Abstract
Animal studies have shown that the increased intravenous pressure stimulates the group III and IV muscle afferent fibres, and in turn induce cardiovascular responses. However, this pathway of autonomic regulation has not been examined in humans. The aim of this study was to examine the hypothesis that infusion of saline into the venous circulation of an arterially occluded vascular bed evokes sympathetic activation in healthy individuals. Blood pressure, heart rate, and muscle sympathetic nerve activity (MSNA) responses were assessed in 19 young healthy subjects during local infusion of 40 ml saline into a forearm vein in the circulatory arrested condition. From baseline (11.8 ± 1.2 bursts min−1), MSNA increased significantly during the saline infusion (22.5 ± 2.6 bursts min−1, P < 0.001). Blood pressure also increased significantly during the saline infusion. Three control trials were performed during separate visits. The results from the control trial show that the observed MSNA and blood pressure responses were not due to muscle ischaemia. The present data show that saline infusion into the venous circulation of an arterially occluded vascular bed induces sympathetic activation and an increase in blood pressure. We speculate that the infusion under such conditions stimulates the afferent endings near the vessels, and evokes the sympathetic activation.
Exercise evokes sympathetic nervous system activation as well as parasympathetic withdrawal, effects which result in increases in heart rate, cardiac output, peripheral vasoconstriction and blood pressure (Alam & Smirk, 1937; Mitchell & Wildenthal, 1974; Mark et al. 1985). Group III and IV receptors respond to mechanical deformation of their receptive fields during contraction (Kniffki et al. 1978; Kaufman et al. 1983), and to accumulation of metabolic by-products of contraction (Kaufman et al. 1983; Mense & Stahnke, 1983; Rotto & Kaufman, 1988; Sinoway et al. 1993). Thus, the group III and IV afferent fibres in muscles are suggested to be involved in the exercise pressor reflex (Coote & Pérez-González, 1970; Coote et al. 1971; McCloskey & Mitchell, 1972). Previous studies demonstrate that group III muscle afferents are mechanically sensitive, whereas unmyelinated group IV muscle afferents are predominantly sensitive to metabolites (Kaufman et al. 1983, 1984; Adreani et al. 1997; Adreani & Kaufman, 1998). However, a significant proportion of both types were capable of responding to both mechanical and metabolic stimuli (Kniffki et al. 1978; Kaufman et al. 1983; Mense & Stahnke, 1983; Kaufman et al. 1984).
Animal studies (Stacey, 1969; Von Düring & Andres, 1984; Andres et al. 1985; Von Düring & Andres, 1990) suggest that a large proportion of group III and IV fibres ended directly within the adventitia of the small venous vessels, and the arterioles (for group III). Some of the nerve fibre endings were found in close proximity to the smooth muscle cells of the blood vessels. Therefore, pressure changes and/or the deformation of the small vessels may directly stimulate these group III and IV afferents. In anaesthetized cats, Haouzi et al. (1999) showed that group III and group IV afferents from endings in the triceps surae muscle responded to vasodilatory agents and venous occlusion. Most of the group IV fibres that discharged in response to intermittent dynamic contractions of the triceps surae also responded to venous distension or to vasodilatory agents (Haouzi et al. 1999). There are no reports regarding the ability of peripheral vascular deformation to evoke systemic sympathetic efferent responses in humans. We speculated that the infusion of saline into the venous circulation of an arterially occluded vascular bed stimulates the muscle afferents near the vessels, which in turn evoke sympathetic efferent responses.
The procedure of intravenous regional anaesthesia can be used as a model to examine this hypothesis. In clinical practice, intravenous regional anaesthesia (Bier block) (Goodwin et al. 1984; Brill et al. 2004) is a simple and effective method providing regional anaesthesia during surgical procedures of the limbs (Goodwin et al. 1984) including treatment of fractures, management of tendon and muscle injuries, and lacerations (Brill et al. 2004). The advantages of a Bier block have made it an attractive method for local infusion of drugs in basic research (Lee et al. 2000; Cui et al. 2007). For example, we recently employed this method to examine the roles of prostaglandins in the exercise pressor reflex (Cui et al. 2007, 2008). Non-steroidal anti-inflammatory agents were infused into the exercising arm to inhibit the synthesis of prostaglandins. In the control trial, the same volume of physiological saline was infused with the same procedure.
