Abstract
Mechanical stimuli impinging on the skin are converted into electrical signals by mechanically gated ion channels located at the peripheral nerve endings of dorsal root ganglion (DRG) neurons. Under inflammatory conditions sensory neurons are commonly sensitised to mechanical stimuli; a putative mechanism that may contribute to such sensitisation of sensory neurons is enhanced responsiveness of mechanotransduction ion channels. Here we show that the algogens UTP and ATP potentiate mechanosensitive RA currents in peptidergic nociceptive DRG neurons and reduce thresholds for mechanically induced action potential firing in these neurones. Pharmacological characterisation suggests that this effect is mediated by the Gq-coupled P2Y2 nucleotide receptor. Moreover, using the in vitro skin nerve technique, we show that UTP also increases action potential firing rates in response to mechanical stimuli in a subpopulation of skin C-fibre nociceptors. Together our findings suggest that UTP sensitises a subpopulation of cutaneous C-fibre nociceptors via a previously undescribed G-protein-dependent potentiation of mechanically activated RA-type currents.
Following inflammation it is thought that sensory neurones from the dorsal root ganglia (DRG) can be sensitised to mechanical stimuli, so that only mildly painful stimuli become more painful (e.g. mechanical hyperalgesia). A major component of mechanical hyperalgesia is at the level of the central nervous system as second order neurones display amplified responses to nociceptor input, a phenomenon referred to as central sensitisation (Woolf & Salter, 2000; Lewin & Moshourab, 2004). Peripheral sensitisation, which is the enhanced responsiveness of primary afferent nociceptors to natural stimuli, also plays a significant role in hyperalgesia, especially from deep tissue and the viscera (Gebhart, 2000; Lewin & Moshourab, 2004; Schaible, 2004). Many workers have also emphasised the contribution of changes in voltage-gated ion channels to enhanced nociceptor excitability (Lai et al. 2004). Peripheral sensitisation may also be due to the sensitisation of transduction channels that convert mechanical energy into graded receptor potentials (Di Castro et al. 2006; Hu & Lewin, 2006). In mammals it has not been possible to use intracellular recording techniques to measure mechanically activated currents at the peripheral endings of sensory afferents in vivo. However, mechanosensitive currents can be measured in acutely isolated DRG neurones and appear to be necessary for the mechanosensitivity of somatic sensory neurones (Hu & Lewin, 2006; Drew & Wood, 2007; Wetzel et al. 2007; Lechner et al. 2009).
Nucleotides like ATP are thought to be released by damaged cells at sites of injury (Cook & McCleskey, 2002), and these molecules can produce pain and hyperalgesia in animals and man (Hamilton et al. 2000, 2001). Nucleotides exert their actions via two major classes of receptors; ionotropic P2X receptors and metabotropic P2Y receptors. P2X receptors are expressed by nociceptors and they are thought to mediate acute effects of ATP under conditions of tissue injury and inflammation (Chizh & Illes, 2001; Hamilton et al. 2001). Four P2Y receptor subtypes (P2Y1, P2Y2, P2Y4 and P2Y6) have been detected in DRG neurones (Gerevich & Illes, 2004), but their role in nociception has only just begun to be investigated (Gerevich & Illes, 2004; Malin et al. 2008). The P2Y agonist UTP can evoke slow onset depolarisation and sustained firing in dissociated rat DRG neurones and excites a subpopulation of C-fibre nociceptors in the mouse (Molliver et al. 2002; Stucky et al. 2004).
Here we show that ATP and UTP can acutely and substantially augment the size of mechanosenitive currents in peptidergic nociceptive sensory neurones. Potentiation of the mechanosensitive current by UTP is rapid, reversible and leads to a substantial decrease in the mechanical threshold for action potential initiation. Pharmacological characterisation suggested that UTP sensitisation is mediated by the Gq-coupled P2Y2 nucleotide receptors. We have also shown here that UTP sensitises C-fibres in the saphenous nerve to mechanical stimuli. Our data provide the first example of a G-protein-mediated sensitisation of the primary mechanotransduction event in nociceptors by an algogenic compound.
Methods
Cell culture
For all experiments overnight DRG cultures from neonatal C57BL/6N mice (3–14 days) were used. DRG neurones taken from neonatal mice possess the same set of mechanosensitive currents that are found in sensory neurones from adult mice (Hu & Lewin, 2006; Lechner et al. 2009). Animal housing and care, as well as protocols for killing, were registered with and approved by the appropriate German state authorities (State of Berlin). Animals were killed by decapitation and DRGs from all spinal segments were collected in Ca2+ and Mg2+-free phosphate-buffered saline (PBS). DRGs were subsequently treated with collagenase IV (1 mg ml−1, Sigma-Aldrich) and trypsin (0.05%, Invitrogen, Karlsruhe, Germany) for 10 and 20 min, respectively, at 37°C. Digested DRGs were washed twice with growth medium (Dulbecco's modified Eagle's medium (DMEM)–F12 (Invitrogen) supplemented with l-glutamine (2 μm, Sigma-Aldrich), glucose (8 mg ml−1, Sigma-Aldrich), penicilin (200 U ml−1)–streptomycin (200 μg ml−1) (both Invitrogen), 5% fetal horse serum (Invitrogen)), triturated using fire-polished Pasteur pipettes and plated in a droplet of growth medium on a glass coverslip precoated with poly-l-lysine (20 μg cm−2, Sigma-Aldrich) and laminin (4 μg cm−2, Invitrogen). To allow neurones to adhere, coverslips were kept for 3–4 h at 37°C in a humidified 5% incubator before being flooded with fresh growth medium. Cultures were used for patch-clamp experiments on the next day.
