Abstract
Size at birth is critical in determining life expectancy and is dependent primarily on the placental supply of nutrients. However, the fetus is not just a passive recipient of nutrients from the placenta. It exerts a significant acquisitive drive for nutrients, which acts through morphological and functional adaptations in the placenta, particularly when the genetically determined drive for fetal growth is compromised by adverse intrauterine conditions. These adaptations alter the efficiency with which the placenta supports fetal growth, which results in optimal growth for prevailing conditions in utero. This review examines placental efficiency as a means of altering fetal growth, the morphological and functional adaptations that influence placental efficiency and the endocrine regulation of these processes.
Fetal growth and size at birth, in particular, are critical in determining mortality and morbidity, both immediately after birth and in later life. Infants that are small or large for gestational age, or show intrauterine growth restriction (IUGR) are less likely to survive at birth and are at greater risk of developing adult-onset degenerative diseases, such as glucose intolerance and Type 2 diabetes (Barker & Clark, 1997). In mammals, the major determinant of intrauterine growth is the placental supply of nutrients to the fetus (Fowden et al. 2006b). Indeed, in many species, fetal weight near term is positively correlated to placental weight, as a proxy measure of the surface area for materno-fetal transport of nutrients (Baur, 1977; Mellor, 1983). In turn, the nutrient transfer capacity of the placenta depends on its size, morphology, blood flow and transporter abundance (Fowden et al. 2006b). In addition, placental synthesis and metabolism of key nutrients and hormones influences the rate of fetal growth (Fowden & Forhead, 2004). Changes in any of these placental factors can, therefore, affect intrauterine growth (Fowden et al. 2006b; Jones et al. 2007). However, the fetus is not just a passive recipient of nutrients from the placenta. In cows, pigs and horses, embryo transfer between breeds of different size has shown that either enhancing or constraining the genetic potential for growth of the transferred embryo can alter placental development relative to the norm for the recipient breed (Ferrell, 1991; Wilson et al. 1998; Allen et al. 2002). The fetal genome, therefore, exerts a significant acquisitive drive for maternal nutrients through adaptations in the placenta, particularly when the potential for feto-placental growth is compromised. These adaptations alter the phenotype of the placenta and the efficiency with which it supports growth of the fetus with potential consequences for adult health and disease (Jones et al. 2006; Fowden et al. 2008). For any given birth weight, adult blood pressure is lower the smaller the placenta so a large baby with a small placenta has the lowest risk of developing adult hypertension (Barker & Clark, 1997). Consequently, over the normal range of birth weights, small efficient placentas appear to confer a health benefit in the long term. This review examines placental efficiency as a means of altering fetal growth, the morphological and functional adaptations that influence placental efficiency and the endocrine regulation of these processes.
Placental efficiency
Placental efficiency is most commonly defined as the grams of fetus produced per gram of placenta (Wilson & Ford, 2001). It can also be calculated as grams fetus produced per unit area of placental exchange surface but this measurement is less widely used because of the difficulty in estimating the exchange area in every placenta (Baur, 1977; Wooding & Burton, 2008). Placental efficiency measured as grams fetus per gram placenta varies widely between species, ranging from 5 g g−1 in human infants to 20 g g−1 in foals at term (Leiser & Kaufmann, 1994). Within species, it also varies with breed, with higher values in hardier sheep and more prolific pig breeds (Wilson et al. 1998; Wilson & Ford, 2001; Dwyer et al. 2005; Vonnahme et al. 2006). Different strains of rats and mice also have different placental efficiencies in late gestation (McClaren, 1965; Kurtz et al. 1999; Buresova et al. 2006). In several species, placental efficiency increases with parity during the early part of reproductive life but then declines with each successive pregnancy as the multiparous mother ages (Dwyer et al. 2005; Wilsher & Allen, 2003; Bravo et al. 2009). In polytocous species like pigs, rats and mice, placental efficiency can vary by 100% or more within a litter and, on average, is related positively to litter size (Kurtz et al. 1999; Wilson & Ford, 2001; Buresova et al. 2006). Even in di- and tri-tocous species, placental efficiency is higher in triplet than twin or single pregnancies (Dwyer et al. 2005; Konyali et al. 2007; Rutherford & Tardif, 2008). In addition to these natural variations in placental efficiency, the fetal to placental weight ratio can be altered experimentally by manipulation of uterine blood flow, oxygen availability and of the intake and composition of the maternal diet (Table 1). These observations suggest that placental efficiency is genetically determined, in part, but is also responsive to environmental conditions during pregnancy.
Table 1.
The effects of nutritional and endocrine manipulations during pregnancy on placental and fetal weights, and on actual and derived placental efficiency (grams fetus per gram placenta) in different laboratory species measured in late gestation (= 80% gestation)
| Treatment | Species | Gestational age (days) at treatment | Placental weight | Fetal weight | Placental efficiency | References | |
|---|---|---|---|---|---|---|---|
| Nutritional manipulations | |||||||
| Over-nutrition | |||||||
| High fat | Mouse | 0–19 | No Δ | ↑ 33% | ↑ 33% | Jones et al. 2009 | |
| High sugar | Rat | 0–22 | ↓ 4% | No Δ | ↑ 4% | Holemans et al. 2004 | |
| Under-nutrition | |||||||
| Protein | 4% | Rat | 2–21 | ↓ 14% | ↓ 21% | ↓ 10% | Jansson et al. 2006 |
| 5% | Rat | 0–20 | ↓ 27% | ↓ 20% | ↑ 10% | Malandro et al. 1996 | |
| 6% | Rat | 2–21 | ↓ 18% | ↓ 24% | ↓ 7% | Rosso 1977a,b; | |
| 8% | Rat | 0–18 | No Δ | ↑ 9% | ↑ 10% | Langley-Evans et al. 1996 | |
| 8% | Rat | 0–21 | ↓ 9% | ↓ 6% | ↑ 8% | Fernandez-Twinn et al. 2003 | |
| Calories | Mouse | 0–16 | ↓ 6% | No Δ | ↑ 9% | Vaughan et al. 2008 | |
| Rat | 0–18 | ↓ 32% | ↓ 23% | ↑ 13% | Woodall et al. 1996 | ||
| Guinea pig | 0–60 | ↓ 31% | ↓ 35% | ↓ 6% | Roberts et al. 2001 | ||
| Guinea pig | 15–65 | ↓ 12% | ↓ 6% | ↑ 10% | Bacon et al. 1984 | ||
| # Iron | Rat | 0–21 | No Δ | ↓ 19% | ↓ 24% | Lewis et al. 2001 | |
| Restricted uterine flow | Rat | 19–21 | ↓ 16% | ↓ 27% | ↓ 14% | Gilbert & Leturque 1982 | |
| Rat | 19–21 | No Δ | ↓ 10% | ↓ 19% | Reid et al. 1999 | ||
| Rat | 18–20 | No Δ | ↓ 15% | ↓ 15% | Wlodek et al. 2005 | ||
| # Hypoxia | Rat | 15–20 | No Δ | ↓ 8% | ↓ 8% | Lueder et al. 1995 | |
| Guinea pig | 15–65 | ↑ 12% | No Δ | ↓ 12% | Bacon et al. 1984 | ||
| Guinea pig | 20–64 | ↓ 5% | ↓ 30% | ↓ 26% | Gilbert et al. 1979 | ||
| Endocrine manipulations | |||||||
| Glucocorticoids | |||||||
| Maternal treatment | Mouse | 15–17 | ↓ 7% | ↓ 19% | ↓ 13% | Baisden et al. 2007 | |
| Rat | 13–20 | ↓ 50% | ↓ 24% | ↑ 60% | Ain et al. 2005 | ||
| Rat | 13–22 | ↓ 34% | ↓ 22% | ↑ 19% | Hewitt et al. 2006 | ||
| Rat | 15–21 | ↓ 50% | ↓ 27% | ↑ 46% | Sugden et al. 2001 | ||
| 11βHSD2−/− | Mouse | 0–15 | ↓ 10% | No Δ | ↑ 22% | Wyrwoll et al. 2009 | |
| Mouse | 0–18 | ↓ 6% | ↓12% | ↓ 5% | Wyrwoll et al. 2009 | ||
| IGF-II | |||||||
| Igf2P0+/− | Mouse | 0–19 | ↓ 34% | ↓ 23% | ↑ 13% | Constancia et al. 2005 | |
| Igf2+/− | Mouse | 0–19 | ↓ 40% | ↓ 51% | ↓ 18% | Coan et al. 2008b | |
| H19 Null | Mouse | 0–19 | ↑ 50% | ↑ 23% | ↓ 15% | Angiolini et al. 2006 | |
| IGF-I | |||||||
| Igf1−/− | Mouse | 0–19 | No Δ | ↓ 40% | ↓ 40% | Efstradiadis, 1998 | |
| Maternal treatment | Guinea pig | 20–38* | No Δ | ↑ 17% | ↑ 17% | Sferruzzi-Perri et al. 2006; 2007a; | |
Gestational age at measurement is the age at the end of treatment except where indicated. ↑ increase, ↓ decrease * gestational age at measurement = 62 days.
