Abstract
G protein activated K+ channels (GIRK, Kir3) are switched on by direct binding of Gβγ following activation of Gi/o proteins via G protein-coupled receptors (GPCRs). Although Gαi subunits do not activate GIRKs, they interact with the channels and regulate the gating pattern of the neuronal heterotetrameric GIRK1/2 channel (composed of GIRK1 and GIRK2 subunits) expressed in Xenopus oocytes. Coexpressed Gαi3 decreases the basal activity (Ibasal) and increases the extent of activation by purified or coexpressed Gβγ. Here we show that this regulation is exerted by the ‘inactive’ GDP-bound Gαi3GDP and involves the formation of Gαi3βγ heterotrimers, by a mechanism distinct from mere sequestration of Gβγ‘away’ from the channel. The regulation of basal and Gβγ-evoked current was produced by the ‘constitutively inactive’ mutant of Gαi3, Gαi3G203A, which strongly binds Gβγ, but not by the ‘constitutively active’ mutant, Gαi3Q204L, or by Gβγ-scavenging proteins. Furthermore, regulation by Gαi3G203A was unique to the GIRK1 subunit; it was not observed in homomeric GIRK2 channels. In vitro protein interaction experiments showed that purified Gβγ enhanced the binding of Gαi3GDP to the cytosolic domain of GIRK1, but not GIRK2. Homomeric GIRK2 channels behaved as a ‘classical’ Gβγ effector, showing low Ibasal and strong Gβγ-dependent activation. Expression of Gαi3G203A did not affect either Ibasal or Gβγ-induced activation. In contrast, homomeric GIRK1* (a pore mutant able to form functional homomeric channels) exhibited large Ibasal and was poorly activated by Gβγ. Expression of Gαi3GDP reduced Ibasal and restored the ability of Gβγ to activate GIRK1*, like in GIRK1/2. Transferring the unique distal segment of the C terminus of GIRK1 to GIRK2 rendered the latter functionally similar to GIRK1*. These results demonstrate that GIRK1 containing channels are regulated by both Gαi3GDP and Gβγ, while GIRK2 is a Gβγ-effector insensitive to Gαi3GDP.
G protein activated K+ channels (GIRK, Kir3) mediate postsynaptic inhibitory effects of many neurotransmitters in the heart and brain. GIRK channels are switched on by direct binding of Gβγ following activation of Gi/o proteins via numerous G protein coupled receptors (GPCRs) (Logothetis et al. 1987; Wickman & Clapham, 1995; Dascal, 2001). Although GIRK is not considered an effector for Gα subunits, fast and specific channel activation was attributed to formation of GPCR–Gαiβγ–GIRK signalling complexes (Huang et al. 1995; Slesinger et al. 1995; Leaney et al. 2000; Peleg et al. 2002; Ivanina et al. 2004). GIRK subunits interact with Gβγ subunits in vitro (Huang et al. 1995; Kunkel & Peralta, 1995; Huang et al. 1997; Ivanina et al. 2003; Finley et al. 2004) and in living cells before and after agonist activation (Rebois et al. 2006; Riven et al. 2006). Cytosolic GIRK segments also bind GαiGDP and GαiGTPγSin vitro, and GIRK channels immunoprecipitate with Gα subunits in native brain membranes (Huang et al. 1995; Ivanina et al. 2004; Clancy et al. 2005), but interaction of Gα with GIRK in vivo is under debate (Fowler et al. 2006; Rebois et al. 2006).
The main neuronal GIRK channel is a GIRK1/2 heterotetramer, composed of GIRK1 and GIRK2 subunits. These subunits can also co-assemble with GIRK3 to form GIRK1/3 and GIRK2/3. GIRK1/2 channels are abundant in the hippocampus and the cerebellum. GIRK2 can also form functional homomeric channels, which form the majority of GIRKs in the substantia nigra (Slesinger et al. 1996; Inanobe et al. 1999; Saenz del Burgo et al. 2008). In hippocampal and locus coeruleus neurons, GIRKs possess a substantial basal activity (Ibasal) (Luscher et al. 1997; Torrecilla et al. 2002; Chen & Johnston, 2005; Wiser et al. 2006). The mechanisms of regulation of Ibasal and its relation to the neurotransmitter-evoked activity (Ievoked) are poorly understood. We have previously presented evidence that in expression systems, Gαi regulates GIRK gating, keeping Ibasal low and preparing the channel for activation by ‘free’ Gβγ (Peleg et al. 2002; Rishal et al. 2005; Rubinstein et al. 2007). We proposed that this action was carried out by GαGDP (classically considered as the inactive form of Gα), possibly as a heterotrimer with Gβγ. However, the involvement of ‘active’ GαGTP could not be ruled out.
Divergent subunit compositions of GIRK channels have not been systematically studied. However, subunit content appears to play a role in coupling of GIRKs to GABAB receptors (Cruz et al. 2004; Fowler et al. 2006) and GIRK1/3 was shown to have higher affinity to Gβγ compared to GIRK2/3 (Jelacic et al. 2000). When expressed as homomers, GIRK1 (GIRK1F137S, a functional pore mutant, denoted GIRK1*) displayed low conductance compared to GIRK1/2 or GIRK1/4, and long bursts of activity (Chan et al. 1996; Vivaudou et al. 1997), whereas GIRK2 showed higher conductance than GIRK1* but low open probability with brief, flickery openings (Yi et al. 2001). In vitro studies suggested a smaller Gβγ and Gα binding surface in N and C termini of GIRK2 compared with GIRK1 (Ivanina et al. 2003; Ivanina et al. 2004). Yet, no definite differences in Gβγ or Gαi regulation of GIRK1 and GIRK2 have been reported so far.
We explored the mechanisms of regulation of GIRK channels (heteromeric GIRK1/2 channel as well as homomeric GIRK1 and GIRK2 channels) by Gαi and Gβγ using in vitro protein interaction assay and functional assays in Xenopus oocytes. To definitely distinguish between the effects of ‘inactive’ GαGDP and ‘active’ GαGTP, we utilized ‘constitutively inactive’ and ‘constitutively active’ Gαi3 mutants. We demonstrate a profound regulation of the heteromeric GIRK1/2 and the homomeric GIRK1* channels by Gαi3GDP, probably in the context of the Gαiβγ heterotrimer. This regulation does not occur in homomeric GIRK2 channels, leading to different regulation of GIRK1 and GIRK2 by Gβγ, and to an asymmetric regulation by Gαiβγ heterotrimer.
Methods
DNA constructs and mRNA
The cDNA constructs were described in previous publications (Peleg et al. 2002; Rishal et al. 2005). cDNA constructs were inserted into pGEX (for production of glutathione-S-transferase (GST) fusion proteins in E. coli) or into high-expression oocyte vectors containing 5′ and 3′ untranslated sequences of Xenopusβ-globin: pGEMHE, pGEMHJ, or pBS-MXT. Rat GIRK1 (U01071), mouse GIRK2a (U11859), and human Gαi3 (J03198) were used for oocytes expression and for deriving mutants, except human GIRK1* (U39195).
Gαi3G203A and Gαi3Q204L were produced using standard PCR-based methods and fully sequenced. The HA-(hemagglutinin)-tagged GIRK2 (Chen et al. 2002; Clancy et al. 2005) was subcloned into pBS-MXT. Yellow fluorescent protein (YFP) was fused in-frame before the N-terminus of GIRK1* or GIRK2, or after the C-terminus of GIRK2, essentially as described by Riven et al. (2003) and Fowler et al. (2006). G1NC and G2NC constructs were made by PCR; the transmembrane segments (GIRK1 a.a 85–184; GIRK2 a.a 96–193) were deleted, and N-terminus and C-terminus (NT and CT, respectively) were connected by a linker encoding amino acids QSTASQST in G1NC and KL in G2NC. The G2CTG1 chimera (GIRK2HA(a.a 1-381)GIRK1(a.a371-501)) was constructed using PCR followed by blunt end ligation. All PCR products were fully sequenced. RNAs were synthesized in vitro as described (Kanevsky & Dascal, 2006) and injected into oocytes at 0.2–20 ng per oocyte. To prevent the formation of GIRK1*/5 heterotetramers, we injected antisense targeted against the oocytes’ endogenous GIRK5 subunits (Hedin et al. 1996) when studying GIRK1*.
Xenopus oocytes preparation and electrophysiology
Experiments were approved by the Tel Aviv University Institutional Animal Care and Use Committee (permit no. 11-05-064). Briefly, female frogs, maintained at 20 ± 2°C on an 11 h light–13 h dark cycle, were anaesthetized in a 0.15% solution of procaine methanesulphonate (MS222), and portions of ovary were removed through a small incision on the abdomen. The incision was sutured, and the animal was returned to a separate tank until it had fully recovered from the anaesthesia, and afterwards was returned to a large tank, together with the other postoperational animals. The animals did not show any signs of postoperational distress and were allowed to recover for at least 8 weeks until the next surgery. Following the final collection of oocytes, the frogs were killed by decapitation and double pithing while under anaesthesia. Oocytes were defolliculated by collagenase, injected with RNA (Peleg et al. 2002) and incubated for 3 days (whole cell studies) or 2–4 days (patch clamp) at 20–22°C in ND96 solution (low K+) containing, in mm: 96 NaCl, 2 KCl, 1 MgCl2, 1 CaCl2, 5 Hepes, pH 7.5, supplemented with 2.5 mm sodium pyruvate, 100 μg ml−1 streptomycin and 62.75 μg ml−1 penicillin (or 50 μg ml−1 gentamycine). Whole-cell GIRK currents were measured using standard two-electrode voltage clamp procedures (Rubinstein et al. 2007) at 20–22°C, in high K+ solutions (24 mm K+ for GIRK1*, and 24, 48 or 96 mm for GIRK2, as indicated). K+ solutions at 24 and 48 mm were obtained by mixing ND96 with a 96 mm K+ solution containing, in mm: 96 KCl, 2 NaCl, 1 CaCl2, 1 MgCl2, 5 Hepes, pH 7.5. Acetylcholine (ACh) was used at 10 μm. Whole cell currents produced by the expression of untagged or YFP-tagged homomeric GIRK1* were similar in amplitude and in regulation by Gβγ, and the data were pooled. The same was observed with untagged, HA- or YFP-tagged GIRK2 channels.
Patch clamp experiments were performed as described (Peleg et al. 2002). Data acquisition and analysis were done using pCLAMP software (Molecular Devices, Sunnyvale, CA, USA) as described (Peleg et al. 2002). Currents were recorded at a holding potential of −80 mV, sampled at 10 kHz and filtered at 2 kHz. Patch pipettes had resistances of 1.2–2.5 MΩ. After seal formation, the patches were excised and exposed to air to prevent the formation of closed membrane vesicles at the tip. Stock solution (10 μm) of the purified Gβ1γ2 protein was diluted into 50 μl of the bath solution (final concentration of 20–40 nm as indicated), added to the 500 μl solution in the bath, and stirred. For patch recordings of heteromeric GIRK1/2 channels, the pipette solution contained, in mm: 144 KCl, 2 NaCl, 1 MgCl2, 1 CaCl2, 1 GdCl3, 10 Hepes/KOH, pH 7.5. Bath solution contained, in mm: 130 KCl, 2 MgCl2, 1 EGTA, 2 Mg-ATP, and 10 Hepes/KOH, pH 7.5. Measurements of homomeric channels were made with a pipette solution that contained, in mm: 146 KCl, 2 NaCl, 1 MgCl2, 1 CaCl2, 1 GdCl3, 10 Hepes/KOH, pH 7.5. Bath solution contained, in mm: 146 KCl, 6 NaCl, 2 MgCl2, 1 EGTA, 2 Mg-ATP, 10 Hepes/KOH, pH 7.5. (The presence of NaCl was found essential to prevent strong rundown of GIRK1* channels in excised patches.) GdCl3 completely inhibited the stretch-activated channels. For the comparison of channel activity in excised patches vs. cell attached patches, currents were also corrected for changes in the single channel amplitude, i. For GIRK1/2, i was taken as 2.4 pA in cell-attached and 2.2 nA in excised patches (authors’ unpublished observations). For GIRK1*, i was 1.24 ± 0.03 pA in cell-attached mode and 1.06 ± 0.03 pA in excised mode. For GIRK2HA, i was 1.4 ± 0.07 pA and 1.03 ± 0.08 pA in cell attached and excised mode, respectively. For GIRK2, i was 1.74 ± 0.05 pA and 1.49 ± 0.05 pA in cell attached and excised mode, respectively.