In these previous studies (Cui et al. 2007; Cui et al. 2008), the drug solution or same volume of saline was infused into a forearm vein while the forearm circulation was occluded with an upper arm cuff. Previous studies showed that the saline infusion during Bier block significantly increased the venous pressure in the isolated limb (Mabee et al. 1997; Mabee & Orlinsky, 2000). Thus, we speculated that the infusion of saline retrograde into a large forearm vein during the Bier block procedure would stimulate the muscle afferents near the vessels, in turn evoking sympathetic and cardiovascular responses. We hypothesized that muscle sympathetic nerve activity (MSNA) and blood pressure would increase during the infusion of saline into the venous circulation of an arterially occluded vascular bed in healthy individuals. Some of the data presented were from subjects reported in previous studies (Cui et al. 2007; Cui et al. 2008). However, previous reports focused on the protocols performed before and after the Bier block. The MSNA and cardiovascular responses during the period of Bier block procedure were not analysed and therefore were not reported previously. Moreover, to verify the observations, three additional trials were performed.
Methods
Subjects
Nineteen subjects (12 male, 7 female) participated in the study. The mean age was 25 ± 2 years (s.d.m.) and all were of normal height (174 ± 8 cm) and weight (71 ± 9 kg). All subjects were normotensive (supine blood pressures <140/90 mmHg), in good health, and none were taking medication. Subjects refrained from caffeine, alcohol and exercise 24 h prior to the study. The experimental protocol was approved by the Institutional Review Board of the Milton S. Hershey Medical Center and conformed with the Declaration of Helsinki. Each subject had the purposes and risks of the protocol explained to them before written informed consent was obtained. Twelve of the 19 subjects were included in previously published reports (Cui et al. 2007, 2008).
Measurements
Systolic, diastolic and mean arterial pressures were obtained by an automated sphygmomanometer (Dinamap, Critikon, Tampa, FL, USA) from the non-treated arm. Blood pressure was also recorded on a beat-by-beat basis from a finger via a Finapres device (Finapres, Ohmeda, Madison, WI, USA) in 12 subjects. A standard electrocardiogram was used to monitor heart rate. Respiratory excursions were monitored with pneumography. Multifibre recordings of MSNA were obtained with a tungsten microelectrode inserted in the peroneal nerve of a leg. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The recording electrode was adjusted until a site was found in which muscle sympathetic bursts were clearly identified using previously established criteria (Vallbo et al. 1979). The nerve signal was amplified, a band-pass filtered with a bandwidth of 500–5000 Hz, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA, USA). The nerve signal was also routed to a loudspeaker and a computer for monitoring throughout the study.
Experimental design
All subjects were tested in the supine position. An intravenous catheter was inserted in the antecubital fossa of the non-dominant arm. After the instrumentation, 6 min baseline data of heart rate, blood pressure, MSNA, and respiratory excursion were collected in the resting condition. Subjects then performed static handgrip exercise until fatigue followed by forearm circulatory occlusion. This exercise protocol was imposed to address unrelated questions, which were presented in previous reports (Cui et al. 2007, 2008). At least 15 min after the exercise protocol was completed, all parameters returned to baselines. Thereafter, the Bier block procedure was performed. The arm was elevated and exsanguinated with a tight elastic wrapping from the wrist to the elbow (∼1–1.5 min). The pneumatic cuff on the upper arm was then inflated to 250 mmHg, and the wrap was removed. After the preparation (∼1–2 min), 40 ml saline was then infused into the occluded arm via the venous catheter (∼1.5–2 min). Circulatory occlusion was then maintained for 20 min before the cuff was deflated. After cuff deflation, subjects rested for 12–20 min until cardiovascular parameters returned to baseline. Another 6 min of data were then collected (as recovery).
A control trial was performed in 7 of the 19 subjects during a second visit. This control trial was to examine whether the observed MSNA and blood pressure responses were related to the infusion, the muscle compression by forearm wrapping, or the forearm circulatory arrest. The study set-up was the same as the first visit. After 6 min baseline data collection, the arm was elevated and exsanguinated. The pneumatic cuff on the upper arm was then inflated to 250 mmHg, and the wrap was removed. Before the infusion, 5 min of data were collected. This period was much longer than during the first visit (∼1–2 min). This period of circulatory occlusion was employed to verify whether the observed responses seen during the first visit were due to the effects of forearm wrapping and/or the effects of pre-infusion ischaemia (∼1–2 min) per se. Thereafter, 40 ml of saline was infused into the occluded arm via the venous catheter. Circulatory occlusion was then maintained for 10 min before the cuff was deflated. Another 6 min of data were then collected 12–20 min after the deflation of the cuff.
In three subjects (1 female and 2 males, age 25–29, height 168–175 cm, and body weight 57–89 kg), blood pressure and heart rate were recorded during infusion of 40 ml saline just after inflating the upper arm cuff to 250 mmHg. The exsanguination procedure was not performed in this control trial in order to determine if ischaemic forearm compression for ∼1–2 min was a necessary and sufficient condition for evoking the observed responses. In an additional three subjects, MSNA, blood pressure and heart rate were recorded during infusion of 40 ml saline under freely perfused condition. This control trial was performed to examine whether forearm occlusion was required in order to observe the sympathetic response.