Changes in placental efficiency can occur by alterations in the weight of the fetus, placenta or both (Table 1). Generally, in normoxic conditions before term (< 90% gestation), lighter placentas are more efficient than those that are heavier, which is consistent with the fetal drive for nutrient acquisition. Over the normal range of birth weights in term human infants, placentas weighing less than 300 g support 20% more fetal mass per gram than those weighing 500 g or more, even though the infant with the small placenta weighs less than average at birth (Molteni et al. 1978; Salafia et al. 2007). Increases in efficiency are also seen in naturally small relative to large placentas in pigs, sheep, goats, rats and mice (Wilson & Ford, 2001; Dwyer et al. 2005; Buresova et al. 2006; Konyah et al. 2007; Coan et al. 2008a). In sheep, restriction of placental growth from early in gestation by removal of implantation sites and adverse environmental conditions, such as heat stress and under- or over-nutrition, retards fetal and placental growth but often increases the fetal to placental weight ratio compared to control animals near term (Owens et al. 1989; Wallace et al. 2002; Regnault et al. 2003; Ogersby et al. 2004). In laboratory species, experimental manipulation of placental growth by maternal dietary restriction from conception can increase or decrease the fetal to placental weight ratio depending on the type, duration and severity of the nutrient deprivation and the gestational age at study (Table 1).
In general before term, restriction of oxygen availability by maternal anaemia, reduction in uterine blood flow or direct hypoxaemia reduces placental efficiency while restriction of substrates for oxidation and growth by dietary calorie or protein deprivation tends to increase placental efficiency (Table 1). On low protein diets, placental efficiency declines towards term with either a direct switch from increased to decreased efficiency or a progressive decline in the degree to which the fetal to placental weight ratio is increased above normal over the last 4–5 days of gestation (Rosso, 1977a,b; Langley-Evans et al. 1996; Jansson et al. 2006). Similarly, in both rats and mice, maternal undernutrition leads to increased placental efficiency at the beginning of the fetal growth spurt (days 15–16) but decreased efficiency closer to term when nutrient availability fails to meet the fetal growth demands and IUGR ensues (Woodall et al 1996; Vaughan et al. 2008). In part, this ontogenic decline in placental efficiency is related to the severity of the insult. For instance at day 21 of pregnancy, placental efficiency is still increased in rats fed an 8% isocalorific low protein diet but is decreased when the protein content is lowered to 4–6% compared to controls on an 18–20% protein diet (Langley-Evans et al. 1996; Fernandez-Twinn et al. 2003; Jansson et al. 2006).
At any given gestational age, measurements of placental efficiency, therefore, provide an indication of the conditions experiences in utero and the extent to which adaptations in feto-placental development have occurred to meet the fetal growth demands. In the laboratory species studied to date, inefficient placentas tend to occur when oxygen availability is limited either by experimental manipulations or poor placental vascularization (Table 1, Fowden et al. 2006a). Conversely, efficient placentas are more commonly observed in normoxic conditions when the availability of oxidative substrates or specific nutrients is altered. In particular, the experimentally induced or naturally occurring small placenta appears to adapt to help maintain fetal growth by increasing its efficiency, although its small size may eventually limit intrauterine growth and lower the apparent efficiency of the placenta shortly before birth. These adaptations may be morphological or functional in origin.
Morphological adaptations
Placental efficiency could be changed by alterations in the surface area for exchange, the thickness of the barrier between the maternal and fetal circulations and/or in the density and architectural arrangements of the fetal and maternal vasculature within the placenta. Amongst species, the differences in placental efficiency may relate, in part, to the differences in gross placental morphology and the number of membrane layers between the maternal and fetal circulations, which can vary from four to eight layers (Leiser & Kaufmann, 1994). However, epitheliochorial placental types with eight layers can be as efficient as the four layered, hemochorial placentas, which emphasises the complexity of placental nutrient transport in relation to fetal growth (Fowden et al. 2006b; Wooding & Burton, 2008). A more likely explanation for the species differences in placental efficiency is the vascular architecture of the placenta. Species with counter current arrangements of the two circulations, such as the horse and the mouse, have higher placental efficiencies than species with multi-villous or cross-current flows, like the sheep and human (Leiser & Kaufmann, 1994).
The natural intra-species variation in placental efficiency may also be related to differences in the placental vasculature. In pigs and sheep, the breed differences in placental efficiency are associated with changes in capillary density with higher values in the more prolific breeds with smaller individual placentas and fetuses but higher fetal to placental weight ratios (Wilson & Ford, 2001; Reynolds et al. 2006). Similarly, within pig litters, the smaller more efficient placenta has a higher vascularity coupled to increased expression of VEGF (Vonnahme et al. 2001). Increased fetal capillary volume density is also seen in the labyrinthine areas responsible for nutrient transfer in the small placenta of guinea pigs exposed to normobaric hypoxia, although the fetal to placental weight ratio is reduced in these circumstances (Bacon et al. 1984). In contrast, vascularity and VEGF expression are decreased in the more efficient, small placenta of heat stressed or overnourished adolescent ewes (Regnault et al. 2003; Reynolds et al. 2006). The smallest placenta within a mouse litter also has a smaller volume of fetal capillaries in the labyrinthine zone than the largest placenta in the litter, despite its increased efficiency (Coan et al. 2008a). No changes in vascularity were observed in the placenta of rats fed an 8% protein diet during pregnancy (Doherty et al. 2003). These observations suggest that changes in placental vascularity can account, in part, for altered placental efficiency but not in all instances.
Less is known about other morphological adaptations that may contribute to changes in placental efficiency. The thickness of the barrier between the fetal and maternal circulations is increased in the less efficient placenta of the undernourished guinea pig (Roberts et al. 2001). In contrast, no changes in barrier thickness were observed in placentas with different efficiencies within mouse litters (Coan et al. 2008a). In small efficient mouse placentas at day 16 of pregnancy, the labyrinthine zone accounts for a greater proportion of the total volume so that there is 35% more surface area per gram of placenta compared to the large placenta in the litter (Coan et al. 2008a). Similarly, the length of fetal-maternal contact and the complexity of its folding per unit area were increased in the more efficient placentas within pig litters from 45 to 105 days of gestation (Vallet & Freking, 2007). Increases in the total surface area of materno-fetal contact per unit volume are also seen in the more efficient placentas of rats fed an 8% protein diet and in the triplet relative to twin placenta of the marmoset (Doherty et al. 2003; Rutherford & Tardif, 2009). Conversely, the percentage of total placental volume that was labyrinthine and the surface area per gram placenta were reduced in the less efficient, growth restricted placenta of the undernourished guinea pig (Roberts et al. 2001). However, morphological changes are unlikely to account entirely for the changes in placental efficiency. For example, in the marmoset, the triplet has 25% less exchange surface area per fetus than the twin, despite the significantly greater surface density of maternal–fetal interface in the total placenta, yet weighs only 13% less than the twin (Rutherford & Tardif, 2008, 2009). The most efficient human placentas are also the thinnest and smallest in area (Salafia et al. 2007). In these instances, there must be functional adaptations in the placenta to increase its efficiency.
Functional adaptations
Functionally, placental efficiency could be altered by changes in the capacity of the placenta to supply nutrients to the fetus or hormones to both the fetus and mother. The placenta synthesises and metabolises a range of hormones with metabolic and growth regulatory actions that could influence feto-placental growth both directly and indirectly by changes in maternal metabolism and partitioning of nutrients between mother and fetus (Fowden & Forhead, 2009). The placenta transports nutrients to the fetus by simple diffusion and transporter mediated processes which can be facilitated or active (Sibley et al. 2005). All these processes depend to a certain extent on morphological characteristics of the placenta, such as surface area, barrier thickness, capillary density and maternal blood supply. However, the transporter mediated processes involved in transferring the nutrients required for fetal growth are also influenced by the abundance, activity and localisation of specific transporters in the placental membranes (Sibley et al. 2005). In addition, simple and facilitated diffusion are affected by the materno-fetal concentration gradient across the placenta (Hay, 1994). The apparent alterations in placental efficiency associated with maternal dietary manipulation and other environmental challenges may, therefore, be due to the innate functional reserve capacity of the placenta and/or to changes in the transplacental nutrient concentration gradients rather than a direct consequence of adaptations in the nutrient transfer capacity of the placenta per unit weight (Fowden et al. 2006b, 2008).
In vivo and in vitro measurements of nutrient transfer across placentas with differing efficiencies have been made in several species using a range of techniques to manipulate feto-placental growth (Rosso, 1977a,b; Bacon et al. 1984; Owens et al. 1989; Malandro et al. 1996; Ashworth et al. 2001; Jansson et al. 2006; Coan et al. 2008a; Jones et al. 2009). In general, these studies show that more efficient placentas transfer more substrate on a weight specific basis than less efficient placentas, although total transfer across the small, efficient placenta may be inadequate to support normal fetal growth (Fowden et al. 2008). For example, in sheep, the small placenta of the carunclectomised ewe leads to fetal growth restriction but supports 10–15% more fetus and transfers 40–50% more 3-O-methyl-d-glucose (MG), a non-metabolisable glucose analogue, per gram than the normal placenta (Owens et al. 1987, 1989). Similarly, the more efficient placenta of the high fat fed mouse transports 5-fold more glucose per gram than the placenta of mice fed a normal diet (Jones et al. 2009). Conversely, glucose transfer is reduced by 50% per gram in the smaller, less efficient placenta of rats fed a 6% protein diet (Rosso, 1977b). Since transplacental glucose transfer is facilitated, these changes may be due, in part, to alterations in the glucose concentration gradient between the maternal and fetal circulations (Rosso, 1977b; Owens et al. 1989; Fernandez-Twinn et al. 2003; Holemans et al. 2004).
