 2007;11:35–46. doi: 10.1016/j.smrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]


