Abstract
The guinea pig bladder is innervated by at least five distinct major classes of extrinsic sensory neurons. In this study, we have examined the mechanisms of mechanotransduction and chemosensitivity of two classes of bladder afferents that have their endings in the vicinity of the urothelium: stretch-sensitive muscle-mucosal mechanoreceptors and stretch-insensitive, mucosal high-responding afferents. The non-selective P2 purinoreceptor antagonist pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid did not affect stretch- or stroking-induced firing of these afferents but significantly reduced the excitatory action of α,β-methylene ATP. Blocking synaptic transmission in Ca2+-free solution did not affect stretch-evoked firing but slightly reduced stretch-induced tension responses. Stroking-induced firing of both classes of afferents was also not affected in Ca2+-free solution. Of blockers of mechano-gated channels, benzamil (100 μm), but not amiloride (100 μm), Gd3+ (100 μm) or SKF 96365 (50 μm), inhibited stretch- and stroking-induced firing. Serotonin (100 μm) applied directly onto receptive fields predominantly activated muscle-mucosal afferents. Muscarine (100 μm) and substance P (100 μm) in 24% and 36% cases activated only mucosal high-responding units. Bradykinin (10 μm), but not prostaglandin E2 (10 μm), excites predominantly mucosal units. High (80 mm) K+ solution activated both afferent classes, but responses of mucosal units were 4 times greater. In contrast to muscle-mucosal units, most mucosal high-responding units were activated by hot Krebs solution (45–46°C), low pH (pH 4) and capsaicin (3 μm). TRPV1 antagonist, capsazepine (10 μm) was without effect on mechanotransduction by mucosal high-responding afferents. The results show that mechanotransduction of these two types of afferents are not dependant upon Ca2+-dependent exocytotic release of mediators, or ATP, and it is likely that benzamil-sensitive stretch-activated ion channels on their endings are involved in direct mechanotransduction. The chemosensitivity to agonists and noxious stimuli differs significantly between these two major classes of bladder afferents that reflects their different physiological and pathophysiological roles in the bladder.
Distension of the bladder activates extrinsic afferent neuronal pathways involved in micturition and continence and may cause conscious sensation such as fullness, the urge to micturate, discomfort and pain. Recent experiments in vitro have identified several distinct functional classes of bladder afferents (Rong et al. 2002; Daly et al. 2007; Zagorodnyuk et al. 2007; Xu & Gebhart, 2008b). However, the functional role of the various types of bladder afferents is still unknown. Furthermore, the mechanisms underlying the activation of mechanosensitive afferents are poorly understood. Two main types of mechanisms have been demonstrated to operate during mechanotransduction in afferent systems: an indirect, chemical transduction mechanism, which relies on activation of afferents by mediators being released from non-neuronal cells by mechanical stimulation, and direct, physical transduction, which is due to mechano-gated ion channels in the afferent endings, without involvement of extracellular mediators (Burnstock, 2001; Hamill & Martinac, 2001; Ernstrom & Chalfie, 2002). In the bladder, ATP has been proposed to be essential for the mechanotransduction of afferent activity (Namasivayam et al. 1999; Burnstock, 2001; Rong et al. 2002). Interestingly, both mechanosensitive release of ATP and mechano-gated channels are ubiquitous in eukaryotic cells (Hamill & McBride, 1996; Nakamura & Strittmatter, 1996; Bodin & Burnstock, 2001). On one hand, in the bladder, P2 purinoreceptors are expressed on mechanosensitive afferent endings and urothelial cells (Birder et al. 2004; Zagorodnyuk et al. 2007) and ATP release has been described from the bladder urothelium in response to mechanical stimuli including bladder distension (Ferguson et al. 1997; Vlaskovska et al. 2001; Birder et al. 2003). Purinoreceptor antagonists, such as suramin, pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) and 2′,3′-O-trinitrophenyl-ATP (TNP-ATP), can reduce mechanosensitivity of afferents innervating rat and mouse urinary bladder (Namasivayam et al. 1999; Rong et al. 2002). Both single (P2X2 or P2X3) or double (P2X2/P2X3) receptor knockout mice demonstrate bladder hyporeflexia and decreased pelvic afferent firing in response to bladder distension (Cockayne et al. 2000, 2005; Vlaskovska et al. 2001). On the other hand, mechano-gated channels belonging to ENaC/ASIC/degenerin sodium and/or TRP (TRPA1, TRPV4, TRPC1 and TRPV1) non-selective cationic ion channel families are likely to be involved in transduction of mechanical stimuli in many cell types including primary afferent neurons (Price et al. 2000; Hamill & Martinac, 2001; Ernstrom & Chalfie, 2002).
In the bladder, an important sensory role of the urothelium is well documented (Birder, 2005). In response to mechanical and chemical stimuli, urothelial cells are capable of releasing a number of transmitters and modulators such as ATP, nitric oxide (NO), prostaglandins (PGs), acetylcholine and substance P (SP) which may influence afferent nerve endings in the vicinity of the urothelium (Birder, 2005). It has been proposed that suburothelial myofibroblasts may act as an amplification stage in the sensory response of bladder wall to stretch (Fry et al. 2007). In addition, there are suggestions that receptors for acetylcholine, tachykinins and prostaglandins are present on bladder afferent endings, although these studies were primarily based on indirect in vivo experiments (Maggi, 1993; Morrison, 1999; Kim et al. 2005; Abrams et al. 2006; Cefalu et al. 2007; Kullmann et al. 2008b). Only few studies have investigated the presence of receptors directly on the bladder afferent nerve fibres. We have recently shown that low threshold stretch-sensitive vagal and spinal mechanoreceptors in mucosa-free preparations of the upper and lower gut use direct mechanisms of mechanotransduction via stretch-activated ion channels (Zagorodnyuk et al. 2003, 2005). In this study we aimed to compare two major classes of bladder mechanoreceptors, stretch-sensitive muscle-mucosal afferents and stretch-insensitive mucosal high-responding bladder afferents, both of which have their receptive fields in the vicinity of the urothelium (Zagorodnyuk et al. 2007), in terms of their mechanotransduction mechanisms and chemosensitivity. These data give us better understandings of the mechanisms of activation and modulation of bladder afferents that may lead to rational pharmacological treatment of hypersensitive bladder disorders such as overactive and painful bladder syndromes.
Preliminary data were presented at the International Society for Autonomic Neuroscience (ISAN) meeting in Marseilles (France) and subsequently published as a conference paper (Zagorodnyuk et al. 2006).
Methods
Extracellular recording
Adult male guinea pigs (N= 64 guinea pigs), weighing between 200–350 g, were killed by a blow to the occipital region and exsanguinated, in a manner approved by the Animal Welfare Committee of Flinders University. The method of extracellular recordings from bladder afferents in guinea-pigs has been described previously (Zagorodnyuk et al. 2007). Briefly, the bladder was removed and opened into a flat sheet and washed with Krebs solution (mm: NaCl, 118; KCl, 4.75; NaH2PO4, 1.0; NaHCO3, 25; MgCl2, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 95% O2–5% CO2). Full thickness, flat sheet preparations (∼15 mm × 15–20 mm which represent about two-thirds of the bladder) were superfused at 3 ml min−1, temperature 33–34°C and studied with the mucosa uppermost. ‘Close-to target’ extracellular recordings (Zagorodnyuk et al. 2007) were made from axons in fine nerve trunks (71 nerve trunks from 64 guinea pigs) entering the bladder trigone. The preparations were pinned down along one edge in a 5 ml organ bath while the other edge was attached to a ‘tissue stretcher’ (a microprocessor-controlled stepper motor) with in-series built isometric force transducer, DSC no.46-1001-01 (Kistler-Morse, Redmond, WA, USA) (Brookes et al. 1999; Zagorodnyuk et al. 2007). The slack was taken up to give a resting tension of 1–1.5 mN and at least 60 min of equilibration was allowed before experiments started. The preparations were stretched at 1 mm s−1 for distances of 1–4 mm and held for 10 s, at 3–4 min intervals. Mean firing rate of afferent units was calculated during 10 s stretches. Stretch-evoked increases in tension were calculated as integrated tension (area under the curve) in units of N s.
