Abstract
Long chain polyunsaturated fatty acids are essential nutrients for a healthy diet. The different kinds consumed by the mother during gestation and lactation may influence pregnancy, fetal and also neonatal outcome. The amount of fatty acids transferred from mother to fetus depends not only on maternal metabolism but also on placental function, i.e. by the uptake, metabolism and then transfer of fatty acids to the fetus. The third trimester of gestation is characterized by an increase of long chain polyunsaturated fatty acids in the fetal circulation, in particular docosahexaenoic acid, especially to support brain growth and visual development. These mechanisms may be altered in pathological conditions, such as intrauterine growth restriction and diabetes, when maternal and fetal plasma levels of long chain polyunsaturated fatty acids undergo significant changes. The aim of this review is to describe the maternal and placental factors involved in determining fetal fatty acid availability and metabolism, focusing on the specific role of long chain polyunsaturated fatty acids in normal and pathological pregnancies.
Fatty acids (FAs), together with glucose, lactate and amino acids, represent essential nutrients during intrauterine life. Amongst fatty acids, polyunsaturated fatty acids (PUFAs) are of particular relevance because of their numerous physiological functions (metabolic, energetic and structural) and for the potential influence of their changes on some pathological conditions.
In particular, docosahexaenoic acid (DHA) is an ω-3 long chain polyunsaturated fatty acid (LC-PUFA) that has been widely investigated for its critical importance on fetal growth and especially on CNS development.
This review summarizes maternal and placental metabolism of fatty acids with a specific focus on PUFAs, the mechanisms involved in their transfer to the fetus, their changes in pathological conditions, and their role in fetal neurodevelopment.
Maternal fatty acids: changes with gestation
Fats are part of a balanced and wholesome diet but the type and total amount consumed is important to maintain the state of health. The recommended fat intake for adults ranges between 20 and 35% of total calories (Dietary guidelines for Americans, 2005; U.S. Department of Health).
The most important lipids usually introduced with diet are triglycerides and to a lesser extent phospholipids and cholesterol esters. These are complex molecules that once introduced into the organism cannot be wholly absorbed by the intestine but they have to be broken into simpler molecules by specific pancreatic enzymes.
According to the number of double bonds in the hydrocarbon chain, fatty acids can be classified as saturated and unsaturated, with no, or one or more double bonds, respectively. LC-PUFAs (18 or more carbons) can be further distinguished as two key families, ω-3 and ω-6 depending on the position of the first double bond in the hydrocarbon chain. They have many distinct functions: energetic, metabolic and structural.
Among polyunsaturated fatty acids, linoleic (LA; 18:2 ω-6) and α-linolenic (αLA; 18:3 ω-3) acids are called ‘essential fatty acids’ (EFAs) because they cannot be synthesized endogenously but they have to be introduced with food, in particular by consumption of vegetable oils.
EFAs are converted into their main metabolites which are able to exert the full range of biological actions (Burdge & Calder, 2005). Derivatives of αLA are represented by DHA (22:6 ω-3), necessary for brain growth and development (Innis, 2005) and eicosapentaenoic acid (EPA; 20:5 ω-3), the precursor of series-3 prostaglandins which inhibit platelet aggregation. LA is converted to arachidonic acid (ARA; 20:4 ω-6), the main precursor of prostaglandins, thromboxanes and leukotrienes, fundamental for immune responses. DHA, EPA and ARA may also be introduced with diet: ω-3 are mainly present in fish, shellfish, and sea mammals and are scarce in land animals and plants, whereas ω-6 mainly derive from vegetable oils (Bézard et al. 1994; Innis, 2003).
The Δ5–Δ6 enzymatic chain promotes the conversion of ARA from LA, and EPA from αLA. Due to a competition mechanism, EPA and DHA are produced more efficiently than ARA in an abundance of αLA. For this reason, higher intakes of ω-3 are recommended for many pathological conditions (heart diseases, atherosis and autoimmune diseases) in order to reduce the production of prostanoids derived from ARA, such as prostaglandin E2 and thromboxane A2.
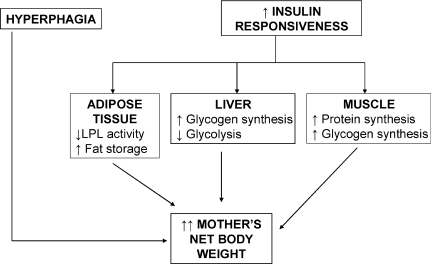
The mother modifies her metabolism from the beginning of pregnancy in order to support the nutritional needs of the feto-placental unit (Fig. 1) (Cetin et al. 2005). The first trimester of pregnancy can be considered an anabolic period during which the mother increases her net body weight through hyperphagia and substance storing in adipose tissue, also under conditions of malnutrition (Prentice & Golberg 2000; Herrera 2002). Fat storage is facilitated by increasing the insulin responsiveness of adipose tissue which reduces lipoprotein lipase activity.
Figure 1. Anabolic maternal metabolism in the first trimester of pregnancy.
In this phase, the mother stores lipids, proteins and carbohydrates thanks to the enhanced insulin sensitivity and hyperphagia.
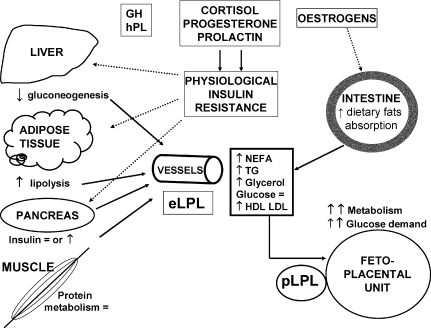
In the third trimester of pregnancy the fetus intensifies its nutrient demands to support exponential tissue growth, so maternal lipid metabolism switches to a catabolic condition. In this stage, even short fasting periods are associated with a condition defined as accelerated starvation, during which the pregnant woman makes available to the fetal–placental unit large quantities of nutrients (Fig. 2) (Felig et al. 1970).
Figure 2. Catabolic maternal metabolism in the third trimester of pregnancy: availability of nutrients to sustain fetal growth.
eLPL: endothelial lipoprotein lipase; pLPL placental lipoprotein lipase.