HEK293 transfection and electrophysiology
HEK293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mm glutamine, 10% fetal calf serum, 100 units ml−1 penicillin-G sodium and 100 μg ml−1 streptomycin sulfate in an atmosphere of 95% air–5% CO2 at 37°C. Cells were transfected using the FugeneR6 Transfection Reagent (Roche Applied Science, USA), in a 24-well dish, with cDNAs of GIRK1 and GIRK2 (0.2–0.6 μg), m2R (0.5 μg), and (when designed) of Gαi3 (0.15–0.3 μg), m-phosducin (0.2 μg), Gβ1 and Gγ2 (0.2 μg each). Total DNA concentration was adjusted to 2.1 μg per 1.5 cm well by adding empty pcDNA3 vector DNA. The CD8 reporter gene system was used to visualize transfected cells (0.5 μg DNA per well). Beads coated with anti-CD8-antibodies were purchased from Invitrogen.
Whole cell recordings were performed at 21–23°C. Patch pipette solution contained, in mm: 130 KCl, 1 MgCl2, 5 EGTA, 3 MgATP, 10 Hepes. Low-K bath solution contained, in mm: 140 NaCl, 4 KCl, 1.8 CaCl2, 1.2 MgCl2, 11 glucose, 2 CdCl2, 5.5 Hepes. High-K bath solution contained 90 mm KCl and 54 mm NaCl; the rest was as in low-K solution. The osmolarity of intracellular and extracellular solutions was adjusted to 290 and 310 mosmol l–1 respectively, with sucrose; pH was 7.4–7.6. Patch clamp was done using an Axopatch 200B (Molecular Devices). Data acquisition and analysis were done using pCLAMP software (Molecular Devices).
Biochemistry
GST-fused Gαi3 (GST-Gαi3) was purified as described (Rishal et al. 2003). [35S]Methionine-labelled G1NC (G1N1-84C183-501) and G2NC (G2N1-95C194-414) proteins were synthesized in rabbit reticulocyte lysate (Promega Corp., Madison, WI, USA). For pull down experiments, 3 μg of GST-Gαi3 was incubated at 30°C with 30 μm of GDP for 30 min in 50 μl of a high-K+ binding buffer (Rishal et al. 2003) containing 0.01% Lubrol. Purified recombinant Gβ1γ2 (3 μg, wild-type or His6-tagged) or vehicle was added for another 30 min. Then, 5 μl of reticulocyte lysate containing [35S]methionine-labelled G1NC or G2NC was added, the total reaction volume was brought to 300 μl, 5 μl was removed and used to measure the loaded protein (‘input’), and the incubation continued for 1 h at room temperature. Binding to glutathione-Sepharose beads and elution with 15 mm glutathione were done as described (Rishal et al. 2003). The eluted proteins were separated on 12% polyacrylamide-SDS gels. The radioactive signals from protein bands of the gels were imaged using PhosphorImager and the software ImageQuaNT (Molecular Dynamics). Western blots were performed using standard procedures, using Gβ antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, c.a., USA) and ECL reagents (Pierce Biotechnology, Inc., Rockford, IL, USA).
Imaging and immunocytochemistry
Imaging of proteins in the plasma membrane (PM) was performed either in giant PM patches or in whole oocytes. Imaging of homomeric GIRK1* in PM was performed using N or C terminally YFP-labelled channels in whole oocytes (Fig. 5A and Supplemental Fig. 2A), or immunolabelling of untagged channels in giant excised PM patches using GIRK1 antibody (Fig. 6A). GIRK2 expression was monitored in intact oocytes with either C terminally labelled YFP channel (Supplemental Fig. 2A), or GIRK2HA channels with an extracellular HA tag (Figs 5A and 8A and C) or giant PM patches using GIRK2 antibody (Fig. 8A). Immunocytochemistry in giant PM patches was done essentially as described (Singer-Lahat et al. 2000; Peleg et al. 2002). In brief, the vitelline membrane was peeled off and the oocytes were placed on plastic coverslips (Thermanox plastic coverslip, Nunc, Naperville, IL, USA). After sticking to the coverslip, the oocyte was removed by washing with a strong jet of solution. Pieces of membrane attached to the coverslip, with their cytosolic leaflet surface exposed to the external solution, were washed until the membrane patch became transparent. Following fixation for 10 min in 1% formaldehyde, the membranes were washed three times with 5% skim milk dissolved in phosphate-buffered saline (PBS). Blocking of non-specific binding sites was done with donkey immunoglobulin G (IgG, whole molecule, 1/400, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 30 min. Each coverslip was incubated for 1 h with antibodies against GIRK1 or GIRK2 (Alomone Labs, Jerusalem, Israel). Residual antibody was washed out with 5% skim milk 3 times, 5 min each. This was followed by a 30 min incubation with secondary antibody (Cy3 donkey anti-rabbit IgG, 1 : 400, Jackson ImmunoResearch Laboratories). Free secondary antibody was then washed out with PBS 3 times, 5 min each in darkness and the coverslips were mounted on a glass slide. The fluorescent labelling was examined by a confocal laser scanning microscope (LSM 510 Meta, Zeiss, Germany) with 20× or 5× objective lens. Cy3 was excited at 514 nm and the emitted light was collected between 540 and 615 nm using the spectral mode of the Zeiss 510 Meta (beam splitter HFT 405/514/633).
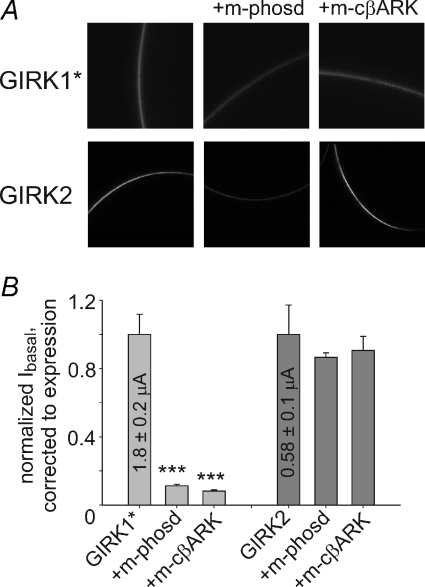
Figure 5. The basal activity is Gβγ dependent in GIRK1*, but Gβγ independent in GIRK2.
A, confocal images of whole oocytes expressing YFP-labelled GIRK1* (visualized using YFP) or GIRK2HA (visualized using anti-HA antibody). B, the effect coexpression of Gβγ scavengers, m-phosducin (10 ng) and m-cβARK (5 ng), on Ibasal of GIRK1* (grey) and GIRK2 (dark grey). Currents were corrected to changes in PM level of the channel (shown in Supplemental Fig. 3). n= 16–20. ***P < 0.001.
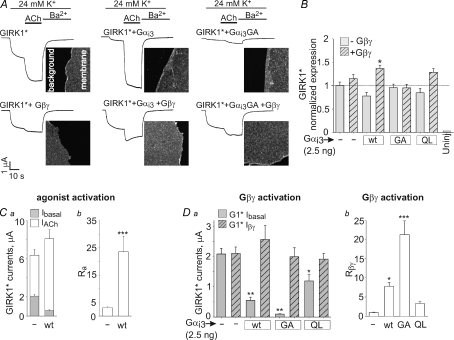
Figure 6. Gαi3 regulates GIRK1* activation in whole oocytes.
A, examples of basal and ACh-evoked currents (continuous lines) of GIRK1* (10–15 ng), with and without coexpression of Gα (2.5 ng) and Gβγ (5 and 1 ng RNA, respectively). The images beside the current traces show the PM expression of GIRK1*, monitored in giant excised PM patches using anti-GIRK1 antibody and a secondary, fluorescently labelled antibody. The images focus on the edges of the PM patches to allow comparison with the background. The image area is 55 × 55 μm. B, summary of GIRK1* expression levels under different conditions. n= 14–19. C, summary of Ibasal and IACh with or without coexpression of Gαi3-wt (2.5 ng RNA per oocyte) (a), and of Ra measured in the same oocytes (b). n= 22–30. D, the effect of coexpression of Gαi3 (wt, GA or QL, 2.5 ng each) on GIRK1*'s Ibasal and Iβγ (a), and the summary of measurements of Rβγ in this series of experiments (b). n= 17–36. Statistical comparisons of multiple groups were done using one-way ANOVA followed by Dunnett's test against the control group, GIRK1* alone.*P < 0.05; **P < 0.01; ***P < 0.001.
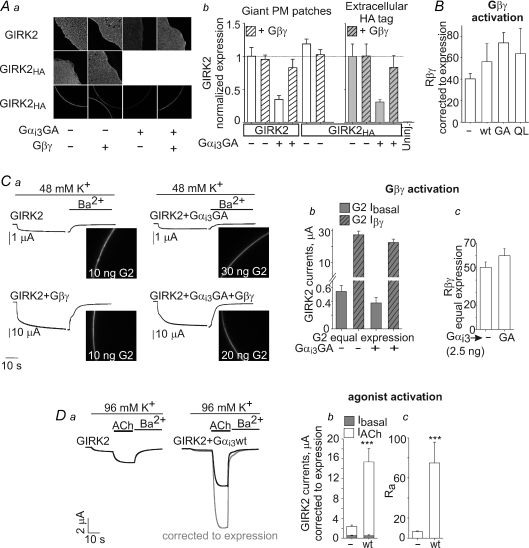
Figure 8. GIRK2 is not regulated by Gαi3 in whole oocytes.
Aa, changes in the amount of GIRK2 channels in PM caused by coexpression of Gαi3 (2.5 ng RNA) and Gβγ (5 and 1 ng RNA, respectively). Data were obtained by measurements in giant PM patches using anti-GIRK2 antibody and a secondary, fluorescently labelled antibody, or in whole oocytes using external HA tag, in the same experiment. b, summary of GIRK2 expression and comparison of the two imaging methods. Open bars show the amount of GIRK2 and GIRK2HA channels, assessed in giant PM patches using the anti-GIRK2 antibody. Grey bars show the PM expression of GIRK2HA measured using the external HA tag. With both methods, the expression level in different groups was normalized to the control group of oocytes expressing the channel alone. Note that both methods provide very similar assessment of the relative effects of expression of Gαi3GA (reduction in PM expression levels) and of Gβγ (no change or recovery of expression). n= 4–11. B, coexpression of Gαi3 (wt, GA or QL, 2.5 ng each) did not significantly affect Rβγ in a series of experiments where the measured currents were corrected to the PM expression of GIRK2 or GIRK2HA measured in the same experiments. The full details of PM expression, Ibasal and Iβγ are presented in Supplemental Fig. 4A. n= 13–28. C, summary of a separate experiment in which GIRK2HA PM expression was titrated, by injected different amounts of RNA as indicated on the images, to produce equal channel expression in the presence of coexpressed Gαi3GA (2.5 ng) with or without Gβγ (5 and 1 ng RNA, respectively). a, the confocal images of whole oocytes obtained with an anti-HA antibody. Examples of currents in representative oocytes are shown to the left of the confocal images. See Supplemental Fig. 4B for further details. b and c, the effect of coexpression of Gαi3GA on GIRK2's Ibasal and Iβγ (b) and the summary of measurements of Rβγ (c) in groups of oocytes with equal PM channel expression. n= 8 oocytes in each group. D, examples of GIRK2 currents (a) and summaries of Ibasal and IACh (b) and Ra (c) with or without coexpression of Gαi3-wt (2.5 ng) in four experiments where the amounts of GIRK2 RNA were not titrated, channel expression in the PM was monitored, and current measurements were corrected for PM level changes. n= 13–28. ***P < 0.001.