Isolectin B4 labelling
To label neurones with the isolectin B4 (IB4) of Griffonia simplicifolia, cultures were incubated with 10 μm IB4 conjugated to Alexa-488 (Molecular Probes) for 15 min and then rinsed three times with standard extracellular patch clamp buffer.
Patch-clamp recordings
Whole cell patch clamp recordings were made at room temperature (20–24°C). Patch pipettes were pulled (Flaming–Brown puller, Sutter Instruments, Novato, CA, USA) from borosilicate glass capillaries (Hilgenberg, Malsfeld, Germany), filled with a solution consisting of (mm): KCl, 110; NaCl, 10; MgCl, 1; EGTA, 1; Hepes, 10; adjusted to pH 7.3 with KOH; they had tip resistances of 6–8 MΩ. Unless otherwise stated, intracellular solution also contained 2 mm GTP and 2 mm ATP. The bathing solution contained (mm): NaCl, 140; KCl, 4; CaCl2, 2; MgCl2, 1; glucose, 4; Hepes, 10; adjusted to pH 7.4 with NaOH. Drugs were applied with a gravity driven multi-barrel perfusion system (WAS-02 custom made by Dr Dittert et al. 2006). All recordings were made using an EPC-10 amplifier (HEKA, Lambrecht, Germany) in combination with Patchmaster and Fitmaster software (HEKA). Pipette and membrane capacitance were compensated using the auto function of Patchmaster and series resistance was compensated by 70% to minimise voltage errors.
Mechanically activated currents were recorded as previously described (Hu & Lewin, 2006). Briefly, neurones were clamped to −60 mV, stimulated mechanically with a fire-polished glass pipette (tip diameter 2–3 μm) that was driven by a piezo based micromanipulator called Nanomotor® (MM3A, Kleindiek Nanotechnik, Reutlingen, Germany) and the evoked whole cell currents were recorded with a sampling frequency of 200 kHz. The stimulation probe was positioned at an angle of 45 deg to the surface of the dish and moved with a velocity of 3.5 μm ms−1. Currents were fitted with single exponential functions and classified as RA-, IA- and SA-type currents according to their inactivation time constant (Hu & Lewin, 2006; Wetzel et al. 2007). For classification of sensory neurones, action potentials were evoked by repetitive 80 ms current injections increasing from 40 pA to 800 pA in increments of 40 pA.
In vitro skin nerve recordings
The skin nerve preparation was used as previously described (Milenkovic et al. 2008). Adult C57BL/6N mice were killed by placing them in a CO2-filled chamber for 2–4 min followed by cervical dislocation. The saphenous-nerve and the shaved skin of the hind limb were dissected free and placed in an organ bath. The chamber was perfused with a synthetic interstitial fluid (SIF buffer) consisting of (in mm): NaCl, 123; KCl, 3.5; MgSO4, 0.7; NaH2PO4, 1.7; CaCl2, 2.0; sodium gluconate, 9.5; glucose, 5.5; sucrose, 7.5; Hepes, 10 at a pH of 7.4. The skin was placed with the corium side up in the organ bath and the nerve was placed in an adjacent chamber for fibre teasing and single-unit recording. Single units were isolated with a mechanical search stimulus applied with a glass rod and classified by conduction velocity, von Frey hair thresholds and adaptation properties to suprathreshold stimuli. A computer-controlled nanomotor (Kleindieck, Reutlingen, Germany) was used to apply controlled displacement stimuli of known amplitude and velocity. Standardised suprathreshold displacement stimuli of 2 s duration were applied to the receptive field at regular intervals (interstimulus period, 58 s) for 15 min. The probe was a stainless steel metal rod and the diameter of the flat circular contact area was 0.8 mm. UTP was added to a stainless steel ring which isolated receptive fields from the surrounding bath and prevented washout of the drug during the experiment. All experiments were carried out at a bath temperature of 32°C.The signal driving the movement of the nanomotor and raw electrophysiological data were collected with a Powerlab 4.0 system (ADInstruments) and spikes were discriminated off-line with the spike histogram extension of the software.