Adipose tissue lipolytic activity increases due to a reduction in insulin sensitivity, under the hormonal control of progesterone, cortisol, prolactin and leptin (Cousins, 1991; Catov et al. 2007). High circulating oestrogens determine a decrease in extra-hepatic lipoprotein lipase (LPL) activity (Alvarez et al. 1996), whereas the intestinal absorption of dietary fats increases (Argiles & Herrera, 1989; Herrera, 2002). All these events result in elevations of free fatty acids (FFAs) and glycerol plasma levels taken up by the liver for the synthesis of Very Low Density Lipoprotein (VLDL), rich in triglycerides, quickly released into the circulation. Therefore, the hyper-triglyceridaemia that characterizes this phase of gestation is mainly due to an increase in VLDL production and a decrease in its peripheral clearance (Alvarez et al. 1996; Herrera, 2002).
During the last weeks of gestation, mainly under fasting conditions, FFAs and glycerol released from the adipose tissue can be used for ketone bodies synthesis (Zorzano et al. 1986; Herrera 2002). Ketone bodies and FFAs, similarly to glucose, may easily cross the placenta to sustain the augmented fetal metabolism. However, this is not very relevant in quantitative terms, since fatty acids are mainly provided to the fetal circulation by placental transport and eventually by elaboration of lipoproteins (Wittmaack et al. 1995; Herrera 2002).
We previously reported an increment in proportion of total saturated fatty acids during normal pregnancy, with a decline in ω-6 probably as a result of a decline in the percentage of ARA, with a stable proportion of LA, the most abundant ω-6 fatty acid in maternal plasma. Concerning ω-3, their total amount is stable during gestation, with an increment in percentage αLA and a reduction in EPA and DHA (Herrera et al. 2004).
Cholesterol and triglyceride levels increase during pregnancy, with an increment in FFAs mostly near delivery (Herrera et al. 2004).
After delivery, LC-PUFA content in circulating lipoproteins shows a greater decline in the triglyceride fraction than in phospholipids or esterified cholesterol. Plasma FFAs fall to pre-pregnancy levels within 3 days and triglycerides in 2 weeks, although this also depends on maternal lactation, being more rapid in women that establish breast feeding (Montelongo et al. 1992).
Placental transfer and fetal levels of fatty acids
During intrauterine life the fetus is dependant for its growth and development on the nutrients provided by the mother. The amount of substrates reaching the placenta strictly depends on maternal diet and metabolism. However, the placenta plays a major role since its transfer ability and metabolism together determine the amount of nutrients in the fetal circulation (Cetin et al. 2005).
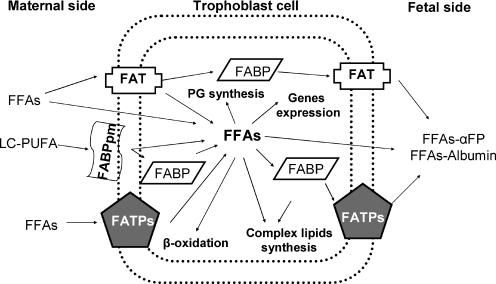
FAs are taken up by the placenta from the maternal circulation as FFAs or as maternal lipoproteins. FAs incorporated in maternal lipoproteins are released by placental lipoprotein lipases. FFAs may be transferred to the fetus by simple diffusion because the FFA concentration gradient between the mother and the fetus increases steadily during pregnancy (Benassayag et al. 1999) or, more importantly, through transport proteins located on the microvillus or basal membranes of trophoblast cells and on endothelial membranes of fetal capillaries (Fig. 3).
Figure 3.
Schematic view of fatty acid transport proteins in the human placenta
A number of studies suggest that the placenta modulates the fatty acids supply for its own metabolism and also that of the fetus. Indeed, leptin produced by the placenta and secreted both in the maternal and fetal circulations stimulates lipolysis (Masuzaki et al. 1997; Hoggard et al. 2001).
Maternal lipoproteins rich in triglycerides do not directly cross the placenta but they require hydrolysis by placental lipoprotein lipase, so that the major source of FAs in the fetal circulation is represented by free fatty acids (Herrera et al. 2006). FFAs can cross the lipid bilayers by simple diffusion or connecting to specific binding proteins or albumin (Herrera et al. 2006).
Generally speaking, fetal fatty acids are correlated to the maternal levels, with significantly lower total levels in the fetal than in the maternal circulation (Cetin et al. 2002). However, numerous studies have proved that the FA profile is different in the fetal compared with the maternal circulation, with higher proportions of LC-PUFAs and a lower percentage of their precursors LA and αLA (Crawford et al. 1976). This phenomenon is called ‘biomagnification’ and seems to be related to the ability of the placenta or the fetus to increase LC-PUFA percentages in fetal blood in order to support central nervous system development. However, the biochemical mechanisms responsible for this are not yet completely clarified. In particular Δ5 and Δ6 desaturase activities are very low in placental tissue (Llanos et al. 2005). Fetal ability to desaturate and elongate fatty acids was demonstrated for LA → ARA conversion, but this capability is less efficient for DHA (Szitanyi et al. 1999).
Biomagnification of ARA and DHA in fetal plasma is therefore probably due to the placental ability to preferentially transfer DHA and then ARA, ALA and LA into fetal circulation as demonstrated by in vitro and in vivo, and by stable isotope experiments (Ruyle et al. 1990; Haggarty et al. 1999; Larquéet al. 2003).
Several FA transfer proteins have been identified in the placenta:
FABPs: different isoforms of these fatty acid binding proteins have been recognized in the placenta. FABPpm is located only on the membrane-facing maternal circulation of trophoblast cells and preferentially binds LC-PUFA (Campbell et al. 1998b) and Liver-FABP (L-FABP) located in the cytoplasm (Campbell et al. 1998a).
FAT/CD36 (FA translocase): this is an integral membrane protein placed in both the placental membranes involved not only in lipid metabolism but also in angiogenesis and inflammation (Campbell et al. 1998a).
