Abstract
The placenta, in general and the physiology of maternofetal nutrient transfer is under-researched compared to other organs with epithelial transport function, as evidenced, for example, by publication numbers. This report provides reasons why more researchers should become involved in this topic. First, the syncytiotrophoblast, the transporting epithelium of the placenta, though having many basic cell physiology properties similar to those of other transporting epithelia, has several properties which are markedly different. Better information on these might help fundamental understanding of how epithelia in general function as well as improving knowledge of how the syncytiotrophoblast operates. Second, the synctiotrophoblast has a key role in controlling fetal growth, not only by transporting nutrients and waste products of metabolism but also because it increasingly appears to be one site, perhaps even the dominant site, in which integration of, sometimes conflicting, signals between mother and fetus takes place. Finally, better understanding of placental nutrient transfer and especially of how it is regulated by maternal and fetal signals could provide better information on the placental phenotype in fetal growth disorders – information which might contribute to providing better biomarkers which the obstetrician could use to improve early diagnosis of these disorders.
The title of this review is deliberately provocative. Its aim is to entice more people to study the transport physiology of the placenta. Considering the fundamental importance of this organ for fetal growth and development, and the impact this has on the health of an individual from prenatal life through to old age (Godfrey & Barker, 2001), it is grossly under-researched. A PubMed search on the word ‘placenta’ for the years 1998–2008 returns 18 024 publications; by comparison the return for ‘kidney’ is 164 943 papers. Delving deeper, search on ‘placenta’ and ‘transport’ gives 1172 papers, whereas ‘kidney’ and ‘transport’ gives 8655 papers.
So, why should anyone bother with studying transport across the placenta? This review will provide an answer on two grounds. (1) The syncytiotrophoblast, the transporting epithelium of the placenta, though having many basic cell physiology properties similar to those of other transporting epithelia, also has several properties which are markedly different. And yet it works well, as most babies are born at the expected size and weight. So the physiologist coming from other fields could gain important insights into the fundamental nature of epithelial transport by studying this unusual epithelium, especially as it enables the study of primary human cells (Clarson et al. 1996; Greenwood et al. 1996). Furthermore, there are still important facets of syncytiotrophoblast transport physiology which remain to be elucidated. (2) In 1942 Flexner posed the question, ‘Does the placenta behave like an inert membrane between the maternal and fetal circulations, or does it contribute energy in the transfer of material from mother to fetus, thereby becoming a secretory organ?’ (Flexner & Gelhorn, 1942). Work since then has clearly shown that the answer is ‘yes’: the placenta is in fact like both a secretory and an absorptive organ. Not only are there an array of transporter proteins, some active, some facilitative, involved in transfer across the synctiotrophoblast, there is good evidence that the activity and, in some cases, expression of these are altered in relation to fetal growth requirements. Furthermore the activity of syncytiotrophoblast transport proteins seems to adapt in relation to both maternal nutrient intake and the demands of fetal growth for a supply of the appropriate nutrients. Synctiotrophoblast increasingly appears to be one site, perhaps even the dominant site, in which integration of, sometimes conflicting, signals between mother and fetus takes place. Understanding the ability of the placenta to adapt to these signals may provide new tools for both diagnosis and treatment of fetal under- and over-growth.
Therefore the intention of this report is not to provide a comprehensive review of placental transport (such recent reviews can be found elsewhere, e.g. Atkinson et al. 2006; Jones et al. 2007), but firstly to consider the syncytiotrophoblast as a transporting epithelium and compare it to other epithelia, indicating where important knowledge is lacking; secondly to consider syncytiotrophoblast transport adaptability as a site of integration of maternal and fetal signals influencing nutrient supply; and finally to speculate on using the information to diagnose the placental phenotype in fetal growth disorders– information which might contribute to providing better biomarkers of fetal growth. I focus on the human placenta, referring only to other species where these provide additional important information. Space limits also exclude consideration of gaseous transfer.
The syncytiotrophoblast as a transporting epithelium
Morphology
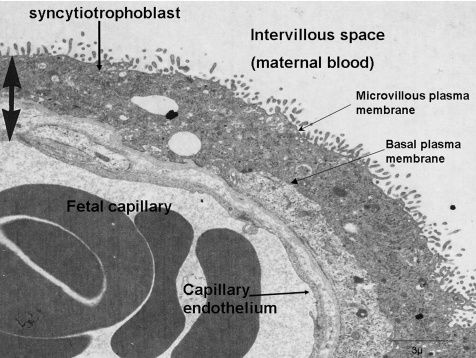
From late first trimester the syncytiotrophoblast has two properties unique amongst transport epithelia: it is perfused by two blood streams, being bathed directly with blood on its maternal face and via a capillary network on its fetal; and it is a true syncytium with no lateral plasma membranes to separate the cytosol associated with each nucleus. The syncytiotrophoblst does have a microvillous, maternal facing, plasma membrane, usually equated to the apical plasma membrane of other epithelia, and its fetal facing plasma membrane is usually taken as equivalent to a basal plasma membrane (Fig. 1). This designation does not, however, appear to be a good guide to the localization of transporter proteins (see below).
Figure 1. Electron micrograph showing the key features of the exchange barrier in the human placenta.
Image kindly provided by Dr Carolyn Jones.
Because it is a syncytium, there is an interesting question as to whether the syncytiotrophoblast forms effectively a single cell covering the whole of the villous placenta. A dye injection study suggests that there is a continuum of syncytiotrophoblast cytosol surrounding the villous (Gaunt & Ockleford, 1986) and this poses special problems for investigating the electrophysiology of the syncytiotrophoblast, as discussed further below.