Results
UTP potentiates mechanically gated currents in a subpopulation of nociceptors
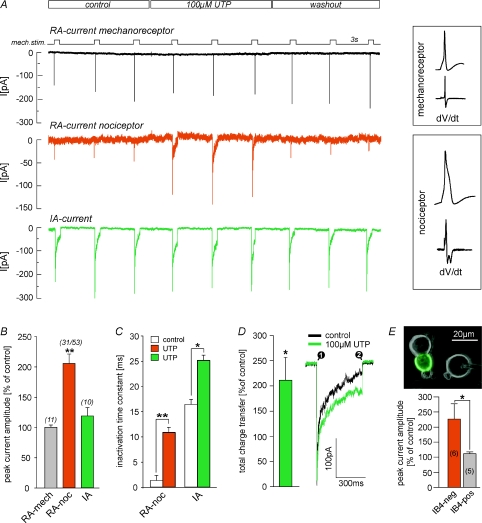
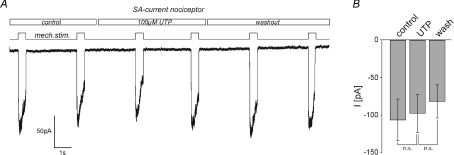
To test whether UTP augments the responses of DRG neurones to mechanical stimuli, whole-cell, voltage-clamp recordings were performed and mechanically gated currents were evoked by repetitive mechanical stimulation (duration 500 ms) in the presence and absence of 100 μm UTP (Fig. 1A). Three types of mechanically activated currents have been described in DRG neurones that can readily be distinguished by their inactivation time constants (Hu & Lewin, 2006; Wetzel et al. 2007): rapidly adapting (RA, inactivation times < 5 ms), intermediately adapting (IA, inactivation time 5–50 ms) and slowly adapting (SA, little or no inactivation, >50 ms). Cells were classified as mechanoreceptors or nociceptors based on their characteristic action potential configuration (Fig. 1A, inset) (Koerber et al. 1988). Only nociceptors have humped action potentials with the exception of low threshold C-fibres, which are rare in rodents (Traub & Mendell, 1988; Lewin & Mendell, 1994). Strikingly, UTP rapidly and reversibly potentiated RA current amplitude and slowed inactivation kinetics in 58% (n= 53) of the tested nociceptors (mean cell diameter 19.7 ± 1.4 μm; mean half-peak duration 2.84 ± 0.37 ms), but the RA current in large diameter low threshold mechanoreceptors was completely unaffected (mean cell diameter 27.3 ± 2.1 μm; mean half-peak duration 1.03 ± 0.21 ms) (Fig. 1A and B). Potentiation of peak current amplitudes amounted to 206 ± 16% (n= 31, Student's paired t test, P < 0.01, Fig. 1B) of control and inactivation time constants – derived from a single exponential fit – increased significantly from 1.3 ± 0.15 ms under control conditions to 10.8 ± 2.02 ms (n= 31, Student's paired t test, P < 0.01, Fig. 1C) in the presence of UTP. Thus the potentiation effect in terms of total charge transfer was much larger than 200% increase in the peak current amplitude. A small number of nociceptors possess an IA mechanosensitive current and we could also show that this current is modulated by UTP (Fig. 1A, C and D green trace). In contrast to the modulation of the RA current the peak amplitude of the IA current was not increased by UTP but the inactivation time constant was slowed from 16.4 ± 3.33 ms to 25.1 ± 5.4 ms (n= 9, Student's paired t test, P < 0.05, Fig. 1C), which resulted in an increase of the total charge transfer to 219 ± 43% of control (n= 9, P < 0.05, Fig. 1D). We next asked whether potentiation of RA currents is confined to a molecularly defined subset of nociceptive sensory neurones, as only 58% of all tested nociceptors with an RA current were sensitive to UTP. There are two major populations of cutaneous nociceptors in mice. One population expresses TrkA, the receptor for nerve growth factor (NGF), and calcitonin gene-related peptide (CGRP) and is thus often referred to as the population of peptidergic neurones. The other population, the non-peptidergic nociceptors, expresses the tyrosine kinase Ret, the receptor for glial-derived neurotrophic factor (GDNF) and is characterised by its ability to bind the isolectin B4 (IB4). Strikingly, potentiation of RA currents was significantly larger in IB4-negative neurones (226 ± 51% of control) than in neurones that were labelled by IB4 (111 ± 6% of control, n= 5–6, Mann–Whitney-test, P < 0.05, Fig. 1E). SA currents, which are present in around 30% of all nociceptors (Hu & Lewin, 2006; Lechner et al. 2009), were not affected by application of UTP (Fig. 2A and B).
Figure 1. UTP potentiates RA-type mechanically activated currents in a subpopulation of nociceptors.