FATPs (FA transporter proteins): integral transmembrane proteins located on both trophoblast membranes (Campbell et al. 1998a).
The location of FAT and FATP on both sides of trophoblast cells and their lack of specificity for a particular type of FFA allows their transport to occur bidirectionally from mother to fetus and vice versa. However, for its exclusive location on the maternal side and its preference for LC-PUFAs, FABPpm seems to be implied in their sequestration in the placenta. (Campbell et al. 1998a; DuttaRoy, 2000, 2008).
Once in the placenta, FAs are in part oxidized in mitochondria to produce energy. Moreover, they are incorporated into phospholipids while a certain proportion of ARA is converted into prostaglandins by cyclooxygenases. The remaining FAs are elongated, desaturated and then released into the fetal circulation. A different fetal blood FA profile is observed, with proportionally higher amounts of LC-PUFAs in comparison with those present in the maternal circulation.
The simultaneous evaluation of lipid and fatty acid profile in mother and fetus during gestation is very difficult under normal conditions. While it is possible to describe this profile at different gestational ages in fetuses that are born prematurely, this event could in itself condition or be conditioned by an alteration in lipid profile (Olsen, 2004).
A few years ago, we described the fetal fatty acid profile in the second half of pregnancy using fetal blood sampling performed in utero to evaluate fetal karyotype (Cetin et al. 2002). In that study, we reported few changes occurring with gestation, namely an increase in saturated (mainly palmitic acid) and polyunsaturated fatty acids, and a decrease in oleic and consequently in the percentage of mono-unsaturated fatty acids. No differences were observed in the profile of LC-PUFAs during gestation.
Relevance of fatty acids in fetal nutrition
The third trimester of pregnancy is characterized by massive fetal growth and consequently a high demand of nutrients by the fetus. During this period and through the early weeks of life it has been estimated that the fetus needs approximately 50 mg/kg/day of ω-3 and 400 mg/kg/day of ω-6 (Clandinin et al. 1980, 1981). The brain is the organ that most of all rapidly incorporates LC-PUFAs for its development. In particular, DHA is selectively incorporated into fetal brain and retina phospholipids more than 10 times faster than LA and αLA, while ARA is mainly used in postnatal life.
DHA confers a high degree of fluidity and flexibility to neural and endothelial membranes of the central nervous system. DHA is involved in neurotransmission, regulates ion-channel activity and gene expression and can be metabolized in neuroprotective metabolites (Innis, 2007a). Recent studies on rats suggest that deficiency of brain ω-3 provokes a reduction of neuron size and dendritic arborization (Ahmad et al. 2002). In rhesus monkeys, however, a deficient ω-3 diet reduces rod phototransduction sensitivity and delays rod recovery but does not influence cone activity (Jeffrey & Neuringer, 2009). In a previous study the same group noticed that in monkeys ω-3 FA deficiency also influences fluid intake and output without affecting serum electrolyte levels; the exact mechanisms of these changes still have to be elucidated (Reisbick et al. 1990).
Considering the main role of DHA for brain and retina function and because the amount of FAs in the fetal circulation largely depends on the FA composition of maternal blood, many studies have investigated the effects on brain activity with increasing maternal dietary intake of ω-3 LC-PUFA during pregnancy and lactation. These studies have proved that supplementation has many favourable effects: increased visual acuity (Smithers et al. 2008), more mature neonatal sleep state (Cheruku et al. 2002), reduced hyperactivity (Gale et al. 2008) and enhanced cognitive functions and attention. It is still not known if these benefits persist to childhood and adult life, but in a trial with 4 years follow up, children born to supplemented mothers seem to have a higher IQ than controls (Helland et al. 2003) (Table 1). Further studies are required to further understand the role of LC-PUFAs in brain development but we have to consider that neuronal processes are complex and multifactorial so that confounding factors such as maternal baseline intake of LC-PUFAs, socioeconomic status or education may influence the results.
Table 1.
Effects of ω-3 on fetal central nervous system development
| Smithers et al. 2008 | Increased visual acuity | (*) VEP at 4 months: high-DHA fetuses had 1.4 cpd higher than controls (P= 0.025) |
| Cheruku et al. 2002 | Favoured mature neonatal sleep state | ($) Infants of high-DHA mothers had a significantly lower AS/QS ratio (P < 0.05) and < AS than infants of low-DHA mothers (P < 0.05) |
| Gale et al. 2008 | Reduced hyperactivity | (°) Reduced risk of hyperactivity in fetuses of FO supplemented mothers (OR = 0.34 95% CI 0.15–0.78) |
| Helland et al. 2003 | Higher IQ at 4 years of age | (§) Mean IQ 106 vs. 102 (P < 0.05) |
(*)VEP: visual evoked potential; cpd: cycle per degree
($) AS: active sleep; QS :quiet sleep
(°) FO: fish oil, OR: odd ratio
(§) Scores on the Mental Processing Composite of the K-ABC (Kaufman Assessment Battery for Children) for children whose mothers had taken cod liver vs. corn oil during pregnancy and lactation.
LC-PUFAs are essential components of membrane phospholipids not only in CNS but also in other organs and tissues and so the potential benefits of their supplementation on pregnancy outcome have been widely investigated in numerous studies (Table 2). In particular, a recent meta-analysis has shown that supplementation with ω-3 prolongs gestation by an average value of 1.6–2.6 days, with a concomitant small increase in birth weight. Moreover, it prevents preterm birth before 34 weeks of gestation mainly in high risk pregnancies (Szajewska et al. 2006). This evidence could be explained by the influence of high concentrations of ω-3 on the production of prostaglandin involved in the initiation of labour.
Table 2.