Imaging of whole oocytes was done using external HA tag (GIRK2HA, G2CTG1) (Kanevsky & Dascal, 2006) or YFP (YFP labelled GIRK1* or GIRK2). To visualize the HA tag, whole oocytes expressing GIRK2HA or G2CTG1 were fixated in 4% formaldehyde (from 37% stock) in Ca2+-free ND96 solution for 30 min. Blocking of non-specific binding sites was done by 5% skim milk for 1 h in Ca2+-free ND96. Then the oocytes were incubated for 1 h with the mouse monoclonal anti-HA antibody (Santa Cruz Biotechnology), diluted 1 : 400 in 2.5% skim milk. Residual antibody was washed out with 2.5% skim milk 3 times, 5 min each. This was followed by 1 h incubation with the secondary antibody (Alexa Fluor 488 conjugated, 1 : 400, Molecular Probes/Invitrogen) in dark. Free secondary antibody was then washed out with Ca2+-free ND96. Oocytes were placed in a chamber with a transparent bottom, and fluorescence imaging was performed with LSM 510 (×20 objective, zoom = 2, pinhole 3 Airy units). Alexa was excited at 488 nm, and the emitted light was collected in the wavelength interval of 508–615 nm in spectral mode (with HFT 405/488 beam splitter).
Imaging of YFP labelled GIRK1* and GIRK2 channels was performed with LSM 510 (×20 objective, zoom = 2, pinhole 3 Airy units). YFP was excited at 514 nm, and the emitted light was collected in the wavelength interval of 524–609 nm in spectral mode (with HFT 405/514/633 beam splitter).
All images were obtained from optical slices from the animal hemisphere close to the oocyte's equator. Quantification of all the images was done using Zeiss LSM software. The intensity of fluorescence in the PM was measured by averaging the signal obtained from three standard regions of interest. Net fluorescence intensity per unit area was obtained by subtracting the background signal measured in uninjected oocytes. In all confocal imaging procedures, care was taken to completely avoid saturation of the signal. In each experiment, all oocytes from the different groups were studied using constant LSM settings.
Data analysis, presentation and statistics
Relative activation by agonist, Ra, was calculated in each cell as Itotal/Ibasal, where Itotal=Ibasal+IACh. Thus, Ra=Itotal/Ibasal= (IACh+Ibasal)/Ibasal= (IACh/Ibasal) + 1. Similarly, the extent of Gβγ activation, Rβγ, was defined as Rβγ=Iβγ/Ibasal, where Iβγ is the total Gβγ-dependent current. The better the activation by Gβγ, the greater is Rβγ; Rβγ= 1 when there is no effect of Gβγ. In patch clamp experiments, Ibasal was measured in a cell-attached mode before excision, and Iβγ in the same patch after excision and addition of purified Gβγ (Figs 3, 7 and 9). In whole cells, Iβγ is the total GIRK current in a cell expressing Gβγ, and Ibasal is the average GIRK current in oocytes of the same batch expressing GIRK alone (Rubinstein et al. 2007). These definitions are essentially the same as the ones used previously (Ra=IACh/Ibasal; Rβγ=Iβγ/Ibasal− 1) (Peleg et al. 2002; Rubinstein et al. 2007) except for the addition of a constant number, 1. The change in definition was motivated by the desire to avoid division by very small numbers during normalization to (division by) Ra or Rβγ of the ‘GIRK only’ groups. Indeed, Ra and Rβγ calculated according to the previous definition (Rubinstein et al. 2007) were often close to zero in these cells at high expression levels of GIRK1/2 or GIRK1*, when the activation by ACh or by Gβγ was weak.
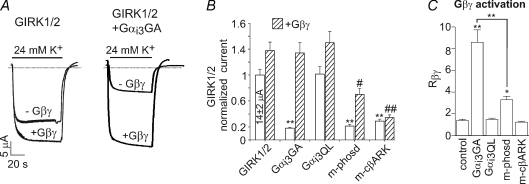
Figure 3. Gαi3GA improves GIRK1/2 activation by added Gβγ protein in excised plasma membrane patches.
A, examples of patch clamp records in oocytes expressing either GIRK1/2 alone (a) or with Gαi3GA (b). RNAs at 1–2.5 ng per oocyte of GIRK1/2, Gαi3GA and Gαi3QL were injected. Approximate amplitudes of Ibasal and Iβγ are indicated for illustration in a; the exact values were calculated from all-points histograms (Yakubovich et al. 2009). B, summary of patch clamp experiments. In view of large batch-to-batch variability, all data (currents, Rβγ) were normalized to those of control group (GIRK1/2 alone) recorded on the same day. Number of measurements is shown above the bars. *P < 0.05; **P < 0.01.
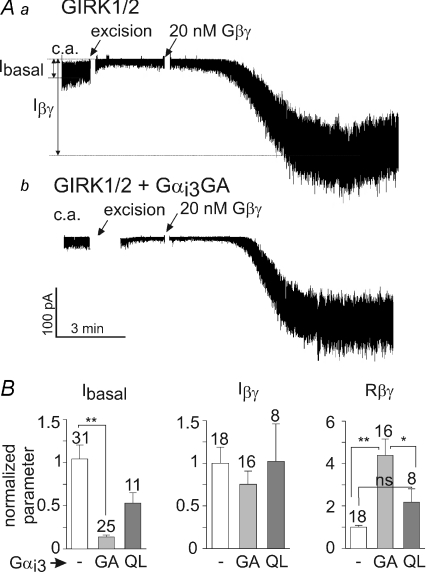
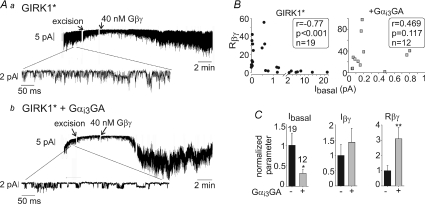
Figure 7. Gαi3GA regulates GIRK1* in excised plasma membrane patches.
GIRK1* (10–17 ng) was expressed with or without Gαi3GA (2.5 ng). A, examples of patch clamp records in oocytes expressing GIRK1* alone (a) or GIRK1* with Gαi3GA (b), with zooms on segments of the c.a. records below the main traces. B, correlation between Ibasal (measured in the cell attached mode) and Rβγ with (right) or without (left) Gαi3GA coexpression. The data were analysed using Spearman's correlation algorithm. Correlation coefficient (r), P value and n are shown in the boxes. C, Summary of patch clamp experiments. In view of large batch-to-batch variability, all data (currents, Rβγ) were normalized to those of control group (GIRK1* alone) recorded on the same day. The number of measurements is shown on top of the Ibasal bars (left graph). *P < 0.05, **P < 0.01.
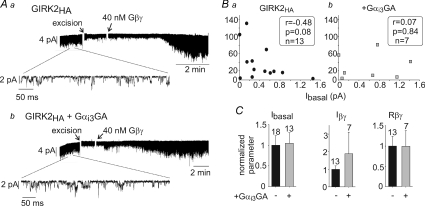
Figure 9. Gαi3GA does not regulate GIRK2 in excised plasma membrane patches.
A, examples of patch clamp records in oocytes expressing GIRK2HA alone (10 ng) (a) or GIRK2HA+ Gαi3GA (Gαi3GA was expressed at 2 ng) (b). B, correlation between Ibasal (measured in the cell attached mode) and Rβγ with (right) or without (left) Gαi3GA coexpression. The data were analysed using Spearman's correlation algorithm. Correlation coefficient (r), P value and n are shown in the boxes. C, summary of patch clamp experiments. In view of large batch-to-batch variability, all data (currents, Rβγ) were normalized to those of control group (GIRK2HA alone) recorded on the same day. The number of measurements is shown above the bars.
Results are shown as means ±s.e.m. When summarizing several experiments, in order to minimize batch-to-batch variations and to enhance the accuracy of the statistical analysis, whole-cell GIRK currents were normalized, in each oocyte, to the average GIRK current of the control group (GIRK alone) of the same experiment (Sharon et al. 1997). Two group comparisons were done using Student's two-tailed t-test. Multiple group comparison was done using one-way analysis of variance (ANOVA) followed by Tukey's, Student–Neumann–Keuls or Dunnet's test. Correlations between two parameters were examined using Spearman's test.
Results
Gαi3 regulates GIRK1/2 gating in HEK 293 cells, like in oocytes
We have previously observed that the Ibasal of GIRK1/2 increased disproportionally as the channel expression level was increased, whereas activation by agonist or Gβγ became weaker; there is a negative correlation between Ibasal and the extent of activation by agonist (Ra) or by Gβγ (Rβγ). This abnormal gating was corrected by coexpressed Gαi3 which reduced Ibasal, increased Ra and Rβγ, but did not reduce the total Gβγ-dependent current (basal + evoked), Itotal, as if the presence of Gαi3 prepares (‘primes’) the channel for activation by Gβγ (Peleg et al. 2002; Rubinstein et al. 2007). The enhancement of agonist-evoked current by coexpressed Gαi appeared self-evident (Vivaudou et al. 1997; He et al. 1999) (free Gβγ is sequestered by GαiGDP but then released after activation of GPCR). However, the enhancement of activation induced by added or coexpressed Gβγ could not be fully explained by a mechanism in which Gα reduces Ibasal by ‘sequestering Gβγ away’ from GIRK (Rusinova et al. 2007), suggesting a more complex mechanism, where the GIRK channel may be regulated by Gαi3GDP (Peleg et al. 2002).
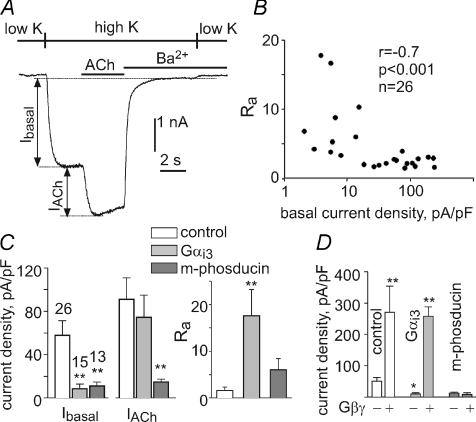
The aforementioned hallmarks of Gαi regulation have been observed so far only in Xenopus oocytes, which are believed to have relatively high levels of Gβγ that help to maintain the meiotic arrest (Sheng et al. 2001; Evaul et al. 2007). Therefore, it was important to show that regulation by Gαi does not arise from some unusual properties of Xenopus oocytes. To this end, we transiently transfected HEK 293 cells with GIRK1, GIRK2 and the muscarinic 2 receptor (m2R). Evoked currents were elicited by acetylcholine (ACh) or by coexpression of Gβγ. In general, Ra and Rβγ were somewhat higher in HEK 293 cells than in oocytes, corroborating higher basal Gβγ levels in the oocytes. Despite this quantitative difference, the fundamental hallmarks of Gαi3 regulation were as in Xenopus oocytes (Fig. 1). There was a strong negative correlation between Ibasal (taken as the indicator of expression level) and Ra or Rβγ; expression of Gαi3 restored the low Ibasal and high activation by agonist and Gβγ, whereas the membrane-attached Gβγ scavenger protein m-phosducin (Rishal et al. 2005; Rubinstein et al. 2007) diminished both Ibasal and the evoked currents. For further functional assays we used Xenopus oocytes, where protein expression levels can be accurately controlled and monitored.
Figure 1. Regulation of GIRK1/2 channels expressed in HEK293 cells.