To identify two major classes of bladder mechanoreceptors, in particular stretch-sensitive muscle-mucosal afferents and stretch-insensitive mucosal high-responding mechanoreceptors (Zagorodnyuk et al. 2007), the following strategy was developed. First, the preparation was stretched by 1–4 mm and stretch-sensitive single units (if any) were identified. Then, the mucosa was stroked with light von Frey hairs (1 mN) to determine the receptive field of mucosal mechano-sensitive units. Receptive fields (‘hotspots’) identified in this way were marked with carbon particles on the von Frey hair. Stimulus–response curves for focal stroking were constructed for muscle-mucosal mechanoreceptors; five consecutive strokes (with 2–3 s interval) were applied with each von Frey hair and the three largest responses were averaged. Since full stroking (0.05–10 mN) response curves for mucosal high-responding mechanoreceptors were difficult to reproduce in preliminary experiments, we used a single stimulus of 1 mN with an interval of 15–20 min which gives rise to consistent responses (see Results).
All experiments were performed in the presence of nicardipine (3 μm) to minimize interference by smooth muscle contractions (Zagorodnyuk et al. 2007). Chemical stimuli including α,β-methylene ATP (α,β-me-ATP, 1 mm), serotonin (5-hydroxytryptamine, 5-HT, 100 μm), SP (100 μm), bradykinin (10 μm), prostaglandin E2 (PGE2; 10 μm), capsaicin (3 μm), high (80 mm) KCl, low pH (pH 4) solution (phosphate-buffered saline, PBS, with pH adjusted by HC1) or hot Krebs solution (45–46°C) were applied either by adding into a small chamber, which was sealed with silicone grease above marked hotspot area, or by direct application from a syringe onto the marked receptive field. To increase penetration of the applied drugs into the bladder tissue a small hole (0.2–0.3 mm i.d.) was cut into the urothelium at the corner of each receptive field within the sealed chamber (3 mm i.d.). In preliminary experiments this procedure did not change the mechanosensitive properties of recorded bladder afferents but increased excitatory effects of high (80 mm) K+ on bladder afferents. In most cases α,β-me-ATP- and low pH-evoked responses showed fast desensitization. To be able to compare their effects with other drugs, mean firing rate of afferent units was calculated during 5 s of maximum firing (mean maximum firing) evoked by these chemical stimuli.
Fine nerve fibres, originating from the pelvic ganglia, were dissected free and, together with a separate strand of connective tissue, were pulled into a second small chamber (∼1 ml volume) separated by a coverslip and silicone grease barrier (Ajax Chemicals, Australia). The small chamber was filled with paraffin oil and differential extracellular recordings were made via platinum electrodes. Signals were amplified (DAM 80, WPI, USA) and recorded at 20 kHz with a MacLab 8sp (ADInstruments, Sydney, NSW, Australia) attached to an Apple iMac G5 computer using Chart 5.4.2 software (ADInstruments). Single units were discriminated by amplitude and duration using Spike Histogram software (ADInstruments).
Drugs
Gadolinium, benzamil, α,β-me-ATP, SKF 96365, bradykinin, SP, PGE2, capsaicin, capsazepine and 5-HT were obtained from Sigma Chemical Co. (St Louis, MO, USA). Gd3+ was used in modified Krebs solution (mm: NaCl, 139; KCl, 4.75; Hepes, 5; MgCl2, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 100% O2). The effect of gadolinium on stretch- and stroking-induced firing was compared to control responses studied also in modified Krebs solution.
Data analysis
Results are expressed as means ±s.e.m. The use of ‘n’ numbers in the results section refers to the number of units and N to the number of animals. Statistical analysis was performed by Student's two-tailed t-test for paired or unpaired data or by repeated measures one-way or two-way analysis of variance (ANOVA) using Prism 4 software (GraphPad Software, Inc., San Diego, CA, USA). Differences were considered significant if P < 0.05.
Results
Muscle-mucosal afferents
Muscle-mucosal afferents in the bladder are stretch-sensitive afferents which also respond to light mucosal stroking with von Frey hairs, hypertonic stimuli and exogenous ATP applied directly to their receptive fields in the mucosa. These afferents have their receptive field endings close to the urothelium since its removal reduced stretch and stroking-sensitive responses (Zagorodnyuk et al. 2007). In the presence of nicardipine (3 μm) the majority of muscle-mucosal afferents (n= 31, N= 27) did not show any spontaneous firing in unstretched full thickness bladder preparations (under 1–1.5 mN basal tension), except three units which intermittently fired at 0.05–0.13 Hz. All muscle-mucosal units studied had low thresholds (1–2 mm) to circumferential distension.
It has been suggested that ATP, acting via P2X3 and P2X2/3 receptors, may be involved in mechanotransduction by spinal afferents in the mouse and rat urinary bladders (Namasivayam et al. 1999; Rong et al. 2002). However, in the guinea pig bladder we found that the non-selective P2 purinoreceptor antagonist, PPADS (30 μm), did not affect stretch (1–4 mm)-induced firing of muscle-mucosal afferents: 1.44 ± 0.28 Hz before and 1.43 ± 0.27 Hz 20 min after PPADS (30 μm) at 3 mm stretch (n= 6, N= 6, NS, 2 way ANOVA) or stretch-induced contractile responses: 0.11 ± 0.03 N s. before and 0.11 ± 0.03 N s 20 min after PPADS at 3 mm stretch (N= 6, NS, 2 way ANOVA, Bonferoni's post hoc test) (Fig. 1A and B). In addition, von Frey hair (1 mN) stroking-induced firing was also not affected by PPADS: 8.33 ± 1.9 spikes before and 8.28 ± 1.9 spikes 20 min after PPADS (30 μm) (n= 6, N= 6, NS, paired t-test) (Fig. 1D). In most cases responses of these afferents to α,β-me-ATP fully desensitized within 5 s and a second application to the receptive field after 10–15 min evoked no response, or the responses were significantly reduced. To avoid sensitization we have studied the effect of α,β-me-ATP only once on the same afferent fibre in the absence or in the presence of PPADS. Without PPADS, spritzing 1 mmα,β-me-ATP directly on the receptive field evoke mean firing of 12 ± 2.4 Hz (n= 9, N= 8). In the presence of PPADS (30 μm for 20 min), responses of muscle-mucosal afferents to spritzing of 1 mmα,β-me-ATP on their receptive field were abolished (n= 5, N= 3) or significantly reduced (n= 2, N= 2), with mean firing significantly reduced to 0.49 ± 0.31 Hz (n= 7, N= 5, P < 0.001, unpaired t test) compared with control (Fig. 1C). These data suggest that ATP is unlikely to be involved in mechanotransduction of these type of afferents in the normal condition.
Figure 1. Effects of α,β-me-ATP and the P2 purinoreceptor antagonist PPADS on firing of muscle-mucosal afferents.
PPADS (30 μm) did not affect either stretch-induced firing of bladder mechanoreceptors (n= 6, N= 6) (A) or stretch-induced responses (N= 6) (B). C, averaged data of the effects of P2X-preferring agonist α,β-me-ATP (100 μm) on muscle-mucosal afferents in control (n= 9, N= 8) or in the presence of PPADS (30 μm, n= 7, N= 5). *P < 0.001. D, averaged data of the effects of PPADS (30 μm, n= 6, N= 6) on firing of muscle-mucosal afferents evoked by stroking of their transduction sites with a von Frey hair (1 mN).