Data analysis
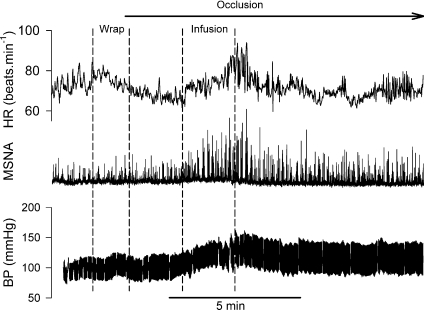
Data were sampled at 200 Hz via a data acquisition system (MacLab, ADInstruments, Castle Hill, NSW, Australia). MSNA bursts were first identified in real time by visual inspection, coupled with the burst sound from the audio amplifier. These bursts were further evaluated via computer software that identified bursts based upon fixed criteria, including an appropriate latency following the R-wave of the electrocardiogram (Cui et al. 2007). Integrated MSNA was normalized by assigning a value of 100 to the mean amplitude of the top 10% largest sympathetic bursts during the rest baseline period. Normalization of the MSNA signal was performed to reduce variability between subjects attributed to factors including needle placement, signal amplification, etc. (Halliwill, 2000). Total MSNA was identified from burst area of the integrated neurogram. Because the maximum MSNA and blood pressure responses occurred toward the end of the infusion (Fig. 1), the analysis was focused on the data around this period (the last minute of the infusion, and the first 30 s of post-infusion).
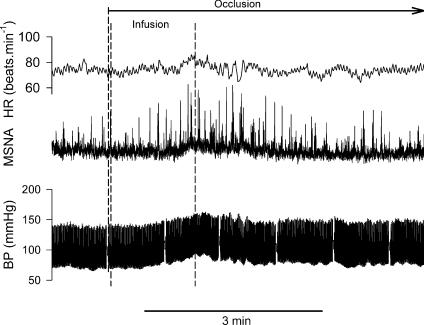
Figure 1. Representative tracing of heart rate (HR), muscle sympathetic nerve activity (MSNA) and arterial blood pressure (BP) obtained from a Finapres during Bier block procedure.
Wrap: the arm was elevated and wrapped with a tight elastic bandage from the wrist to the elbow. The pneumatic cuff on the upper arm was then inflated to 250 mmHg. Infusion: 40 ml saline was infused into the occluded arm via the venous catheter. Circulatory occlusion was then maintained for 20 min before the cuff was deflated.
Statistical analysis
Differences in the mean values of haemodynamic parameters during the first minute of wrapping, the last minute of infusion, the first 30 s of the post-infusion ischaemia, the fifth to sixth minute (2 min) of the post-infusion ischaemia, and recovery after cuff deflation from the baseline were evaluated via Tukey's post hoc analysis after repeated measures one-way ANOVA. SigmaStat software was used for statistical analysis (Systat Software Inc., San Jose, CA, USA). All values are reported as means ± standard error of the mean (s.e.m.). P values of <0.05 were considered statistically significant.
Results
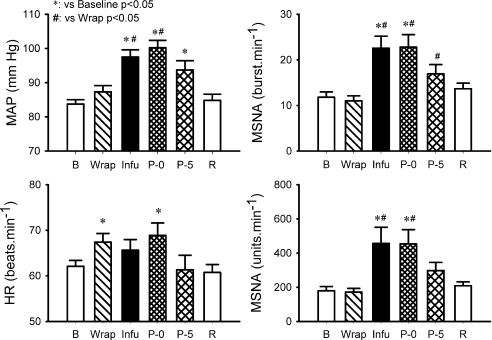
The baselines of the haemodynamic variables are shown in Table 1. Recordings of heart rate, integrated MSNA and blood pressure via Finapres during the Bier block procedure in a representative subject are shown in Fig. 1. The heart rate rose before the onset of forearm wrapping. Three seconds prior to forearm wrapping, the heart rate was significantly greater than the baseline value (67.8 ± 1.7 vs. 62.1 ± 1.3 beats min−1, P < 0.05). During the wrapping, heart rate was significantly greater than the baseline, while the blood pressure and MSNA were not significantly different from the baseline (Fig. 2). During the last minute of the infusion and the first 30 s of post-infusion, MSNA and blood pressure were significantly elevated from baseline (Fig. 2). Moreover, the MSNA and blood pressure were significantly greater than those during the wrapping. The heart rate during the first 30 s of the post-infusion was significantly greater than the baseline. Thereafter, MSNA, heart rate and blood pressure decreased (Fig. 1). During the fifth to sixth minute of post-infusion ischaemia, MSNA and heart rate were not significantly different from the baseline, although the blood pressure was still higher than the baseline (Fig. 2).