Effects of ω-3 on pregnancy and fetal outcome
| Szajewska et al. 2006 | Prolong gestation Increase of birth weight | Mean 1.57 days (95% CI: 0.35, 2.78) Mean 47 or 54 g |
| Horvath et al. 2007 | Prevent preterm birth (< 34 weeks) in high risk patients | RR 0.39 (95% CI 0.18, 0.84) |
| Enke et al. 2008 | Influence fetal immune response | DHA reduces inflammatory cytokines |
| Hibbeln, 2002 | Deficiency increases risk of post-partum depression (PPD) | Prevalence of PPD is higher in countries with lower consumption of seafood (r=−0.81 P < 0.0001) and lower concentration of DHA in mother's milk (r=−0.84; P < 0.0001). |
| Weisinger et al. 2001 | Deficiency increases blood pressure | Rats fed with a deficient ω-3 diet had a higher blood pressure than controls (P < 0.05) |
RR: risk ratio
A recent study has correlated LC-PUFA levels in the uterus with the immune phenotype in childhood due to their ability to influence Th1 and Th2 balance. In particular ARA enhances the inflammatory process while DHA decreases inflammatory cytokines; consequently DHA supplementation seems to have a potential role in preventing future allergies (Enke et al. 2008).
Among many effects of LC-PUFAs on fetal and pregnancy outcomes, they seem to have an influence also on maternal depression. Epidemiological studies have shown that the prevalence of post-partum depression is higher in countries with low ω-3 intake than in those with highest intake (Hibbeln, 2002). In particular, post-partum depression (PPD) seems to be related to a higher ω-6/ω-3 ratio (De Vriese et al. 2003). The mechanism underlying this observation could be the physiological reduction of DHA contents in maternal brain lipids in the late stage of pregnancy and during lactation. This physiological event, associated with the evidence that pregnant women with a current episode of major depression seem to have lower ω-3 PUFA levels than healthy controls, seems to predispose these patients to an elevated risk of developing PPD (Rees et al. 2009). Few studies and with different dosages have investigated the role of ω-3 supplementation on PPD incidence with discordant results (Llorente et al. 2003; Freeman et al. 2006).
Besides the positive role of a suitable maternal diet with a sufficient intake of LC-PUFAs, it is important to underline that an unbalanced maternal diet may also have potential dramatic consequences on the developing fetus. In rats, abnormal maternal consumption of saturated fatty acids may permanently alter fetal lipid and glucose metabolism in the offspring, increasing the risk for cardiovascular disease in adult life (Siemelink et al. 2002; Chechi & Cheema 2006; Chechi et al. 2009). Hypertension in particular seems to be related to an excessive intake of saturated FAs or to an inadequate intake of LC-PUFAs in the perinatal period (Weisinger et al. 2001; Khan et al. 2005).
Fatty acid changes in intrauterine growth restriction and diabetes
Fetal growth depends on a combination of different factors: endocrine maternal state, nutritional maternal environment and genetic potential of the fetus. The alteration of one or more of these factors can lead to a modification in the fetal growth trajectory. Intrauterine growth restriction (IUGR) results from insufficient growth of the fetus, while gestational diabetes is associated with increased nutrient availability and fetal overgrowth.
IUGR, which affects approximately 7–15% of pregnancies, is a condition characterized by an inadequate ability of the placenta to provide nutrients and oxygen to the growing conceptus (Cetin et al. 2005; Cetin & Alvino, 2009). IUGR represents one of the principal causes of perinatal morbidity and mortality and is also associated with cardiovascular disease and glucose intolerance in adult life (De Rooij et al. 2007).
Many studies have proved that the placenta of IUGR fetuses has a specific phenotype characterized by altered metabolism and decreased nutrient transport. In particular, supply of amino acids is reduced with an increased maternal–fetal gradient of glucose (Sibley et al. 2005).
In normal pregnancy, total plasma fatty acid concentrations are significantly higher in mothers than in fetuses with differences in the relative amounts of some fatty acids. Although the total amount of fatty acids is unchanged in IUGR (Cetin et al. 2002), we have reported changes in the LC-PUFA fetal/maternal relationship in IUGR at the time of fetal blood sampling, with significantly higher F/M ratios for LA and significantly lower for DHA and ARA. We also demonstrated that fetuses with IUGR have a lower proportion of ARA and DHA relative to their precursors LA and αLA in comparison with control infants of similar gestational age. These data can explain the high susceptibility of IUGR infants to brain and vascular damage.
In a more recent study (Alvino et al. 2008) we have evaluated the maternal and fetal blood FA profile in pregnancies complicated by IUGR with or without pre-eclampsia.
Pre-eclampsia is a syndrome typically correlated with placental insufficiency beginning with a shallow trophoblast invasion of spiral arteries similar to the pathogenesis of IUGR (Pardi et al. 2002). However, this condition is also characterized by an escalation in inflammatory response to ischaemic damage that succeeds in involving different areas of the maternal circulation. The role of fatty acids, particularly EPA (ω-3) and ARA (ω-6), which are precursors of prostanoids, could be very important in limiting (ω-3 prostanoids) or enhancing (ω-6 prostanoids) this inflammatory condition (Innis, 2005).
We reported a lower ARA/LA ratio in IUGR compared to normal fetuses (Alvino et al. 2008). Moreover, IUGR fetuses in pregnancies complicated with pre-eclampsia (IUGR-PE) showed a lower percentage of ARA in venous plasma. Their mothers had significantly higher concentrations of triglycerides in comparison with IUGR only and normal mothers and this could represent a risk factor for hypertensive disorders and development of the metabolic syndrome in later life (Carpenter, 2007).
Both in IUGR and IUGR-PE mothers we reported higher concentrations of FFAs associated with higher fetal concentrations. Our group previously demonstrated a higher expression of LPL (lipoprotein lipase) and EL (endothelial lipase) genes in IUGR placentas that could be associated with these results but also with the different distribution of fatty acids in these pathological conditions (Tabano et al. 2006; Gauster et al. 2007).