An electron micrograph of the exchange barrier of the human placenta is shown in Fig. 1. It should be noted that there is a continuous capillary endothelium on the fetal side and that, whilst this is not considered further here, it does make a significant contribution to the barrier properties of the placenta, restricting the permeability to molecules above the size of about 3.0 nm molecular radius (Firth & Leach, 1996).
Passive permeability of the placenta to inert hydrophilic solutes
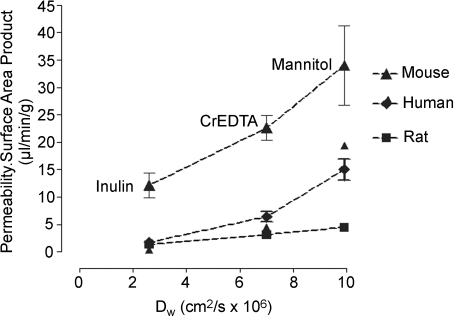
The absence of lateral plasma membranes from the human syncytiotrophoblast would suggest that it might have a relatively low passive permeability. In fact measurements of the unidirectional maternofetal clearance of series of inert hydrophilic solutes (‘inert’ in this context meaning that the solutes are not substrates for any transporter systems) of a range of sizes suggest that the opposite is the case in the human. As shown in Fig. 2, in vivo measurements find an inverse correlation between clearance and molecular radii of these solutes. Furthermore, there is a significant permeability to proteins of the size of alphafetoprotein (AFP) and horseradish peroxidase (used as a tracer in electron microscopy studies) (Edwards et al. 1993; Brownbill et al. 1995). These data with regard to AFP are consistent with the fact that it is synthesized only in the fetus but easily measurable in maternal plasma (Brownbill et al. 2000).
Figure 2. Permeability–surface area product for placentas as measured in vivo in the mouse, human and rat for three inert hydrophilic tracers plotted against their diffusion coefficient in water (Dw).
Data taken from Robinson et al. (1988); Bain et al. (1990) and Sibley et al. (2004).
Across species there does seem to be a relationship between the number of cell layers in the placental exchange barrier and permeability: the epitheliochorial placenta of the sheep, which has four layers between the fetal and maternal blood (fetal capillary endothelium, chorionic epithelium, uterine epithelium and maternal endothelium), is calculated to have a permeability at least one order of magnitude less than that of the human (Boyd et al. 1976). This does not appear to hold within species with haemochorial placentas (where, as in the human, there is only fetal endothelium and trophoblast between maternal and fetal blood). The hameotrichorial (three trophoblast layers) rat placenta does have a slightly lower permeability than the hameomonochorial human but the hameotrichorial mouse placenta has a slightly higher similar permeability (Fig. 2). Although these comparisons may be complicated by the effects of blood flow (Schroder et al. 1985) and water filtration (Faber, 1995), it is far from clear how additional trophoblast layers contribute to placental permeability.
The linear relationship found between clearance of inert hydrophilic solutes across the human placenta and their calculated aqueous diffusion coefficient implies the presence of water filled channels or pores across the syncytiotrophoblast sufficiently wide for there to be no ‘steric hindrance’ to their movement and enabling them to by-pass the ‘cell’ membrane of the syncytiotrophoblast. But how can this be, if the synctium is a single cell with no obvious lateral intercellular spaces? This question has vexed placental physiologists and morphologists for many years and is still not clearly answered, although some possible explanations have been provided.
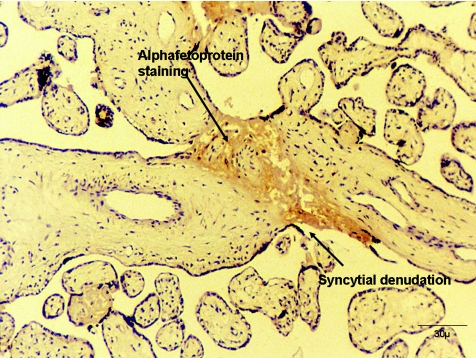
In the guinea-pig placenta, which is haemeomonochorial and syncytial like the human, with a similar permeability to the inert hydrophilic solutes, tubules or bag-like structures in the syncytiotrophoblast can be visualized under the electron microscope but only if the placentas are fixed following imposition of a significant hydrostatic pressure (Kaufmann et al. 1982). Similar structures may also be visualized in the placenta of the degu (Kertschanska et al. 1997). However, it has not been possible to demonstrate whether these structures really are channels which span the width of the syncytiotrophoblast. A different possible aqueous pathway across human placenta might be through areas of denudation of the syncytiotrophoblast which can be observed frequently in all placentas (Edwards et al. 1993). What causes these denudations is not certain but they may arise from damage or from the localized apoptosis which occurs in the syncytiotrophoblast (Nelson, 1996). They are usually observed filled with fibrin-containing fibrinoid, which may provide a foundation for re-syncytialisation of the denudation by cytotrophoblast cells (Nelson, 1996). Using the perfused human placental cotyledon we found that AFP could be localized to such fibrin-containing fibrinoid areas (Fig. 3), suggesting they could provide a paracellular route (Brownbill et al. 1995). In a later study we also found a correlation between the unidirectional maternofetal clearance of creatinine across the perfused placenta and a morphometric measurement of the volume of fibrin-containing fibrinoid filled denudations, but there was no such correlation between AFP clearance and the denudations (Brownbill et al. 2000). The latter result could arise from steric hindrance to the large AFP molecule at the level of diffusion across the lateral intercellular spaces in the fetal capillary endothelium. Therefore, the denudations may provide a channel across the human syncytiotrophoblast, but this is far from proven. Furthermore, there is only evidence for the presence of denudations in the syncytiotrophoblast of villous placentas (Nelson et al. 1997). However, the quantitatively large flux of solutes by paracellular diffusion across these placentas, and the importance of understanding how electrochemical gradients are maintained across the placental barrier in the face of such low resistance pathways, makes this uncertainty one that needs to be resolved.