Table 1.
Haemodynamic baselines during the two visits
| Visit 1 | Visit 2 | |
|---|---|---|
| Systolic pressure (mmHg) | 125 ± 2 | 121 ± 3 |
| Diastolic pressure (mmHg) | 63 ± 1 | 64 ± 2 |
| Mean blood pressure (mmHg) | 84 ± 1 | 82 ± 3 |
| Heart rate (beats min−1) | 62 ± 1 | 64 ± 2 |
| MSNA (bursts min−1) | 11.8 ± 1.2 | 12.5 ± 2.6 |
| MSNA (units min−1) | 179 ± 25 | 172 ± 29 |
| Respiration (cycles min−1) | 17.4 ± 0.5 | 16. 7 ± 1.3 |
| Subject number | 19 | 7 |
Values are means ±s.e.m. Arterial blood pressures were measured by an automated sphygmomanometer from an upper arm. There is no significant difference in the measurements between the trials.
Figure 2. Mean arterial blood pressure (MAP), heart rate (HR) and MSNA measured as burst rate (right upper panel) and total activity (right lower panel) during the Bier block procedure.
B: baseline (6 min). Wrap: the first min of wrapping. Infu: the last min of the saline infusion. P-0: the first 30 s of the post-infusion. P-5: the 5th to 6th min from the end of the infusion (2 min). R: recovery (6 min). Subject number = 19. Data are means ±s.e.m.
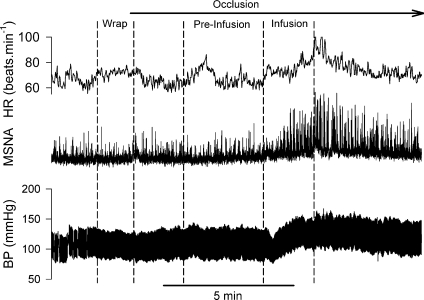
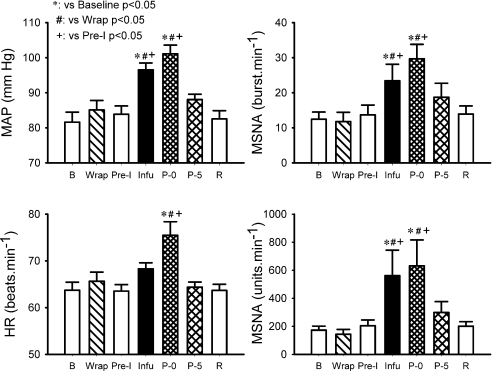
Recordings of heart rate, integrated MSNA and blood pressure in the second visit in the same subject for Fig. 1 are shown in Fig. 3. The wrapping did not induce significant changes in MSNA, blood pressure and heart rate (Fig. 4). From the upper arm occlusion, the 3 min pre-infusion period was at the approximate same time as when the infusion was performed in the first visit (see Figs 1 and 3). During this 3 min pre-infusion period, the MSNA, blood pressure and heart rate were not different from the baseline (Fig. 4). During the last minute of the infusion and the first 30 s of post-infusion, MSNA and blood pressure were significantly elevated from baseline (Fig. 4). Moreover, the MSNA and blood pressure were significantly greater than those during the 3 min of the pre-infusion period. The heart rate during the first 30 s of the post-infusion was significantly greater than the baseline.
Figure 3. Representative tracing of heart rate (HR), MSNA and arterial blood pressure (BP) obtained from the same subject as in Fig. 1 during the second visit.
Pre-Infusion: 3 min period just before the onset of the saline infusion. The interval between the wrap and the infusion during the second visit was much longer than during the first visit. From the onset of the occlusion, the Pre-Infusion period was at the approximate same time when the infusion was performed in the first visit. After the end of the infusion, circulatory occlusion was maintained for 10 min before the cuff was deflated.
Figure 4. Mean arterial blood pressure (MAP), heart rate (HR) and MSNA measured as burst rate (right upper panel) and total activity (right lower panel) during the Bier block procedure in the second visit.
Pre-I: pre-infusion, a 3 min period just before the onset of the saline infusion. Subject number = 7.