Mechanically induced release of ATP from urothelium is likely to be dependent upon exocytosis and Ca2+ dependent (Birder et al. 2003). We investigated the effect of blocking all Ca2+ dependent exocytosis on stretch- and stroking-induced responses by muscle-mucosal afferents using Ca2+-free and high Mg2+ Krebs solution. Ca2+-free solution (containing 1 mm EDTA and 6 mm Mg2+) did not affect stretch-evoked firing: 2.3 ± 0.62 Hz (n= 8, N= 6) at 3 mm stretch before and 3.0 ± 0.75 Hz 30 min after in Ca2+-free solution (n= 8, N= 6, NS, 2 way ANOVA) (Fig. 2C). Stretch-induced tension responses at 3 and 4 mm stretch were slightly reduced in Ca2+-free solution (Fig. 2D): 0.23 ± 0.07 N s before and 0.15 ± 0.03 N s 30 min after in Ca2+-free solution (for 3 mm stretch, N= 6, P < 0.05, 2 way ANOVA, Bonferoni's post hoc test) (Fig. 2D). Von Frey hair stroking (1 mN)-induced firing was not affected in Ca2+-free solution: 11.9 ± 1.4 spikes before and 11.9 ± 1 spikes 30 min after in Ca2+-free Krebs solution (n= 5, N= 5, NS, paired t-test) (Fig. 2E). These data indicate that Ca2+-dependent exocytotic release mechanisms are not required for mechanosensory transduction by muscle-mucosal afferents in the guinea-pig bladder.
Figure 2. Effects of Ca2+-free Krebs solution on firing of muscle-mucosal afferents.
Response of muscle-mucosal unit evoked by a 4 mm stretch in normal Krebs solution (A) was not significantly affected after 30 min of Ca2+-free (containing 1 mm EDTA, 6 mm Mg2+) Krebs solution (B). C and D, averaged data of the effects of Ca2+-free Krebs solution on stretch-induced firing (C, n= 8, N= 6) and stretch-induced contractions (D, N= 6). Note that Ca2+-free Krebs solution reduced the tension response slightly at 3 and 4 mm stretch. *P < 0.05. E, Ca2+-free Krebs solution failed to affect stroking (1 mN)-induced firing of muscle-mucosal afferents (n= 5, N= 5).
Stretch and swelling-induced ATP release in the bladder can be significantly reduced by the ENAC-sodium-channel blocker amiloride and by a blocker of stretch-activated channels, gadolinium (Birder et al. 2003). Amiloride (100 μm for 25 min) did not affect either stretch-induced firing or tension responses: −2 ± 3% (n= 5, N= 3, NS, 2 way ANOVA) and −6 ± 6% (N= 3, NS, 2 way ANOVA) for 4 mm stretch, respectively. Similarly von Frey hair (1 mN) stroking-induced firing of muscle-mucosal mechanoreceptors not affected by amiloride (+1 ± 0.5%, n= 5, N= 3, NS, paired t-test). A more potent analogue of amiloride, benzamil, is a well-established blocker of mechano-gated channels in many cells including vagal and spinal afferents (Hamill & McBride, 1996; Zagorodnyuk et al. 2003, 2005; Page et al. 2007). Benzamil dose-dependently inhibited stretch- and stroking-induced firing of muscle-mucosal mechanoreceptors in the guinea pig bladder (Fig. 3). It is noteworthy that benzamil (at a concentration of 100 μm) had a greater inhibitory effect on stretch- and stroke-induced firing elicited by higher intensity stimulation compared with less intense stimuli (Fig. 3). For example, 4 mm stretch-induced firing was inhibited by 76 ± 8% (n= 5, N= 3, P < 0.001, 2 way ANOVA, Bonferoni's post hoc test) while 2 mm stretch-induced firing was not significantly changed by benzamil (+37 ± 35%, n= 7, N= 5, NS, 2 way ANOVA Bonferoni's post hoc test). Thus the effects of benzamil were highly stretch dependent (interaction: F = 6.72, P < 0.0001, 2 way ANOVA, n= 7, N= 5). There was no significant reduction in the amplitude of stretch-induced action potentials by benzamil at 10–100 μm (for 20–25 min) and 300 μm (for 10–15 min) (NS, 1 way ANOVA, Bonferoni's post hoc test). After 20 min of application of benzamil (300 μm) all stretch-induced firing was abolished. Despite its inhibitory effect on firing, benzamil did not significantly alter stretch-induced intramural tension responses (N= 5, NS, 2 way ANOVA Bonferoni's post hoc test) (Fig. 3). Responses evoked by 10 mN von Frey hair stroking were significantly inhibited by benzamil (100 μm) by 61 ± 7% (n= 7, N= 5, P < 0.001, 2 way ANOVA, Bonferoni's post hoc test) while responses evoked by 0.1 mN von Frey hair stroking were not significantly affected (−18 ± 15%, n= 7, N= 5, NS, 2 way ANOVA, Bonferoni's post hoc test) (Fig. 3H).
Figure 3. Effects of benzamil on stretch-evoked firing and tension responses and stroking-induced firing of muscle-mucosal afferents.
Typical traces showing 2 and 4 mm stretch-induced firing of muscle-mucosal afferent in control (A and B) and after 20 min of application of benzamil (100 μm) (C and D). G, the shape of superimposed seven action potentials for muscle-mucosal big amplitude unit evoked by 2 mm stretch in control (left panel) and in the presence of benzamil (100 μm for 20 min) (right panel). Averaged data showing the effects of benzamil (10–300 μm) on stretch-induced firing (n= 5–7, N= 3–5) (E) and on stretch-induced tension responses (N= 5) (F). Note that inhibition of stretch-induced firing by benzamil (100 μm) was stretch dependent. *P < 0.001. H, averaged data of the effects of benzamil (10–300 μm) on firing evoked by stroking of receptive field with von Frey hairs (0.5–10 mN). *P < 0.001.
The blocker of stretch-activated channels, gadolinium (Gd3+, 100 μm for 20 min), did not affect significantly stretch-induced responses: 1 ± 0.16 Hz (n= 4, N= 3) before and 0.87 ± 0.46 Hz (n= 4, N= 3, NS, 2 way ANOVA, Bonferoni's post hoc test) 20 min after 100 μm Gd3+ for 3 mm stretch (Fig. 4A). Gd3+ did not affect significantly stretch-induced tension responses (measured as area under the curve): 0.16 ± 0.03 mN (N= 3) before and 0.19 ± 0.03 mN (N= 3, NS, 2 way ANOVA, Bonferoni's post hoc test) 20 min after 100 μm Gd3+ for 3 mm stretch. Similarly, Gd3+ did not change significantly von Frey hair (1 mN) stroking-induced firing of muscle-mucosal afferents: 8.1 ± 0.88 spikes (n= 5, N= 4) before and 8.6 ± 1.7 spikes (n= 5, N= 4, paired t-test) 20 min after 100 μm Gd3+ (Fig. 4C). Next, we tested the effects of the non-selective cationic channel blocker, SKF 96365. SKF 96365 (50 μm for 20 min) did not affect significantly stretch-induced firing of muscle-mucosal afferents: 2.3 ± 0.49 Hz (n= 4, N= 3) before and 2.2 ± 0.62 Hz (n= 4, N= 3, NS, 2 way ANOVA) 20 min after SKF 96365 (50 μm) for 3 mm stretch (Fig. 5A). Stretch-induced tension responses were also not significantly changed: 0.18 ± 0.06 N s before and 0.15 ± 0.05 N s (N= 3, NS, 2 way ANOVA, Bonferoni's post hoc test) 20 min after SKF 96365 (50 μm) at 3 mm stretch. Stroking-induced responses were not significantly affected as well 20 min after application of SKF 96365 (50 μm, n= 5, N= 4) (Fig. 5C, NS, 2 way ANOVA, Bonferoni's post hoc test).