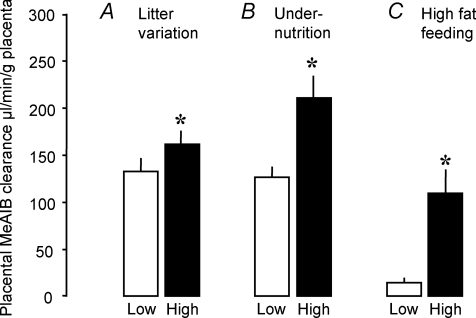
Unidirectional materno-fetal transfer of [14C]methylaminoisobutryic acid (MeAIB), an amino acid analogue transported primarily by the System A amino acid transporters, is increased per gram of mouse placenta near term when efficiency is elevated by maternal high fat feeding, undernutrition or natural variation within litters (Fig. 1). In contrast, MeAIB transfer was decreased in the rat placenta when the fetal to placental weight ratio was reduced by feeding diets with a 4–6% protein content (Rosso, 1977a; Malandro et al. 1996; Jansson et al. 2006). Indeed, the reduction in weight specific MeAIB transport across the placenta preceded IUGR in protein deprived rats (Jansson et al. 2006). In pregnant rats fed 6% protein diets, there are also reductions in the placental activity of the System y+ and X–AG amino acid transporters (Malandro et al. 1996). Furthermore, there is reduced transplacental flux of amino acids using the System L amino acid transporters in the small placenta of heat stressed ewes and runt piglets, despite their increased fetal to placental weight ratios (Finch et al. 2004; Regnault et al. 2005). The different amino acid transporter systems are, therefore, affected differentially when placental growth is restricted. However, transfer of amino acids across the placenta by the System A amino acid transporters, in particular, appears to be closely related to placental efficiency, at least in rodents (Jansson et al. 2006; Coan et al. 2008a; Jones et al. 2009; Wyrwoll et al. 2009). Since the amino acids transported by the System A transporters are often raised in concentration in the maternal circulation during adverse conditions (Regnault et al. 2005; Jones et al. 2007), upregulation of the placental abundance and activity of this transporter system offers a strategy to improve nutrient delivery to the fetus when its growth potential is impaired.
Figure 1. Unidirectional materno-fetal clearance of [14C]methylaminoisobutryic acid clearance across near term mouse placentas.
Mean (±s.e.m.) unidirectional materno-fetal clearance of [14C]methylaminoisobutryic acid (MeAIB) clearance across near term mouse placentas with low (open columns) and high efficiencies (filled columns) induced by natural variations between the lightest and heaviest placentas within a litter (n= 11 litters) (A), undernutrition (UN, n= 10 litters) to 80% of ad libitum fed controls (n= 11 litters) (B) and feeding a high fat diet compared to controls (n= 5 litters per diet) (C). Data from Coan et al. 2008a; Jones et al. 2009 and P. M. Coan & A. L. Fowden unpublished observations.
The differences in glucose and amino acid transport with placental efficiency are paralleled by changes in the abundance of the glucose and amino acid transporters in the placenta (Table 2). The placenta has two principal glucose transporters, Slc2a1/GLUT1 and Slc2a3/GLUT3, the abundance of which tends to increase in more efficient placentas and decrease in less efficient ones, irrespective of species or the cause of the altered efficiency (Table 2). Likewise, abundance of specific isoforms of the System A family of transporters (Slc38a1/SNAT1, Slc38a2/SNAT2 and Slc38a4/SNAT4) varies in parallel with placental efficiency across species and treatments (Table 2). The more efficient placentas, therefore, tend to have increased expression of both glucose and amino acid transporters, particularly of the Slc2a1 and Slc38a2 isoforms (Table 2). These observations are consistent with the suggestion that placental efficiency is an index of feto-placental adaptation and show that the placenta can make functional and morphological adaptations to help match the actual supply of nutrients to the fetal nutrient demand for growth. The mechanisms responsible for modifying placental phenotype in this way may involve nutrient and/or hormonal signals (Jansson & Powell, 2006; Fowden & Forhead, 2009).
Table 2.
The relationship between placental efficiency measured as gram fetus per gram placenta during natural and experimental conditions and placental expression of glucose and amino acid transporter genes or protein in different species.
| Species | Treatment | Glucose transporters | Amino acid transporters | References | |
|---|---|---|---|---|---|
| Efficient | Mouse | Naturally small | ↑Slc2al, NoΔSlc2a3 | ↑Slc 38a2 | Coan et al. 2008a |
| placentas | Mouse | Igf2P0+/− | ↑Slc2a3, NoΔSlc2a1 | ↑Slc38a4 | Constancia et al. 2005 |
| Mouse | High fat | ↑Slc2a1 | ↑Slc38a2 | Jones et al. 2009 | |
| Mouse | 11β HSD−/− Day 15 | NoΔSlc2al or Slc2a3 | ↑Slc38a2 & Slc38a4 | Wyrwoll et al. 2009 | |
| Mouse | hTRX-1 overexpression | ↑Slc2a1 | ? | Umekawa et al. 2008 | |
| Rat | Dexamethasone | ↑ GLUT1 & GLUT3 | ? | Langdown & Sugden, 2001 | |
| Guinea pig | Maternal IGF-I | NoΔSlc2a1 | ↑Slc38a2 | Sferruzzi-Perri et al. 2007a | |
| Inefficient | Mouse | 11β HSD−/− Day 18 | ↓Slc2a3 | NoΔSlc38a1, a2 & a4 | Wyrwoll et al. 2009 |
| placentas | Mouse | Igf2+/− | ? | ↓Slc38a2, | Constancia et al. 2005; |
| ↓ System X−AG & y+ | Matthew et al. 1999 | ||||
| Mouse | GH overexpression | ? | ↓ System X−AG & y+ | Matthew et al. 1999 | |
| Rat | Protein restriction | ? | ↓ SNAT2 | Jansson et al. 2006 | |
| Rat | Restricted uterine blood flow | ↓Slc2a1 | ? | Das et al. 1998 | |
| Rat | Undernutrition | ↓ GLUT3, NoΔ GLUT1 | ? | Lesage et al. 2002 | |
| Human | High altitude | ↓ GLUT1 | ? | Zamudio et al. 2006 |
↑ increased abundance, ↓ decreased abundance, ? no information.
Endocrine regulation of placental phenotype
In both maternal and fetal circulations, hormone concentrations change in response to environmental challenges that adapt placental efficiency, such as manipulation of maternal dietary intake, uterine blood flow and hypoxaemia. In the fetus, environmental conditions favourable for fetal growth increase the concentrations of anabolic hormones, such as insulin, insulin-like growth factors (IGFs) and the thyroid hormones, and lower concentrations of catabolic hormones, like cortisol and the catecholamines (Fowden & Forhead, 2004). Conversely, adverse conditions that constrain fetal growth tend to raise catabolic and lower anabolic hormone concentrations in the fetal circulation. In the mother, dietary and other manipulations that alter placental efficiency commonly affect the concentrations of glucocorticoids, IGF-I, leptin and insulin (Sugden et al. 2001; Lesage et al. 2002; Fernandez-Twinn et al. 2003; Jansson et al. 2006). These fetal and maternal endocrine changes act as signals of nutrient availability and match the rate of fetal growth to the rate of nutrient supply (Fowden & Forhead, 2004, 2009). Of the hormones regulating development in utero, the glucocorticoids and IGFs are the most likely to be involved in modifying placental phenotype as they are responsive to all the naturally occurring and experimentally induced conditions known to alter placental efficiency (Fowden & Forhead, 2009).
Glucocorticoids
Maternal glucocorticoid administration in late pregnancy increases placental efficiency in sheep, rats and non-human primates, despite reductions in both placental and fetal weight (Table 1; Johnson et al. 1979; Jensen et al. 2002; Braun et al. 2007). In sheep, this effect is seen in response to both natural cortisol and synthetic glucocorticoids used to treat threatened pre-term delivery in human clinical practise (Jensen et al. 2002; Braun et al. 2007). The effect depends, in part, on gestational age at administration and is more pronounced with treatment at mid to early-late gestation before the final fetal growth spurt than closer to term in both rats and sheep (Shafrir et al. 1994; Braun et al. 2007; Kutzler et al. 2004; Lesage et al. 2002; Hewitt et al. 2006).