A, HEK 293 cells were transiently transfected with GIRK1, GIRK2 and the muscarinic 2 receptor (m2R). GIRK1/2 currents were studied using whole-cell voltage clamp at −80 mV. Switching from a physiological low-K+ solution to a high-K+ solution (90 mm K+) revealed GIRK1/2 Ibasal. Addition of the agonist acetylcholine (ACh; 10 μm) evoked IACh. Ba2+ at 5 mm was then added to inhibit GIRK currents, to calculate the net Ibasal and IACh. B, Ra is negatively correlated with Ibasal (to account for differences in HEK cell size, we used current densities (in pA pF−1) in all calculations rather than net currents). Correlation coefficient (r), statistical significance (P), and number of measurements (n) are indicated. Ra in HEK cells was usually greater than in Xenopus oocytes, up to 18 (in the oocytes it is usually below 5), corroborating higher basal Gβγ levels in the oocytes. C, coexpression of Gαi3 and m-phosducin reduced Ibasal similarly, but only Gαi3 enhanced the relative activation by ACh, Ra. D, coexpressed Gαi3, but not m-phosducin, improves activation of GIRK1/2 by expressed Gβγ. n= 3–7 cells in each group. The extent of direct activation by Gβγ (Rβγ), calculated here as the ratio of average currents in cells expressing Gβγ to those without Gβγ, was 5.4 for GIRK alone, 26 in the presence of Gαi3, and 0.68 in the presence of m-phosducin. *P < 0.05; **P < 0.01 compared to control by ANOVA.
‘Constitutively inactive’ mutant of Gαi3, Gαi3GA, regulates the basal activity and Gβγ activation of heteromeric GIRK1/2
We envisaged that coexpressed Gαi3 regulated GIRK1/2 in its GDP-bound form, but the involvement of GαiGTP could not be excluded (Peleg et al. 2002; Rubinstein et al. 2007). In order to decisively distinguish between effects of GαiGDP and GαiGTP, we used two mutants of Gα. One is the ‘constitutively active’ Gαi3Q204L (Gαi3QL), which poorly hydrolyses GTP, has a reduced affinity for Gβγ and regulates effectors of GαiGTP in a GPCR-independent manner (Masters et al. 1989). The second one is the ‘constitutively inactive’ Gαi3G203A (Gαi3GA), which strongly binds Gβγ (Ogier-Denis et al. 1996). GαGA mutants can bind GTP but do not regulate known effectors of GαGTP (Lee et al. 1992).
Upon injection of a standard dose of 1–2 ng RNA per oocyte, Gαi3-wild-type (wt) and its two mutants were expressed in the PM at similar levels, more than 4-fold over the endogenous Gαi/o, with no effect on GIRK1/2 expression level (data not shown). As described in other cells (Fishburn et al. 1999; Evanko et al. 2000), Gα and Gβγ mutually affected each other's PM levels. Expression of Gβγ further increased the PM levels of expressed Gαi3, Gαi3GA and Gαi3QL by ∼ 35%, and expression of Gαi3 or Gαi3GA increased the PM level of coexpressed Gβγ by about 50%, whereas Gαi3QL was without effect (data not shown).
Since both Gα mutants disrupt the G protein cycle, we activated GIRK by coexpressed Gβγ (in whole oocytes; see Reuveny et al. 1994; Rubinstein et al. 2007) or by purified Gβγ (in excised patches), thus bypassing the GPCR. Here, the channels are activated by the externally added Gβγ, without the Gαiβγ heterotrimer dissociation and without formation of GαiGTP. As controls for Gαi3 mutants, we expressed two genetically engineered Gβγ scavengers that accumulate at the PM because they are N-terminally myristoylated, like Gαi, and act as ‘sinks’ for Gβγ: m-phosducin and m-cβARK (a modified C-terminal part of β-adrenergic receptor kinase 1) (Rishal et al. 2005). We found that m-phosducin moderately reduced the total amount of detectable coexpressed Gβγ in the PM, strengthening the notion of its being a strong Gβγ sink, whereas cβARK had no effect (data not shown).
With the high GIRK1/2 levels used, Ibasal was large (14 ± 2 μA, n= 31) and the expression of Gβγ produced little additional activation (examples in Fig. 2A, summarized in Fig. 2B); the average relative activation by Gβγ (Rβγ) in this series of experiments was 1.4 ± 0.1 (Fig. 2C). Gαi3GA, m-phosducin and m-cβARK reduced Ibasal, whereas Gαi3QL did not alter Ibasal (Fig. 2B). We titrated the RNA dosage of Gαi3GA, phosducin and m-cβARK to attain a comparable 72–82% reduction in Ibasal (at RNA doses of 2, 5 and 10 ng RNA per oocyte, respectively; data not shown) and used these doses in experiments of Fig. 2. Despite the similar decrease in Ibasal, the three proteins had disparate effects on GIRK1/2's activation by Gβγ. Gαi3GA did not reduce the total Gβγ-dependent current, Iβγ, and significantly enhanced Rβγ (by 6.15 ± 0.83-fold compared to control; n= 25). In contrast, c-βARK reduced Iβγ by ∼75%, almost completely preventing channel activation by the coexpressed Gβγ (Fig. 2B). Accordingly, in the presence of m-cβARK, Rβγ was the lowest among all conditions, only 1.21 ± 0.1 (compare with 8.6 ± 1.2 with Gαi3GA; Fig. 2C). m-Phosducin slightly increased Rβγ, but significantly less than Gαi3GA. Furthermore, m-phosducin also significantly reduced Iβγ, in contrast to Gαi3GA (Fig. 2B and C). The disparate effects of Gαi3GA and the two Gβγ scavengers confirm that the effect of Gαi3GA is distinct from simple Gβγ sequestration away from GIRK.
Figure 2. Gαi3GA improves GIRK1/2 activation by coexpressed Gβγ in whole oocytes.
A, examples of GIRK1/2 currents in four individual oocytes of one batch expressing GIRK1/2 with or without Gβγ (left panel), or GIRK1/2 with 2 ng Gαi3GA, with or without Gβγ (right panel). Five nanograms of RNA of Gβ1 and 1 ng RNA of Gγ2 were injected. Zero current is indicated by dashed lines (note that an outward Ibasal is present in the low, 2 mm K+ solution). B, the effects of coexpression of Gαi3GA or Gαi3QL (2 ng RNA), m-phosducin (5–10 ng) and m-cβARK (5 ng) on basal and Gβγ-induced whole-cell currents. (The data with 5 and 10 ng m-phosducin were pooled as they produced quite similar effects.) **P < 0.01 compared to control Ibasal; #P < 0.05 or ##P < 0.01 compared with control Iβγ. C, Rβγ of the experiments summarized in B. *P < 0.05 and **P < 0.01 against control or between indicated groups. n= 8–31.
We next studied GIRK1/2 regulation by Gα mutants in excised patches, which has the advantage of strictly controlling the concentration of added Gβγ and measuring Ibasal and Iβγ in the same oocyte. GIRK1/2 was expressed alone or with Gαi3GA or Gαi3QL. After 1–3 min of cell-attached (c.a.) recording of Ibasal, patches were excised. The activity declined, reaching a new steady level within 0.5–2 min (Peleg et al. 2002). Approximately 3 min after excision, purified Gβ1γ2 (20 nm) was added to the bath solution to activate the channel (Fig. 3A). Coexpressed Gαi3GA strongly reduced Ibasal but significantly enhanced Rβγ compared to control group (Fig. 3B). Gαi3QL caused a mild reduction in Ibasal (P > 0.05) and no significant changes in Rβγ. Iβγ was unchanged by either Gαi3GA or Gαi3QL. This result (and also the similar result with GIRK1*, Fig. 7) rules out the possibility that the enhanced activation observed with Gαi3GA coexpression was due to Gα-dependent changes in the level of Gβγ, as could potentially happen with coexpressed proteins in whole cell experiments. The effect of Gαi3GA was in sharp contrast to that of m-cβARK, which greatly reduced Gβγ-induced activation of GIRK1/2 in excised patches (Peleg et al. 2002). Thus, in excised patches Gαi3GA, but not Gαi3QL, regulates the channel basal activity, and improves the relative activation by added Gβγ.
To summarize, Gαi3GA has a unique effect on GIRK1/2 gating: similarly to Gαi3-wt, it reduces Ibasal, enhances Rβγ, and does not reduce the total Iβγ. Neither the two Gβγ scavengers tested nor Gαi3QL can reproduce these effects. We conclude that the regulation of Ibasal and of the Gβγ-evoked activation of GIRK1/2 is produced by the GDP-bound form of Gαi3.
Gβγ enhances the interaction of Gαi3GDP with GIRK1 but not GIRK2
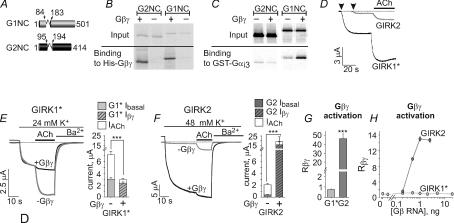
We have examined the interaction of in vitro translated cytosolic domain of GIRK1 (a tandem of full-length N- and C-termini, G1NC, which lacks the transmembrane domain; see Fig. 4A) with Gαi3 in the presence of purified Gβγ. Interestingly, the binding of Gαi3GDP to G1NC was enhanced in the presence of Gβγ (Fig. 4C and unpublished observations). To examine whether this phenomenon also takes place in GIRK2, we constructed a similar tandem, G2NC, encoding the full-length cytosolic domain of GIRK2 (Fig. 4A). The in vitro translated, 35S-labelled G1NC and G2NC gave a single protein band each on SDS-polyacrylamide gels (Fig. 4B and C). Furthermore, both G1NC and G2NC bound Gβγ similarly (Fig. 4B). These results suggest that in vitro synthesized G1NC and G2NC are stable, well folded proteins.
Figure 4. Biochemical and functional differences between GIRK1 and GIRK2.
A, schematic representation of the G1NC and G2NC tandems. The transmembrane portion of the channel was replaced by a short linker (shown by a loop connecting the cylinders). B, His-Gβγ binds the 35S-labelled, in vitro synthesized G1NC and G2NC. Pull down experiments were performed using Ni2+ affinity beads. Autoradiograms of loaded (upper panel, ‘input’; 1/60 of total loaded protein) and bound 35S-labelled G1NC and G2NC proteins (lower panel, ‘binding’) are shown. C, GST-Gαi3 binds G1NC and G2NC. The binding of G1NC, but not G2NC, is enhanced by added purified Gβ1γ2. Pull down experiments were performed using glutathion sepharose affinity beads. These results are representative of 3 independent experiments. D, examples of basal and ACh-evoked currents in two representative oocytes expressing homomeric GIRK1* and GIRK2. Arrowheads indicate the exchange of the external solution from low, 2 mm K+ to high, 24 mm K+. The addition of 10 μm ACh is indicated by the horizontal bar. E and F, examples of currents from four different oocytes expressing homomeric GIRK1* (E, grey), GIRK2 (F, grey) or homomeric channels with coexpression of Gβγ (black traces in E and F). Holding potential was set to −80 mV. Solutions were switches as indicated. Summaries of the basal, ACh-evoked and Gβγ-evoked currents are depicted to the right of the traces in E and F. GIRK1* is depicted in light grey (E) and GIRK2 in dark grey (F). In each bar chart, the bottom bar shows Ibasal, the top open bar shows IACh, and the total height of each bar represents Itotal. G1* stands for GIRK1*, G2 for GIRK2. Diagonal strips indicate coexpression of Gβγ. n= 20–40. G, Rβγ, the extent of activation by coexpressed Gβγ. H, titrated expression of Gβγ revealed dose-dependent activation of GIRK2 (one experiment, n= 5–6) but no activation of GIRK1* (two experiments, n= 5–18). The dose of Gγ was 0.5 of that of Gβ. ***P < 0.001.
Next, binding of the in vitro translated G1NC and G2NC to a GST-fused Gαi3 (GST-Gαi3) (Rishal et al. 2003) was examined in the presence of GDP, and in the presence or absence of purified Gβ1γ2. In the absence of Gβγ, both G1NC and G2NC bound Gαi3 (Fig. 4C). In the presence of Gβγ, the binding of G1NC to GST-Gαi3 was enhanced ∼6-fold. In contrast, G2NC binding to GST-Gαi3 was unaffected by the addition of Gβγ (Fig. 4C). These data suggest the formation of a strong complex between Gαi3βγ heterotrimers and GIRK1, but not GIRK2.