Figure 4. Lack of the effects of Gd3+ on stretch-evoked firing and tension responses and stroking-induced firing of muscle-mucosal afferents.
Averaged data (4 units in 3 guinea pigs) of the effect of Gd3+ (100 μm) on stretch-induced firing (A) and on stretch-induced tension responses (B). C, averaged data (n= 5, N= 4) of the effect of Gd3+ (100 μm) on the firing of muscle-mucosal afferents evoked by stroking of their receptive field with a von Frey hair (1 mN).
Figure 5. Lack of the effects of SKF 96365 on stretch-evoked firing and tension responses and stroking-induced firing of muscle-mucosal afferents.
Averaged data (4 units in 3 guinea pigs) of the effect of SKF 96365 (50 μm) on stretch-induced firing (A) and on stretch-induced tension responses (B). C, averaged data of the effect of SKF 96365 (50 μm, n= 5, N= 4) on the firing of muscle-mucosal afferents evoked by stroking of their receptive field with von Frey hairs (0.5–10 mN).
The chemosensitivity of muscle-mucosal afferents were assessed by spritzing various agonists directly onto their receptive fields, or by placing agonists in a small chamber sealed over the receptive field. 5-HT (100 μm spritzed onto the receptive fields) in most cases activated muscle-mucosal units: they usually responded in a bursting type pattern with mean maximum firing of 3.8 ± 0.8 Hz, (n= 10, N= 8) (Fig. 8A). Only 3 out of 13 units (N= 3 out of 8) did not respond to application of 5-HT. The muscarinic agonist, muscarine (100 μm), in most cases (8 out of 12 units, 5 out of 7 preparations) did not activate muscle-mucosal units (n= 8, N= 5). In two preparations (n= 4), muscarine induced small contractions (∼2 mN) followed by transient firing (4.4 ± 1.1 Hz, n= 4, N= 2). It is worth mentioning that the tension threshold of activation of these particular units evoked by 1 mm stretch was in the same range (1.2–2 mN) as muscarine-induced contractions. The application of the tachykinin SP (100 μm) in 11 out of 12 units (N= 7) failed to activate muscle-mucosal afferents. Similarly, low pH solution (pH 4, n= 11, N= 7) and the autacoid PGE2 (10 μm, n= 9, N= 5) did not excite these units. Bradykinin (10 μm) in 3 out of 12 units (N= 2 out of 7 preparations) evoked activation of muscle-mucosal units with a mean maximum firing of 3.6 ± 1.3 Hz, (n= 3, N= 2), in 2 out of 12 units (N= 2) evoked small contractions (5–8 mN) followed by a few afferent spikes at 0.4–1.2 Hz. The remaining seven units (N= 4) did not respond to bradykinin application. High (80 mm) KCl in the majority of cases (7 out of 12 units, N= 6 out of 7) evoked small but long-lasting excitation of muscle-mucosal units with a mean maximum firing of 1.7 ± 0.4 Hz (n= 7, N= 6). As a tonicity control, 80 mm NaCl did not activate the majority of muscle-mucosal afferents (n= 4, N= 3). In one unit 80 mm NaCl evoked small transient contraction (3 mN) followed by a few afferent spikes at 0.6 Hz. All muscle-mucosal units studied were not activated by capsaicin (3 μm, n= 12, N= 8). Hot Krebs solution (45–46°C, n= 6, N= 4) spritzed over their receptive fields in 6 out of 11 units (N= 4 out of 7 preparations) did not activate these units. In 3 out of 11 units (N= 3) spritzing of hot Krebs (45–46°C), but not bath temperature Krebs, evoked small transient contractions (3–4 mN) followed by a few afferent spikes at mean maximum firing of 0.9 ± 0.3 Hz (n= 3, N= 3). In the remaining two units (N= 2), spritizing of hot Krebs solution evoked a small number of spikes at 0.8 Hz which were most likely evoked by the flow of spritzing Krebs solution since spritzing of bath temperature Krebs solution also evoked similar firing (0.4–1.2 Hz) on the same preparations.
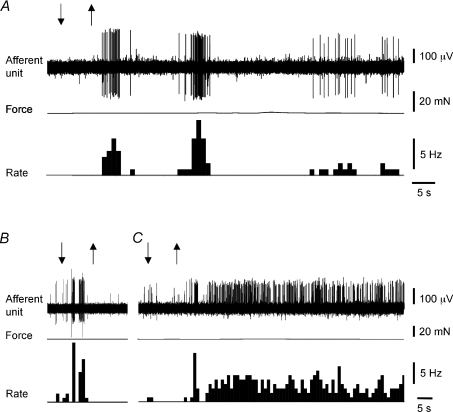
Figure 8. Chemosensitivity of bladder afferents.
Responses of muscle-mucosal afferent to spritzing of 5-HT (100 μm) onto receptive field (A). B, transient response of mucosal high-responding unit to low pH (pH 4) solution. C, sustained activation of mucosal high-responding unit by spritzing of bradykinin (10 μm) onto receptive field.
Mucosal high-responding mechanoreceptors
In a recent study (Zagorodnyuk et al. 2007) we characterized two distinct classes of mucosal afferents in the guinea-pig bladder that responded differential to mucosal stimulation. One class responded weakly to mucosal stimulation firing only a few action potentials, which we have termed ‘low responders’ and another class responded with an intense discharge of action potentials that we have termed ‘high responders’. In contrast to ‘low responders’, capsaicin, hypertonic solutions and ATP activated the majority of high-responding mucosal units. All of the mucosal high-responding afferents investigated in this study were spontaneously active in unstretched bladder preparations firing at an average of 1.1 ± 0.1 Hz (n= 33, N= 26). To activate mechanically mucosal high-responding mechanoreceptors, we used a single von Frey stroking of 1 mN, which evoked approximately 50% of the maximal response (Zagorodnyuk et al. 2007). In control experiments, six consecutive stimuli (at an interval of 15–20 min between strokes) with a 1 mN von Frey hair showed consistent and well-reproducible responses (n= 4, N= 4, NS, 1 way ANOVA) (Fig. 6B).
Figure 6. Responses of mucosal high-responding afferents to mucosal stroking, hot Krebs solution and capsaicin.
Typical tracing of the stroking-induced firing with a von Frey hair (1 mN, indicated by bar) (A) and averaged data (4 units in 4 guinea pigs) for 6 consecutive strokes (B). Note that the stroking-induced responses were not significantly different for 6 consecutive strokes with an interval of 15–20 min. Spritzing hot Krebs solution (46°C) (C), but not bath temperature Krebs solution (D), onto their receptive field evoked activation of mucosal high-responding unit. The same unit was activated by capsaicin (3 μm) spritzed onto their receptive field (E). Arrows show the onset and end of spritzing.
The P2 purinoreceptor antagonist PPADS did not affect stroking (1 mN)-induced firing of mucosal high-responding afferents: 28 ± 3 spikes (n= 3, N= 3) before and 31 ± 7 spikes (n= 3, N= 3, NS, paired t-test) 20 min after PPADS (30 μm) (Fig. 7A). Similarly, Ca2+-free Krebs solution (containing 1 mm EDTA and 6 mm Mg) did not affect stroking-induced responses: 40 ± 2 spikes (n= 3, N= 3) before and 43 ± 4 spikes (n= 3, N= 3, NS, paired t-test) 30 min after in Ca2+-free Krebs solution (Fig. 7B). The blocker of ENAC channels, amiloride (100 μm for 25–30 min), did not affect stroking (1 mN)-induced firing (−6 ± 1%, n= 4, N= 3, paired t-test). The blocker of mechano-gated channels, benzamil, at a concentration of 100 μm significantly reduced stroking-induced firing of mucosal high-responding mechanoreceptors by 31 ± 7% (n= 5, N= 5, P < 0.05, 1 way ANOVA, Bonferoni's post hoc test) (Fig. 7C). It is noteworthy that benzamil at 100 μm did not affect amplitude of stroking-induced action potentials (−2 ± 3%, n= 5, N= 5, NS, 1 way ANOVA, Dunnett's post hoc test).