The opposite phenotype of IUGR is represented by the fetal overgrowth that characterizes pregnancies complicated by diabetes types I and II, and GDM (gestational diabetes mellitus) (Catalano et al. 2007). Intrauterine exposure to these pathologies is a significant determinant of the development of obesity, dyslipidaemia and early onset of type 2 diabetes mellitus in the offspring (Schaefer-Graf et al. 2005; Harder et al. 2007). It is known that in these conditions there is an altered placental transport activity with an increase in glucose and amino acid transfer (Jansson et al. 2006). Moreover, since diabetic women have higher plasma levels of triglycerides-lipoproteins and FFAs than healthy controls, lipid transfer is also increased by the rising of the maternal–fetal concentration gradient (Hollingsworth et al. 1982; Montelongo et al. 1992). Recent evidence showed that placenta LPL activity is increased by 39% in Insulin Dependent Diabetes Mellitus (IDDM), but not in GDM patients, while the expression of L-FABP (cytoplasmic FA binding protein) is increased both in IDDM and GDM by 112% and 69%, respectively (Magnusson et al. 2004). In diabetic pregnancy, a large proportion of lipids that cross the placenta are stored directly in the placental unit while the remainder are released into the fetal circulation and contribute to fetal fat deposition together with glucose (Magnusson et al. 2004).
GDM pregnancies are also associated with higher placental/fetal weight ratios (Taricco et al. 2003) reflecting a greater layer of exchange and/or a major density of nutrient transporters. This leads to higher availability of nutrients for the fetus with more tissue deposition, particularly fat. Moreover, the quality of fatty acids that reach the fetus could be different, because of the great enhancement of lipolysis observed in GDM mothers affecting the fatty acids pattern (Wijendran et al. 1999; Thomas et al. 2004).
In non-pregnant women, diabetes determines a reduced synthesis of ARA and DHA probably due to an impaired activity of Δ5 and Δ6 desaturases (Brenner et al. 2000). Although this evidence has not been found in patients with GDM (Thomas et al. 2004), both diabetic and GDM patients show depressed levels of ARA and DHA in red blood cell phospholipids (Wijendran et al. 1999).
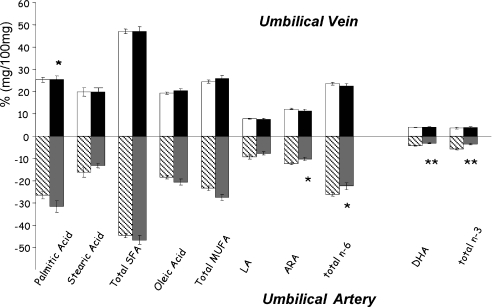
We recently reported lower percentages of both ARA and DHA in umbilical arterial plasma but not in the umbilical vein of GDM neonates (Ortega-Senovilla et al. 2009) (Fig. 4). These data suggest that a lower concentration of LC-PUFAs in neonates of GDM mothers seems to be due to altered fetal metabolism rather than limited placental transfer. This finding could have potential implications for fetal and postnatal growth that need to be investigated.
Figure 4. Fatty acid profile in arterial and venous plasma of AGA (adequate for gestational age) and GDM fetuses.
*P < 0.05, **P < 0.01 ANOVA and Tukey's test between u.v. (umbilical vein) and u.a. (umbilical artery). Open columns, u.v. AGA; shaded columns, u.a. AGA; filled columns, u.v. GDM; grey columns, u.a. GDM. SFA, saturated fatty acids; MUFA, monounsaturated fatty acids. Data adapted from Ortega-Senovilla et al. (2009).
There are also important metabolic changes that characterize pregnancies complicated by maternal obesity but with normal glucose tolerance. Maternal obesity is associated with a higher risk of complications like pre-eclampsia, GDM, fetal anomalies and high birth weight (King, 2006). In obese women, the physiological metabolic adjustments are amplified: insulin sensitivity is 40% lower with a consequent increase in plasma glucose, amino acids and plasma FFAs (King, 2006). Therefore obesity, like GDM, leads to an excess of available nutrients for the fetus, heavily influencing its growth and development.
Conclusions and recommendations
In conclusion, it is clear that LC-PUFA intake may influence fetal, maternal and child health. It is therefore advisable that pregnant and lactating women aim to achieve an average intake of at least 200 mg/day of DHA to achieve a desirable outcome (Koletzko et al. 2007). For this purpose, women should consume oily fish two times a week during pregnancy, because of its beneficial effects on vascular function and on the developing fetal brain and retina. Only Carlson et al. (1996) reported that supplementation of premature infants with marine oils can adversely affect neonatal growth because of an altered balance of ω-3 (EPA) and ω-6 (reduction of ARA → prostanoids → growth factors), but usually the supply of ARA with the diet and from maternal adipose tissue is sufficient to maintain a correct ω-3/ω-6 ratio. The beneficial effect of DHA on prolonging gestation and consequently fetal weight is very consistent in different studies (Smuts et al. 2003; Olsen et al. 2007).
Of note, since marine fish may contain large quantities of dangerous contaminants such as polychlorinated biphenols, dioxins and mercurial derivatives (Grandjean & Weihe, 2003) women should be advised to consume non-predator fish with a lower content of contaminants (Koletzko et al. 2008).
Moreover, dietary fat intake during pregnancy and lactation, as a proportion of total energy intake, should be the same as that recommended for the general population.
However, further research is needed in order to better characterize the mechanisms regulating the placental transfer of long chain fatty acids.
Finally, maternal intake of dietary fats in the periconceptional period should be investigated in future studies in order to determine a potential role in implantation and in early embryo development.
Glossary
Abbreviations
- ARA
arachidonic acid
- αLA
α-linolenic acid
- CNS
central nervous system
- DHA
docosahexaenoic acid
- EFAs
essential fatty acids
- EL
endothelial lipase
- EPA
eicosapentaenoic acid
- FAs
fatty acids
- FABP
fatty acid binding protein
- FFA
free fatty acid
- FAT
fatty acid translocase
- GDM
gestational diabetes mellitus
- IUGR
intrauterine growth restriction
- LA
linoleic acid
- LC-PUFAs
long chain polyunsaturated fatty acids
- LPL
lipoprotein lipase
- PE
preeclampsia
- PPD
post-partum depression
- PUFAs
polyunsaturated fatty acids
- Th1 and Th2
Thelper 1 and 2.