Functional differences between homomeric GIRK1 and GIRK2
The asymmetric interaction of GIRK1 and GIRK2 with Gαi3βγ suggested a different regulation of function of each subunit within the heteromeric channel. We therefore sought to investigate these differences using homomeric GIRK channels expressed in Xenopus oocytes. GIRK2 subunits form functional homomers, whereas GIRK1 does not. Thus, we used the GIRK1F137S (GIRK1*), a pore mutant of GIRK1, able to form functional channels in the plasma membrane (PM) (Vivaudou et al. 1997). The presence of a single mutation in the pore is not likely to affect the regulation of the channel by Gα and Gβγ, which bind to the cytosolic segments. In support, heterotetrameric GIRK1*/GIRK2 functioned similarly to wild-type GIRK1/2, showing an excessive Ibasal and reduced activation by agonist and Gβγ at high expression levels, as well as the typical regulation by Gαi3GA (data not shown).
Homomeric GIRK channels were activated by ACh (via coexpressed m2R), by coexpression of Gβγ, or by the addition of purified Gβγ to excised patches. For the whole cell experiments K+ currents were measured using two electrode voltage clamp, concurrently with measurements of protein level of the channels in the PM. GIRK1* currents were always measured in 24 mm K+, whereas most of GIRK2 recordings were done in 48 mm K+, to allow for a better resolution of Ibasal of the GIRK2.
Titrated expression of the two channels revealed striking differences which became especially prominent at high expression levels (10–15 ng RNA per oocyte) (see Table 1). The first difference was the magnitude of Ibasal, which was many-fold larger in GIRK1* than GIRK2 (3 ± 0.2 μA, n= 44 vs. 0.38 ± 0.06, n= 40; P < 0.001, Fig. 4D–F). This occurred despite the fact that GIRK2 expression was higher compared to GIRK1* (Supplemental Fig. 2), and despite the fact that we used higher K+ concentration in GIRK2 measurements. Moreover, Ibasal of GIRK1* was strongly RNA dose dependent, whereas Ibasal of GIRK2 grew much less with channel density (Supplemental Fig. 1A and B).
Table 1.
Comparison between GIRK1/2, GIRK1* homomers and GIRK2 homomers
| Parameter | GIRK1/2 | GIRK1* | GIRK2 |
|---|---|---|---|
| Ibasal | High, Gβγ dependent | High, Gβγ dependent | Low, largely Gβγ independent |
| Activation by Gβγ | Weak or medium at high channel densities (Rβγ 1.4–1.8) | None or negative at high channel densities (Rβγ≤ 1) | Strong (Rβγ > 10) |
| Gαi3GDP on Ibasal | Decreases Ibasal | Decreases Ibasal | Almost no effect |
| Gαi3GDP on Rβγ | Strong regulation (increases Rβγ with no decrease in Iβγ) | Strong regulation (increases Rβγ with no decrease in Iβγ) | Weak regulation (no increase in Rβγ and Iβγ) |
| Gαi3-wt on IACh | Increases IACh and Ra with no change in Itotal | Increases IACh and Ra with no change in Itotal | Increases IAch, Ra and Itotal |
Ibasal is the agonist-independent basal activity. IACh is the agonist-evoked response, revealed upon application of ACh via activation of coexpressed m2R. Itotal=Ibasal+IACh. Rβγ, the relative activation by Gβγ, calculated as the ratio of Iβγ measured in a given oocyte which coexpressed Gβγ, to the average Ibasal of the control group lacking coexpressed Gβγ (Rβγ=Iβγ/Ibasal). Note that Iβγ is the total GIRK current, comprising the basal and the Gβγ-evoked current. See (Peleg et al. 2002; Rubinstein et al. 2007) for additional data regarding GIRK1/2.
The second difference was the effect of coexpression of Gβγ. GIRK1* or GIRK2 currents recorded in control and Gβγ-expressing oocytes are shown in Fig. 4E and F, left panels; the right panels show summaries of the experiments of this series. Surprisingly, coexpression of Gβγ failed to increase whole-cell GIRK1* currents at high channel expression levels (Figs 4E and 6D), whereas GIRK2 was strongly activated by the coexpressed Gβγ (Figs 4F and 8C). Titrated Gβγ expression demonstrated a clear dose-dependent activation of GIRK2 by Gβγ, which contrasted with lack of activation of GIRK1* by Gβγ at all RNA doses tested (Fig. 4H). The differences in the effects of Gβγ were not caused by differential changes in channel expression level (Supplemental Fig. 2). Despite lack of activation of GIRK1*, Gβγ expression almost completely abolished the agonist-evoked response of GIRK1* (Fig. 4E). Interestingly, when GIRK1* was expressed at low levels (1–5 ng RNA per oocyte; Ibasal < 0.6μA), the coexpressed Gβγ activated the channel ∼5-fold (Supplemental Fig. 1D), supporting the data reported by (Vivaudou et al. 1997). The mechanism of the complex, even paradoxical, behaviour of GIRK1* is still unclear but seems to be connected with its regulation by Gα (see below).
Inversely, as mentioned above, GIRK2 was strongly activated by the coexpressed Gβγ, as expected from a Gβγ effector (Fig. 4F and H). Rβγ was 46.6 ± 5.17 (Fig. 4G), dramatically higher compared to GIRK1* (0.75 ± 0.05) or even the neuronal GIRK1/2 channel (1.4 ± 0.1, Fig. 2C; Table 1). Notably, Iβγ of GIRK2 was ∼6-fold higher compared to the total current (Ibasal+IACh) observed without Gβγ expression (Fig. 4F; 16.3 ± 3.1 μA vs. 2.6 ± 0.2 μA) (see Discussion).
Previously, using the Gβγ scavengers m-phosducin and m-cβARK, we showed that up to 90% of the basal current of the neuronal GIRK1/2 channel is Gβγ dependent (Rishal et al. 2005). These two Gβγ scavengers were used to characterize the basal currents of the homomeric channels (Fig. 5 and Supplemental Fig. 3). The PM expression of GIRK1* and especially GIRK2 was substantially reduced by coexpression of m-phosducin (Supplemental Fig. 3), and therefore the currents in Fig. 5 are presented after correction to the relative PM expression. Coexpression of m-phosducin or m-cβARK reduced Ibasal of GIRK1* by 89–95%, suggesting that the basal activity of GIRK1* is mostly Gβγ dependent, like in GIRK1/2 (see Fig. 2). In contrast, GIRK2 basal activity appeared largely Gβγ independent as its Ibasal was not inhibited by coexpressed m-cβARK or m-phosducin.
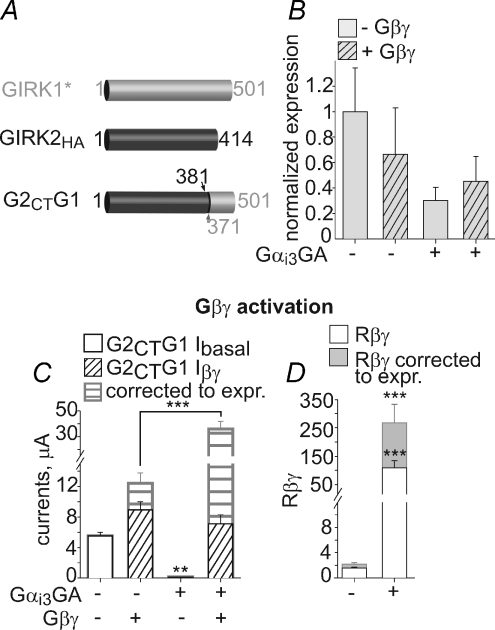
GIRK1* is regulated by Gαi3
The distinct patterns of basal and Gβγ-evoked activities in GIRK1* and GIRK2 and the difference in binding of GIRK subunits to Gαi in the presence of Gβγ led us to hypothesize that GIRK1* and GIRK2 may be differentially regulated by Gαi. To test this, homomeric GIRK1* channels were coexpressed with Gαi3-wt, Gαi3GA or Gαi3QL, with or without the coexpression of Gβγ, and the emerging changes in Ibasal, IACh, Iβγ, Ra, Rβγ and the channel's surface expression were monitored. Analysis of the effects of Gαi3 were performed at high GIRK1* expression levels (Ibasal > 1.4 μA) to minimize the potential interference of endogenous Gαi (Peleg et al. 2002). GIRK1* PM expression was measured in giant excised PM patches (Singer-Lahat et al. 2000; Peleg et al. 2002; Kanevsky & Dascal, 2006) (Fig. 6A) and found to be largely unaffected by either Gαi3 variant or by Gβγ (summarized in Fig. 6B). Therefore, the recorded whole-cell currents under different treatments were usually compared without correcting them for changes in PM expression.
Coexpression of Gαi3-wt or Gαi3GA reduced GIRK1*Ibasal by 73% or 95%, respectively (see Fig. 6A for representative current records, and Fig. 6Da for summary), consistent with the observation that GIRK1*Ibasal is Gβγ dependent, as in GIRK1/2. Also, like in GIRK1/2 (Ivanina et al. 2004), coexpression of Gαi3-wt dramatically increased the relative activation by agonist, Ra (7.6-fold, Fig. 6C). In addition, the extent of activation by Gβγ (Rβγ) was greatly increased by Gαi3: ∼8-fold by Gαi3-wt (or ∼5-fold if corrected for PM expression changes) and ∼22-fold by Gαi3GA (Fig. 6Db). Neither Gαi3-wt nor Gαi3GA reduced Iβγ, despite their ability to sequester free Gβγ. Coexpression of Gαi3QL reduced Ibasal by ∼40% (Fig. 6Da), possibly due to this mutant's ability to bind Gβγ (Majumdar et al. 2006); however, Rβγ was not significantly improved by Gαi3QL (Fig. 6Db). Thus, Gαi3GDP regulates GIRK1* and GIRK1/2 similarly: it decreases Ibasal and improves Rβγ, while preserving Iβγ (Rubinstein et al. 2007).
Regulation of GIRK1* by Gβγ and Gαi3GA was further examined in excised patches (Fig. 7A). When oocytes were injected with 10–17 ng of GIRK1* RNA, we observed high cell-to-cell and patch-to-patch variability of channel density (range of Ibasal in the absence of Gαi3GA: 0.03–22 pA; Fig. 7B), therefore not all patches could be defined as having high channel density (Peleg et al. 2002). Nevertheless, GIRK1* showed the main hallmarks of regulation by Gβγ and Gαi3GA, as in whole cells. (1) Like GIRK1/2, homomeric GIRK1* channels exhibited strong negative correlation between Ibasal and Rβγ (Fig. 7B, left panel, P < 0.001), and this correlation was absent in the presence of Gαi3GA (Fig. 7B, right panel). The negative correlation between Ibasal and Rβγ is an important hallmark of a concerted regulation of Ibasal by Gα and Gβγ (Peleg et al. 2002; Rubinstein et al. 2007). (2) Gαi3GA expression reduced Ibasal by 70% and improved Rβγ (Fig. 7C). (3) The total Gβγ-evoked current was not reduced by Gαi3GA.
There were, however, quantitative differences with whole-cell data: the average activation of GIRK1* by added Gβγ in excised patches was stronger than by coexpressed Gβγ in whole cells (Rβγ of 12 ± 3.7), and the improvement in Rβγ by Gαi3GA expression was milder (3-fold). These differences could, at least in part, result from the inclusion in the analysis of low-density patches, which show high Rβγ in the absence of exogenous Gα. Indeed, in seven patches with high Ibasal, above 2 pA, Rβγ was 1.01 ± 0.27 (range: 0.27–2.04; Fig. 7B), resembling the low relative activation of Gβγ in whole cells.