Figure 3. Immunocytochemical staining of alphafetoprotein in a fibin-containing fibrin deposit within a denuded area of syncytiotrophoblast.
Micrograph kindly provided by Dr D. Michael Nelson.
Electrochemical gradients
A comprehensive review of solute concentrations in maternal and fetal blood is provided in Atkinson et al. (2006). Of particular note is the number of nutrients, on which the fetus depends, for which the fetal plasma concentration is higher than the maternal plasma concentration. This suggests a transport process requiring energy to pump against the concentration gradient, or preferential binding to a component of fetal blood, and/or, for ions, an electrical potential gradient balancing the chemical potential. As mentioned above, these gradients are maintained across the placental exchange barrier despite its apparent high permeability (low resistance).
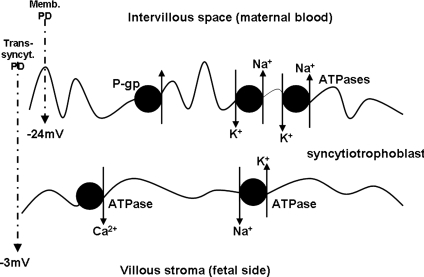
The magnitude and polarity of the electrical potential difference (PD) across the placental exchange barrier has been a topic of much work and debate over many years. It is known that in several species, including possibly human, there is a PD between maternal plasma or extracellular fluid and fetal plasma or extracellular fluid (reviewed in Atkinson et al. 2006), and this has recently been demonstrated again in the mouse (Dilworth et al. 2008). However, there is uncertainty as to whether this maternofetal PD is equivalent to a transplacental PD, i.e. is generated in the exchange barrier of the placenta. In women the maternofetal PD measured in vivo was reported to be 0 mV at term (Mellor et al. 1969), −2.7 ± 0.4 mV, fetus negative in mid-gestation (Stulc et al. 1978) and, as measured between the coelomic cavity and mother, −8.7 ± 1 mV in first trimester (Ward et al. 1998). The last two values especially are (bearing in mind vast experimental differences and likely individual variation) reasonably consistent with the value of −6 mV (range −2.5 to −10 mV) found in the only direct measurement of transplacental PD, using microelectrode techniques on fragments of term placenta in vitro (Greenwood et al. 1993) (Fig. 4). The latter study also reported the trans-syncytiotrophoblast PD to be −3 mV (range 0 to −15 mV) (Greenwood et al. 1993). In similar experiments the microvillous membrane PD (i.e. the PD with just microvillous plasma membrane impaled by microelectrode) was found to decrease from early first trimester (median −32 mV) to late first trimester (median −24 mV) with the term value being only slightly lower than that (−21 mV) (Birdsey et al. 1997). These are all low compared to intracellular PDs measured in other epithelia. There is no reported measurement of PD across the basal plasma membrane of the syncytiotrophoblast. The technical challenge involved in making all of these measurements cannot be underestimated but they badly need to be verified or refuted by others so that ion transfer across the placenta can be accurately modelled (it needs to be remembered that a wide variety of transport processes are affected by PD including those which are clearly directly involved in fetal growth control, e.g. the Na+-dependent transport of some amino acids, as discussed later). In doing so more information will be gleaned as to how a true syncytium can separate charge and maintain resistance (and indeed if the syncytiotrophoblast is electrically a true syncytium). The transporters and membrane properties underlying these PD measurements also need to be understood: the Na+/K+-ATPase appears to make little contribution to PD and, whilst K+ and Cl− conductances certainly do (Birdsey et al. 1997), the channels involved have not been explored. Such functional identification of specific channels has been hampered by another technical challenge in working with a syncytiotrophoblast: the impossibility of whole cell patch clamp recording in a syncytium where capacitance is several orders of magnitude greater than that in a single cell.
Figure 4. Diagram illustrating the polarity of the syncytiotrophoblast in regard to localization of Ca2+-ATPase, P-glycoprotein (P-gp) and Na+/K+-ATPase.
Also shown is the microvillous membrane potential difference (Memb. PD) and trans-syncytiotrophoblast (trans-syncyt.) PD. See text for references.
Distribution of transporter proteins and polarity of the syncytiotrophoblast
In general terms, vectorial transport across an epithelium is usually enabled by electrochemical gradients and/or by the localization of transporter proteins being polarized to either apical or basolateral plasma membranes. Consequently, the mechanisms by which transporter proteins are trafficked to either apical or basolateral plasma membranes has been the subject of much, ongoing, investigation in many epithelia, not least because there is pathophysiology of these processes in diseases (Tanos & Rodriguez-Boulan, 2008).