A, example traces showing the effect of 100 μm UTP on RA and IA currents. Neurones were clamped to −60 mV and currents were evoked by mechanical stimuli (500 ms duration) applied at 3 s intervals (black trace; mech.stim.). UTP was present as indicated. Small neurones with wide action potentials that exhibited a hump or inflection on the falling phase (two minima in first derivative dV/dt) were classified as nociceptors and large cells with narrow uninflected APs were classified as mechanoreceptors (see inset). B, UTP induced potentiation of peak current amplitudes of different types of mechanically gated currents. Potentiation is expressed as a percentage of control, where control is the mean amplitude of currents evoked before (control) and after (washout) UTP was applied. Note, RA current amplitudes are significantly potentiated in nociceptors but not in mechanoreceptors. C, inactivation was fitted with a single exponential equation. Time constants of control currents are compared with those in the presence of UTP. Error bars represent s.e.m. (*P < 0.05; **P < 0.01, Students paired t test). D, the increase of the total charge transfer (measured between points 1 and 2, see inset) in the presence of UTP. E, cultures were labelled with IB4 to discriminate between peptidergic and non-peptidergic nociceptors. Note, only IB4-negative (trkA+/peptidergic) nociceptors are susceptible to potentiation by UTP (*P < 0.05, Mann–Whitney test).
Figure 2. SA currents are insensitive to modulation by UTP.
A, example traces showing the effect of 100 μm UTP on SA currents. Neurones were clamped to −60 mV and currents were evoked by mechanical stimuli (500 ms duration) applied at 3 s intervals (black trace). UTP was present as indicated. All cells with an SA current had action potentials characteristic of nociceptors (inflection in the falling phase). B, bars represent mean SA current amplitudes measured before (control), during (UTP) and after (wash) application of UTP. Note, SA currents in the presence of UTP did not differ from those recorded under control/wash conditions (n.s., P > 0.4, Student's paired t test, n= 8).
The effect of UTP is mediated by metabotropic P2Y2 receptors
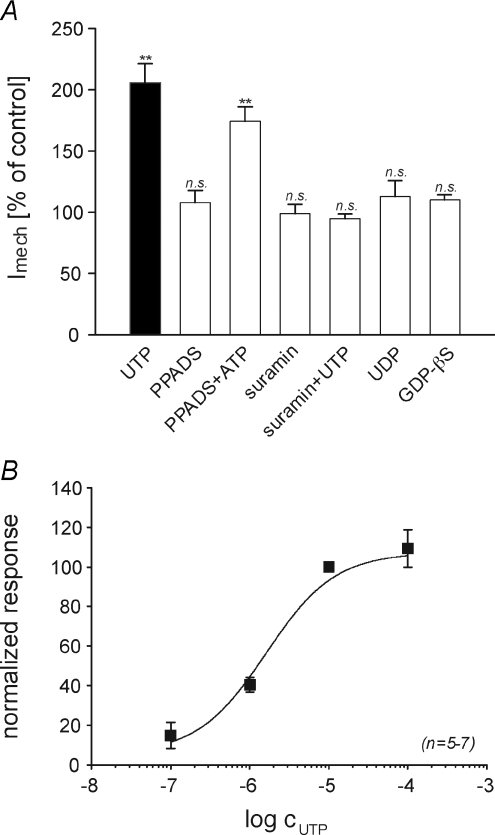
RA currents recorded in mechanoreceptors, which are pharmacologically indistinguishable from RA currents in nociceptors (Hu & Lewin, 2006), were not potentiated by UTP (Fig. 1B). This suggests that the effect of UTP is mediated by binding to a purine receptor which may not be present in mechanoreceptive neurones. Uridine nucleotides have very low affinities for P2X receptors (Khakh et al. 2001), and thus we first investigated the possible role of G-protein-coupled P2Y receptors. Replacement of intracellular GTP with the G-protein inhibitor guanosine-5′-O-(2-thio-diphosphate) (GDP-βS, 2 mm), completely abolished UTP induced potentiation of RA currents (110.2 ± 3.8%; n= 11; Fig. 2A), indicating the involvement of metabotropic P2Y receptors. UTP is a selective agonist for P2Y2 and P2Y4 receptors, which are both equally activated by ATP (von Kugelgen & Wetter, 2000; Suarez-Huerta et al. 2001). When applied alone, ATP elicited large inward currents in many of the neurones recorded (4/7, data not shown), most likely due to the activation of P2X receptors. In order to isolate the effect of ATP on RA currents, P2X currents were blocked with the non-selective P2X antagonist pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS, 25 μm). PPADS alone did not affect RA currents (107.9 ± 9.9% of control; n= 5, Student's paired t test, P > 0.05); however, ATP applied in the continuous presence of PPADS potentiated RA currents to 174.3 ± 11.8% of control (n= 7, Student's paired t test, P < 0.01). Since PPADS not only blocks P2X receptors but also antagonises P2Y4 mediated effects (von Kugelgen & Wetter, 2000; Suarez-Huerta et al. 2001) these results strongly suggest that the potentiation of RA currents is mediated by P2Y2 receptors. A P2Y2 mechanism is further supported by the fact that suramin (100 μm), an antagonist at P2Y1 and P2Y2 receptors, completely abolishes UTP induced potentiation of RA currents (98.8 ± 7.7%, n= 7; Fig. 3A). Moreover UDP, which is produced by enzymatic degradation of UTP by ectonucleotidases and preferentially activates P2Y6, did not mimic the effect of UTP. Finally, the concentration dependence of UTP induced potentiation (EC50= 1.6 μm; Fig. 3B) is comparable to published EC50 values for P2Y2 receptors (Lustig et al. 1993). Taken together, the pharmacology of the potentiation of RA currents by UTP and ATP is highly suggestive of a P2Y2 subtype.