In the control studies without forearm exsanguination, MSNA and blood pressure clearly increased in all three of the subjects during the saline infusion, which was initiated just after inflating the upper arm cuff to 250 mmHg. The prior occlusion MSNA baseline was 15.6 ± 4.7 bursts min−1 and 288 ± 86 units min−1. The peak MSNA response during the last 30 s of the infusion and the first 30 s post-infusion (total 1 min) was 24.3 ± 5.4 bursts min−1 and 506 ± 125 units min−1. The mean blood pressure increased from 81.2 ± 3.6 mmHg (baseline) to 89.6 ± 3.1 mmHg (peak response). Representative recordings from one subject are shown in Fig. 5. Saline infusion under freely perfused condition did not induce changes in MSNA and haemodynamic variables.
Figure 5. Representative tracing of heart rate (HR), MSNA and arterial blood pressure (BP) obtained from another subject during the saline infusion under the circulatory occlusion condition.
The exsanguination procedure (i.e. wrapping forearm, etc.) was not performed before upper arm cuff inflation in this control trial.
Discussion
The main finding of the present study was that MSNA and blood pressure rose during the saline infusion into a forearm vein when the arterial circulation was occluded. This observation confirmed our hypothesis that infusion of saline into the local peripheral venous circulation of an arterially occluded vascular bed evokes systemic sympathetic activation and induces the increases in blood pressure in healthy humans.
A previous study (Williamson et al. 1994) showed that compression of leg muscles under circulatory occlusion condition increases blood pressure. The authors concluded that this intervention could stimulate the muscle mechanoreceptors. It should be emphasized that leg muscle compression evoked a pressor response only when circulatory occlusion was present. Removal of the circulatory occlusion abolished the pressor response even when leg compression was sustained (Williamson et al. 1994). Limb compression of occluded vessels would be expected to cause a much larger increase in tissue pressure than when the compression was performed without circulatory occlusion. Compression with occlusion may also cause greater ischaemia. However, direct pressure applied to the forearm musculature during ischaemia had no similar effect (McClain et al. 1994). In the present study, wrapping the arm from the wrist to the elbow did not increase the MSNA. The blood pressure was not significantly greater than the baseline. The difference in muscle mass (forearm vs. leg) can be one of the factors which cause the different response from the previous study (Williamson et al. 1994). Because the upper arm cuff was not inflated during the wrapping, the vessel pressure change evoked by wrapping in this fashion may be less than that noted during compression of an occluded vessel. The heart rate increased by ∼4 beats min−1 during the wrapping during the first visit. We speculate that mental stress contributed to this small increase in heart rate during forearm wrapping as this procedure requires two or three investigators to surround the subject's raised forearm. We suspect this served to ‘arouse’ the subjects. This speculation was supported by the fact that heart rate rose just before the wrapping was initialled on the first visit. Moreover, the heart rate did not rise during the second trial, suggesting extinction of the arousal response (Silber et al. 2000).
The MSNA and blood pressure increased greatly from the baseline during the last minute of the infusion. Moreover, the MSNA and blood pressure during the infusion were also greater than those during the wrapping period. We believe the observed rise in MSNA was not due to muscle ischaemia but to the saline infusion for the following reasons. (1) During the prolonged interval between wrapping and the infusion in the second visit, no significant MSNA and blood pressure response was observed. This suggests that muscle ischaemia of ∼1–2 min does not evoke significant increases in MSNA and blood pressure under the condition of the present study. (2) Similar MSNA and blood pressure responses were observed during the saline infusion during the second visit. (3) the MSNA and blood pressure increases were also observed during the infusion after only inflation of the upper arm cuff. Thus, these data demonstrate that the infusion of saline into a vein of an isolated limb induces increases in MSNA and blood pressure. Furthermore, we suspect that the elevated MSNA and blood pressure seen during the first 30 s after the infusion were due to the effects of the infusion. Importantly, no pain was reported by the volunteers after the saline infusion was completed.
Previous studies have shown that the saline infusion via the intravenous catheter during Bier block significantly increased the venous pressure in the isolated limb (Mabee & Orlinsky, 2000), although tissue pressure might not be increased (Mabee et al. 1997). Thus, the increase in peripheral venous pressure and/or peripheral vascular distension by the saline infusion into the occluded limb may stimulate sensors on or near the blood vessels. An early anatomical study (Stacey, 1969) demonstrated that group III and IV muscle afferents were predominantly seen in the vascular nerve trunks that formed anastomosing plexi closely associated with the branching arterioles and venules. Many group III and IV fibres are found in the adventitia of the blood vessels, including the arterioles and venules. Other studies (Von Düring & Andres, 1984; Andres et al. 1985; Von Düring & Andres, 1990) also showed that a large proportion of group III and IV fibres ended directly within the adventitia of the small venous vessels (for both groups), and the arterioles (for group III). Some of the nerve fibre endings were found in close proximity to the smooth muscle cells of the blood vessels. We suspect that the saline infusion stimulated these muscle afferents.