The syncytiotrophoblast is polarized in terms of both transporters and localization of other proteins to microvillous and basal plasma membranes (examples shown in Fig. 5). However, to my knowledge, there has been no investigation of how this localization is effected and certainly no consideration of whether this is an important component of the pathophysiology of any of the placenta related pregnancy diseases. It is, again, a particularly interesting question to answer in a true synctium without lateral plasma membranes, as in some epithelia proteins are initially trafficked to one plasma membrane and then redirected to the other via lateral movement (Tanos & Rodriguez-Boulan, 2008): this does not seem a possibility for the syncytiotrophoblast. The importance of understanding trafficking in the syncytiotrophoblast can be exemplified with regard to the sodium pump (Na+/K+-ATPase). In most epithelia (a notable exception being the choroid plexus) this transporter is localized to the basolateral plasma membrane whereby it provides the energy, through generation of a Na+ gradient, for the vectorial transport of ions as well as contributing to osmotic gradients for water transfer in the apical to basolateral direction (Brown et al. 2004). It has now been clearly demonstrated that the opposite localization is found in the syncytiotrophoblast – there is more than twice as much sodium pump activity on the microvillous plasma membrane as on the basal plasma membrane (Johansson et al. 2000). Furthermore, data show that this localization is disrupted in idiopathic fetal growth restriction (FGR): microvillous plasma membrane sodium pump expression and activity is reduced in placentas from FGR babies as compared to those from normally grown babies without any effect on basal plasma membrane expression and activity and without any change in overall sodium pump mRNA expression (Johansson et al. 2003). This raises the questions (1) how is this unusual polarity brought about: other transporters are on the expected plasma membrane of the syncytiotrophoblast by analogy with other epithelia (e.g. P-glycoprotein, Ca2+-ATPase; Fisher et al. 1987; Atkinson et al. 2003) (Fig. 5)? (2) How is net flux of ions and water to the fetus brought about when Na+ is likely being pumped in the opposite direction? (3) What goes wrong with trafficking in FGR, and is it only the sodium pump which is affected?
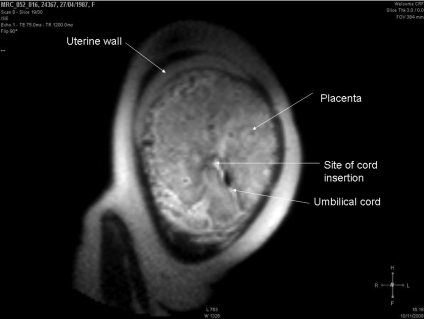
Figure 5. Magnetic resonance image of a placenta.
Image was acquired at 1.5 T from a woman at 36 weeks gestation having a normal pregnancy. Image kindly provided by Dr Caroline Wright and Dr David Morris.
Most epithelia are either secretory or absorptive at any one time; the syncytiotrophoblast has to subserve both these functions simultaneously in dealing with nutrient transfer to the fetus and waste product transfer back to the mother. This, and the requirements of its own cellular homeostasis, might result in the apparently unusual polarity of this epithelium. It should also be remembered that hormonal influences in vivo might alter the apparent polarity as determined in vitro.
Syncytiotrophoblast transport adaptability to fetal nutrient demand signals and integration with maternal supply signals
Fetal growth restriction and placental transfer
The placenta is central to normal fetal growth and it is becoming increasingly apparent that pathophysiology of placental function is a major cause of growth abnormalites, both FGR (failure of a fetus to achieve its genetic growth potential) and macrosomia (where a fetus exceeds its genetic growth potential). These are important issues because FGR is associated with increased risk of antenatal and neonatal death and other morbidities (Thornton et al. 2004) and there is now a well documented association between size at birth and a range of morbidities experienced in adulthood (Godfrey & Barker, 2001). Similarly the macrosomic baby is at great risk of birth difficulties and of obesity in later life (Catalano & Ehrenberg, 2006).
All aspects of placental physiology that have been studied and which contribute to the capacity to exchange nutrients and waste products between mother and fetus are now known to be altered in relation to FGR. Both uterine and umbilical artery blood flows are abnormal in association with FGR as demonstrated with Doppler ultrasound velocity measurements (Cohen-Overbeek et al. 1985). On the uterine side this is related to abnormal trophoblast invasion of the spiral arteries in early pregnancy (Pijnenborg et al. 2006) and on the umbilical side is likely to be due to abnormalities in the architecture and function of the fetoplacental vessels (Krebs et al. 1996; Mills et al. 2005). Although the permeability of the placenta to inert hydrophilic solutes has not been measured in FGR, the related structural variables of exchange barrier surface area and thickness have been shown to decrease and increase, respectively, in this condition (Mayhew et al. 2004). Finally, there is burdgeoning literature describing a wide variety of changes in transporter expression and activity in both the syncytiotrophoblast microvillous and basal plasma membranes in association with FGR (Table 1).
Table 1.