Figure 3. Pharmacological characterisation of the receptor mediating potentiation of RA currents.
A, potentiation of RA currents by the indicated substances. For better comparability, data for UTP was re-plotted from Fig. 1. Drugs were applied and potentiation (% of control) was calculated as described in Fig. 1. When antagonists were used, PPADS+ATP and suramin+UTP, they were also present before and after application of ATP and UTP, respectively. GDP-βS was applied intracellularly via the patch electrode; recordings were started 5 min after membrane rupture to allow GDP-βS to sufficiently dialyse the cytosol. (**P < 0.01; n.s., P > 0.5). B, the concentration–response curve of UTP induced potentiation fitted with the Hill equation (EC50= 1.6 μm). Data points were normalised to the potentiation induced by 10 μm UTP in the very same neurone (error bars represent s.e.m.). Abbreviations: PPADS, pyridoxal-5′-phosphate-6-azophenyl-2′,4′-disulfonate; GDP-βS, guanosine-5′-O-(2-thio-diphosphate).
UTP reduces the mechanical threshold for action potential firing
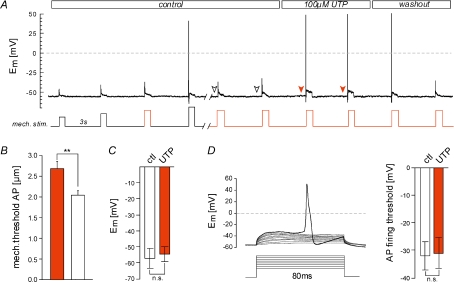
We next tested whether potentiation of RA currents by UTP was sufficient to significantly alter mechanical thresholds for action potential (AP) generation in nociceptors. Therefore, the mechanical threshold for AP generation was determined by applying increasing mechanical stimuli in increments of 350 nm (= 1 step) in current-clamp mode. The same cell was superfused with UTP and sub-threshold stimuli (threshold-1 step, -2 steps, etc.) were tested for their ability to evoke an AP (Fig. 4A). Strikingly, UTP significantly reduced mechanical thresholds by ∼25% from 2.68 ± 0.17 μm to 2.04 ± 0.11 μm (n= 6, P < 0.01, Student's paired t test; Fig. 4B). P2Y receptors are well known to modulate voltage-gated ion channels in many types of neurones (Lechner & Boehm, 2004), and thus we tested whether the effects of UTP could be explained by modulation of the electrical excitability of nociceptors. In rat DRG neurones, UTP was previously shown to produce a slow onset, slowly developing depolarisation of the membrane in a subpopulation of small diameter nociceptors (Molliver et al. 2002). However, during the short applications of UTP used here (≤10 s), we noted no significant change in the resting membrane potentials (measured directly before each mechanical stimulation, indicated by arrowheads, Fig. 4A and D). We also observed that during the time course of RA current sensitisation the magnitude of current injection required to evoke an AP was not changed in the presence of UTP (Fig. 4D).
Figure 4. UTP reduces mechanical thresholds for action potentials.
A, mechanical thresholds for action potentials under control conditions were determined by applying increasing mechanical stimuli (left side/before break). Thresholds in the presence of UTP were determined by applying series of decreasing subthreshold mechanical stimuli (threshold-1, -2 steps etc.; highlighted in red). UTP was present as indicated. B, comparison of mean mechanical AP thresholds determined as described in A, under control conditions (white bar) with those in the presence of UTP (n= 7, **P < 0.01, Students paired t test). C, bars show the mean resting membrane potentials measured before each mechanical stimulus under control conditions (white arrowheads, A) and in the presence of UTP (red arrowheads, A). D, inset shows the stimulation protocol used to determine electrical AP thresholds. Brief current injections of increasing amplitude (starting from 30 pA in increments of 20 pA) were applied. Mean membrane potentials measured 5–1 ms before the AP were considered as the AP threshold. Results are summarised in the bar graph.