Animal studies have shown that distension of peripheral veins evokes activation of group III and IV afferents. For example, the local distension of the femoral-saphenous vein (large vein) by a relatively large range of venous pressures evokes activities in group III afferents in cats (Davenport & Thompson, 1987; Michaelis et al. 1994). For the small vessels in muscles, Haouzi et al. (1999) showed that groups III and IV afferents from endings in the triceps surae muscle responded to vasodilatory agents and venous occlusion. Most of the group IV fibres responding to intermittent dynamic triceps surae contractions also responded to venous distension or to vasodilatory agents (Haouzi et al. 1999). Using a similar approach in sheep, Haouzi et al. (1996) demonstrated that limb peripheral venous distension increased ventilation and arterial pressure. The present result supports the observations in animals. Therefore, the systemic sympathetic activation observed in the present study could be evoked by the stimulation of muscle afferents near the blood vessels by the infusion. In a recent study performed in humans by Green et al. (2007), the forearm was exposed to sub-atmospheric pressures without prior circulatory occlusion. The hypobaric pressure evoked by suction induced significant increases in the hydrostatic pressure in the forearm veins. In this prior report, the mean arterial pressure significantly increased when the hypobaric pressure was equal to and greater than 160 mmHg. Because the suction was applied on the surface without forearm circulatory occlusion, the physical changes evoked by this intervention (e.g. pressure gradient in the forearm) are likely to be different from those seen in the present report where a relatively small volume of saline is infused into a closed system.
Group III muscle afferents are mechanically sensitive, whereas unmyelinated group IV muscle afferents are predominantly sensitive to metabolites (Kaufman et al. 1983, 1984; Adreani et al. 1997; Adreani & Kaufman, 1998). However, a significant proportion of both types were capable of responding to both mechanical and metabolic stimuli (Kniffki et al. 1978; Kaufman et al. 1983, 1984; Mense & Stahnke, 1983). Although chemical stimulation cannot be excluded in the present study, we postulate that the mechanical distension was a necessary fact in the responses observed. This hypothesis is based on the following observations. First, the response was not observed during the infusion under the freely perfused condition. Under the freely perfused condition, the infusion would induce far less vascular deformation and less of a rise in forearm venous pressure. Second, the MSNA and blood pressure increased during the infusion of saline performed after forearm cuff inflation alone (i.e. no 2 min occlusion or forearm wrap). This result suggests that the muscle compression and the period of ischaemia were not necessary to evoke the reflex responses. Third, the maximal MSNA response occurred towards the end of the infusion. Thereafter, MSNA decreased gradually. A previous study (Mabee & Orlinsky, 2000) showed that the intravenous pressure during the Bier block procedure increased during the infusion. The pressure gradually decreased after the end of the infusion along with saline diffusing into tissues, although the pressure was still higher than the baseline during the later period of the Bier block. Fourth, the time courses of MSNA and blood pressure responses by the saline infusion were different from those by chemical stimulation during occlusion. The MSNA levels evoked by the chemical stimulation, as is seen during post exercise muscle ischaemia (Cui et al. 2008), generally remains elevated (and in some cases increases) as long as ischaemia is maintained. The above observations suggest that mechanical stimulation is a key factor in evoking the observed responses. On the other hand, previous studies (Rotto & Kaufman, 1988; Adreani & Kaufman, 1998; Cui et al. 2008) have suggested the responses seen with mechanical stimulation are influenced by the prevailing local metabolic conditions. Thus, the changed chemical milieu evoked by the pre-muscle compression and a period of ischaemia are likely to have influenced the magnitude of the responses seen in trial 1 and 2. Further studies are necessary to examine the roles played by various chemicals in evoking the observed response.
Heart rate increased toward the end of the infusion. After the infusion was completed, the heart rate decreased rapidly (see Figs 1 and 3). The significant heart rate elevation was observed during the first 30 s of the post infusion period. Thereafter, heart rate was not significantly different from baseline. Previous studies demonstrated that stimulation of the muscle mechanoreceptors can evoke increases in heart rate (Hollander & Bouman, 1975; Gelsema et al. 1985; Nobrega & Araujo, 1993; Gladwell & Coote, 2002; Fisher et al. 2005). Gladwell & Coote, (2002) reported that 1 min of sustained passive triceps surae stretch increased heart rate. These authors concluded that stimulation of mechanoreceptors in muscle inhibits cardiac vagal activity and increases heart rate. Passive rhythmic movement of leg muscles rapidly increases the heart rate (Nobrega & Araujo, 1993). Our previous study also showed that dynamic passive stretch evokes a transient increase in heart rate (Cui et al. 2006). Thus, the heart responses seen at the end of the infusion could be evoked by the stimulation of mechano-sensitive afferents on and/or near the blood vessels. It should be noted that stimulation of metaboreceptors during the post exercise muscle ischaemia does not alter heart rate (Mark et al. 1985).