Unfortunately, the same definition of channel density could not be used in cells expressing Gαi3GA because it strongly reduced Ibasal. Thus, we attempted to sort out the cells with high expression levels according to high total current, Iβγ (>2 pA). This criterion is applicable to all patches since Iβγ does not depend on the presence of Gαi3GA (Fig. 7C). Under this definition, Rβγ was 5 ± 3 (n= 10) in control and significantly higher (44 ± 12, n= 7, P < 0.01) in Gαi3GA-expressing cells (Rβγ was 32 ± 8, n= 12, in all Gαi3GA-containing patches). We conclude that Gαi3GA genuinely improves the relative activation of GIRK1* by Gβγ, as in whole cells. However, in general, activation of GIRK1* by Gβγ in excised patches was better than in whole cells, and therefore an involvement of unidentified cytosolic factors in regulating the Gβγ-dependent gating of GIRK1* cannot be ruled out.
GIRK2 is not regulated by Gαi3GDP
Wild-type GIRK2 channels or GIRK2HA channels containing an extracellular HA tag (Clancy et al. 2005) were expressed at 10–15 ng RNA per oocyte with Gαi3-wt, Gαi3GA and Gαi3QL, with or without Gβγ. GIRK2 expression was very sensitive to Gαi3 and Gβγ: coexpression of Gαi3-wt and Gαi3GA reduced PM levels of GIRK2 by up to 80%, while Gαi3QL had a mild effect (Fig. 8A and Supplemental Fig. 4Aa). Interestingly, coexpression of Gβγ partly restored GIRK2 expression. Thus, PM expression of GIRK2 was monitored in every experiment. GIRK2 and GIRK2HA PM levels were measured by imaging in giant PM patches using anti-GIRK2 antibody, and in intact oocytes with an anti-HA antibody. We compared the two methods for assessment of the relative PM expression of GIRK2 and GIRK2HA under various experimental conditions in the same experiment, and found them to give almost identical results (Fig. 8A). These results further validate the notion that the measurements in giant excised PM patches reliably report the surface expression of membrane or membrane-associated proteins (Kanevsky & Dascal, 2006). The results with GIRK2 and GIRK2HA were pooled as there were no substantial differences in PM expression or the effects of Gαi3 and Gβγ on channel currents.
After correcting GIRK2 currents for changes in PM expression, we found that Ibasal was largely unaffected by Gαi3-wt or Gαi3GA (Supplemental Fig. 4Ab). Iβγ, the total Gβγ-evoked current, and Rβγ were also unaffected by coexpression of Gαi3 (Fig. 8B and Supplemental Fig. 4Ab). However, the above correction assumes a linear relation between channel PM levels and whole-cell currents, which may not always hold. Therefore, we also titrated the channel's expression by increasing the amount of injected RNA in order to get equal PM expression with or without Gαi3GA and Gβγ (Fig. 8C; see additional details in Supplemental Fig. 4B). When groups of oocytes with equal PM expression levels were compared, expression of Gαi3GA only slightly decreased Ibasal (P > 0.05) with no effect on Iβγ or Rβγ (Fig. 8C). Thus, Gαi3GDP does not alter either basal or Gβγ-evoked activity of GIRK2.
In contrast, expression of Gαi3-wt dramatically increased the agonist response (Fig. 8D): Itotal was increased by ∼7-fold and Ra was improved by ∼12-fold (note that Ra is calculated for each oocyte and is therefore independent of the channel expression variations). In this experimental protocol Gαi3 serves as the donor of Gβγ following the activation by GPCR. It is plausible that overexpression of Gαi3 increases the fraction of GIRK2 channels associated with Gαiβγ trimers prior to activation, and in effect increased the number of functionally responsive channels (see Discussion). The increase in Itotal by Gαi3-wt was not observed with GIRK1/2 or GIRK1* (Fig. 6C and Ivanina et al. 2004).
GIRK2 channels were also tested for activation by purified Gβγ in excised patches. Examples of the currents with or without Gαi3GA are shown in Fig. 9A. Unlike GIRK1* (Fig. 7) or GIRK1/2 (Peleg et al. 2002), the negative correlation between Ibasal and Rβγ, if any, was weak and did not reach statistical significance either in wt GIRK2 (data not shown) or GIRK2HA, with or without Gαi3GA (Fig. 9B). Further, in agreement with the whole-cell results, coexpression of Gαi3GA had no effect on GIRK2 Ibasal, Iβγ or Rβγ (Fig. 9C).
In conclusion, although Gαi3 binds both GIRK1 and GIRK2 subunits, regulation by Gαi3GDP of Ibasal and of Gβγ-induced activation is a unique feature of GIRK1-containing channels.
The C terminus of GIRK1 is important for Gαi modulation
GIRK1 and GIRK2 differ in sequence and length, especially in the CT, which is ∼320 amino acids long in GIRK1 and only 220 in GIRK2 (see Ivanina et al. 2003). The distal CT, starting approximately at amino acid (a.a.) 370 of GIRK1 (381 in GIRK2), is the region of lowest homology between the two subunits. Logothetis and collaborators pointed out the importance of the unique CT of GIRK1 for large currents in the context of a GIRK1/GIRK4 chimera (Chan et al. 1997). Accordingly, we hypothesized that this segment may play a role in the unique modulation of GIRK1 by Gαi3. To this end, we constructed a chimeric channel, G2CTG1, composed of GIRK2HA in which the distal CT (a.a. 382–414) was replaced with that of GIRK1, a.a. 371–501 (Fig. 10A). Monitoring of the PM expression was done in whole oocytes using an external HA tag. Like GIRK1*, the chimeric G2CTG1 displayed large basal currents, which were strongly RNA dose dependent, reaching 5.5 μA at 2 ng RNA per oocyte (Fig. 10C and Supplemental Fig. 1C). On the other hand, coexpression of Gαi3GA reduced the PM expression levels of G2CTG1 by ∼60%, resembling in this respect the GIRK2 (Fig. 10B). Coexpression of Gβγ induced weak activation with Rβγ of ∼2 and Iβγ of 8.9 μA (or 12.4 μA if corrected to PM expression, Fig. 10C and D). Gαi3GA coexpression reduced Ibasal by 96–98% but enabled full activation by Gβγ, or even enlargement of the Iβγ if corrected for the reduced expression. Rβγ with Gαi3GA coexpression was increased from 2 to ∼70 (Fig. 10D). Therefore, the distal part of GIRK1 CT is important for Gα and Gβγ regulation. Transferring this segment confers upon GIRK2 most (though not all) of the unique qualities of GIRK1*: high, Gβγ-dependent basal activity and regulation by Gαi3GA.
Figure 10. The C terminus of GIRK1 is important for Gαi modulation.
A, schematic presentation of the G2CTG1 chimera. B, summary of G2CTG1 expression levels (1–2 ng RNA per oocyte), as measured in whole oocytes using external HA tag. n= 4–7. C, Gαi3GA (2.5 ng) regulates Ibasal and Iβγ of the G2CTG1 chimera. Grey bars with horizontal hatching indicate the currents after correction to the PM expression levels (from B), diagonal black hatching indicate coexpression of Gβγ. D, Rβγ with (grey) or without (white) correction to the changes in the PM expression. n= 7–24. **P < 0.05; ***P < 0.001.
Discussion
Here we show that Gαi regulates the Gβγ gating of the neuronal GIRK1/2 channels in Xenopus oocytes and mammalian (HEK 293) cells. Utilizing ‘constitutively inactive’ and ‘constitutively active’ Gαi3 mutants in functional assays, we find that the ‘inactive’ Gαi3 in its GDP-bound form reduces Ibasal and predisposes GIRK1/2 to Gβγ activation. This action of GαiGDP is Gβγ dependent and involves the formation of Gαiβγ heterotrimers. We further explored previously unrecognized differences between GIRK1 and GIRK2 in their interaction with, and functional regulation by, Gαi and Gβγ. GIRK1 channels, in contrast to GIRK2 homomers, are regulated by Gαi3, and consequently show a strikingly different pattern of regulation by Gβγ. Our results support the hypothesis that the Gαi/oβγ heterotrimers or free GαiGDP are regulators of gating of GIRK channels, but potentially limit the list of Gαi/oGDP-regulated GIRK channels to those containing the GIRK1 subunit.
‘Constitutively inactive’ mutant of Gα, Gαi3GA, improves the activation of the neuronal GIRK1/2 by Gβγ
We have previously reported that coexpression of Gαi3-wt reduced the basal activity of GIRK1/2 and enhanced the direct activation of the channels by added Gβγ, bypassing the GPCR and the Gαiβγ heterotrimer dissociation. We initially called this phenomenon ‘priming by Gαi’ and proposed that Gαi regulates GIRK gating, keeping Ibasal low and preparing the channel for activation by ‘free’ Gβγ (Peleg et al. 2002; Rishal et al. 2005; Rubinstein et al. 2007). We suggested that GIRK1/2 modulation was mediated by GαiGDP, or Gαiβγ heterotrimers. Yet, it was also essential to decisively distinguish between effects of GαiGDP and GαiGTP. Xenopus oocytes reportedly contain high basal levels of ‘free’ Gβγ that help to maintain the meiotic arrest. An unidentified constitutively active GPCR has been hypothesized to cause this condition (Evaul et al. 2007), and in such cases a high level of free GαGTP is also expected. Here we showed that only the ‘constitutively inactive’ mutant Gαi3GA improves GIRK1/2 activation by Gβγ. As with Gαi3-wt (Rubinstein et al. 2007), the improvement is only on the background of a concomitant decrease in basal activity; the total Gβγ-induced activation is not changed, both in whole cells and in excised patches. The constitutively active Gαi3QL did not significantly affect Gβγ-induced activation of GIRK1/2 (Figs 2 and 3). However, at present we cannot fully rule out a minor direct effect of Gαi3QL on GIRK1/2.
Differences in interaction between GIRK subunits and Gαi3βγ
Despite a larger Gβγ and Gα-interacting surface in GIRK1 compared to GIRK2 (Ivanina et al. 2003; Ivanina et al. 2004), no functional asymmetry was previously reported. Full cytosolic domains of GIRK1 and GIRK2 interact with Gβγ and Gαi3. However, regulation (enhancement) by Gβγ of the Gαi3GDP–GIRK interaction is observed only with GIRK1 (Fig. 4). This implies a central role for the GIRK1 subunit in mediating the effects of Gαi (or Gαiβγ) on heterotetrameric GIRK1/2 channels, and possibly on other GIRK1-containing heterotetramers. The enhanced binding of Gαi3GDP in the presence of Gβγ could be due to stronger binding of Gαiβγ heterotrimer to GIRK via Gβγ, or an enhancement of the direct binding of Gαi3 to a site separate from that of Gβγ. In support, strong binding of Gαi1βγ heterotrimer to the NT of GIRK1 was reported in the past, and this was suggested to reflect the existence of preformed GIRK-G protein signalling complexes (Huang et al. 1995).
Homomeric GIRK2 and GIRK1* channels are distinctly regulated by Gβγ and Gαi3GDP
The homomeric GIRK2 channel seems to behave as a ‘classical’ Gβγ effector, displaying very low basal activity in the absence of agonist and strong activation by added Gβγ (see Table 1). Its gating by Gβγ is not regulated by Gαi3GDP, remaining unaltered in the presence of coexpressed Gαi3-wt or Gαi3GA in whole cells or excised patches. In agreement with the biochemical data, several functional results suggest a weak interaction with Gαiβγ heterotrimers. (1) GIRK2's basal currents are small and insensitive to Gβγ scavengers or to the coexpression of Gαi3. (2) Coexpression of either Gαi3-wt or Gβγ enhanced the overall GIRK2 currents (Itotal and Iβγ, respectively) by ∼7-fold, compared to control cells expressing the channel alone (Figs 4F and 8D). Since Gαi3GDP does not alter the extent of the channel's activation caused by direct addition of Gβγ, it is most likely that the addition of Gαi3 simply increases the amount of Gαi3βγ heterotrimers available for the activation of this channel. These results indicate that the endogenously present Gαiβγ heterotrimers cannot fully activate all expressed GIRK2 channels, and the majority of GIRK2 homomers (probably more than 80%) lack pre-associated G-proteins (in contrast to GIRK1*, see below). In summary, GIRK2 channels display very strong activation by added Gβγ and no regulation by Gαi3GDP. Lack of regulation of GIRK2 by Gαi3GDP further emphasizes the authenticity and uniqueness of this regulation in GIRK1-containing channels.