Figure 7. Effects of PPADS, Ca2+-free Krebs solution and benzamil on stroking-induced firing of mucosal high-responding afferents.
PPADS (30 μm) did not affect firing evoked by stroking of receptive field with a von Frey hair (1 mN, n= 3, N= 3). B, Ca2+-free (1 mm EDTA, 6 mm Mg2+) Krebs solution (3 units, N= 3) failed to affect significantly stroking (1 mN)-induced firing. C, averaged data of the effects of benzamil (10–100 μm, n= 5, N= 5) on firing evoked by stroking of receptive field with a von Frey hair (1 mN). *P < 0.01.
The chemosensitivity of mucosal high-responding afferents was assessed in most cases by spritzing various agonists directly onto their receptive fields. In contrast to muscle-mucosal units, most mucosal units (19 out of 20, N= 15) were activated by spritzing of hot Krebs solution (45–46°C) on their receptive fields generating mean maximum firing of 9.5 ± 1.1 Hz (n= 19, N= 15) (Fig. 6C). As for control responses, spritzing of bath temperature Krebs solution evoked a slight transient activation in only 2 out of 19 units studied (N= 15). All temperature sensitive mucosal high-responding units were also activated by capsaicin (3 μm, n= 10, N= 7). Spritzing 3 μm capsaicin onto receptive fields induces 11.1 ± 1.3 Hz mean firing (n= 15, N= 11) (Fig. 6E). Bath application of the TRPV1 antagonist capsazepine (10 μm for 30 min) did not affect stroking-induced responses of these units (+8 ± 9%, n= 5, N= 3, NS, paired t-test). In contrast to muscle-mucosal units, 5-HT (100 μm) in most cases did not activate (9 out of 12 units, N= 8 out of 10) mucosal high-responding units. Only in two guinea pigs was it found that 5-HT (100 μm) evoked a response with mean maximum firing of 4.9 ± 1.9 Hz (n= 3, N= 2). Application of muscarine (100 μm) in most cases (13 out of 17 units, N= 9 out of 12) did not activate mucosal units. However in 4 out of 17 units (4 out of 12 preparations) muscarine evoked activation of mucosal afferents at a frequency of 4.8 ± 1.1 Hz (n= 4, N= 4). Similarly, application of SP (100 μm for 2 min into the well or spritzed directly on the receptive field) in the majority of cases (9 out of 14 units, N= 7 out of 10) failed to activate mucosal units. However, in 5 out of 14 units (4 out of 10 preparations), SP was found to slightly activate mucosal units at a frequency of 3.3 ± 0.7 Hz (n= 5, N= 4). Bradykinin (10 μm), but not PGE2 (10 μm, n= 7, N= 6), evoked sustained activation of all mucosal units studied inducing mean maximum firing of 6.9 ± 1 Hz (n= 11, N= 9) (Fig. 8C). Low pH (pH = 4) solution activated all units studied inducing a fast and transient firing of 5.3 ± 0.7 Hz (n= 8, N= 6) (Fig. 8B). High KCl (80 mm) in the majority of cases (11 out 13 units, 8 out of 10 preparations) evoked excitation of mucosal high-responding units (6.7 ± 0.7 Hz, n= 11, N= 8) while application of 80 mm NaCl (as a tonicity control) solution did not have any effects (n= 4, N= 3).
Discussion
The present study demonstrates that the mechanotransduction of muscle-mucosal and mucosal high-responding afferents in the guinea pig bladder in vitro does not require a rapid Ca2+ dependent process of exocytosis, or endogenous ATP. Rather, our data do suggest that in the guinea pig bladder benzamil-sensitive stretch-activated ion channels are involved in direct mechanotransduction by these classes of afferents.
Both stretch-sensitive muscle-mucosal and stretch-insensitive mucosal high-responding mechanoreceptors have their receptive fields in the vicinity of the urothelium (Zagorodnyuk et al. 2007), thus positioning them well to respond to the various physiological stimuli and pathophysiological changes originating from the suburothelium/urothelium area. Muscle-mucosal bladder afferents and muscle afferents represent two classes of low threshold stretch-sensitive afferents in the guinea pig bladder (Zagorodnyuk et al. 2007) whose function is to detect increasing volume and tension of the bladder during the bladder filling and micturition. Excitability of muscle-mucosal afferents can be also modulated by changes in the urine composition (for example, change in tonicity) probably via transmitters release from urothelial and/or other cells in the lamina propria. Activity of these afferents can be significantly changed in pathophysiological states when the lining of the urothelium is compromised. It has been demonstrated that in feline interstitial cystitis, intravesical high (150 mm) KCl was able to penetrate through the urothelium and causes a reduction, or complete block of activity of Aδ stretch-sensitive bladder afferents (Roppolo et al. 2005).
It is likely that mucosal high-responding afferents found in vitro in this and previous studies (Zagorodnyuk et al. 2006, 2007) correspond to so-called bladder chemoreceptors which can be excited in vivo by capsaicin and by distension with isotonic KCl, but not NaCl (Moss et al. 1997). These afferents are activated by chemical stimuli (capsaicin, hypertonic solution, α,β-methylene ATP) but also respond vigorously to gentle mucosal stroking indicating that they are actually polymodal afferents (Zagorodnyuk et al. 2007). In further support of this are the present findings indicating that these afferents are also activated by high temperature (45–46°C).
The hypothesis of purine-mediated mechanosensory transduction
It is well established that ATP is released from the urothelium by mechanical stimuli as well as from endothelial and epithelial cells of lungs and gut (Nakamura & Strittmatter, 1996; Bodin & Burnstock, 2001; Burnstock, 2001). Various subtypes of both P2X and P2Y purinoreceptors have been shown to be expressed on urothelial cells and bladder sensory neurons (Cockayne et al. 2000; Birder et al. 2004; Dang et al. 2005). Furthermore, exogenous ATP, or its analogues, activate several types of visceral afferents in the gut and bladder and sensitize their mechanosensory responses (Namasivayam et al. 1999; Vlaskovska et al. 2001; Rong et al. 2002; Wynn et al. 2003; Zagorodnyuk et al. 2003, 2007). Transgenic mice with single (P2X3 or P2X2) or double (P2X2/P2X3) mutations show reduced urinary bladder reflexes and decreased pelvic afferent firing in response to bladder distension using multiunit recordings (Cockayne et al. 2000, 2005; Vlaskovska et al. 2001). In addition, it has been recently suggested that suburothelial myofibroblasts expressing P2Y6 purinoreceptors may be involved in the response of the bladder wall to stretch, via ATP release from urothelial cells (Fry et al. 2007). Purinoreceptor antagonists, such as the non-selective P2X/P2Y purinoreceptor antagonists suramin and PPADS, and the P2X1, P2X3 and P2X2/3 receptor antagonist TNP-ATP reduce distension induced firing of pelvic afferents innervating the rat and mouse bladders in both multiunit and single unit recordings from high threshold afferents (Namasivayam et al. 1999; Vlaskovska et al. 2001; Rong et al. 2002). All these data indicate that ATP is the signalling molecule playing a role in the mechanosensory function of spinal afferents innervating the bladder. It is hypothesized that in response to bladder distension, ATP is released from urothelial cells and evokes firing of suburothelial afferent nerve endings via P2X2 or P2X2/3 receptors. Activation of these afferents then facilitates micturition and/or induces sensations of pain (Namasivayam et al. 1999; Burnstock, 2001; Chizh & Illes, 2001).