Dexamethasone at clinical doses has little apparent effect on the gross morphology of the rat placenta, although, at higher doses, it causes loss of trophoblast cells in the mouse placenta (Ain et al. 2005; Baisden et al. 2007). In sheep, cortisol administration to either the fetus or mother reduces eversion of the placentomes during late gestation and decreases the frequency of the more everted placentome, although the functional significance of the different placentome types remains obscure (Jensen et al. 2002; Kutzler et al. 2004; Ward et al. 2006). In rats, the reduction in placental weight induced by maternal dexamethasone treatment in late gestation is accompanied by decreased labyrinthine vascularity and VEGF expression, which may compromise nutrient transfer (Hewitt et al. 2006). However, acute administration of dexamethasone leads to a transient increase in umbilical blood flow in the sheep during late gestation due to a rise in fetal blood pressure (Jellyman et al. 2004). Ultrastructurally, both fetal and maternal glucocorticoid administration reduces the number of binucleate cells (BNCs) in the ovine placenta, which reduces maternal placental lactogen concentrations with implications for maternal metabolism (Ward et al. 2002; Braun et al. 2007). Since fetal BNCs fuse with the maternal epithelium to form a feto-maternal syncytium in the ovine placenta (Wooding & Burton, 2008), changes in BNC number may influence expansion of this syncytial layer, which, in turn, could alter both the transport and endocrine functions of the placenta (Ward et al. 2002). Dysregulated expression of the prolactin family of genes also occurs in the rat placenta after maternal dexamethasone treatment late in gestation (Ain et al. 2005). In addition, expression of leptin and its transporter receptor are down-regulated while its signalling receptor is up-regulated in the dexamethasone treated rat placenta (Sugden et al. 2001; Smith & Waddell, 2002). Furthermore, glucocorticoids affect placental production of the eicosanoids, sex steroids and active thyroid hormones (see Fowden & Forhead, 2009). Moreover, glucocorticoids can influence their own bioavailability in feto-placental tissues by down-regulating placental activity of 11β-hydroxysteroid dehydrogenase type 2 which converts active glucocoticoids to their inactive metabolites (Clarke et al. 2002; Kerzner et al. 2002). The maternal and fetal endocrine environments are, therefore, altered by these glucocorticoid induced changes in placental hormone synthesis and metabolism with potential consequences for the partitioning of maternal nutrients to fetal growth.
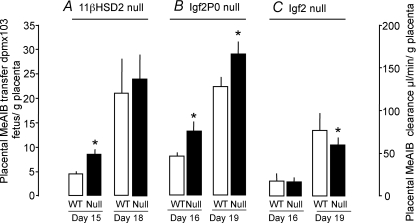
The effects of glucocorticoids on placental nutrient transfer are less well established. Cortisol has little effect on glucose or MeAIB transport in human placental villous fragments but increases MeAIB transport in association with increased SNAT2 abundance in BeWo cells, a human placental cell line (Ericsson et al. 2005; Jones et al. 2006). Dexamethasone treatment also affects transplacental transfer of glutamate in the sheep during late gestation (Timmerman et al 2000). Administration of cortisol directly to fetal sheep reduces umbilical uptake of glucose per gram placenta primarily by increasing the rate of uteroplacental glucose consumption (Ward et al. 2004). This effect was more pronounced in placentas with a greater number of more everted placentome types (Ward et al. 2006). Glucocorticoids down-regulate GLUT1 expression in the human placenta and up- and/or down-regulate GLUT1 and GLUT3 expression in rat placenta depending on the degree of IUGR (Hahn et al. 1999; Langdown & Sugden, 2001), but have little, if any, effect on expression of either GLUT in the ovine placenta (Ward et al. 2004). Increasing feto-placental exposure to bioactive glucocorticoids in mice by deleting the 11βHSD2 gene up-regulates placental MeAIB transport at day 15 of pregnancy (Fig. 2A), in association with an increased fetal to placental weight ratio and up-regulated placental expression of the Slc38a2 and Slc38a4 isoforms of the System A transporters (Tables 1 and 2). However, by day 18, this effect is lost (Fig. 2A), and placental glucose transfer and Slc2a3 glucose transporter expression are decreased along with a reduced fetal to placental weight ratio compared to their wild-type littermates (Tables 1 and 2). Glucocorticoids, therefore, appear to influence placental efficiency through changes in placental morphology, hormone synthesis and transport physiology.
Figure 2. Unidirectional materno-fetal transfer of [14C]methylaminoisobutryic acid across wild-type and null placentas.
Mean (±s.e.m.) unidirectional materno-fetal transfer of [14C]methylaminoisobutryic acid (MeAIB) across wild-type (WT, open columns) and null placentas (filled columns) of 11β-HSD2−/− (11β-hydroxysteroid dehydrogenase type 2)(A), Igf2P0+/− (B), and Igf2+/− mutant mice (C) at Day 15 or 16 and Day 18 or 19 of gestation calculated as counts transferred to the fetus per gram placenta in A and as clearance in B and C. Number of litters = 7–15 for each mutant at each gestational age. Data from Constancia et al. 2005, Sferruzzi-Perri et al. 2009 and Wyrwoll et al. 2009.
Insulin-like growth factors
The IGFs have an important role in feto-placental development and have morphological and functional effects on placental efficiency (Fowden, 2003, Forbes & Westwood, 2008). They are expressed in a wide range of feto-placental tissues and act as signals of nutrient availability in both the fetus and the mother. Their expression in fetal and placental tissues is also affected by the glucocorticoids and other hormones including insulin and the thyroid hormones (Fowden & Forhead, 2004). The IGFs may, therefore, be responsible for the changes in placental efficiency and nutrient transfer capacity induced by nutritional and other challenges that alter the endocrine milieu in utero.
In guinea pigs, maternal administration of IGF-I from days 20 to 38 of pregnancy increases the fetal to placental weight ratio at both mid and late gestation by increasing fetal and placental weight at mid gestation and fetal weight alone at term (Sferruzzi-Perri et al. 2006, 2007a,b). Placental weight, labyrinthine surface area, trophoblast abundance, thinness and vascularity were all directly related to maternal IGF-I concentrations in late gestation, although direct administration of IGF-I between days 20 and 38 of pregnancy had little effect on labyrinthine morphology in late gestation (Roberts et al. 2002; Sferruzzi-Perri et al. 2006). At 35 days of gestation, 15 days of maternal IGF-I treatment increased transplacental transport of MG and MeAIB per gram placenta in association with increased placental Slc38a2 gene expression (Sferruzzi-Perri et al. 2007b). By 62 days of pregnancy, 25 days after cessation of IGF-I treatment, transport of glucose was still elevated per gram placenta relative to vehicle treated controls and there was a trend for increased MeAIB transport in association with increased fetal tissue MeAIB uptake and elevated fetal α-amino nitrogen concentrations (Sferruzzi-Perri et al. 2007a). Transplacental transfer of MG, but not MeAIB, was positively correlated to maternal IGF-I concentrations at this age (Sferruzzi-Perri et al. 2007a).
In sheep, short term maternal administration of IGF-I in late gestation increased placental lactate production by 56% but had little effect on placental blood flow or transfer by simple on facilitated diffusion (Liu et al. 1994). When maternal IGF-I levels were elevated for 10 days by GH treatment in mid to late gestation, there was an increase in the placental capacity for simple diffusion and a trend towards increased placental clearance of MG without a change in placental weight (Harding et al. 1997; Jenkinson et al. 1999). In contrast, short term administration of IGF-I directly to fetal sheep reduced placental lactate production by 30% but had no effect on simple diffusion measured as urea clearance (Harding et al. 1994). Longer fetal infusions of IGF-I altered the frequency distribution of the different placentome types and reduced placental clearance of MG and MeAIB from the fetal circulation, without a change in urea clearance or weight of the fetus and placenta (Lok et al. 1996; Bloomfield et al. 2002). Similarly, in the mouse, elevation of maternal IGF-I by overexpression of GH leads to down regulation of placental expression of specific isoforms of the System X–AG and y+ amino acid transporters in late gestation coupled with a reduced fetal to placental weight ratio caused by fetal not placental growth retardation (Matthews et al. 1999). Deletion of the Igf1 gene also retards fetal but not placental growth but little is known about placental nutrient transfer in the Igf1knockout mouse. In contrast, in human first trimester and term placentas, IGF-I increases amino acid uptake into cultured and freshly isolated trophoblast cells (Kniss et al. 1994; Karl, 1995). Taken together, these findings suggest that fetal IGF-I is not directly involved in the growth of the placenta but can alter its transport characteristics while maternal IGF-I affects both placental development and function, perhaps by altering nutrient partitioning between the mother and fetus. Indeed, maternal IGF-I treatment during early to mid pregnancy reduces maternal adipose stores near term, indicative of altered materno-fetal resource allocation (Sferruzzi-Perri et al. 2006).
Like IGF-I, IGF-II also affects placental development (Fowden, 2003). The Igf2 gene is widely expressed in placental tissues of many species, particularly during the early stages of development (Carter & Enders, 2004; Forbes & Westwood, 2008). In the mouse, deletion of the Igf2 gene leads to placental and fetal growth retardation while overexpression of IGF-II by imprint relaxation through deletion of the H19 gene or by direct deletion of the Igf2r clearance receptor causes fetal and placental overgrowth (Efstradiadis, 1998). Deletion of the labyrinthine specific transcript of the Igf2 gene (Igf2P0) also leads to placental and fetal growth retardation, although this is less severe than seen in the compete Igf2 null (Constancia et al. 2005). Conversely, in chimeric embryos with normal Igf2 expression in the placenta but Igf2 deficiency in the fetal tissues, placental weight is reduced by 14% (Gardner et al. 1999). Growth of the mouse placenta, therefore, appears to depend on both the paracrine and endocrine actions of the Igf2 gene.