GIRK1* is similar to GIRK1/2 and differs from GIRK2 in two major aspects (Table 1): (1) GIRK1* exhibits a considerable Gβγ-dependent basal activity; and (2) it is strongly regulated by Gαi3. Ibasal of GIRK1* is highly Gβγ dependent; expression of Gβγ-binding proteins m-phosducin, m-cβARK or Gαi3 reduces Ibasal by more than 70%. It is highly unlikely that the high Ibasal of GIRK1* (or GIRK1/2) depends on ‘free’ Gβγ elevated by some ‘constitutively active’ GPCR present in the oocyte, because GIRK2 (which is highly sensitive to both Gβγ and ACh) shows almost no Gβγ-dependent Ibasal. Recent studies conducted in vivo using fluorescent energy transfer techniques suggest that GIRK1-containing channels are associated with Gβγ both in endoplasmic reticulum (Robitaille et al. 2009) and in the PM (Riven et al. 2006). Taken together, these considerations suggest that the excessive basal activity of GIRK1* at high expression levels is due to an excess of bound Gβγ, and to an insufficient amount of Gα, as in the case of GIRK1/2 (Peleg et al. 2002; Rishal et al. 2005).
The data presented here establish that Gαi3GDP, and not Gαi3GTP, is responsible for the regulation of Ibasal and Rβγ in GIRK1/2 and GIRK1*. Several facts support regulation by GαiGDP, probably via the formation of Gαi3βγ heterotrimers. (1) Gαi3-wt and Gαi3GA reduced Ibasal with no reduction in Iβγ, and greatly improved the extent of activation by Gβγ (Rβγ). (2) In excised patches, Rβγ of GIRK1* exhibited strong negative correlation with Ibasal, with almost no activation when Ibasal is high. Expression of Gαi3GA abolished the negative correlation and improved Rβγ by ∼9-fold. (3) Expression of Gαi3-wt improved the relative activation by agonist, Ra, by ∼8-fold, but Itotal did not change. Thus, the improvement of IACh and Ra correlated with the decrease in Ibasal, indicating that the expressed Gαi3 enhanced the amount of GIRK1-associated Gαi3 and corrected the balance between the bound Gαi3 and Gβγ. Together with the biochemical data, these considerations suggest that most of GIRK1* homomers and GIRK1/2 heterotetramers arrive at the PM in complex with Gαiβγ or with Gβγ alone.
A plausible model to describe the regulation of GIRK1/2 and GIRK1* by Gαi3GDP would be one which includes two sites for G protein binding, somewhat similar to the model proposed by Logothetis and colleagues (He et al. 1999). In our two-site model the GIRK channel would have a docking, or anchoring, site for Gαiβγ, and a separate activation site that binds Gβγ. In a normal physiological situation, Gαi/oβγ is anchored to GIRK either via Gαi (Clancy et al. 2005) or via Gβγ; the Gβγ activation site is free. Activated GPCR triggers partial or full separation of Gαi/o from Gβγ, causing a shift of Gβγ to the activation site and opening of the channel. For reasons as yet unclear, the overexpressed channel is trafficked to the PM with excess Gβγ over Gαi/o (Rishal et al. 2005), and Gβγ is free to interact with the activation site, and hence the high Ibasal. We further propose that coexpressed Gαi3 binds Gβγ, and the attachment of the resulting Gαi3βγ heterotrimer to the anchoring site reduces the excessive Ibasal and renders the channel activatable by added Gβγ, which can bind to the activation site. In contrast to GIRK1, it appears that GIRK2 does not possess an anchoring site. In a heterotetrameric GIRK1/2, GIRK1 may serve mainly as the Gαiβγ-anchoring subunit and GIRK2 as the Gβγ acceptor responsible for channel activation.
Intricate regulation of gating within the GIRK1–G protein complex
While in general GIRK1* behaved similarly to GIRK1/2 (Table 1), it displayed several features unexpected from a Gβγ effector, which further indicate a complex mechanism of regulation by G protein subunits. The most unique and unexpected feature of GIRK1* homomers is the reduction in the total current upon coexpression of Gβγ (Fig. 4E). When the channel was expressed alone, ACh evoked substantial currents. However, Gβγ coexpression failed to activate GIRK1* channels, while completely suppressing the agonist response. The disappearance of IACh in cells overexpressing Gβγ is observed also with heterotetrameric GIRK1/4 and GIRK1/2 (Reuveny et al. 1994; Lim et al. 1995; Rubinstein et al. 2007) and has been interpreted as indicating full stimulation of GIRK channels by Gβγ. This is not the case with GIRK1* (as there is no stimulation). These properties are entirely incompatible with the classical idea of activation of GIRK1* by ‘free’ Gβγ.
Although the molecular mechanisms underlying the peculiar behaviour of GIRK1* are currently unclear, several scenarios based upon the existence of a preformed GIRK1–Gαiβγ complex seem plausible. GIRK1's CT has been proposed to inhibit channel activation by Gβγ without directly competing with Gβγ for binding to GIRK (Dascal et al. 1995; Luchian et al. 1997). Such a cytosolic ‘lock’ may hinder the access of external Gβγ while allowing activation by Gβγ derived from the pre-docked Gαiβγ heterotrimer. Alternatively (or in addition), Gαiβγ might allosterically regulate GIRK1* activation, if the proper gating of GIRK1* by Gβγ (via the activation site) requires the docking of Gαiβγ at the anchoring site. Consequently, overexpression of ‘free’ Gβγ may deplete the channels of docked Gαiβγ by sequestering Gαi away from the channel (the ‘lock’ may allow the exit of pre-docked Gαi), weakening the activation by Gβγ when it binds to the activation site. Coexpression of Gαi would correct the gating both by restoring the reserve of docked Gαiβγ, and by regulating Ibasal. Allosteric interactions between the ‘lock’, Gαi and Gβγ, and the existence of two Gβγ-binding sites may produce a complex regulation pattern. Furthermore, additional factors may be involved. We deem it unlikely that the GPCR itself is important in GαiGDP-mediated effects, as regulation of GIRK1/2 by Gαi3 and Gβγ was identical with or without coexpressed GPCRs (Peleg et al. 2002; Rubinstein et al. 2007). Modulators of Gα activity such as Regulators and Activators of G Protein Signaling (RGSs and AGSs, respectively) were proposed to form complexes with and modulate GIRK channels (Jaen & Doupnik, 2006; Wiser et al. 2006). Yet, the level of endogenous RGSs in the oocytes is low (Doupnik et al. 1997), and titrated coexpression of RGS4 and RGS7 has only marginal effects on Ibasal of GIRK1/2 (Keren-Raifman et al. 2001). A full understanding of the molecular details of regulation of GIRK1-containing channels by Gα, Gβγ and other factors and the testing of the model proposed above and its alternatives present a challenge for future work.
The unique distal CT of GIRK1 is essential for Gαi3-dependent regulations
The distal third of the CT of GIRK1 (a.a. 371–501) does not bind Gαi and does not strongly interact with Gαi3 or Gβγ, though it probably possesses a low-affinity Gβγ binding site (Ivanina et al. 2003, 2004). Nevertheless, we find that regulation of GIRK1 by Gαi3 involves this segment of the CT. Its transfer from GIRK1 to GIRK2 conveyed upon GIRK2 most of GIRK1 properties, notably high Ibasal and strong modulation by Gαi3GA (a high Ibasal in a similar GIRK4–GIRK1 chimera has been previously noted by Chan et al. (1997)). These results imply that the end of GIRK1 CT (a.a 371–501) may be important for the anchoring of Gαiβγ (possibly by interacting with the other parts of the channel rather than with Gαiβγ itself). Yet, the G2CTG1 chimera could still be activated by coexpressed Gβγ (weak activation, Rβγ of ∼2), and thus other parts of GIRK1 may be additionally involved in its unique properties. It is also possible that in G2CTG1 as well as in GIRK1/2 activation is enabled due to the preservation of Gβγ binding sites of GIRK2, as the latter appears to be an excellent sensor of free ‘added’ Gβγ.
Possible physiological consequences of GIRK1/2 asymmetry
The brain expresses all GIRK subunits, predominantly heterotetrameric GIRK1/2 (hippocampus, cerebellum, cortex), GIRK1/3 and GIRK2/3, and homomeric GIRK2 (substantia nigra) (Koyrakh et al. 2005). Variable regional distribution of GIRK2 homomers vs. GIRK1/2 implies distinct properties and roles of these channels in different neurons, rendering the discovered differences between GIRK1 and GIRK2 physiologically relevant. We propose that GIRK1-containing channels contribute to the regulation of both basal and neurotransmitter-induced excitability in neurons (indeed, in hippocampal neurons GIRKs were found to contribute to the resting potential; Chen & Johnston, 2005; Wiser et al. 2006), whereas GIRK2 homomers serve as a low-noise, high-gain neurotransmitter-induced inhibitory relay.
Acknowledgments
This work is a part of fulfilment of Ph.D. Thesis requirements of S.P., M.R. and S.B. The work was supported by grants from NIH (GM68493 (N.D.) and GM60419 (C.D.)) and the Israel Science Foundation (grants 1396/05 and 49/08 (N.D.)), and a Segol Fellowship from Tel Aviv University Adams Brain Research Center (M.R). We thank Drs D. Logothetis, E. Peralta, E. Reuveny, M. Lohse and P. Slesinger for kindly providing the original cDNAs of human GIRK1*, human m2R, rat m-cβARK (aa 452–689end), bovine phosducin and mouse GIRK2aHA, respectively.
Glossary
Abbreviations
- CT
C-terminus
- GPCR
G protein-coupled receptor
- GST
glutathione-S-transferase
- HA
hemagglutinin
- NT
N-terminus
- PM
plasma membrane
- YFP
yellow fluorescent protein
- wt
wild-type
Author contributions
N.D., M.R., T.I., S.P., S.B. and C.W.D. conceived the main ideas; N.D., M.R., S.P., T.I., D.B, T.K.-R. and S.B. designed and performed the experiments; M.R. and N.D. wrote the paper; all authors participated in the analysis and interpretation of data, and critically revised and approved the paper.