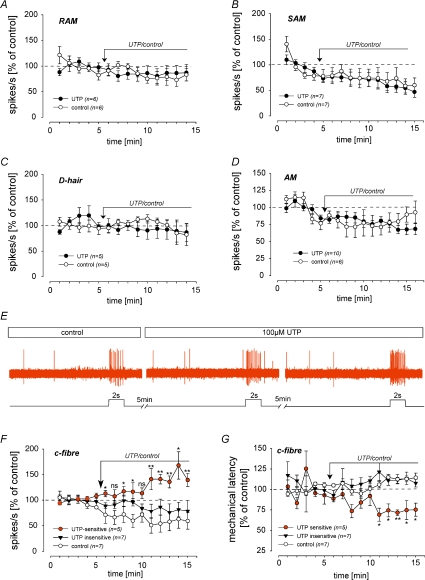
UTP sensitises a subpopulation of C-fibre nociceptors to mechanical stimuli
In order to determine whether sensitisation of RA currents by UTP in the cell soma is relevant to peripheral sensitisation we made teased fibre recordings from the saphenous nerve (Milenkovic et al. 2008). We identified single units as previously described and could classify them into low threshold mechanoreceptors and nociceptors with thinly myelinated Aδ-fibre or unmyelinated C-fibre axons (Wetzel et al. 2007; Milenkovic et al. 2008). After initial classification suprathreshold mechanical stimuli were applied at 1 min intervals, using a computer controlled mechanical stimulator, for a period of 15 min, and 100 μm UTP was applied locally to the receptive field for the last 10 min (Fig. 5). Repeated mechanical stimulation in the presence of Ringer solution has no effect on the response amplitude over time in any of the tested afferent types (Fig. 5A–D, F and G; white circles). The responses of low-threshold mechanoreceptors, including slowly adapting (SAMs), rapidly adapting (RAMs) and D-hair mechanoreceptors were not altered by the addition of 100 μm UTP (Fig. 5A–C). However, 41% (n= 5/12) of the tested C-fibre nociceptors exhibited significant sensitisation to mechanical stimuli after exposure to 100 μm UTP. Thus the mean firing rate in response to the standardised mechanical stimulus was significantly increased relative to the control period (spike response 5 min after UTP was 145±12%, compared to control stimuli 1–5; Mann–Whitney rank test, P < 0.05; Fig. 5E and F) and, more importantly, was also significantly higher than that measured in control experiments at the same time points (red and white circles in Fig. 5F). Aδ mechanonociceptors (AMs) were not significantly affected by the application of UTP (Fig. 5D). The spike rates during the mechanical stimuli were corrected for ongoing activity, as all fibres that exhibited sensitisation to mechanical stimuli also showed UTP-induced excitation (Stucky et al. 2004). Mechanical latency is the time from the onset of the mechanical stimulus until the first spike corrected for conduction delay and reflects displacement threshold for single fibres (Milenkovic et al. 2008). During the UTP application we observed a reduction in mechanical latency only in the population that displayed UTP-induced sensitization, while latencies in the UTP-insensitive population and in the buffer controls slightly increased during the course of the experiment (Fig. 5G). In the UTP-sensitive population, the mean mechanical latency during stimuli 11–15 was significantly shorter (73 ± 3% of control) than in the control population (112.0 ± 3.6% of control) (n= 5–7, Mann–Whitney test, P < 0.05, Fig. 5G).
Figure 5. UTP sensitises a subpopulation of cutaneous C-fibre nociceptors.
A–D and F, receptive fields of identified fibres were stimulated with a computer controlled mechanical stimulator for 2 s at 1 min intervals and UTP or buffer was applied after 5 min as indicated by the arrow. Mechanically induced activity was normalised to the mean spike rate obtained during the first three mechanical stimuli and is plotted as a function of time. Note, only a subpopulation of C-fibres (red circles, F) are sensitised in the presence of UTP (spike frequencies in the presence of UTP were compared with those of controls; ns, not significant; *P < 0.05; **P < 0.01; n= 5–7, Student's t test); in all other fibres mechanically induced activity decreased after repetitive stimulation both in controls and in UTP treated fibres. E, example trace of a UTP-sensitive C-fibre. G, mechanical latency (time from the onset of the mechanical stimulus until the first spike corrected for conduction delay) is plotted as a function of time. Note, in UTP-sensitive C-fibres (red circles) mechanical latencies (measured at stimuli 11–15) were reduced to ∼75% of control, which was significantly different from latencies in controls (white circles) and UTP-insensitive fibres (black triangles) measured at the same time points (n= 5–7, Mann–Whitney test, *P < 0.05, **P < 0.01).
Discussion
In the present study we show that activation of P2Y2 receptors by UTP potentiates mechanically activated RA currents in peptidergic nociceptive DRG neurones. We also provide evidence that the potentiation of the RA mechanosensitive current and not changes in membrane excitability reduces the mechanical threshold for action potential firing in these nociceptors. The sensitisation of the mechanosensitive current that probably underlies the generator potential in nociceptors may also underlie UTP-induced mechanical sensitisation of cutaneous C-fibre nociceptors. Together these findings suggest that P2Y2 mediated potentiation of mechanically activated RA-type currents contributes to peripheral sensitisation of cutaneous C-fibre nociceptors.
ATP is a potent algogen that together with other nucleotides is present at high concentrations in the extracellular environment of inflamed and injured tissue. Nucleotides are not only released from the cytosol of damaged cells during injury and from secretory cells such as activated platelets and sympathetic neurones, but also from non-excitatory cells including fibroblasts and keratinocytes (Cook & McCleskey, 2002; Lazarowski et al. 2003). Here we show that both ATP and UTP enhance mechanically gated RA-type currents in a major subpopulation of nociceptors, but not in low threshold mechanoreceptors (Fig. 1B). The rank order of agonist potencies (UTP = ATP ≫ UDP) for the potentiation effect and the observed sensitivity to antagonists (suramin prevents potentiation, PPADS is ineffective) is consistent with the pharmacological profile of the metabotropic P2Y2 nucleotide receptor (von Kugelgen & Wetter, 2000; Khakh et al. 2001). A role for P2Y2 receptors is also in good agreement with previous reports showing high levels of P2Y2 mRNA in almost 90% of all small diameter nociceptors but only in one-third of large diameter sensory neurones the identity of which is unknown (Molliver et al. 2002).