Lack of involvement of ATP in mechanosensory function of low threshold bladder mechanoreceptors
Despite strong evidence supporting the purine hypothesis of afferent responses to mechanical distension, a role of ATP in mechanosensory function of low threshold mechanoreceptors is less well established. For example, low threshold stretch-sensitive afferents in the guinea pig oesophagus and rectum do not require ATP for mechanotransduction (Zagorodnyuk et al. 2003, 2005) even though they can respond to exogenous ATP in the former case. In contrast to effects on high threshold afferents, distension-induced firing of single unit low threshold mechanoreceptors in mouse bladder was little affected by the purinergic antagonist TNP-ATP (Rong et al. 2002).
In the present study, the non-selective P2X and P2Y purinoreceptor antagonist PPADS effectively antagonized the excitatory effects of α,β-me-ATP, but did not affect mechanotransduction by either muscle-mucosal or mucosal high-responding low threshold mechanoreceptors in the guinea pig bladder. In support of this, it has recently been reported that TNP-ATP and PPADS do not affect multiunit firing evoked by the bladder distention in the rat (Yu & de Groat, 2008). This is contrasts with earlier studies in rats, in which suramin was used as a purinoreceptor antagonist (Namasivayam et al. 1999). It is possible that low threshold mechanoreceptors do not require ATP for mechanotransduction and that the modest mechanical stimuli needed to activate them do not release sufficient ATP to alter their excitability. In most studies of ATP release from urothelial cells, mechanical stimuli which evoked significant release of ATP were intense: 10–20% elongation of urothelial cells for 50–90 h (Sun et al. 2001), or strongly hypotonic conditions (Birder et al. 2003), or intravesical pressure of 30 cmH2O (Birder et al. 2002). Secondly, the concentrations of α,β-me-ATP used to activate of low threshold mechanoreceptors are relatively high: usually from 100 μm to 1 mm (Vlaskovska et al. 2001; Rong et al. 2002; Wynn et al. 2004; Zagorodnyuk et al. 2007). Thirdly, probably ATP plays a more prominent role in mechanosensory transduction during chronic inflammation. A significant increase in the mechanically induced release of ATP from urothelium has been reported in pathological conditions, such as interstitial cystitis (Sun et al. 2001; Kumar et al. 2007) or from epithelial cells in experimentally induced colitis (Wynn et al. 2004). In addition, expression and/or function of P2X3 purinoreceptors is augmented in spinal afferent neurons that innervate the bladder or colon during chronic inflammation by cyclophosphamide, acetic acid or trinitrobenzenesulfonic acid (TNBS) (Wynn et al. 2004; Dang et al. 2008; Xu et al. 2008a). Indeed, in cyclophosphamide-induced cystitis, but not in normal bladder, the increase in pelvic afferent firing evoked by small (10 cm H2O) isotonic distensions was PPADS and TNP-ATP sensitive (Yu & de Groat, 2008). Similarly, the modest inhibitory effects of these antagonists on colonic mechanoreceptors were significantly increased in experimental colitis (Wynn et al. 2004). Since both low threshold muscle-mucosal and mucosal high responding mechanoreceptors in the guinea pig bladder have excitatory P2 purinoreceptors on their endings (Zagorodnyuk et al. 2007), endogenous ATP may play an important role in setting their excitability during pathological conditions. However, it does not appear to play a significant role in their sensitivity to mechanical stimuli. We cannot rule out additional contribution by species or experimental condition differences to the discrepancies between the present studies and previous reports.
Role of exocytotic transmission in bladder afferents activation
Mechanically induced release of ATP from endothelium and urothelium is probably exocytotic and Ca2+ dependent (Bodin & Burnstock, 2001; Burnstock, 2001; Birder et al. 2003). In the present study, a Ca2+-free solution was found to have no significant effect on stretch- or stroking-induced firing in either type of afferents. Thus, mechanotransduction by two major classes of bladder afferents appears to be independent of any exocytotic release of neurotransmitter. These results are consistent with our previous findings obtained on vagal oesophageal and spinal rectal low threshold mechanoreceptors (Zagorodnyuk et al. 2003, 2005). These data further argue against ATP and other transmitters being required for mechanotransduction by low threshold mechanoreceptors in the bladder. Alternatively, release of transmitters, including ATP, from urothelium could be Ca2+ independent and therefore non-vesicular (Ferguson et al. 1997). However, in this study Ca2+ free conditions were not fully established since the authors did not use a Ca2+ chelator and ATP release was measured only 3 min after switching to Ca2+ free solution.
It is noteworthy that all experiments in this study were performed in the presence of nicardipine. This dihydropyridine is unlikely to affect the excitability of afferents endings or urothelial cells since previous studies reported no significant effects on stretch- or stroking-induced firing for the two classes of low threshold bladder afferents (Zagorodnyuk et al. 2006, 2007).
Mechano-gated channels responsible for mechanotransduction by muscle-mucosal and mucosal high-responding afferents
One of the likely candidates for mechano-gated channels of extrinsic primary afferent neurons in mammals belong to the ENaC/ASIC/degenerin sodium and/or TRP families of non-selective cationic ion channels (Price et al. 2000; Hamill & Martinac, 2001; Ernstrom & Chalfie, 2002; Clapham et al. 2003). In the present study, the stretch-activated channel blocker benzamil dose-dependently inhibited stretch- and stroking-induced firing of bladder muscle-mucosal mechanoreceptors. This effect was not due to its effects on bladder contractility since bladder wall tension responses (studied in the presence of nicardipine) were not significantly changed. The inhibitory effect of benzamil at 100 μm on stretch-induced firing was highly stretch dependent with proportionally greater inhibition seen with larger stretches. This is unlikely to be explained by a reduction in afferent fibre excitability as we detected no significant attenuation in the action potential amplitude. This stretch-dependent feature of benzamil inhibition is in agreement with its open channel blockade mechanism (Hamill & McBride, 1996; Hamill & Martinac, 2001) and was similar to previous findings obtained for low threshold mechanoreceptors in the guinea pig oesophagus (Zagorodnyuk et al. 2003). Inhibition of stretch- and stroking-induced firing at a higher concentration of benzamil (300 μm, after 20 min perfusion) is likely to be due to its non-specific inhibitory effects on voltage-dependent Na+ currents (Carr et al. 2001). Another known blocker of stretch-activated channels, Gd3+, did not affect stretch- or stroking-induced firing of bladder afferents, which is in agreement with previous observations for vagal and spinal mechanoreceptors innervating upper and lower gut (Zagorodnyuk et al. 2003, 2005). In contrast to rectal afferents (Zagorodnyuk et al. 2005), a non-selective blocker of cationic TRP channels, SKF 96365 (Inoue et al. 2001), did not affect both stretch- and stroking-induced firing of muscle-mucosal afferents in the bladder.
By using ASIC2−/− and ASIC3−/− knockout mice it has been concluded that these channels did not participate in mechanically activated currents in DRG neurons (Drew et al. 2004). However, when mechanosensitive endings of spinal afferents were studied in ASIC3−/− mutant mice, it has been reported that a decrease in mechanosensitivity occurs in muscular-mucosal (Jones et al. 2005) and serosal colonic afferents (Page et al. 2007). The inhibitory effect of benzamil (10–100 μm) on mechanosensitvity of serosal colonic afferent endings in mice was markedly diminished in ASIC2−/−and ASIC3−/− mice suggesting that benzamil is acting on ENaC/ASIC/degenerin sodium channels of nerve endings (Page et al. 2007). Recently, the same group has established a selective role for TRPV4 ion channels for mechanotransduction of serosal colonic afferents (Brierley et al. 2008). The ENaC/ASIC/degenerin channels are selectively inhibited by low micromolar concentrations of amiloride or submicromolar concentration of benzamil. In the bladder, amiloride at a concentration of 100 μm was ineffective in reducing mechanosensitivity for both classes of bladder mechanoreceptors. It is noteworthy that amiloride and benzamil at high concentrations can block both ENaC/ASIC/degenerin and TRP families of ionic channels (Hamill & McBride, 1996; Drummond et al. 2001; Inoue et al. 2001; Trebak et al. 2002), and therefore the exact nature of mechano-gated channels (ENaC/ASIC/degenerin or TRP, or both) involved in the mechanotransduction by bladder afferents still remains to be identified.