Change in the activity of transporter proteins in syncytiotrophoblast microvillous (MVM) and basal (BM) plasma membranes in fetal growth restriction
| Transporter | MVM Change | BM Change | Reference |
|---|---|---|---|
| System A (alanine/glycine) | Decrease | No change | (Glazier et al. 1997; Jansson et al. 2002) |
| Leucine | Decrease | Decrease | (Jansson et al. 1998) |
| Lysine | No change | Decrease | (Jansson et al. 1998) |
| Na+-dependent taurine | Decrease | No change | (Norberg et al. 1998; Roos et al. 2004) |
| Na+-independent taurine | No change | Decrease | (Norberg et al. 1998) |
| GLUT1 (glucose) | No change | No change | (Jansson et al. 1993; Jansson et al. 2001) |
| Na+/K+-ATPase | Decrease | No change | (Johansson et al. 2003) |
| Ca2+ (ATP dependent) | (not present) | Increase | (Strid et al. 2003) |
| Na+/H+ exchanger | Decrease | Activity not present | (Glazier et al. 1997; Johansson et al. 2002) |
| Lipoprotein lipase (cf FFA transport) | Decrease | Not known | (Magnusson et al. 2004) |
| H+/lactate transporter | No change | Decrease | (Settle et al. 2006) |
Adaptability of syncytiotrophoblast transporter expression and activity to fetal growth demand
As shown in Table 1 the activity of some transporters (measured per mg membrane protein) in the syncytiotrophoblast goes down in relation to FGR, that of at least one transporter goes up, and others do not change at all. This range of changes suggests at least a degree of regulation or adaptation in FGR which could contribute to the disease or which could be responses to maintained fetal demand in the face of decreased placental supply. There is some epidemiological data to support the concept of adaptation in placental supply capacity to meet fetal demand. At any given birth weight, placental weight of small for gestational age (SGA) babies was found to be significantly lower than that of appropriately grown for gestational age babies (Heinonen et al. 2001). This suggests that the placental transfer efficiency (weight of baby per weight of placenta) was actually greater in the SGA group. Further evidence for adaptability comes from a study of System A amino acid transporter in microvillous plasma membranes of placentas from babies across the range of normal birthweights; we found that System A activity, per mg placental membrane protein, was highest in the smallest babies (Godfrey et al. 1998). This contrasts with the situation in frank FGR (Table 1) and suggests that placental efficiency has been increased by upregulating the activity of the transporter per unit of placental membrane enabling fetal growth to continue along the lower limit of the normal range.
Further evidence for this hypothesis comes from mouse studies. Knockout of the placental specific transcript (P0) of the insulin-like growth factor 2 gene (Igf-2) results in placental growth restriction at e14 onwards (term is e20), but fetal growth restriction does not occur until around e18/e19 (Constancia et al. 2002, 2005). Consequently, the fetal : placental weight ratio at e16 is 50% higher in the P0 knockout conceptuses compared to wild-type. Importantly maternofetal transfer of [14C]methylaminoisobutyric acid (MeAIB, a specific non-metabolizable substrate of System A), measured in vivo, was also 50% increased, per unit weight of placenta, in the P0 versus wild-type and the same change was reflected in an increased expression of the Slc38a4 isoform of the System A gene in the P0 knockout placentas (Constancia et al. 2002, 2005). At e19, when the P0 pups as well as placentas were growth restricted, all of these variables, fetal : placental weight ratio, [14C]MeAIB transfer and Slc38a4 mRNA were similar in P0 knockout and wild-type conceptuses. These data suggest that, as in the human study, System A activity in the placenta can be upregulated to increase placental efficiency when the placental size per se is too low to meet the demands of the normally growing fetus. However, this upregulation and increased efficiency does not appear to be sustainable in this mouse knockout, so that fetal growth restriction does finally ensue.
Adaptation and increases in placental efficiency can also be observed in normal mice. Coan and colleagues (2008) compared the smallest and the largest placentas in mouse litters at e16 and e19. They found that there were increases in labyrinth zone (exchange region of the mouse placenta) volume fraction and increases in [14C]MeAIB transfer per unit weight of placenta, as well as gene expression changes (interestingly including upregulation of the Slc38a2 isoform of System A rather than the Slc38a4 isoform as found in the study described above), which enabled the small placentas to maintain normal fetal growth until term. Interestingly the morphological changes occurred earlier (e16) than the transport changes (e19), suggesting that the adaptation in placental phenotype enabling increased efficiency can alter; this may well affect final fetal body composition as the mix of solute transferred following the morphological change will almost certainly not be the same as that transferred following a change in transporter activity (Coan et al. 2008).
These data taken together support the hypothesis that the capacity of the placenta to supply nutrient can be modulated, to some extent, by the demands of the fetus so enabling it to maintain a normal growth trajectory. It could be that in some cases it is a failure of this adaptation which leads to FGR rather than defective exchange capability per se. Understanding the adapted, and mal-adapted, placental phenotype could be of major benefit in diagnosing the fetus at risk of fatal growth restriction. Furthermore, if we understood the nature of the signal between fetus and placenta, then this might provide new therapeutic strategies.
Relationship between syncytiotrophoblast transporter expression and activity and maternal nutrition
Maternal nutrition undoubtedly affects placental and fetal growth. A recent series of studies on the rat from Jansson, Powell and colleagues also suggests that placental transport function might be modulated in relation to maternal diet. Rats fed a low protein diet during pregnancy had placentas and fetuses with the same weights as those fed an isocaloric, control diet up until day 19 (term is day 21/22 in rat), but which then showed growth restriction at day 21 (Jansson et al. 2006). However, placental System A transport activity was reduced at day 19 in response to the low protein diet, i.e. preceding the growth restriction. This time course of events suggests that it was the downregulation of the transporter activity which was a key cause of the growth restriction.
Ericsson et al. (2007) investigated the effects of hyperglycaemia in early and late pregnancy on placental and fetal weights. They found that three bolus injections of glucose in early pregnancy (but not when given later on) resulted in a small increase in placental and fetal weights at term, whereas six bolus injections in early pregnancy resulted in no change in placental weight, a decrease in placental System A transport and increased fetal weight at term. These data are somewhat difficult to interpret but the authors suggest that changes in maternal metabolism in early pregnancy are sensed by the placenta which responds to match maternal nutrient resources to the fetal growth trajectory.