These changes in placental growth with manipulation of Igf2 gene expression are accompanied by alterations in placental efficiency. Both the overgrown H19 null placenta and the growth retarded complete Igf2 null placenta are inefficient while the growth retarded IgfP0 null placenta is more efficient and supports 15–25% more fetus per gram than their respective wild-type placentas. In part, these differences in efficiency are due to morphological changes in the placenta (Sibley et al. 2004; Coan et al. 2008b). In both, the complete Igf2 and Igf2P0 null placentas, there is less surface area for exchange and a thicker barrier between the maternal and fetal vessels in the labyrinthine zone compared to their wild-type counterparts (Coan et al. 2008b). This results in a significant reduction in the theoretical diffusion capacity of the null placentas, which is greater in the Igf2P0 than complete Igf2 null mutants, contrary to predictions from their respective efficiencies. Measurements of the actual passive permeability of these mutant placentas using radio-labelled solutes also indicate that the IgfP0 null placenta is less permeable than the complete Igf2 null (Coan et al. 2008b). These observations suggest that interactions between the various fetal and placental Igf2 transcripts control not only development of the trophoblast surface and capillary density but also the number and size of the pores in the interhaemal membrane involved in simple diffusion (Coan et al. 2008b). Morphological changes in the placenta, therefore, provide an explanation for the decreased efficiency of the complete Igf2 null placenta but cannot account for the increased efficiency of the Igf2P0 mutant placenta.
Although the passive diffusion capacity of the Igf2P0 null placenta is decreased, transporter-mediated transport processes are up-regulated (Constancia et al. 2005). Both the facilitated diffusion of MG and the active transport of MeAIB are increased by 30–60% per gram of Igf2P0 null relative to wild-type placenta (Fig. 2B; Constancia et al. 2005). These increases in transfer are accompanied by increased placental expression of Slc38a4 and Slc2a1 (Table 2). Up-regulation of nutrient transfer, therefore, compensates for the small size and reduced passive permeability of the Igf2P0 null placenta and leads to increased placental efficiency (Fowden et al. 2006a). In the complete Igf2 null placenta, less MeAIB is transported per gram compared to their wild-type counterparts at day 19 of gestation (Fig. 2C), in part, due to reduced placental expression of Slc38a2 (Table 2). This, together with the reduced expression of the System X–AG and Y+ amino acid transporters will contribute to the reduced efficiency of the complete Igf2 null placenta (Table 2; Matthews et al. 1999). Preliminary data also suggest that the reduced efficiency of the overgrown H19 null placenta is associated with reduced MeAIB transport per gram placenta close to term (Angiolini et al. 2006).
Comparison of the complete Igf2, Igf2P0 and H19 null placentas shows that interaction between placental and fetal Igf2 has an important role in regulating placental efficiency and the capacity for nutrient transfer. In the Igf2P0 mutant, the small placenta responds to the nutrient demand signals produced by the fetal tissues still expressing Igf2 by increasing its area for exchange and abundance of nutrient transporters. When the drive for fetal growth is reduced in the complete Igf2 null mouse, a smaller placental supply of nutrients is required to meet the demands of the lower growth rate and placental amino acid transfer declines. In contrast, when the fetal growth demand rises in the heavier H19 null fetuses, the increased nutrient requirements will be met by the larger placenta. The fall in MeAIB transfer across the large H19 null placenta close to term may, therefore, be a strategy to limit nutrient allocation to the fetuses when the drain on maternal resources is becoming excessive with the higher fetal growth demands in late gestation. A similar situation may occur in human infants, as placental System A activity is reduced in severely growth retarded infants but is inversely related to placental and infant weight over the normal range of weights at birth (Godfrey et al. 1998; Jansson et al. 2002; Pardi et al. 2002). Since placental Igf2 gene expression is reduced by maternal undernutrition and dexamethasone administration in rats (Price et al. 1992; Ain et al. 2005), this gene may be responsible for the changes in placental efficiency induced by these treatments. Indeed, preliminary findings suggest that the Igf2P0 transcript mediates the placental adaptations to undernutrition in the mouse (Sferruzzi-Perri et al. 2009).
Placental morphology and transport characteristics are also affected by maternal IGF-II concentrations. Maternal infusion of IGF-II at mid gestation had no effect on placental weight at either mid or late gestation but increased fetal weight close to term (Sferruzzi-Perri et al. 2006, 2007a,b). However, there were changes in the morphology of the placenta near term after maternal IGF-II treatment earlier in gestation with 25–40% increases in trophoblast volume and surface area within the labyrinthine zone (Sferruzzi-Perri et al. 2007a). Maternal IGF-II concentrations are also positively correlated with labyrinthine trophoblast volume density, barrier thinness and vascularity at 62 days of gestation (Roberts et al. 2001). Transport of MG, but not MeAIB, was increased per gram of IGF-II treated placenta near term (Sferruzzi-Perri et al. 2007a). In addition, when data from all groups were combined there was a direct relationship between MG transport per gram term placenta and the maternal IGF-II concentration at early to mid pregnancy (Sferruzzi-Perri et al. 2006, 2007a). In part, these action of maternal IGF-II may be mediated by the IGF type 2 receptor as Leu27-IGF-II, an IGF analogue that cannot bind to the IGF type 1 or insulin receptor, up-regulates MG and MeAIB transfer per gram term placenta after maternal treatment at 20–38 days of pregnancy (Sferruzzi-Perris et al. 2008). Changes in maternal IGF-II concentrations early in pregnancy may, therefore, programme the nutrient transport capacity of the guinea pig placenta much closer to term.
Other hormones
A range of other hormones, such as angiotensin, leptin and tri-iodothronine (T3), have been shown to alter the transport capacity of the placenta by effects on placental metabolism and transporter expression (Jones et al. 2007; Fowden & Forhead, 2009). For example, leptin increases MeAIB transport across human microvillous membranes in association with increased Slc38a2 expresssion (Jansson et al. 2003). Indeed, changes in maternal and fetal leptin concentrations in response to dietary manipulations, such as protein deprivation and high fat feeding, may explain, in part, the concomitant changes in placental amino acid transport and efficiency (Jansson et al. 2006; Jones et al. 2009; Forhead & Fowden, 2009). Since hormones, like the glucocorticoids, alter bioavailability of the IGFs, leptin, T3, placental lactogen and the glucocorticoids themselves (Fowden & Forhead, 2004), endocrine regulation of placental efficiency will be multifactorial and dependent on the combined endocrine changes in the mother, fetus and placenta itself.
Conclusions and implications
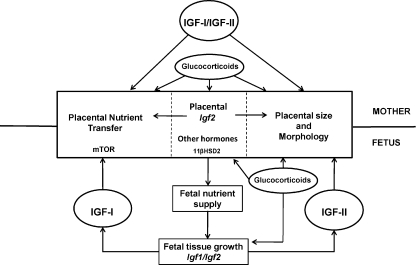
The efficiency of the placenta in supporting fetal growth can be varied in response to environmental conditions particularly when these lead to a mismatch between the available supply of nutrients and the genetically determined fetal drive for growth. This mismatch causes morphological and functional adaptations in the placenta, which help to rectify the perceived imbalance and optimise fetal growth in the prevailing conditions in utero. The specific adaptations to the placenta vary with species, gestational age and the type, duration and severity of the environmental insult. For instance, when fetal availability of oxidative substrates but not oxygen is restricted, placental efficiency and nutrient transporter abundance tend to increase to maximise delivery of the limited resources to the fetus for growth. However, in hypoxic conditions, this response would be inappropriate as low oxygen availability is the ultimate constraint on fetal growth. Reduced placental efficiency and nutrient transporter abundance in these circumstances ensures that fetal availability of oxygen and oxidative substrates are better matched with benefits for survival in utero. By responding to the availability of nutrients and oxygen, hormones like the glucocorticoids and IGFs signal the degree of mismatch and adapt placental phenotype accordingly (Fig. 3). In particular, interplay between maternal, fetal and placental IGF may have a pivotal role in mediating the adaptations in placental nutrient transfer capacity that alter placental efficiency (Fig. 3). However, the mechanisms by which IGFs and other hormones alter placental transport characteristics remain unknown but may involve changes in oxidative stress and/or intracellular signalling through the mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-phosphate (PI3P) or mammalian target of rapamycin (mTOR) pathways (Forbes & Westwood, 2008; Roos et al. 2009).
Figure 3. Schematic diagram of the regulation of placental phenotype by glucocorticoids and insulin like growth factors in relation to fetal growth and development.
Circular profiles, circulating hormones; square profiles, regulated processes. Gene expression is shown in italics. IGF, insulin like growth factor; 11βHSD2, 11β-hydroxysteroid dehydrogenase type 2; mTOR, mammalian target of rapamycin.
The changes in the absolute and relative amount of nutrients supplied to the fetus as a result of altered placental phenotype are likely to have long term consequences for adult health and morbidity (Fowden et al. 2008). Although more studies across a range of species are required to establish the predictive value of placental efficiency in determining adult physiological phenotype (Sibley, 2009), the prognosis for infants with efficient and inefficient placentas probably differs. Measurement of placental weight and transporter phenotype in relation to birth weight may provide a better index of subsequent disease risk than birth weight alone or any of the other surrogate markers used to assess environmental quality during intrauterine development.
Acknowledgments
We would like to thank all the members of the Department of Physiology, Development and Neuroscience who have helped with our studies presented here and with the preparation of the manuscript. We are indebted to the BBSRC, Horserace Betting Levy Board and Wellcome Trust for funding.