Supplemental material
References
- Chan KW, Sui JL, Vivaudou M, Logothetis DE. Control of channel activity through a unique amino acid residue of a G protein-gated inwardly rectifying K+ channel subunit. Proc Natl Acad Sci U S A. 1996;93:14193–14198. doi: 10.1073/pnas.93.24.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KW, Sui JL, Vivaudou M, Logothetis DE. Specific regions of heteromeric subunits involved in enhancement of G protein-gated K+ channel activity. J Biol Chem. 1997;272:6548–6555. doi: 10.1074/jbc.272.10.6548. [DOI] [PubMed] [Google Scholar]
- Chen L, Kawano T, Bajic S, Kaziro Y, Itoh H, Art JJ, Nakajima Y, Nakajima S. A glutamate residue at the C terminus regulates activity of inward rectifier K+ channels: Implication for Andersen's syndrome. Proc Natl Acad Sci U S A. 2002;99:8430–8435. doi: 10.1073/pnas.122682899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:3787–3792. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy SM, Fowler CE, Finley M, Suen KF, Arrabit C, Berton F, Kosaza T, Casey PJ, Slesinger PA. Pertussis-toxin-sensitive Gα subunits selectively bind to C-terminal domain of neuronal GIRK channels: evidence for a heterotrimeric G-protein-channel complex. Mol Cell Neurosci. 2005;28:375–389. doi: 10.1016/j.mcn.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Dascal N. Ion-channel regulation by G proteins. Trends Endocrinol Metab. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- Dascal N, Doupnik CA, Ivanina T, Bausch S, Wang W, Lin C, Garvey J, Chavkin C, Lester HA, Davidson N. Inhibition of function in Xenopus oocytes of the inwardly rectifying G- protein-activated atrial K channel (GIRK1) by overexpression of a membrane-attached form of the C-terminal tail. Proc Natl Acad Sci U S A. 1995;92:6758–6762. doi: 10.1073/pnas.92.15.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc Natl Acad Sci U S A. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko DS, Thiyagarajan MM, Wedegaertner PB. Interaction with Gβγ is required for membrane targeting and palmitoylation of Gαs and Gαq. J Biol Chem. 2000;275:1327–1336. doi: 10.1074/jbc.275.2.1327. [DOI] [PubMed] [Google Scholar]
- Evaul K, Jamnongjit M, Bhagavath B, Hammes SR. Testosterone and progesterone rapidly attenuate plasma membrane Gβγ-mediated signalling in Xenopus laevis oocytes by signalling through classical steroid receptors. Mol Endocrinol. 2007;21:186–196. doi: 10.1210/me.2006-0301. [DOI] [PubMed] [Google Scholar]
- Finley M, Arrabit C, Fowler C, Suen KF, Slesinger PA. βL-βM loop in the C-terminal domain of G protein-activated inwardly rectifying K+ channels is important for Gβγ subunit activation. J Physiol. 2004;555:643–657. doi: 10.1113/jphysiol.2003.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn CS, Herzmark P, Morales J, Bourne HR. Gβγ and palmitate target newly synthesized Gαz to the plasma membrane. J Biol Chem. 1999;274:18793–18800. doi: 10.1074/jbc.274.26.18793. [DOI] [PubMed] [Google Scholar]
- Fowler CE, Aryal P, Suen KF, Slesinger PA. Evidence for association of GABAB receptors with Kir3 channels and RGS4 proteins. J Physiol. 2006;580:51–65. doi: 10.1113/jphysiol.2006.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Zhang H, Mirshahi T, Logothetis DE. Identification of a potassium channel site that interacts with G protein βγ subunits to mediate agonist-induced signalling. J Biol Chem. 1999;274:12517–12524. doi: 10.1074/jbc.274.18.12517. [DOI] [PubMed] [Google Scholar]
- Hedin KE, Lim NF, Clapham DE. Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IKACh currents in oocytes. Neuron. 1996;16:423–429. doi: 10.1016/s0896-6273(00)80060-4. [DOI] [PubMed] [Google Scholar]
- Huang CL, Jan YN, Jan LY. Binding of the G protein βγ subunit to multiple regions of G protein-gated inward-rectifying K+ channels. FEBS Lett. 1997;405:291–298. doi: 10.1016/s0014-5793(97)00197-x. [DOI] [PubMed] [Google Scholar]
- Huang CL, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of Gβγ to the GIRK1 G protein- gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Yoshimoto Y, Horio Y, Morishige KI, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanina T, Rishal I, Varon D, Mullner C, Frohnwieser-Steinecke B, Schreibmayer W, Dessauer CW, Dascal N. Mapping the Gβγ-binding sites in GIRK1 and GIRK2 subunits of the G protein-activated K+ channel. J Biol Chem. 2003;278:29174–29183. doi: 10.1074/jbc.M304518200. [DOI] [PubMed] [Google Scholar]
- Ivanina T, Varon D, Peleg S, Rishal I, Porozov Y, Dessauer CW, Keren-Raifman T, Dascal N. Gαi1 and Gαi3 differentially interact with, and regulate, the G protein-activated K+ channel. J Biol Chem. 2004;279:17260–17268. doi: 10.1074/jbc.M313425200. [DOI] [PubMed] [Google Scholar]
- Jaen C, Doupnik CA. RGS3s and RGS4 differentially associate with GPCR-Kir3 channel signalling complexes revealing 2 modes of RGS modulation: precoupling and collision-coupling. J Biol Chem. 2006;281:34549–34560. doi: 10.1074/jbc.M603177200. [DOI] [PubMed] [Google Scholar]
- Jelacic TM, Kennedy ME, Wickman K, Clapham DE. Functional and biochemical evidence for G protein-gated inwardly rectifying potassium (GIRK) channels composed of GIRK2 and GIRK3. J Biol Chem. 2000;275:36211–36216. doi: 10.1074/jbc.M007087200. [DOI] [PubMed] [Google Scholar]
- Kanevsky N, Dascal N. Regulation of maximal open probability is a separable function of Cavβ subunit in L-type Ca2+ channel, dependent on NH2 terminus of α1C (Cav1.2α) J Gen Physiol. 2006;128:15–36. doi: 10.1085/jgp.200609485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Raifman T, Bera AK, Zveig D, Peleg S, Witherow DS, Slepak VZ, Dascal N. Expression levels of RGS7 and RGS4 proteins determine the mode of regulation of the G protein-activated K+ channel and control regulation of RGS7 by Gβ5. FEBS Lett. 2001;492:20–28. doi: 10.1016/s0014-5793(01)02220-7. [DOI] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel MT, Peralta EG. Identification of domains conferring G protein regulation on inward rectifier potassium channels. Cell. 1995;83:443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- Leaney JL, Milligan G, Tinker A. The G protein α subunit has a key role in determining the specificity of coupling to, but not the activation of, G protein-gated inwardly rectifying K+ channels. J Biol Chem. 2000;275:921–929. doi: 10.1074/jbc.275.2.921. [DOI] [PubMed] [Google Scholar]
- Lee E, Taussig R, Gilman AG. The G226A mutant of Gsα highlights the requirement for dissociation of G protein subunits. J Biol Chem. 1992;267:1212–1218. [PubMed] [Google Scholar]
- Lim NF, Dascal N, Labarca C, Davidson N, Lester HA. A G protein-gated K channel is activated via β2-adrenergic receptors and Gβγ subunits in Xenopus oocytes. J Gen Physiol. 1995;105:421–439. doi: 10.1085/jgp.105.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Luchian T, Dascal N, Dessauer C, Platzer D, Davidson N, Lester HA, Schreibmayer W. A C-terminal peptide of the GIRK1 subunit directly blocks the G protein-activated K+ channel (GIRK) expressed in Xenopus oocytes. J Physiol. 1997;505:13–22. doi: 10.1111/j.1469-7793.1997.013bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Ramachandran S, Cerione RA. New insights into the role of conserved, essential residues in the GTP binding/GTP hydrolytic cycle of large G proteins. J Biol Chem. 2006;281:9219–9226. doi: 10.1074/jbc.M513837200. [DOI] [PubMed] [Google Scholar]
- Masters SB, Miller RT, Chi MH, Chang FH, Beiderman B, Lopez NG, Bourne HR. Mutations in the GTP-binding site of Gsα alter stimulation of adenylyl cyclase. J Biol Chem. 1989;264:15467–15474. [PubMed] [Google Scholar]
- Ogier-Denis E, Houri J-J, Bauvy C, Codogno P. Guanine nucleotide exchange on heterotrimeric Gi3 protein controls autophagic sequestration in HT-29 cells. J Biol Chem. 1996;271:28593–28600. doi: 10.1074/jbc.271.45.28593. [DOI] [PubMed] [Google Scholar]
- Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. Gαi controls the gating of the G-protein-activated K+ channel, GIRK. Neuron. 2002;33:87–99. doi: 10.1016/s0896-6273(01)00567-0. [DOI] [PubMed] [Google Scholar]
- Rebois RV, Robitaille M, Gales C, Dupre DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hebert TE. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci. 2006;119:2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Rishal I, Keren-Raifman T, Yakubovich D, Ivanina T, Dessauer CW, Slepak VZ, Dascal N. Na+ promotes the dissociation between Gα-GDP and Gβγ, activating G-protein-gated K+ channels. J Biol Chem. 2003;278:3840–3845. doi: 10.1074/jbc.C200605200. [DOI] [PubMed] [Google Scholar]
- Rishal I, Porozov Y, Yakubovich D, Varon D, Dascal N. Gβγ-dependent and Gβγ-independent basal activity of G protein-activated K+ channels. J Biol Chem. 2005;280:16685–16694. doi: 10.1074/jbc.M412196200. [DOI] [PubMed] [Google Scholar]
- Riven I, Iwanir S, Reuveny E. GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron. 2006;51:561–573. doi: 10.1016/j.neuron.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Riven I, Kalmanzon E, Segev L, Reuveny E. Conformational rearrangements associated with the gating of the G protein-coupled potassium channel revealed by FRET microscopy. Neuron. 2003;38:225–235. doi: 10.1016/s0896-6273(03)00193-4. [DOI] [PubMed] [Google Scholar]
- Robitaille M, Ramakrishnan N, Baragli A, Hebert TE. Intracellular trafficking and assembly of specific Kir3 channel/G protein complexes. Cell Signal. 2009;21:488–501. doi: 10.1016/j.cellsig.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Peleg S, Berlin S, Brass D, Dascal N. Gαi3 primes the G protein-activated K+ channels for activation by coexpressed Gβγ in intact Xenopus oocytes. J Physiol. 2007;581:17–32. doi: 10.1113/jphysiol.2006.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinova R, Mirshahi T, Logothetis DE. Specificity of Gβγ signalling to Kir3 channels depends on the helical domain of pertussis toxin-sensitive Gα subunits. J Biol Chem. 2007;282:34019–34030. doi: 10.1074/jbc.M704928200. [DOI] [PubMed] [Google Scholar]
- Saenz del Burgo L, Cortes R, Mengod G, Zarate J, Echevarria E, Salles J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J Comp Neurol. 2008;510:581–606. doi: 10.1002/cne.21810. [DOI] [PubMed] [Google Scholar]
- Sharon D, Vorobiov D, Dascal N. Positive and negative coupling of the metabotropic glutamate receptors to a G protein-activated K+ channel, GIRK, in Xenopus oocytes. J Gen Physiol. 1997;109:477–490. doi: 10.1085/jgp.109.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Tiberi M, Booth RA, Ma C, Liu XJ. Regulation of Xenopus oocyte meiosis arrest by G protein βγ subunits. Curr Biol. 2001;11:405–416. doi: 10.1016/s0960-9822(01)00123-3. [DOI] [PubMed] [Google Scholar]
- Singer-Lahat D, Dascal N, Mittelman L, Peleg S, Lotan I. Imaging plasma membrane proteins in large membrane patches of Xenopus oocytes. Pflugers Arch. 2000;440:627–633. doi: 10.1007/s004240000341. [DOI] [PubMed] [Google Scholar]
- Slesinger PA, Patil N, Liao YJ, Jan YN, Jan LY, Cox DR. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996;16:321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Slesinger PA, Reuveny E, Jan YN, Jan LY. Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron. 1995;15:1145–1156. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaudou M, Chan KW, Sui JL, Jan LY, Reuveny E, Logothetis DE. Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the KACh channel, using functional homomeric mutants. J Biol Chem. 1997;272:31553–31560. doi: 10.1074/jbc.272.50.31553. [DOI] [PubMed] [Google Scholar]
- Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- Wiser O, Qian X, Ehlers M, Ja WW, Roberts RW, Reuveny E, Jan YN, Jan LY. Modulation of basal and receptor-induced GIRK potassium channel activity and neuronal excitability by the mammalian PINS homolog LGN. Neuron. 2006;50:561–573. doi: 10.1016/j.neuron.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Yakubovich D, Rishal I, Dessauer CW, Dascal N. Amplitude histogram-based method of analysis of patch clamp recordings that involve extreme changes in channel activity levels. J Mol Neurosci. 2009;37:201–211. doi: 10.1007/s12031-008-9117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi BA, Lin Y, Jan YN, Jan LY. Yeast screen for constitutively active mutant G protein-activated potassium channels. Neuron. 2001;29:657–667. doi: 10.1016/s0896-6273(01)00241-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.