References
- Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr Neurol. 2002;26:210–218. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- Alvarez JJ, Montelongo A, Iglesias A, Lasunciòn MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and post heparin lipases during gestation in women. J Lipid Res. 1996;37:299–308. [PubMed] [Google Scholar]
- Alvino G, Cozzi V, Radaelli T, Ortega H, Herrera E, Cetin I. Maternal and fetal fatty acid profile in normal and intrauterine growth restriction pregnancies with and without preeclampsia. Pediatr Res. 2008;64:615–620. doi: 10.1203/PDR.0b013e31818702a2. [DOI] [PubMed] [Google Scholar]
- Argiles J, Herrera E. Appearance of circulating and tissue 14C-lipids after oral 14C-tripalmitate administration in the late pregnant rat. Metabolism. 1989;38:104–108. doi: 10.1016/0026-0495(89)90247-3. [DOI] [PubMed] [Google Scholar]
- Benassayag C, Rigourd V, Mignot TM, Hassid J, Leroy MJ, Robert B, Civel C, Grangé G, Dallot E, Tanguy J, Nunez EA, Ferré F. Does high polyunsaturated free fatty acid level at the feto-maternal interface alter steroid hormone message during pregnancy? Prostaglandins Leukot Essent Fatty Acids. 1999;60:393–399. doi: 10.1016/s0952-3278(99)80019-2. [DOI] [PubMed] [Google Scholar]
- Bézard J, Blond JP, Bernard A. The metabolism and availability of essential fatty acids in animal and human tissues. Reprod Nutr Dev. 1994;34:539–568. doi: 10.1051/rnd:19940603. [DOI] [PubMed] [Google Scholar]
- Brenner RR, Bernasconi AM, Garda HA. Effect of experimental diabetes on the fatty acid composition, molecular species of phosphatidyl-choline and physical properties of hepatic microsomal membranes. Prostaglandins Leukot Essent Fatty Acids. 2000;63:167–176. doi: 10.1054/plef.2000.0175. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Calder PC. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- Campbell FM, Bush PG, Veerkamp JH, Duttaroy AK. Detection and cellular localization of plasma membraneassociated and cytoplasmic fatty acid binding proteins in human placenta. Placenta. 1998a;19:409–415. doi: 10.1016/s0143-4004(98)90081-9. [DOI] [PubMed] [Google Scholar]
- Campbell FM, Gordon MJ, Dutta-Roy AK. Placental membrane fatty acid binding protein preferentially binds arachidonic and docosahexaenoic acids. Life Sci. 1998b;63:235–240. doi: 10.1016/s0024-3205(98)00267-7. [DOI] [PubMed] [Google Scholar]
- Carlson SE, Werkman SH, Tolley EA. Effect of long-chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. Am J Clin Nutr. 1996;63:687–697. doi: 10.1093/ajcn/63.5.687. [DOI] [PubMed] [Google Scholar]
- Carpenter MW. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care. 2007;30(Suppl. 2):S246–250. doi: 10.2337/dc07-s224. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Thomas A, Huston-Presley L, Amini SB. Phenotype of infants of mothers with gestational diabetes. Diabetes Care. 2007;30(Suppl. 2):S156–160. doi: 10.2337/dc07-s209. [DOI] [PubMed] [Google Scholar]
- Catov JM, Patrick TE, Powers RW, Ness RB, Harger G, Roberts JM. Maternal leptin across pregnancy in women with small-for-gestational-age infants. Am J Obstet Gynecol. 2007;196:558.e1–8. doi: 10.1016/j.ajog.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Cetin I, Alvino G. Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta. 2009 doi: 10.1016/j.placenta.2008.12.006. DOI 10.1016/J.placenta.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Cetin I, Alvino G, Radaelli T, Pardi G. Fetal nutrition: a review. Acta Paediatr Suppl. 2005;94:7–13. doi: 10.1111/j.1651-2227.2005.tb02147.x. [DOI] [PubMed] [Google Scholar]
- Cetin I, Giovannini N, Alvino G, Agostoni C, Riva E, Giovannini M. Intrauterine growth restriction is associated with changes in polyunsaturated fatty acid fetal-maternal relationships. Pediatr Res. 2002;52:750–755. doi: 10.1203/00006450-200211000-00023. [DOI] [PubMed] [Google Scholar]
- Chechi K, Cheema SK. Maternal diet rich in saturated fats has deleterious effects on plasma lipids of mice. Exp Clin Cardiol. 2006;11:129–135. [PMC free article] [PubMed] [Google Scholar]
- Chechi K, McGuire JJ, Cheema SK. Developmental programming of lipid metabolism and aortic vascular function in C57Bl/6 mice: a novel study suggesting an involvement of LDL-receptor. Am J Physiol Regul Integr Comp Physiol. 2009;296:1029–1040. doi: 10.1152/ajpregu.90932.2008. [DOI] [PubMed] [Google Scholar]
- Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J Clin Nutr. 2002;76:608–613. doi: 10.1093/ajcn/76.3.608. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Chappell JE, Heim T, Swyer PR, Chance GW. Fatty acid utilization in perinatal de novo synthesis of tissue. Early Hum Dev. 1981;5:355–366. doi: 10.1016/0378-3782(81)90016-5. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- Cousins L. Insulin sensitivity in pregnancy. Diabetes. 1991;40:39–43. doi: 10.2337/diab.40.2.s39. [DOI] [PubMed] [Google Scholar]
- Crawford MA, Hassan AG, Williams G, Whitehouse WL. Essential fatty acids and fetal brain growth. Lancet. 1976;1:452–453. doi: 10.1016/s0140-6736(76)91476-8. [DOI] [PubMed] [Google Scholar]
- De Rooij SR, Painter RC, Holleman F, Bossuyt PM, Roseboom TJ. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am J Clin Nutr. 2007;86:1219–1224. doi: 10.1093/ajcn/86.4.1219. [DOI] [PubMed] [Google Scholar]
- De Vriese SR, Christophe AB, Maes M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sci. 2003;73:3181–3187. doi: 10.1016/j.lfs.2003.02.001. [DOI] [PubMed] [Google Scholar]
- DuttaRoy AK. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am J Clin Nutr. 2000;71:315S–322S. doi: 10.1093/ajcn/71.1.315s. [DOI] [PubMed] [Google Scholar]