Glossary
Abbreviations
- 11βHSD2
11β-hydroxysteroid dehydrogenase type 2
- IGF-I and -II
insulin-like growth factor 1 and 2
- MAPK
mitogen-activated protein kinase
- MeAIB
methylaminoisobutyric acid
- MG
methylglucose
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinosotol-3-phosphate
Author contributions
A.F. has responsibility for manuscript design, data summary and writing the text. A.S.-P. and P.C. provided data included in the text. M.C. and G.B. contributed to the text and to the development of the ideas presented in the paper.
References
- Ain R, Canham LN, Soares MJ. Dexamethasone induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signalling pathway. J Endocrinol. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- Allen WR, Wilsher S, Turnbull C, Stewart F, Ousey J, Rossdale PD, Fowden AL. The influence of maternal size on placental, fetal and postnatal growth: Development in utero. Reproduction. 2002;123:454–465. [PubMed] [Google Scholar]
- Angiolini E, Fowden AL, Coan PM, Sandovici I, Smith P, Dean W, Burton GJ, Tycko B, Reik W, Sibley C, Constancia M. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl A):S98–102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Ashworth CJ, Finch AM, Page KR, Nwagwu MO, McArdle HJ. Causes and consequences of fetal growth retardation in pigs. Reproduction Suppl. 2001;58:233–246. [PubMed] [Google Scholar]
- Bacon BJ, Gilbert RD, Kaufmann P, Smith AD, Trevino FT, Longo LD. Placental anatomy and diffusing capacity in guinea pigs following long-term maternal hypoxia. Placenta. 1984;5:475–488. doi: 10.1016/s0143-4004(84)80002-8. [DOI] [PubMed] [Google Scholar]
- Baisden B, Sonne S, Joshi RM, Ganapathy V, Shekhawat PS. Antenatal dexamethasone treatment leads to changes in gene expression in a murine late placenta. Placenta. 2007;28:1082–1090. doi: 10.1016/j.placenta.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2:105–112. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- Baur R. Morphology of the placental exchange area. Adv Anat Emb Cell Bio. 1977:401–410. doi: 10.1007/978-3-642-66603-2. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, van Zijl PL, Bauer MK, Harding JE. A chronic low dose infusion of insulin-like growth factor I alters placental function but does not affect fetal growth. Reprod Fert Dev. 2002;14:393–400. doi: 10.1071/rd02022. [DOI] [PubMed] [Google Scholar]
- Braun T, Li S, Moss TJM, Newhan JP, Challis JRG, Gluckman PD, Sloboda DM. Maternal betamethasone administration reduces binucleate cell number and placental lactogen in sheep. J Endocrinol. 2007;194:337–347. doi: 10.1677/JOE-07-0123. [DOI] [PubMed] [Google Scholar]
- Bravo PW, Garnica J, Puma G. Cria alpaca body weight and perinatal survival in relation to age of the dam. Anim Reprod Sci. 2009;111:214–219. doi: 10.1016/j.anireprosci.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Buresova M, Zidek V, Musilova A, Simakova M, Fucikova A, Bila V, Kren V, Kazdova L, Di Nicolantonio R, Pravenec M. Genetic relationship between placental and fetal weights and markers of metabolic syndrome in rat recombinant inbred strains. Physiol Genomics. 2006;26:226–231. doi: 10.1152/physiolgenomics.00056.2006. [DOI] [PubMed] [Google Scholar]
- Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. doi: 10.1186/1477-7827-2-46. DOI 10.1186/1477-7827-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K, Ward JW, Forhead AJ, Giussani DA, Fowden AL. Regulation of 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2) activity in ovine placenta by fetal cortisol. J Endocrinol. 2002;172:527–534. doi: 10.1677/joe.0.1720527. [DOI] [PubMed] [Google Scholar]
- Coan PM, Angiolini E, Sandovici I, Burton GJ, Constancia M, Fowden AL. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol. 2008a;586:4567–4576. doi: 10.1113/jphysiol.2008.156133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan PM, Fowden AL, Constancia M, Ferguson-Smith AC, Burton GJ, Sibley CP. Disproportional effects of Igf2 knockout on placental morphology and diffusional exchange characteristics in the mouse. J Physiol. 2008b;586:5023–5032. doi: 10.1113/jphysiol.2008.157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith AC, Sibley CP, Reik W, Fowden AL. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transport systems. Proc Natl Acad Sci U S A. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U, Sadiq HF, Soares MJ, Hay WW, Devaskar SU. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Physiol Regul Integr Comp Physiol. 1998;274:R339–347. doi: 10.1152/ajpregu.1998.274.2.R339. [DOI] [PubMed] [Google Scholar]
- Doherty CB, Lewis RM, Sharkey A, Burton GJ. Placental composition and surface area but not vascularization are altered by maternal protein restriction in the rat. Placenta. 2003;24:34–38. doi: 10.1053/plac.2002.0858. [DOI] [PubMed] [Google Scholar]
- Dwyer CM, Calcert SK, Farish M, Donbavand J, Pickup He. Breed, litter and parity effects on placental weight and placentome number, and consequences for the neonatal behaviour of the lamb. Theriogeniology. 2005;63:1092–1110. doi: 10.1016/j.theriogenology.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Efstradiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- Ericsson A, Hamark B, Jannson N, Johansson BR, Powell TL, Jansson T. Hormonal regulation of glucose and System A amino acid transport in first trimester placental villous fragments. Am J Physiol Regul Integr Comp Physiol. 2005;288:R656–662. doi: 10.1152/ajpregu.00407.2004. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ozanne SE, Ekizoglou S, Doherty C, James L, Gusterson B, Hales CN. The maternal endocrine environment in the low protein model of intrauterine growth retardation. Br J Nutr. 2003;90:815–822. doi: 10.1079/bjn2003967. [DOI] [PubMed] [Google Scholar]
- Ferrell CL. Maternal and fetal influences on uterine and conceptus development in the cow: I Growth of tissues of the gravid uterus. J Anim Sci. 1991;69:1945–1953. doi: 10.2527/1991.6951945x. [DOI] [PubMed] [Google Scholar]
- Finch AM, Yang LG, Nwagwu MO, Page KR, McArdle HJ, Ashworth CJ. Placental transport of leucine in a porcine model of low birth weight. Reproduction. 2004;128:229–235. doi: 10.1530/rep.1.00193. [DOI] [PubMed] [Google Scholar]
- Forbes K, Westwood M. The IGF axis and placental function. Horm Res. 2008;69:129–137. doi: 10.1159/000112585. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Fowden AL. The hungry fetus? Role of leptin as a nutritional signal before birth. J Physiol. 2009;587:1145–1152. doi: 10.1113/jphysiol.2008.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–812. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Horm Res. 2006a;65:50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Ward JW, Wooding FBP, Forhead AJ, Constancia M. Programming placental nutrient transfer capacity. J Physiol. 2006b;572:5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20:439–450. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Hormones as epigenetic signals in developmental programming. Exp Physiol. 2009;94:607–625. doi: 10.1113/expphysiol.2008.046359. [DOI] [PubMed] [Google Scholar]
- Gardner RL, Squire S, Zaina S, Hills S, Grahan CF. Insulin-like growth factor-2 regulation of conceptus composition: effects of the trophectoderm and inner cell mass genotypes in the mouse. Biol Reprod. 1999;60:190–195. doi: 10.1095/biolreprod60.1.190. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Leturque A. Fetal weight and its relationship to placenta blood flow and placental weight in experimental intrauterine growth retardation in the rat. J Dev Physiol. 1982;4:237–246. [PubMed] [Google Scholar]
- Gilbert RD, Cummings LA, Juchan MR, Longo LD. Placental diffusing capacity and fetal development in exercising or hypoxic guinea pig. J Appl Physiol. 1979;46:828–834. doi: 10.1152/jappl.1979.46.4.828. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Matthew N, Glazier J, Jackson A, Wilman C, Sibley CP. Neutral amino acid uptake by the microvillous plasma membrane of the human placenta is inversely related to fetal size at birth in normal pregnancy. J Clin Endocrinol Metab. 1998;83:3320–3326. doi: 10.1210/jcem.83.9.5132. [DOI] [PubMed] [Google Scholar]
- Hahn T, Barth S, Grat R, Engelmann M, Beslagic D, Reul JMHM, Holsboer F, Dohr G, Desoye G. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endocrinol Metab. 1999;84:1445–1452. doi: 10.1210/jcem.84.4.5607. [DOI] [PubMed] [Google Scholar]
- Harding JE, Evans PC, Gluckman Maternal growth treatment increases placental diffusion capacity but not fetal or placental growth in sheep. Endocrinology. 1997;138:5352–5358. doi: 10.1210/endo.138.12.5584. [DOI] [PubMed] [Google Scholar]
- Harding JE, Lin L, Evans PC, Gluckman PD. Insulin-like growth factor 1 alters feto-placental protein and carbohydrate metabolism in fetal sheep. Endocrinology. 1994;134:1509–1514. doi: 10.1210/endo.134.3.8119193. [DOI] [PubMed] [Google Scholar]
- Hay WW. Regulation of placental metabolism by glucose supply. Reprod Fert Develop. 1994;7:365–375. doi: 10.1071/rd9950365. [DOI] [PubMed] [Google Scholar]
- Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147:5566–5574. doi: 10.1210/en.2006-0825. [DOI] [PubMed] [Google Scholar]
- Holemans K, Caluwaerts S, Poston L, Van Assche FA. Diet-induced obesity in the rat: a model of gestational diabetes meelitus. Am J Obstet Gynec. 2004;190:858–865. doi: 10.1016/j.ajog.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88:1205–1211. doi: 10.1210/jc.2002-021332. [DOI] [PubMed] [Google Scholar]
- Jansson N, Petterson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Human placental transport in altered fetal growth: does the placenta act as a nutrient sensor?– a review. Placenta. 2006;27(Suppl.):S91–97. doi: 10.1016/j.placenta.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ylven K, Wennergre M, Powell TL. Glucose transport system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- Jellyman JK, Gardner DS, Fowden AL, Giussani DA. Effects of dexamethasone on the uterine and umbilical vascular beds during basal and hypoxemic conditions in sheep. Am J Obstet Gynec. 2004;190:825–835. doi: 10.1016/j.ajog.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Jenkinson CMC, Min SH, Mackenzie DDS, McCutcheon SN, Breier BH, Gluckman PD. Placental development and fetal growth in growth hormone-treated ewes. Growth Horm IGF Res. 1999;9:11–17. doi: 10.1054/ghir.1998.0065. [DOI] [PubMed] [Google Scholar]
- Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of chronic maternal cortisol infusion on the late gestation fetal sheep. J Endocrinol. 2002;174:27–36. doi: 10.1677/joe.0.1740027. [DOI] [PubMed] [Google Scholar]
- Johnson JWC, Mitzer W, London WT, Palmer AE, Scott R. Betamethasone and the rhesus fetus: multisystemic effects. Am J Obstet Gynecol. 1979;133:677–684. doi: 10.1016/0002-9378(79)90018-8. [DOI] [PubMed] [Google Scholar]
- Jones HN, Ashworth CJ, Page KR, McArdle Cortisol stimulates System A amino acid transport and SNAT2 expression in human placental cell line (BeWo) Am J Physiol Endocrinol Metab. 2006;291:E596–603. doi: 10.1152/ajpendo.00359.2005. [DOI] [PubMed] [Google Scholar]
- Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport – a review. Placenta. 2007;28:763–774. doi: 10.1016/j.placenta.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Jones HN, Woollett LA, Barbow N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marker up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23:271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl PI. Insulin-like growth factor-I stimulates amino acid uptake by the cultured human placental trophoblast. J Cell Physiol. 1995;165:83–88. doi: 10.1002/jcp.1041650111. [DOI] [PubMed] [Google Scholar]
- Kerzner LS, Stonestreet BS, Wu KY, Sadowska G, Malee MP. Antenatal dexamethasone: effect on ovine 11 beta-hydroxysteroid dehydrogenase type 2 expression and fetal growth. Pediatr Res. 2002;52:706–712. doi: 10.1203/00006450-200211000-00016. [DOI] [PubMed] [Google Scholar]
- Kniss DA, Shubert PJ, Zimmerman PD, Landon MB, Grabbe SG. Insulin-like growth factors: their regulation of glucose and amino acid transport in placental trophoblast isolated from first-trimester chorionic villi. J Reprod Med. 1994;39:249–256. [PubMed] [Google Scholar]
- Konyali A, Tölü C, Das G, Savas T. Factors affecting placental traits and relationships of placental traits with neonatal behaviours in goat. Anim Reprod Sci. 2007;97:394–401. doi: 10.1016/j.anireprosci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Kurtz H, Zechner U, Orth A, Fundele R. Lack of correlation between placenta and offspring size in mouse interspecific crosses. Anat Embryol. 1999;200:335–343. doi: 10.1007/s004290050284. [DOI] [PubMed] [Google Scholar]
- Kutzler MA, Ruane EK, Coksaygan T, Vincent SE, Nathanielsz PW. Effects of three courses of maternally administered dexamethasone at 0.7, 0.75 and 0.8 of gestation on prenatal and postnatal growth in sheep. Pediatr Res. 2004;113:313–319. doi: 10.1542/peds.113.2.313. [DOI] [PubMed] [Google Scholar]
- Langdown ML, Sugden MC. Enhanced GLUT1 and GLUT3 expression in dexamethasone-induced fetal growth retardation. Mol Cell Endocrinol. 2001;185:109–117. doi: 10.1016/s0303-7207(01)00629-3. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Gardner DS, Jackson AA. Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J Reprod Fert. 1996;106:307–312. doi: 10.1530/jrf.0.1060307. [DOI] [PubMed] [Google Scholar]
- Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol. 1994;109:122–134. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- Lesage J, Hann D, Léonhardt M, Blondeau B, Breant B, Dupony JP. Maternal undernutrition during late gestation-induced intrauterine growth retardation in the rat is associated with impaired placental GLUT3 expression, but does not correlate with endogenous corticosterone levels. J Endocrinol. 2002;174:37–43. doi: 10.1677/joe.0.1740037. [DOI] [PubMed] [Google Scholar]
- Lewis RM, James LA, Zhang J, Byrne C, Hales CN. Effects of maternal iron restriction in the rat on hypoxia-induced gene expression and fetal metabolites levels. Br J Nutr. 2001;85:193–201. doi: 10.1079/bjn2000247. [DOI] [PubMed] [Google Scholar]
- Liu L, Harding JE, Evans PC, Gluckman PD. Maternal insulin-like growth factor-I infusion alters feto-placental carbohydrate and protein metabolism in pregnant sheep. Endocrinology. 1994;135:895–900. doi: 10.1210/endo.135.3.8070384. [DOI] [PubMed] [Google Scholar]
- Lok F, Owens JA, Mundy L, Robinson JS, Owens PC. Insulin-like growth factor-I promotes growth selectively in fetal sheep in late gestation. Am J Physiol Regul Integr Comp Physiol. 1996;270:R1148–R1155. doi: 10.1152/ajpregu.1996.270.5.R1148. [DOI] [PubMed] [Google Scholar]
- Lueder FL, Kim S-B, Buroker CA, Bangalore SA, Ogata ES. Chronic maternal hypoxia retards fetal growth and increases glucose utilization of select fetal tissues in the rat. Metabolism. 1995;44:532–537. doi: 10.1016/0026-0495(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Malandro MS, Beveridge MJ, Kilberg MS, Novak DA. Effect of low protein diet-induced intrauterine growth retardation on rat placental amino acid transport. Am J Physiol Cell Physiol. 1996;271:C295–303. doi: 10.1152/ajpcell.1996.271.1.C295. [DOI] [PubMed] [Google Scholar]
- Matthews JC, Beveridge MJ, Dialynas E, Bartke A, Kilberg MS, Novak DA. Placental anionic and cationic amino acid transporter expression in growth hormone overexpressing and null IGF-II on null IGF-I receptor mice. Placenta. 1999;20:639–650. doi: 10.1053/plac.1999.0421. [DOI] [PubMed] [Google Scholar]
- McLaren A. Genetic and environmental effects on foetal and placental growth in mice. J Reprod Fertil. 1965;9:79–98. doi: 10.1530/jrf.0.0090079. [DOI] [PubMed] [Google Scholar]
- Mellor DJ. Nutritional and placental determinants of foetal growth rate in sheep and consequences for the newborn lamb. Br Vet J. 1983;139:307–324. doi: 10.1016/s0007-1935(17)30436-0. [DOI] [PubMed] [Google Scholar]
- Molteni RA, Stys S, Battaglia FC. Relationship of fetal and placental weight in human beings: Fetal/placental weight ratios at various gestational ages and birth weight distributions. J Reprod Med. 1978;21:327–334. [PubMed] [Google Scholar]
- Osgerby JC, Wathes DC, Howard D, Gadd TS. The effect of maternal undernutrition on the placental growth trajectory and the uterine insulin-like growth factor axis in the pregnant ewe. J Endocrinol. 2004;182:89–103. doi: 10.1677/joe.0.1820089. [DOI] [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Restriction of placental size in sheep enhances efficiency of placental transfer of antipyrine, 3-O-methyl-D-glucose but not urea. J Dev Physiol. 1987;9:457–464. [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Glucose metabolism in pregnant sheep when placental growth is restricted. Am J Physiol Regul Integr Comp Physiol. 1989;257:R350–357. doi: 10.1152/ajpregu.1989.257.2.R350. [DOI] [PubMed] [Google Scholar]
- Pardi G, Marconi AM, Cetin I. Placental-fetal interrelationships in IUGR fetuses – a review. Placenta. 2002;23(Suppl A):S136–141. doi: 10.1053/plac.2002.0802. [DOI] [PubMed] [Google Scholar]
- Price WA, Roog L, Stiles AD, D’Ercole J. Changes in IGF-I and -II, IGF binding protein and IGF receptor transcript abundance after uterine artery ligation. Pediatr Res. 1992;32:291–295. doi: 10.1203/00006450-199209000-00009. [DOI] [PubMed] [Google Scholar]