It is well known that muscle blood flow increases with exercise (Delp & Laughlin, 1998; Saltin et al. 1998). Moreover, dynamic exercise induces venous opening and refilling (muscle pump) (Laughlin, 1987). Skeletal muscle venules dilate during the hyperaemia of exercise (Koller et al. 1998). Therefore, the sensory afferent fibres on and/or near the blood vessels can be stimulated by the deformation of the vessels during exercise. The present study suggests that these sensory afferent fibres can be stimulated by infusing saline into the venous circulation of an arterially occluded forearm. We speculate that the volume infusion into a vein in an isolated limb can be used as a model to study the role these afferents play during physiological interventions that evoke venous distension such as during exercise.
Study limitations
In the present study, the saline infusion was performed manually. The period for the infusion varied somewhat in the different subjects (∼1.5–2 min). This makes it difficult to summarize the mean response curves during the whole infusion period. Because the maximum responses occurred toward the end of the infusion, data during the last minute of infusion were analysed. In the present study, only one volume of the saline was infused. In a previous study (Mabee & Orlinsky, 2000) a total of 60 ml of saline was infused into an exsanguinated arm at 2.5 ml/5 s. After 40 ml saline was infused, the peak increase in the venous pressure was reached in both male and female subjects. Thus, the volume of the saline in the present study (40 ml) was reasonable. The question about whether the magnitude of the responses observed is volume and/or rate dependent will require further study.
In conclusion, saline infusion into the venous circulation of an arterially occluded vascular bed induces sympathetic activation and an increase in blood pressure. We speculate that the infusion under such conditions stimulates the muscle afferent endings near the vessels, and evokes the sympathetic activation.
Acknowledgments
We are pleased to acknowledge the nursing and technical assistance of Cheryl Blaha and Natalia Gonzalez. We are grateful to Jennifer Stoner for secretarial help in preparing this manuscript. This work was supported by National Institutes of Health Grant P01 HL077670 (Sinoway), NIH/NCRR grant M01 RR010732 (GCRC Grant), Tobacco Settlement Funds and the American Heart Association Grant 0635245 N (Cui).
Author contributions
C.P. contributed to data collection, analysis and drafting of the manuscript. All of the other authors contributed to the conception, design and interpretation of data, data collection and analysis, drafting and revision of manuscript critically for important intellectual content and final approval of the version to be published.
References
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–1817. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol. 1998;84:1827–1833. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres KH, von During M, Schmidt RF. Sensory innervation of the Achilles tendon by group III and IV afferent fibres. Anat Embryol. 1985;172:145–156. doi: 10.1007/BF00319597. [DOI] [PubMed] [Google Scholar]
- Brill S, Middleton W, Brill G, Fisher A. Bier's block: 100 years old and still going strong! Acta Anaesthesiol Scand. 2004;48:117–122. doi: 10.1111/j.1399-6576.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Pérez-González JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol. 1970;208:261–278. doi: 10.1113/jphysiol.1970.sp009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Blaha C, Moradkhan M, Gray K, Sinoway L. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol. 2006;576:625–634. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Mascarenhas V, Moradkhan R, Blaha C, Sinoway LI. Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R458–R466. doi: 10.1152/ajpregu.00475.2007. [DOI] [PubMed] [Google Scholar]
- Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman CS, Krishnan A, Sinoway LI. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol. 2007;293:H1861–H1868. doi: 10.1152/ajpheart.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Moradkhan R, Mascarenhas V, Momen A, Sinoway L. Cyclooxygenase inhibition attenuates sympathetic responses to muscle stretch in humans. Am J Physiol Heart Circ Physiol. 2008;294:H2693–H2700. doi: 10.1152/ajpheart.91505.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport PW, Thompson FJ. Mechanosensitive afferents of femoral-saphenous vein. Am J Physiol Regul Integr Comp Physiol. 1987;252:R367–R370. doi: 10.1152/ajpregu.1987.252.2.R367. [DOI] [PubMed] [Google Scholar]
- Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand. 1998;162:411–419. doi: 10.1046/j.1365-201X.1998.0324e.x. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Bell MP, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol. 2005;90:773–781. doi: 10.1113/expphysiol.2005.030577. [DOI] [PubMed] [Google Scholar]