- Duttaroy AK. Transport of fatty acids across the human placenta: A review. Prog Lipid Res. 2008;48:52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Enke U, Seyfarth L, Schleussner E, Markert UR. Impact of PUFA on early immune and fetal development. Br J Nutr. 2008;100:1158–1168. doi: 10.1017/S000711450801413X. [DOI] [PubMed] [Google Scholar]
- Felig P, Lynch V. Starvation in human pregnancy: hypoglycemia, hypoinsulinemia and hyperketonemia. Science. 1970;170:990–992. doi: 10.1126/science.170.3961.990. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr Scand. 2006;113:31–35. doi: 10.1111/j.1600-0447.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Gale CR, Robinson SM, Godfrey KM, Law CM, Schlotz W, O'Callaghan FJ. Oily fish intake during pregnancy-association with lower hyperactivity but not with higher full-scale IQ in offspring. J Child Psychol Psychiatry. 2008;49:1061–1068. doi: 10.1111/j.1469-7610.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- Gauster M, Hiden U, Blaschitz A, Frank S, Lang U, Alvino G, Cetin I, Desoye G, Wadsack C. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2007;92:2256–2263. doi: 10.1210/jc.2006-2403. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P. Arachidonic acid status during pregnancy is associated with polychlorinated biphenyl exposure. Am J Clin Nutr. 2003;77:715–719. doi: 10.1093/ajcn/77.3.715. [DOI] [PubMed] [Google Scholar]
- Haggarty P, Ashton J, Joynson M, Abramovich DR, Page K. Effect of maternal polyunsaturated fatty acid concentration on transport by the human placenta. Biol Neonate. 1999;75:350–359. doi: 10.1159/000014115. [DOI] [PubMed] [Google Scholar]
- Harder T, Rodekamp E, Schellong K, Dudenhausen J, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849–857. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:39–44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- Herrera E. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development – a review. Placenta. 2002;23(Suppl. A):S9–S19. doi: 10.1053/plac.2002.0771. [DOI] [PubMed] [Google Scholar]
- Herrera E, Amusquivar E, Lopez-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res. 2006;65(Suppl. 3):59–64. doi: 10.1159/000091507. [DOI] [PubMed] [Google Scholar]
- Herrera E, Ortega H, Alvino G, Giovannini N, Amusquivar E, Cetin I. Relationship between plasma fatty acid profile and antioxidant vitamins during normal pregnancy. EJCN. 2004;58:1231–1238. doi: 10.1038/sj.ejcn.1601954. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- Hoggard N, Haggarty P, Thomas L, Lea RG. Leptin expression in placental and fetal tissues: does leptin have a functional role? Biochem Soc Trans. 2001;29:57–63. doi: 10.1042/0300-5127:0290057. [DOI] [PubMed] [Google Scholar]
- Hollingsworth DR, Grundy SM. Pregnancy-associated hypertriglyceridemia in normal and diabetic women. Differences in insulin-dependent, noninsulin-dependent, and gestational diabetes. Diabetes. 1982;31:1092–1097. doi: 10.2337/diacare.31.12.1092. [DOI] [PubMed] [Google Scholar]
- Horvath, Koletzko B, Szajewska H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Br J Nutr. 2007;98:253–259. doi: 10.1017/S0007114507709078. [DOI] [PubMed] [Google Scholar]
- Innis SM. Perinatal biochemistry and physiology of long chain polyunsaturated fatty acids. J Pediatr. 2003;143(2 suppl. 1):S1–8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl. A):S70–75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Innis SM. Dietary (n 3) fatty acids and brain development. J Nutr. 2007a;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- Innis SM. Fatty acids and early human development. Early Hum Dev. 2007b;83:761–766. doi: 10.1016/j.earlhumdev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Jansson T, Cetin I, Powell TL, Desoye G, Radaelli T, Ericsson A, Sibley CP. Placental transport and metabolism in fetal overgrowth – a workshop report. Placenta. 2006;27(Suppl. A):S109–113. doi: 10.1016/j.placenta.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Jeffrey BG, Neuringer M. Age-related decline in rod phototransduction sensitivity in rhesus monkeys fed an n-3 fatty acid deficient diet. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-3640. DOI 10.1167/iovs.09-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127–R133. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr. 2006;26:271–291. doi: 10.1146/annurev.nutr.24.012003.132249. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Cetin I, Brenna JT, Perinatal Lipid Intake Working Group; Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenterology, Hepatology and Nutrition, Committee on Nutrition; International Federation of Placenta Associations; International Society for the Study of Fatty Acids and Lipids. Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98:873–877. doi: 10.1017/S0007114507764747. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Lien E, Agostoni C, Böhles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S, Hoesli I, Holzgreve W, Lapillonne A, Putet G, Secher NJ, Symonds M, Szajewska H, Willatts P, Uauy R. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendationsWorld Association of Perinatal Medicine Dietary Guidelines Working Group. J Perinat Med. 2008;36:5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- Larqué E, Demmelmair H, Berger B, Hasbargen U, Koletzko B. In vivo investigation of the placental transfer of 13C-labeled fatty acids in humans. J Lipid Res. 2003;44:49–55. doi: 10.1194/jlr.m200067-jlr200. [DOI] [PubMed] [Google Scholar]
- Llanos A, Li Y, Mena P, Salem N. Infants with intra uterine growth restriction have impaired formation of docosahexaenoic acid in early neonatal life: a stable isotope study. Pediatric Res. 2005;58:735–740. doi: 10.1203/01.PDR.0000180542.68526.A2. [DOI] [PubMed] [Google Scholar]