It has been shown in two reports that treatment of sensory neurones with NGF is also capable of potentiating mechanosensitive currents (Di Castro et al. 2006; Lechner et al. 2009). Cesare and colleagues showed that NGF and activators of protein kinase C (PKC) can increase the density of mechanosensitive currents in the membrane of isolated nociceptors (Di Castro et al. 2006). In our recent study we noted that overnight incubation with NGF dramatically increases the number of nociceptors with an intermediately adapting (IA) mechanosensitive current (Lechner et al. 2009). This effect was very reminiscent of the rapidly induced slowing of the inactivation kinetics of the RA mechanosensitive current that we observed here in nociceptors following UTP treatment (Fig. 1A). P2Y2 receptors are coupled to Gq-proteins and can thus in principal also activate PKC pathways (von Kugelgen & Wetter, 2000). Moreover, UTP induced potentiation of RA currents is also confined to putative NGF-responsive neurones (IB4-negative, Fig. 1E). However, our data suggest that the mechanism underlying potentiation by UTP differs fundamentally from that described for the pharmacological activation of PKC in two important aspects. First, UTP potentiates currents within seconds (≤3 s) while stimulation of PKC only enhanced mechanosensitive currents after more than 1 h (Di Castro et al. 2006). Moreover, in contrast to PKC mediated potentiation, which persisted for hours after removal of PKC activators, the effect of UTP was rapidly reversible (Fig. 1A). Thus it is likely that UTP activation of P2Y2 leads to a Gq-dependent potentiation of the RA current which does not require prior activation of PKC.
The present study is the first to show a G-protein-dependent potentiation of mechanically activated currents. Thus blockade of G-protein signalling with intracellular dialysis of GDP-γS completely prevented UTP mediated potentiation of RA mechanosensitive currents (Fig. 3A). The mechanism of ion channel modulation that underlies this effect must be rapid and reversible. However the molecular mechanism of G-protein modulation of mechanosensitive channels remains unclear and difficult to study directly so long as the identity of the mechanosensitive channel is not known. Nevertheless, the present demonstration of modulation adds a new and important feature to the physiological characteristics of this important mechanosensory channel. Classically algogens that exert their actions via G-protein-coupled receptors, such as prostanoids and bradykinin, can produce pain and sensitisation (Martin et al. 1987; Wang et al. 2006). In particular, prostaglandin E2 (PGE2) can sensitise C-fibres to mechanical stimuli when applied locally (Martin et al. 1987). However, the cellular mechanisms by which PGE2 may sensitise neurones to mechanical stimuli are poorly understood although it has been shown that stretch activated channels present in the membrane of sensory neurones can be sensitised by PGE2 (Cho et al. 2002).
We show here that the potentiation of RA currents is sufficient to sensitise isolated nociceptors to mechanical stimuli (Fig. 4). Thus the threshold displacement needed to evoke action potentials in nociceptors is lowered during UTP mediated potentiation of the mechanosensitive current. In isolated cells the increased action potential firing to mechanical stimuli does not seem to be accounted for by parallel changes in the membrane excitability initiated by UTP (Fig. 4D). In many cases acutely isolated sensory neurones are good models to study the physiological events that happen at the sensory ending in the skin in vivo. However, we also went on to show that the modulation of the mechanosensitive current in isolated cells is also reflected in modulation of the mechanosensory responses of nociceptors innervating the skin (Fig. 5). As in isolated sensory neurones, we found that the responses of low threshold mechanoreceptors to repeated mechanical stimuli were not modulated by local application of UTP. We also found that only a sub-population of the C-fibre nociceptors were sensitised to mechanical stimuli by application of UTP (Fig. 5). All putative nociceptive sensory neurones with an IA current and ∼60% of neurones with a RA current were subject to modulation by UTP in vitro. According to previous estimates of the incidence of RA and IA currents in isolated nociceptors (Hu & Lewin, 2006), we calculate that around 30% of C-fibre nociceptors may display modulation by UTP. The proportion of C-fibre nociceptors that in teased fibre recordings were sensitised to mechanical stimuli in the presence of UTP (∼40%) was in very good agreement with this calculation made from the in vitro results (Fig. 5F and G). The sensitisation that we observed in the skin developed relatively slowly compared to the potentiation of the mechanosensitive current observed in isolated cells. However, in the skin responses may occur with a marked delay caused by limited access to the receptors on sensory endings deep in the skin. We did note that in all the C-fibres in which mechanosensory responses were sensitised by UTP there was also a moderate UTP induced excitation as reflected by low rates (∼0.3 spikes s−1) of action potential firing in the absence of a stimulus (Stucky et al. 2004) (see Fig. 5E). The proportion of C-fibres showing UTP induced excitation (41%) is very similar to that previously reported (54%) by Stucky et al. (2004). Thus it is in principle possible that the enhanced mechanosensitivity of C-fibres can be partly attributed to changes in membrane excitability. However, Stucky et al. also showed that most UTP-sensitive C-fibres co-express ionotropic P2X receptors identified by responses to the selective P2X agonist α,β-methylene-ATP. Our in vitro results were in agreement with a P2X/P2Y2 co-expresion as we observed large inward transients in response to ATP in patch clamp recordings in ∼60% (4/7 tested neurones, data not shown) of UTP-sensitive neurones.