Feeding mice a high fat diet (32% energy from fat) during pregnancy resulted in a 43% increase in fetal weight, as compared to rats fed a control (11% energy from fat) diet, accompanied by increased maternal adiposity, increased maternal leptin plasma concentrations and decreased adiponectin (Jones et al. 2009). Transplacental transport of glucose and neutral amino acids was increased, accompanied by increased expression of the GLUT1 glucose transporter protein and of SNAT2. These data suggest that a high fat diet causes fetal overgrowth, at least partially through upregulation of placental transport activity.
There are hints that the human placenta also modulates transport in relation to maternal nutrition and metabolism. Lewis and colleagues (2005) report preliminary data showing that placental System A activity, per mg membrane protein, is lower in women with lower pre-pregnancy arm muscle area and, independently, is lower in women who report strenuous exercise pre-pregnancy. Furthermore, in a study of teenage pregnancy it was found that System A activity in placental fragments was higher in girls with a normal BMI versus that in girls with a below average BMI in early pregnancy (R. L. Jones, C. Hayward and S. L. Greenwood, personal communication), consistent with the report of Lewis et al. (2005).
The signals linking the maternal nutritional and metabolic environment with placental transport may be manyfold and include the metabolites themselves. However, there are data to suggest, in particular, the involvement of leptin, insulin-like growth factor binding protein 1 and IGF-I REF. At the cellular level Roos and colleagues (2009) have provided evidence that the mammalian target of the rapamycin (mTOR) pathway in the placenta may be a link between maternal nutritional status, placental transport and fetal growth.
These studies all suggest a direct correlation between maternal nutrition, placental transport and fetal growth: if a mother is undernourished, placental transport goes down and fetal growth decreases. However, the previous section reviewed the data showing that the fetus may be able to upregulate, up to a point, placental transport capacity so as to maintain the necessary nutrient supply to meet its genetic growth potential. It will be important to understand how these potential conflicts between maternal nutrient supply and fetal growth demand are integrated in the placenta. Epigenetic mechanisms are likely to have a major role in this integration (Godfrey et al. 2007).
Using the information – diagnosing the placental phenotype in fetal growth disorders
As the importance of all facets of placental structure and function in regulating growth has become clearer, it has been hypothesized that placental phenotypes (that is particular mixes of blood flow, structure, endocrine, transport and other functional variables) may be used in early diagnosis of fetal growth disorders and other placental related pregnancy diseases such as pre-eclampsia (Sibley et al. 2005). Kingdom and colleagues (Toal et al. 2007) have now begun to investigate this possibility in a Placenta Clinic, where measurement, in maternal plasma, of placental hormones and fetal products such as alphafetoprotein, are combined with ultrasound assessment of placental shape and size and ultrasound Doppler measurement of uterine blood flow velocities to provide an assessment of placental phenotype and, on this basis, the risk of a poor pregnancy outcome. Better understanding of placental transport and the signals by which it is linked to maternal nutrition and fetal growth demand should provide additional or new biomarkers. This may be enhanced further by techniques such as magnetic resonance imaging providing a non-invasive assessment of a range of the key structural and functional variables of the placenta (Figure 6) associated with fetal growth and other pregnancy disorders (Toal et al. 2007).
There are therefore very many excellent and exciting reasons to continue to bother about placental transport: come and join us!
Acknowledgments
I am very grateful to all colleagues past and present in Manchester, and in many parts of the world, for their contributions to work cited in this review. I am particularly grateful to Professor Sir Robert Boyd, Dr Susan Greenwood and Dr Jocelyn Glazier for their collaboration and for their critical appraisal of this manuscript. Our work is supported by Tommy's (Lets Talk Baby) Charity, the Medical Research Council, the Wellcome Trust and an the Action Research Endowment Fund.
References
- Atkinson D, Boyd R, Sibley C. Placental transfer. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd edn. Elsevier; 2006. pp. 2787–2846. [Google Scholar]
- Atkinson DE, Greenwood SL, Sibley CP, Glazier JD, Fairbairn LJ. Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer. Am J Physiol Cell Physiol. 2003;285:C584–591. doi: 10.1152/ajpcell.00418.2002. [DOI] [PubMed] [Google Scholar]
- Bain MD, Copas DK, Taylor A, Landon MJ, Stacey TE. Permeability of the human placenta in vivo to four non-metabolized hydrophilic molecules. J Physiol. 1990;431:505–513. doi: 10.1113/jphysiol.1990.sp018343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsey TJ, Boyd RD, Sibley CP, Greenwood SL. Microvillous membrane potential (Em) in villi from first trimester human placenta: comparison to Em at term. Am J Physiol Regul Integr Comp Physiol. 1997;273:R1519–1528. doi: 10.1152/ajpregu.1997.273.4.R1519. [DOI] [PubMed] [Google Scholar]