It has been recently suggested (Daly et al. 2007) that TRPV1 channels could be involved in the mechanosensitivity of low threshold stretch-sensitive mechanoreceptors in the mouse bladder since afferent responses to distension were attenuated in TRPV1 knockout mice. In addition, the TRPV1 antagonist capsazepine significantly attenuated distension-evoked discharge in wild-type mice. The present data indicate that TRPV1 channels are unlikely to be involved in mechanotransduction of stretch-insensitive, capsaicin-sensitive mucosal high-responding afferents in the guinea pig bladder since capsazepine did not have a significant effects on stroking-induced responses of these afferents. In contrast to mice, all low threshold stretch-sensitive bladder afferents including muscle-mucosal mechanoreceptors in guinea pigs do not respond to capsaicin (3–10 μm) (Zagorodnyuk et al. 2007; and present data). High temperatures (45–46°C) activated capsaicin-sensitive high-responding mucosal afferents. TRPV1 ion channels are most probably involved in the transduction of these noxious heat stimuli, since these TRP channel are activated by both capsaicin and high (≥43°C) temperatures (Schepers & Ringkamp, 2009). Interestingly, the same noxious temperatures did not activate muscle-mucosal afferents. This and lack of effects of capsaicin on muscle-mucosal afferents indicates that these afferents have no TRPV1 channels on their endings.
Chemosensitivity of bladder muscle-mucosal and mucosal high-responding afferents
Previous studies (Zagorodnyuk et al. 2007) have demonstrated that both muscle-mucosal and mucosal high-responding afferents were activated by the purinergic agonists α,β-me-ATP and ATP, and by hypertonic stimuli applied to their receptive fields in the mucosa. In the present study significant differences were noted in the chemosensitivity between these two classes of bladder afferents.
A number of previous studies have suggested the presence of muscarinic, tachykinergic, serotoninergic and other receptors on bladder afferents (Maggi, 1993; Kim et al. 2005; Abrams et al. 2006; Cefalu et al. 2007; Kullmann et al. 2008b). However, few studies have applied agonists and antagonists to the bladder from which afferent recordings were made (Morrison, 1999; Iijima et al. 2007; Ustinova et al. 2007). In the present study, most afferents did not respond to muscarine. In fact, in only 24% of recordings were mucosal stretch-insensitive high-responding afferents directly excited by muscarine, indicating the presence of muscarinic receptors on their terminal endings. M2 and M3 immunoreactivity was observed in some nerve bundles in human bladder although the sensory origin of these nerve fibres was not established (Mukerji et al. 2006). Stretch-sensitive muscle-mucosal afferents of the guinea pig bladder most likely do not have excitatory muscarinic receptors. The slight transient activation seen in two preparations was likely to have been due to a small contraction evoked by muscarine (in the presence of nicardipine) since the tension threshold for activation of these units evoked by stretch was in the same range as muscarine-induced contractions. It is unlikely that the lack of effect of muscarine was due to any penetration problems since other agonists such as 5-HT and bradykinin had excitatory effects when tested on the same bladder preparations. The data suggest that some of the previously thought effects of muscarinic agonists and antagonists on bladder afferents were rather due to their effects on contractile activity of the bladder. However, it has been recently reported that a selective M3 muscarinic receptor antagonist (darifenacin) reduces distension-evoked C and Aδ afferent firing in rat bladder without affecting voiding threshold or bladder compliance (Iijima et al. 2007). Similar conclusions were made when tolterodine, an anti-muscarinic, was administered intravesically in low doses and was found to significantly reduce bladder capacity in control but not in resiniferatoxin-treated rats (Yokoyama et al. 2005). It is possible, therefore, that species differences account for the apparent discrepancies. Alternatively, muscarinic antagonists could act indirectly on the excitability of stretch-sensitive afferent fibres. For example, it is well established that acetylcholine evoked release of ATP from the urothelium (Kullmann et al. 2008a), so apparent inhibitory effects of muscarinic antagonist could be due by reducing concentration of ATP, thus diminishing its excitatory modulatory effect on afferents. Another distinct difference in the chemosensitivity of the two major classes of bladder afferents was in their response to 5-HT, which can modulate afferent activity in many visceral organs (Grundy, 2008). 5-HT, applied directly to the receptive fields, predominately activates muscle-mucosal but not mucosal high-responding afferents in the guinea pig bladder. In contrast to 5-HT, the well-known inflammatory mediator bradykinin activated predominantly mucosal high-responding afferents but not muscle-mucosal afferents. It has been previously shown that responses of bladder afferents to intravesical bradykinin or SP are enhanced after intrarectal TNBS-induced inflammation (Ustinova et al. 2007). Both BK1 and BK2 receptor antagonists reduced bladder hyperactivity in experimentally induced cystitis (Chopra et al. 2005). More data are needed to establish whether expression of bradykinin receptors is increased on stretch-sensitive afferents in cystitis, or whether the algogenic effects of bradykinin are mainly due to its action on capsaicin-sensitive mucosal afferents. The autacoid PGE2 is another major pro-inflammatory agent that can activate reflex micturition by stimulation or sensitization of capsaicin-sensitive C-afferent fibres in the bladder (Maggi, 1993; Yoshimura et al. 2002). PGE2 can directly activate mesenteric afferents supplying rat jejunum (Haupt et al. 2000). In the present study, PGE2 did not directly activate bladder mechanoreceptors. Similar to the present findings, in the lower gut, PGE2, in contrast to bradykinin, was unable to activate directly low threshold rectal mechanoreceptors (Lynn et al. 2008). It remains to be established whether PGE2 is capable of sensitizing mechanically induced responses of bladder afferents. In contrast to muscle-mucosal afferents, activity of 36% of mucosal high-responding afferents was increased by SP application. These data are in agreement with previous studies which have shown that SP acting via NK2 receptors increased excitability of capsaicin-responsive DRG neurons and sensitized capsaicin-evoked current and mechanosensitive bladder afferents (Morrison, 1999; Sculptoreanu & de Groat, 2007; Sculptoreanu et al. 2008). Unlike muscle-mucosal afferents, most mucosal high-responding afferents were activated by capsaicin, high temperature and low pH stimuli. High (80 mm) K+ solution evoked 4 times stronger excitation of mucosal high-responding afferents compared with muscle-mucosal afferents. This excitatory effect of high K+ was not due to its hypertonicity since 80 mm NaCl failed to activate these afferents. Thus, the data indicate that various algogenic mediators and potentially harmful stimuli (such as bradykinin, SP, capsaicin, heat, low pH and high K+) mainly act on mucosal high-responding bladder afferents but not muscle-mucosal afferents. This strongly indicates that capsaicin-sensitive mucosal afferents are likely to be involved in the transmission of nociceptive information to the CNS. The present data support the idea that over-activation of capsaicin-sensitive afferents is responsible for many bladder diseases including overactive and painful bladder syndromes (Yoshimura et al. 2002; de Groat, 2006).