- Regnault TRH, de Vrijer B, Galan HL, Daridsen ML, Trembler KA, Battaglia FC, Wilkening RB, Anthony RV. The relationship between transplacental O2 diffusion and placental expression of PIGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J Physiol. 2003;550:641–656. doi: 10.1113/jphysiol.2003.039511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault TRH, Friedman JE, Wilkening RB, Anthony RV, Hay WW. Fetoplacental transport and utilization of amino acids in IUGR – a review. Placenta. 2005;26(Suppl A):S52–62. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Reid GJ, Lane RH, Flozak AS, Simmons RA. Placental expression of glucose transporter proteins 1 and 3 in growth restricted fetal rats. Am J Obstet Gynecol. 1999;180:1017–1023. doi: 10.1016/s0002-9378(99)70675-7. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572:51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CT, Kind KL, Earl RA, Grant PA, Robinson JS, Sohlstrom A, Owens PC, Owens JA. Circulating insulin-like growth factor (IFG)-1 and IGF binding proteins-1 and -3 and placental development in the guinea pig. Placenta. 2002;23:763–770. doi: 10.1016/s0143-4004(02)90849-0. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Sohlshom A, Kind KL, Earl RA, Khong TY, Robinson JS, Owens PC, Owens JA. Maternal food restriction reduces the exchange surface area and increases the barrier thickness of the placenta of the guinea pig. Placenta. 2001;22:177–185. doi: 10.1053/plac.2000.0602. [DOI] [PubMed] [Google Scholar]
- Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochem Soc Trans. 2009;37:295–298. doi: 10.1042/BST0370295. [DOI] [PubMed] [Google Scholar]
- Rosso P. Maternal-fetal exchange during protein malnutrition in the rat. Placental transfer of α-amino isobutyric acid. J Nutr. 1977a;107:2002–2005. doi: 10.1093/jn/107.11.2002. [DOI] [PubMed] [Google Scholar]
- Rosso P. Materno-fetal exchange during protein malnutrition in the rat. Placental transfer of glucose and a non metabolizable glucose analog. J Nutr. 1977b;107:2006–2010. doi: 10.1093/jn/107.11.2006. [DOI] [PubMed] [Google Scholar]
- Rutherford JN, Tardif SD. Placental efficiency and intrauterine resource allocation strategies in the common marmoset pregnancy. Am J Phys Anthropol. 2008;137:60–68. doi: 10.1002/ajpa.20846. [DOI] [PubMed] [Google Scholar]
- Rutherford JN, Tardif SD. Developmental plasticity of the microscopic placental architecture in relation to litter size variation in the common marmoset monkey (Callithrix jacchus) Placenta. 2009;30:105–110. doi: 10.1016/j.placenta.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salafia CM, Zhang J, Miller RK, Chrles AK, Shrout P, Sun W. Placental growth patterns affect birth weight for given placental weight. Birth Defects Res A Clin Mol Teratol. 2007;79:281–288. doi: 10.1002/bdra.20345. [DOI] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Coan PM, Vaughan OR, Constancia M, Burton GJ, Fowden AL. Igf2 deficiency modifies placental adaptation to maternal undernutrition during mouse pregnancy. Reproduct Sci. 2009;16:Suppl 165A. [Google Scholar]
- Sferruzzi-Perri AN, Owens JA, Pringle KG, Robinson JS, Roberts CT. Maternal insulin-like growth factor-I and -II act via different pathways to promote fetal growth. Endocrinology. 2006;147:3344–3355. doi: 10.1210/en.2005-1328. [DOI] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Owens JA, Standen P, Roberts CT. Maternal insulin-like growth factor-II promotes placental functional development via the Type 2 IGF receptor in the guinea pig. Placenta. 2008;29:347–355. doi: 10.1016/j.placenta.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Owens JA, Standen P, Taylor RL, Heinemann GK, Robinson JS, Roberts CT. Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrient partitioning near term. Am J Physiol Endocrinol Metab. 2007a;292:E668–676. doi: 10.1152/ajpendo.00320.2006. [DOI] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Owens JA, Standen P, Taylor RL, Robinson JS, Roberts CT. Early pregnancy maternal endocrine insulin-like growth factor 1 programs the placenta for increased functional capacity throughout gestation. Endocrinology. 2007b;148:4362–4370. doi: 10.1210/en.2007-0411. [DOI] [PubMed] [Google Scholar]
- Shafrir E, Barash V, Zederman R, Kissilevitz R, Diamant YZ. Modulation of fetal and placental metabolic pathways in response to maternal thyroid and glucocorticoid hormone excess. Isr J Med Sci. 1994;30:32–41. [PubMed] [Google Scholar]
- Sibley CP. Understanding placental nutrient transfer – why bother? New biomarkers of fetal growth. J Physiol. 2009;587:3431–3440. doi: 10.1113/jphysiol.2009.172403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CP, Coan PM, Ferguson-Smith AC, Dean W, Hughes J, Smith P, Reik W, Burton GJ, Fowden AL, Constancia M. Placental-specific insulin-like growth factor-2 (Igf2) regulates the diffusional exchange characteristics of the mouse placenta. Proc Natl Acad Sci U S A. 2004;101:8204–8208. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CAR, D'Souza SW, Glazier TC, Greenwood SL, Janson T, Powell TL. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58:827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- Smith JT, Waddell Leptin receptor expression in the rat placenta: changes in Ob-Ra, Ob-Rb and Ob-Re with gestational age and suppression by glucocorticoids. Biol Reprod. 2002;67:1204–1210. doi: 10.1095/biolreprod67.4.1204. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early growth retarded adult offspring. Eur J Endocrinol. 2001;145:529–539. doi: 10.1530/eje.0.1450529. [DOI] [PubMed] [Google Scholar]
- Timmerman M, Teng C, Wilkensup RB, Fennessy P, Battaplia FC, Meschia G. Effect of dexamethasone on fetal hepatic glutamine-glutamate exchange. Am J Physiol Endocrinol Metab. 2000;278:E839–845. doi: 10.1152/ajpendo.2000.278.5.E839. [DOI] [PubMed] [Google Scholar]
- Umekawa T, Sugiyama T, Kihira T, Murabayashi N, Zhang L, Nagao K, Kamimoto Y, Ma N, Yodoi J, Sagawa N. Overexpression of thioredoxinal reduces oxidative stress in the placenta of transgenic mice and promotes fetal growth via glucose metabolism. Endocrinology. 2008;149:3980–3988. doi: 10.1210/en.2007-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet JL, Freking BA. Differences in placental structure during gestation associated with large & small pig foetuses. J Anim Sci. 2007;85:3267–3275. doi: 10.2527/jas.2007-0368. [DOI] [PubMed] [Google Scholar]
- Vaughan OR, Fowden AL, Coan PM. Morphological adaptations of the mouse placenta with maternal undernutrition. Proc Physiol Soc. 2008;11:PC38. [Google Scholar]
- Vonnahme KA, Hess BW, Nijland MJ, Nathanielsz PW, Ford SP. Placentomal differentiation may compensate for maternal nutrient restriction in ewes adapted to harsh range conditions. J Anim Sci. 2006;84:3451–3459. doi: 10.2527/jas.2006-132. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Wilson ME, Ford SP. Relationship between placental vascular endothelial growth factor expression and placental/endometrial vascularity in the pig. Biol Reprod. 2001;64:1821–1825. doi: 10.1095/biolreprod64.6.1821. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Milner S, Hay WW. Placental glucose transport in growth restricted pregnancies induced by overnourishing adolescent sheep. J Physiol. 2002;547:85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JW, Forhead AJ, Wooding FBP, Fowden AL. Functional significance and cortisol dependence of the gross morphology of ovine placentomes during late gestation. Biol Reprod. 2006;74:137–145. doi: 10.1095/biolreprod.105.046342. [DOI] [PubMed] [Google Scholar]
- Ward JW, Wooding FBP, Fowden AL. The effect of cortisol on the binucleate cell population in the ovine placenta during late gestation. Placenta. 2002;23:451–458. doi: 10.1053/plac.2002.0834. [DOI] [PubMed] [Google Scholar]
- Ward JW, Wooding FBP, Fowden AL. Ovine feto-placental metabolism. J Physiol. 2004;554:529–541. doi: 10.1113/jphysiol.2003.054577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsher S, Allen WR. The effects of maternal age and parity on placental and fetal development in the mare. Equine Vet J. 2003;35:476–483. doi: 10.2746/042516403775600550. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Ford SP. Comparative aspects of placental efficiency. Reproduction Suppl. 2001;58:223–232. [PubMed] [Google Scholar]
- Wilson ME, Biensen NJ, Youngs CR, Ford SP. Development of Meishan and Yorkshire littermate co-conceptuses in either a Meishan or Yorkshire uterine environment to day 90 of gestation and to term. Biol Reprod. 1998;58:905–910. doi: 10.1095/biolreprod58.4.905. [DOI] [PubMed] [Google Scholar]
- Wlodek ME, Westcott KT, O’Dowd R, Serruto A, Wassef L, Moritz KM, Moseley JM. Uteroplacental restriction in the rat impairs fetal growth in association with alterations in placental growth factors including PTHrP. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1620–1627. doi: 10.1152/ajpregu.00789.2004. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Breier BM, Johnston BM, Gluckman PD. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. J Endocrinol. 1996;150:231–242. doi: 10.1677/joe.0.1500231. [DOI] [PubMed] [Google Scholar]
- Wooding FBP, Burton GJ. Comparative Placentation: Structure, Function and Evolution. Heidelberg: Springer-Verlag; 2008. [Google Scholar]
- Wyrwoll CS, Seck JR, Holmes MC. Altered placental function of 11β-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology. 2009;150:1287–1293. doi: 10.1210/en.2008-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta. 2006;27:49–55. doi: 10.1016/j.placenta.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]