Inflammation and tissue injury are well known to produce mechanical hyperalgesia. The contribution of peripheral sensitisation to primary mechanical hyperalgesia in the skin has been a subject of some debate (Lewin & Moshourab, 2004), as there have been few direct demonstrations of mechanical sensitisation of cutaneous nociceptors following inflammation (Andrew & Greenspan, 1999; Milenkovic et al. 2008). We have shown that UTP sensitises a major subpopulation of cutaneous C-fibre nociceptors to mechanical stimuli. Our results strongly suggest that this sensitisation results primarily from the enhancement of RA and IA mechanosensitive currents in nociceptors by a novel G-protein-dependent mechanism.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinsschaft (DFG) within collaborative research centre 665 to GRL. We would like to thank Ewan St. J Smith and Kate Poole for helpful comments on the MS.
Glossary
Abbreviations
- DRG
dorsal root ganglion
- NGF
nerve growth factor
- GPCR
G-protein-coupled receptor
- IA
intermediately adapting
- RA
rapidly adapting
- SA
slowly adapting.
Author contributions
S.G.L. and G.R.L. designed and planned the experiments and wrote the manuscript. SGL acquired and analysed electrophysiological data.
References
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- Cho H, Shin J, Shin CY, Lee SY, Oh U. Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci. 2002;22:1238–1247. doi: 10.1523/JNEUROSCI.22-04-01238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Di Castro A, Drew LJ, Wood JN, Cesare P. Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc Natl Acad Sci U S A. 2006;103:4699–4704. doi: 10.1073/pnas.0508005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittert I, Benedikt J, Vyklicky L, Zimmermann K, Reeh PW, Vlachova V. Improved superfusion technique for rapid cooling or heating of cultured cells under patch-clamp conditions. J Neurosci Methods. 2006;151:178–185. doi: 10.1016/j.jneumeth.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Wood JN. FM1–43 is a permeant blocker of mechanosensitive ion channels in sensory neurons and inhibits behavioural responses to mechanical stimuli. Mol Pain. 2007;3:1. doi: 10.1186/1744-8069-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G834–838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Illes P. P2Y receptors and pain transmission. Purinergic Signal. 2004;1:3–10. doi: 10.1007/s11302-004-4740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgesia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol. 2006;577:815–828. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371–397. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lechner SG, Boehm S. Regulation of neuronal ion channels via P2Y receptors. Purinergic Signal. 2004;1:31–41. doi: 10.1007/s11302-004-4746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Frenzel H, Wang R, Lewin GR. Developmental waves of mechanosensitivity acquisition in sensory neuron subtypes during embryonic development. EMBO J. 2009;28:1479–1491. doi: 10.1038/emboj.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Mendell LM. Regulation of cutaneous C-fiber heat nociceptors by nerve growth factor in the developing rat. J Neurophysiol. 1994;71:941–949. doi: 10.1152/jn.1994.71.3.941. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Moshourab R. Mechanosensation and pain. J Neurobiol. 2004;61:30–44. doi: 10.1002/neu.20078. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y(2) Pain. 2008;138:484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HA, Basbaum AI, Kwiat GC, Goetzl EJ, Levine JD. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987;22:651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- Milenkovic N, Wetzel C, Moshourab R, Lewin GR. Speed and temperature dependences of mechanotransduction in afferent fibers recorded from the mouse saphenous nerve. J Neurophysiol. 2008;100:2771–2783. doi: 10.1152/jn.90799.2008. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Cook SP, Carlsten JA, Wright DE, McCleskey EW. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci. 2002;16:1850–1860. doi: 10.1046/j.1460-9568.2002.02253.x. [DOI] [PubMed] [Google Scholar]
- Schaible HG. Basic mechanisms of deep somatic pain. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. Elsevier; 2004. [Google Scholar]
- Stucky CL, Medler KA, Molliver DC. The P2Y agonist UTP activates cutaneous afferent fibers. Pain. 2004;109:36–44. doi: 10.1016/j.pain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Suarez-Huerta N, Pouillon V, Boeynaems J, Robaye B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur J Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Mendell LM. The spinal projection of individual identified Aδ- and C-fibers. J Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- Wang H, Ehnert C, Brenner GJ, Woolf CJ. Bradykinin and peripheral sensitization. Biol Chem. 2006;387:11–14. doi: 10.1515/BC.2006.003. [DOI] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, Erdmann B, Machelska H, Heppenstall PA, Lewin GR. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]