In conclusion, the results of the current study show that mechanotransduction by muscle-mucosal and mucosal high-responding mechanoreceptors in vitro is not dependent on Ca2+-dependent exocytotic release of mediators, or endogenous release of ATP. Rather we show that benzamil-sensitive stretch-activated ion channels are likely to be present on the mechanosensitive nerve endings and are involved in direct mechanotransduction by these two distinct classes of bladder mechanoreceptors. The data demonstrate a significant difference in the expression of different receptors for agonists and noxious agents in these two major afferent classes that are likely to reflect their different physiological and pathophysiological roles in the bladder.
Acknowledgments
This study was funded by the National Health and Medical Research Council of Australia grant no. 375123. V.P.Z. was supported by Bellberry Ltd (Australia) research award.
Glossary
Abbreviations
- α,β-me-ATP
α,β-methylene ATP
- PG
prostaglandin
- PGE2
prostaglandin E2
- PPADS
pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid
- SP
substance P
- TNP-ATP
2′,3′-O-trinitrophenyl-ATP
Author contributions
Experiments were designed by V.P.Z. and were performed by V.P.Z. and S.G. at Flinders University of South Australia. The paper was written by V.P.Z. S.J.B. and N.J.S. contributed to design and interpretation of data as well as to revising the article. All authors approve the final version.
References
- Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005;289:F489–495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Chen BN, Costa M, Humphreys CM. Initiation of peristalsis by circumferential stretch of flat sheets of guinea-pig ileum. J Physiol. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Gover TD, Weinreich D, Undem BJ. Inhibition of mechanical activation of guinea-pig airway afferent neurons by amiloride analogues. Br J Pharmacol. 2001;133:1255–1262. doi: 10.1038/sj.bjp.0704197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cefalu JS, Zhu QM, Eggers AC, Kaan TK, Ho MJ, Jett MF, Cockayne DA, Ford AP, Nunn PA. Effects of the selective prostacyclin receptor antagonist RO3244019 on the micturition reflex in rats. J Urol. 2007;178:2683–2688. doi: 10.1016/j.juro.2007.07.122. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Illes P. P2X receptors and nociception. Pharmacol Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- Chopra B, Barrick SR, Meyers S, Beckel JM, Zeidel ML, Ford AP, de Groat WC, Birder LA. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859–871. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Montell C, Schultz G, Julius D, International Union of Pharmacology International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev. 2003;55:591–596. doi: 10.1124/pr.55.4.6. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol. 2008;99:49–59. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147(Suppl. 2):S25–40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond HA, Welsh MJ, Abboud FM. ENaC subunits are molecular components of the arterial baroreceptor complex. Ann N Y Acad Sci. 2001;940:42–47. doi: 10.1111/j.1749-6632.2001.tb03665.x. [DOI] [PubMed] [Google Scholar]
- Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Annu Rev Genet. 2002;36:411–453. doi: 10.1146/annurev.genet.36.061802.101708. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CH, Sui GP, Kanai AJ, Wu C. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn. 2007;26:914–919. doi: 10.1002/nau.20483. [DOI] [PubMed] [Google Scholar]
- Grundy D. 5-HT system in the gut: roles in the regulation of visceral sensitivity and motor functions. Eur Rev Med Pharmacol Sci. 2008;12(Suppl. 1):63–67. [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- Haupt W, Jiang W, Kreis ME, Grundy D. Prostaglandin EP receptor subtypes have distinctive effects on jejunal afferent sensitivity in the rat. Gastroenterology. 2000;119:1580–1589. doi: 10.1053/gast.2000.20337. [DOI] [PubMed] [Google Scholar]
- Iijima K, De Wachter S, Wyndaele JJ. Effects of the M3 receptor selective muscarinic antagonist darifenacin on bladder afferent activity of the rat pelvic nerve. Eur Urol. 2007;52:842–847. doi: 10.1016/j.eururo.2007.02.057. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibres and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Yoshimura N, Masuda H, de Miguel F, Chancellor MB. Antimuscarinic agents exhibit local inhibitory effects on muscarinic receptors in bladder-afferent pathways. Urology. 2005;65:238–242. doi: 10.1016/j.urology.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Beckel J, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol. 2008a;294:F971–981. doi: 10.1152/ajprenal.00313.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008b;28:1977–1987. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Chapple CR, Surprenant AM, Chess-Williams R. Enhanced adenosine triphosphate release from the urothelium of patients with painful bladder syndrome: a possible pathophysiological explanation. J Urol. 2007;178:1533–1536. doi: 10.1016/j.juro.2007.05.116. [DOI] [PubMed] [Google Scholar]
- Lynn PA, Chen BN, Zagorodnyuk VP, Costa M, Brookes SJ. TNBS-induced inflammation modulates the function of one class of low-threshold rectal mechanoreceptors in the guinea pig. Am J Physiol Gastrointest Liver Physiol. 2008;295:G862–871. doi: 10.1152/ajpgi.00585.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA. The dual sensory and “efferent” function of the capsaicin-sensitive primary sensory neurons in the urinary bladder and urethra. In: Maggi CA, editor. Nervous Control of the Urogenital System. Switzerland: Harwood Academic; 1993. pp. 383–423. [Google Scholar]
- Morrison J. The activation of bladder wall afferent nerves. Exp Physiol. 1999;84:131–136. doi: 10.1111/j.1469-445x.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Moss NG, Harrington WW, Tucker MS. Pressure, volume, and chemosensitivity in afferent innervation of urinary bladder in rats. Am J Physiol Regul Integr Comp Physiol. 1997;272:R695–703. doi: 10.1152/ajpregu.1997.272.2.R695. [DOI] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Grogono J, Underwood J, Agarwal SK, Khullar V, Anand P. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol. 2006;176:367–373. doi: 10.1016/S0022-5347(06)00563-5. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci U S A. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namasivayam S, Eardley I, Morrison JF. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int. 1999;84:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Hughes PA, Blackshaw LA. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain. 2007;133:150–160. doi: 10.1016/j.pain.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo JR, Tai C, Booth AM, Buffington CA, de Groat WC, Birder LA. Bladder Aδ afferent nerve activity in normal cats and cats with feline interstitial cystitis. J Urol. 2005;173:1011–1015. doi: 10.1097/01.ju.0000145591.35569.9e. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neurosci Biobehav Rev. 2009;33:205–212. doi: 10.1016/j.neubiorev.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, Aura Kullmann F, de Groat WC. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur J Neurosci. 2008;27:3171–3181. doi: 10.1111/j.1460-9568.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC. Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp Neurol. 2007;205:92–100. doi: 10.1016/j.expneurol.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol. 2001;166:1951–1956. [PubMed] [Google Scholar]
- Trebak M, Bird GS, McKay RR, Putney JW., Jr Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem. 2002;277:21617–21623. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Gutkin DW, Pezzone MA. Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. Am J Physiol Renal Physiol. 2007;292:F123–130. doi: 10.1152/ajprenal.00162.2006. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–1409. doi: 10.1016/j.gastro.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008a;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008b;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama O, Yusup A, Miwa Y, Oyama N, Aoki Y, Akino H. Effects of tolterodine on an overactive bladder depend on suppression of C-fibre bladder afferent activity in rats. J Urol. 2005;174:2032–2036. doi: 10.1097/01.ju.0000176793.50410.9e. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002;59:61–67. doi: 10.1016/s0090-4295(01)01639-9. [DOI] [PubMed] [Google Scholar]
- Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294:F1146–1156. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Costa M, Brookes SJ. Major classes of sensory neurons to the urinary bladder. Auton Neurosci. 2006;126–127:390–397. doi: 10.1016/j.autneu.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Gibbins IL, Costa M, Brookes SJ, Gregory SJ. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol. 2007;585:147–163. doi: 10.1113/jphysiol.2007.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol. 2005;289:G397–406. doi: 10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]