- Gelsema AJ, Bouman LN, Karemaker JM. Short-latency tachycardia evoked by stimulation of muscle and cutaneous afferents. Am J Physiol Regul Integr Comp Physiol. 1985;248:R426–R433. doi: 10.1152/ajpregu.1985.248.4.R426. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol. 2002;540:1095–1102. doi: 10.1113/jphysiol.2001.013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A, Rubenstein N, Otremski I. Intravenous anaesthesia in the upper limb: a review of 225 cases. Int Orthop. 1984;8:51–54. doi: 10.1007/BF00267740. [DOI] [PubMed] [Google Scholar]
- Green ND, Brown MD, Coote JH. Pain and changes in peripheral resistance at high vascular transmural pressure in the human forearm. Eur J Appl Physiol. 2007;100:627–635. doi: 10.1007/s00421-007-0466-z. [DOI] [PubMed] [Google Scholar]
- Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Hill JM, Lewis BK, Kaufman MP. Responses of group III and IV muscle afferents to distension of the peripheral vascular bed. J Appl Physiol. 1999;87:545–553. doi: 10.1152/jappl.1999.87.2.545. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Hirsh JJ, Gille JP, Marchal F, Crance JP, Huszczuk A. Papaverine injection into the hindlimb circulation stimulates ventilation in sheep. Respir Physiol. 1996;105:143–153. doi: 10.1016/0034-5687(96)00012-6. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol. 1975;38:272–278. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of group III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol. 1984;57:644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978;31:511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Koller A, Dornyei G, Kaley G. Flow-induced responses in skeletal muscle venules: modulation by nitric oxide and prostaglandins. Am J Physiol Heart Circ Physiol. 1998;275:H831–H836. doi: 10.1152/ajpheart.1998.275.3.H831. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol. 1987;253:H993–H1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- Lee F, Shoemaker JK, McQuillan PM, Kunselman AR, Smith MB, Yang QX, Smith H, Gray K, Sinoway LI. Effects of forearm Bier block with bretylium on the hemodynamic and metabolic responses to handgrip. Am J Physiol Heart Circ Physiol. 2000;279:H586–H593. doi: 10.1152/ajpheart.2000.279.2.H586. [DOI] [PubMed] [Google Scholar]
- Mabee J, Orlinsky M. Bier block exsanguination: a volumetric comparison and venous pressure study. Acad Emerg Med. 2000;7:105–113. doi: 10.1111/j.1553-2712.2000.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Mabee JR, Shean C, Orlinsky M, Androy L, Carter V. The effects of simulated Bier block IVRA on intracompartmental tissue pressure. Acta Anaesthesiol Scand. 1997;41:208–213. doi: 10.1111/j.1399-6576.1997.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- McClain J, Hardy JC, Sinoway LI. Forearm compression during exercise increases sympathetic nerve traffic. J Appl Physiol. 1994;77:2612–2617. doi: 10.1152/jappl.1994.77.6.2612. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Goder R, Habler HJ, Janig W. Properties of afferent nerve fibres supplying the saphenous vein in the cat. J Physiol. 1994;474:233–243. doi: 10.1113/jphysiol.1994.sp020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Wildenthal K. Static (isometric) exercise and the heart: Physiological and clinical considerations. Annu Rev Med. 1974;25:369–381. doi: 10.1146/annurev.me.25.020174.002101. [DOI] [PubMed] [Google Scholar]
- Nobrega ACL, Araujo CGS. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc. 1993;25:37–41. [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Silber DH, Sinoway LI, Leuenberger UA, Amassian VE. Magnetic stimulation of the human motor cortex evokes skin sympathetic nerve activity. J Appl Physiol. 2000;88:126–134. doi: 10.1152/jappl.2000.88.1.126. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Stacey MJ. Free nerve endings in skeletal muscle of the cat. J Anat. 1969;105:231–254. [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth K-E, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Von Düring M, Andres KH. Ultrastructure of fine afferent fibre terminations in muscle and tendon of the cat. In: Hamann W, Iggo A, editors. Sensory Receptor Mechanisms. Singapore: World Scientific; 1984. pp. 15–23. [Google Scholar]
- Von Düring M, Andres KH. Topography and ultrastructure of group III and IV nerve terminals of cat's gastrocnemius-soleus muscle. In: Zenker W, Neuhuber WL, editors. The Primary Afferent Neuron: A Survey of Recent Morpho-functional Aspects. New York: Plenum Press; 1990. pp. 35–41. [Google Scholar]
- Williamson JW, Mitchell JH, Olesen HL, Raven PB, Secher NH. Reflex increase in blood pressure induced by leg compression in man. J Physiol. 1994;475:351–357. doi: 10.1113/jphysiol.1994.sp020076. [DOI] [PMC free article] [PubMed] [Google Scholar]