- Llorente AM, Jensen CL, Voigt RG, Fraley JK, Berretta MC, Heird WC. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. Am J Obstet Gynecol. 2003;188:1348–1353. doi: 10.1067/mob.2003.275. [DOI] [PubMed] [Google Scholar]
- Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in the human placenta in pregnancies complicated by intrauterine growth restriction and diabetes. J Clin Endocrinol Metab. 2004;89:4607–4614. doi: 10.1210/jc.2003-032234. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Non adipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- Montelongo A, Lasunción MA, Pallardo LF, Herrera E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes. 1992;41:1651–1659. doi: 10.2337/diab.41.12.1651. [DOI] [PubMed] [Google Scholar]
- Olsen SF. Is supplementation with marine omega-3 fatty acids during pregnancy a useful tool in the prevention of preterm birth? Clin Obstet Gynecol. 2004;47:768–774. doi: 10.1097/01.grf.0000141430.57412.56. [DOI] [PubMed] [Google Scholar]
- Olsen SF, Østerdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. Eur J Clin Nutr. 2007;61:976–985. doi: 10.1038/sj.ejcn.1602609. [DOI] [PubMed] [Google Scholar]
- Ortega-Senovilla H, Alvino G, Taricco E, Cetin I, Herrera E. Gestational diabetes mellitus upsets the proportion of fatty acids in umbilical arterial but not venous plasma. Diabetes Care. 2009;32:120–122. doi: 10.2337/dc08-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi G, Marconi AM, Cetin I. Placental-fetal interrelationship in IUGR fetuses – a review. Placenta. 2002;23(Suppl. A):S136–141. doi: 10.1053/plac.2002.0802. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Golberg R. Energy adaptations in human pregnancy: limits and long term consequences. Am J Clin Nutr. 2000;71:1226S–1232S. doi: 10.1093/ajcn/71.5.1226s. [DOI] [PubMed] [Google Scholar]
- Rees AM, Austin MP, Owen C, Parker G. Omega-3 deficiency associated with perinatal depression: Case control study. Psychiatry Res. 2009;166:254–259. doi: 10.1016/j.psychres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Reisbick S, Neuringer M, Hasnain R, Connor WE. Polydipsia in rhesus monkeys deficient in omega 3 fatty acids. Physiol Behav. 1990;47:315–323. doi: 10.1016/0031-9384(90)90149-x. [DOI] [PubMed] [Google Scholar]
- Ruyle M, Connor WE, Anderson GJ, Lowensohn RI. Placental transfer of essential fatty acids in humans: venous-arterial difference for docosahexaenoic acid in fetal umbilical erythrocytes. Proc Natl Acad Sci U S A. 1990;87:7902–7906. doi: 10.1073/pnas.87.20.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer-Graf UM, Pawliczek J, Passow D, Hartmann R, Rossi R, Bührer C, Harder T, Plagemann A, Vetter K, Kordonouri O. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005;28:1745–1750. doi: 10.2337/diacare.28.7.1745. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D'Souza SW. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58:827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- Siemelink M, Verhoef A, Dormans JA, Span PN, Piersma AH. Dietary fatty acid composition during pregnancy and lactation in the rat programs growth and glucose metabolism in the offspring. Diabetologia. 2002;45:1397–1403. doi: 10.1007/s00125-002-0918-2. [DOI] [PubMed] [Google Scholar]
- Smithers LG, Gibson RA, McPhee A, Makrides M. Higher dose of docosahexaenoic acid in the neonatal period improves visual acuity of preterm infants: results of a randomized controlled trial. Am J Clin Nutr. 2008;88:1049–1056. doi: 10.1093/ajcn/88.4.1049. [DOI] [PubMed] [Google Scholar]
- Smuts CM, Borod E, Peeples JM, Carlson SE. High-DHA eggs: feasibility as a means to enhance circulating DHA in mother and infant. Lipids. 2003;38:407–414. doi: 10.1007/s11745-003-1076-y. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83:1337–1344. doi: 10.1093/ajcn/83.6.1337. [DOI] [PubMed] [Google Scholar]
- Szitanyi P, Koletzko B, Mydlilova A, Demmelmair H. Metabolism of 13C-labeled linoleic acid in newborn infants during the first week of life. Pediatr Res. 1999;45:669–673. doi: 10.1203/00006450-199905010-00010. [DOI] [PubMed] [Google Scholar]
- Tabano S, Alvino G, Antonazzo P, Grati FR, Miozzo M, Cetin I. Placental LPL gene expression is increased in severe intrauterine growth-restricted pregnancies. Pediatr Res. 2006;59:250–253. doi: 10.1203/01.pdr.0000199441.62045.a1. [DOI] [PubMed] [Google Scholar]
- Taricco E, Radaelli T, Nobile de Santis MS, Cetin I. Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta. 2003;24:343–347. doi: 10.1053/plac.2002.0913. [DOI] [PubMed] [Google Scholar]
- Thomas B, Ghebremeskel K, Lowy C, Min Y, Crawford MA. Plasma AA and DHA levels are not compromised in newly diagnosed gestational diabetic women. Eur J Clin Nutr. 2004;58:1492–1497. doi: 10.1038/sj.ejcn.1601996. [DOI] [PubMed] [Google Scholar]
- Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS. Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nat Med. 2001;7:258–259. doi: 10.1038/85354. [DOI] [PubMed] [Google Scholar]
- Wijendran V, Bendel RB, Couch SC, Philipson EH, Thomsen K, Zhang X, Lammi-Keefe CJ. Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: relations with maternal factors. Am J Clin Nutr. 1999;70:53–61. doi: 10.1093/ajcn/70.1.53. [DOI] [PubMed] [Google Scholar]
- Wittmaack FM, Gafvels ME, Bronner M, Matsuo H, McCrae KR, Tomaszewski JE, Robinson SL, Strickland DK, Strauss JF. Localization and regulation of the human very low density lipoprotein/apolipoprotein-E receptor: Trophoblast expression predicts a role for the receptor in placental lipid transport. Endocrinology. 1995;136:340–348. doi: 10.1210/endo.136.1.7828550. [DOI] [PubMed] [Google Scholar]
- Zorzano A, Lasunción MA, Herrera E. Role of the availability of substrates on hepatic and renal gluconeogenesis in the fasted late pregnant rat. Metabolism. 1986;35:297–303. doi: 10.1016/0026-0495(86)90144-7. [DOI] [PubMed] [Google Scholar]