- Boyd RD, Haworth C, Stacey TE, Ward HT. Permeability of the sheep placenta to unmetabolized polar non-electrolytes. J Physiol. 1976;256:617–634. doi: 10.1113/jphysiol.1976.sp011342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–970. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownbill P, Edwards D, Jones C, Mahendran D, Owen D, Sibley C, Johnson R, Swanson P, Nelson DM. Mechanisms of alphafetoprotein transfer in the perfused human placental cotyledon from uncomplicated pregnancy. J Clin Invest. 1995;96:2220–2226. doi: 10.1172/JCI118277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownbill P, Mahendran D, Owen D, Swanson P, Thornburg KL, Nelson DM, Sibley CP. Denudations as paracellular routes for alphafetoprotein and creatinine across the human syncytiotrophoblast. Am J Physiol Regul Integr Comp Physiol. 2000;278:R677–683. doi: 10.1152/ajpregu.2000.278.3.R677. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- Clarson LH, Glazier JD, Greenwood SL, Jones CJ, Sides MK, Sibley CP. Activity and expression of Na+–K+-ATPase in human placental cytotrophoblast cells in culture. J Physiol. 1996;497:735–743. doi: 10.1113/jphysiol.1996.sp021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan PM, Angiolini E, Sandovici I, Burton GJ, Constancia M, Fowden AL. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol. 2008;586:4567–4576. doi: 10.1113/jphysiol.2008.156133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Overbeek T, Pearce JM, Campbell S. The antenatal assessment of utero-placental and feto-placental blood flow using Doppler ultrasound. Ultrasound Med Biol. 1985;11:329–339. doi: 10.1016/0301-5629(85)90132-2. [DOI] [PubMed] [Google Scholar]
- Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- Dilworth MR, Glazier JD, Cowley EC, Boyd RD, Husain SM, Sibley CP, Ward BS. Measurement of maternofetal potential difference in parathyroid hormone related protein (PTHrP) knockout mice. Placenta. 2008;29:1041–1042. doi: 10.1016/j.placenta.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Edwards D, Jones CJ, Sibley CP, Nelson DM. Paracellular permeability pathways in the human placenta: a quantitative and morphological study of maternal-fetal transfer of horseradish peroxidase. Placenta. 1993;14:63–73. doi: 10.1016/s0143-4004(05)80249-8. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Saljo K, Sjostrand E, Jansson N, Prasad PD, Powell TL, Jansson T. Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J Physiol. 2007;581:1323–1332. doi: 10.1113/jphysiol.2007.131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber JJ. Transplacental clearances of inert hydrophilic tracers in rabbits of 18 days gestation. Placenta. 1995;16:403–412. doi: 10.1016/0143-4004(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Firth JA, Leach L. Not trophoblast alone: a review of the contribution of the fetal microvasculature to transplacental exchange. Placenta. 1996;17:89–96. doi: 10.1016/s0143-4004(96)80001-4. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Kelley LK, Smith CH. ATP-dependent calcium transport across basal plasma membranes of human placental trophoblast. Am J Physiol Cell Physiol. 1987;252:C38–46. doi: 10.1152/ajpcell.1987.252.1.C38. [DOI] [PubMed] [Google Scholar]
- Flexner LB, Gelhorn A. The comparative physiology of placental transfer. Am J Obstet Gynecol. 1942;43:965–974. [Google Scholar]
- Gaunt M, Ockleford CD. Microinjection of human placenta. II: Biological application. Placenta. 1986;7:325–331. doi: 10.1016/s0143-4004(86)80150-3. [DOI] [PubMed] [Google Scholar]
- Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. 2007;61:5R–10R. doi: 10.1203/pdr.0b013e318045bedb. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Matthews N, Glazier J, Jackson A, Wilman C, Sibley CP. Neutral amino acid uptake by the microvillous plasma membrane of the human placenta is inversely related to fetal size at birth in normal pregnancy. J Clin Endocrinol Metab. 1998;83:3320–3326. doi: 10.1210/jcem.83.9.5132. [DOI] [PubMed] [Google Scholar]
- Greenwood SL, Boyd RD, Sibley CP. Transtrophoblast and microvillus membrane potential difference in mature intermediate human placental villi. Am J Physiol Cell Physiol. 1993;265:C460–466. doi: 10.1152/ajpcell.1993.265.2.C460. [DOI] [PubMed] [Google Scholar]
- Greenwood SL, Clarson LH, Sides MK, Sibley CP. Membrane potential difference and intracellular cation concentrations in human placental trophoblast cells in culture. J Physiol. 1996;492:629–640. doi: 10.1113/jphysiol.1996.sp021333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen S, Taipale P, Saarikoski S. Weights of placentae from small-for-gestational age infants revisited. Placenta. 2001;22:399–404. doi: 10.1053/plac.2001.0630. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Ekstrand Y, Wennergren M, Powell TL. Placental glucose transport in gestational diabetes mellitus. Am J Obstet Gynecol. 2001;184:111–116. doi: 10.1067/mob.2001.108075. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77:1554–1562. doi: 10.1210/jcem.77.6.8263141. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- Johansson M, Glazier JD, Sibley CP, Jansson T, Powell TL. Activity and protein expression of the Na+/H+ exchanger is reduced in syncytiotrophoblast microvillous plasma membranes isolated from preterm intrauterine growth restriction pregnancies. J Clin Endocrinol Metab. 2002;87:5686–5694. doi: 10.1210/jc.2002-020214. [DOI] [PubMed] [Google Scholar]
- Johansson M, Jansson T, Powell TL. Na+-K+-ATPase is distributed to microvillous and basal membrane of the syncytiotrophoblast in human placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279:R287–294. doi: 10.1152/ajpregu.2000.279.1.R287. [DOI] [PubMed] [Google Scholar]
- Johansson M, Karlsson L, Wennergren M, Jansson T, Powell TL. Activity and protein expression of Na+/K+ ATPase are reduced in microvillous syncytiotrophoblast plasma membranes isolated from pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab. 2003;88:2831–2837. doi: 10.1210/jc.2002-021926. [DOI] [PubMed] [Google Scholar]
- Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport – a review. Placenta. 2007;28:763–774. doi: 10.1016/j.placenta.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23:271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann P, Schroder H, Leichtweiss HP. Fluid shift across the placenta: II. Fetomaternal transfer of horseradish peroxidase in the guinea pig. Placenta. 1982;3:339–348. doi: 10.1016/s0143-4004(82)80027-1. [DOI] [PubMed] [Google Scholar]
- Kertschanska S, Schroder H, Kaufmann P. The ultrastructure of the trophoblastic layer of the degu (Octodon degus) placenta: a re-evaluation of the ‘channel problem’. Placenta. 1997;18:219–225. doi: 10.1016/s0143-4004(97)90096-5. [DOI] [PubMed] [Google Scholar]
- Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175:1534–1542. doi: 10.1016/s0002-9378(96)70103-5. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Greenwood SL, Crozier S, Inskip H, Hanson MA, Cameron IT, Sibley CP, Godfrey KM. Maternal pre-pregnancy body composition influences system A activity. Placenta. 2005;26:A.46. doi: 10.1016/j.placenta.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab. 2004;89:4607–4614. doi: 10.1210/jc.2003-032234. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Wijesekara J, Baker PN, Ong SS. Morphometric evidence that villous development and fetoplacental angiogenesis are compromised by intrauterine growth restriction but not by pre-eclampsia. Placenta. 2004;25:829–833. doi: 10.1016/j.placenta.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Mellor DJ, Cockburn F, Lees MM, Blagden A. Distribution of ions and electrical potential differences between mother and fetus in the human at term. J Obstet Gynaecol Br Commonw. 1969;76:993–998. doi: 10.1111/j.1471-0528.1969.tb09465.x. [DOI] [PubMed] [Google Scholar]
- Mills TA, Wareing M, Bugg GJ, Greenwood SL, Baker PN. Chorionic plate artery function and Doppler indices in normal pregnancy and intrauterine growth restriction. Eur J Clin Invest. 2005;35:758–764. doi: 10.1111/j.1365-2362.2005.01577.x. [DOI] [PubMed] [Google Scholar]
- Nelson DM. Apoptotic changes occur in syncytiotrophoblast of human placental villi where fibrin type fibrinoid is deposited at discontinuities in the villous trophoblast. Placenta. 1996;17:387–391. doi: 10.1016/s0143-4004(96)90019-3. [DOI] [PubMed] [Google Scholar]
- Nelson DM, Swanson PE, Davison BB, Baskin GB, Enders AC. Ontogenetic and phylogenetic evaluation of the presence of fibrin-type fibrinoid in the villous haemochorial placenta. Placenta. 1997;18:605–608. doi: 10.1016/0143-4004(77)90017-0. [DOI] [PubMed] [Google Scholar]
- Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res. 1998;44:233–238. doi: 10.1203/00006450-199808000-00016. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Robinson NR, Atkinson DE, Jones CJ, Sibley CP. Permeability of the near-term rat placenta to hydrophilic solutes. Placenta. 1988;9:361–372. doi: 10.1016/0143-4004(88)90049-5. [DOI] [PubMed] [Google Scholar]
- Roos S, Powell TL, Jansson T. Human placental taurine transporter in uncomplicated and IUGR pregnancies: cellular localization, protein expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R886–893. doi: 10.1152/ajpregu.00232.2004. [DOI] [PubMed] [Google Scholar]
- Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochem Soc Trans. 2009;37:295–298. doi: 10.1042/BST0370295. [DOI] [PubMed] [Google Scholar]
- Schroder H, Leichtweiss HP, Rachor D. Passive exchange and the distribution of flows in the isolated human placenta. Contrib Gynecol Obstet. 1985;13:106–113. [PubMed] [Google Scholar]
- Settle P, Sibley CP, Doughty IM, Johnston T, Glazier JD, Powell TL, Jansson T, D'Souza SW. Placental lactate transporter activity and expression in intrauterine growth restriction. J Soc Gynecol Investig. 2006;13:357–363. doi: 10.1016/j.jsgi.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Coan PM, Ferguson-Smith AC, Dean W, Hughes J, Smith P, Reik W, Burton GJ, Fowden AL, Constancia M. Placental-specific insulin-like growth factor 2 (Igf2) regulates the diffusional exchange characteristics of the mouse placenta. Proc Natl Acad Sci U S A. 2004;101:8204–8208. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D'Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58:827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- Strid H, Bucht E, Jansson T, Wennergren M, Powell TL. ATP dependent Ca2+ transport across basal membrane of human syncytiotrophoblast in pregnancies complicated by intrauterine growth restriction or diabetes. Placenta. 2003;24:445–452. doi: 10.1053/plac.2002.0941. [DOI] [PubMed] [Google Scholar]
- Stulc J, Svihovec J, Drabkova J, Stribrny J, Kobilkova J, Vido I, Dolezal A. Electrical potential difference across the mid-term human placenta. Acta Obstet Gynecol Scand. 1978;57:125–126. doi: 10.3109/00016347809155889. [DOI] [PubMed] [Google Scholar]
- Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet. 2004;364:513–520. doi: 10.1016/S0140-6736(04)16809-8. [DOI] [PubMed] [Google Scholar]
- Toal M, Chan C, Fallah S, Alkazaleh F, Chaddha V, Windrim RC, Kingdom JC. Usefulness of a placental profile in high-risk pregnancies. Am J Obstet Gynecol. 2007;196:363, e361–367. doi: 10.1016/j.ajog.2006.10.897. [DOI] [PubMed] [Google Scholar]
- Ward S, Jauniaux E, Shannon C, Rodeck C, Boyd R, Sibley C. Electrical potential difference between exocelomic fluid and maternal blood in early pregnancy. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1492–1495. doi: 10.1152/ajpregu.1998.274.5.R1492. [DOI] [PubMed] [Google Scholar]