Abstract
Short-term over-expression of neuronal nitric oxide synthase (nNOS) with adenoviral gene transfer into peripheral cardiac autonomic neurons can facilitate cholinergic neurotransmission, and inhibit sympathetic transmission, by regulating cyclic nucleotide-dependent pathways coupled to neuronal calcium entry. We tested the idea whether cardiac neuromodulation by nNOS could be sustained by long-term over-expression of the enzyme following lentiviral gene transfer. We developed a lentiviral vector with an elongation factor 1 (EF1α) promoter to drive nNOS or enhanced green fluorescent protein (eGFP) expression. Lenti.EF1α-nNOS or Lenti.EF1α-eGFP was transferred to the right atrium of Spague–Dawley (SD) rats and acetylcholine (ACh) or noradrenaline (NA) release to field stimulation was measured 4 months after gene transfer. Atria transduced with Lenti.EF1α-nNOS had higher nNOS expression compared to the atria treated with Lenti.EF1α-eGFP (P < 0.05). We also detected significant increases (P < 0.05) in atrial cGMP and cAMP levels in the same tissue. Immunohistochemistry revealed co-localisation of eGFP in intrinsic cholinergic neurons (choline acetyltransferase positive) and intrinsic adrenergic neurons (tyrosine hydroxylase positive) following gene transfer. nNOS-transduced animals displayed enhanced ACh release (P < 0.05) and reduced NA release (P < 0.05) compared to the eGFP-treated group. nNOS-specific inhibition reversed the enhanced ACh release. Persistent nNOS over-expression mediated by a lentiviral vector can modulate sympatho-vagal control of cardiac excitability. This approach may provide a new tool to target impaired cardiac autonomic phenotypes that are disrupted by several cardiovascular pathologies.
Several cardiovascular pathologies involving oxidative stress are known to impair nitric oxide–cyclic nucleuotide coupled pathways linked to regulation of intracellular calcium (Danson et al. 2009). In particular, nitric oxide (NO) derived from neuronal nitric oxide synthase (nNOS) has been demonstrated to facilitate cardiac parasympathetic neurotransmission (Conlon & Kidd, 1999; Herring & Paterson, 2001) and to inhibit sympathetic neurotransmission (Schwarz et al. 1995; Choate & Paterson, 1999; Wang et al. 2006; Zucker, 2006) via cGMP-dependent pathways that modulate neuronal calcium levels to effect exocytosis (Wang et al. 2007). In hypertensive rats (Heaton et al. 2006, 2007; Li et al. 2007) and following acute myocardial infarction in the guinea-pig (Dawson et al. 2008) sympatho-vagal control of cardiac excitability is impaired leading to enhanced release of noradrenaline (norepinephrine) and diminished vagal responsiveness. Whilst NO donors and the cGMP analogues augment vagal-induced bradycardia (Herring & Paterson, 2001) or conversely inhibit cardiac sympathetic neurotransmission (Sears et al. 1998; Mohan & Paterson, 2000), their pharmacological targeting is relatively non-specific with the actions being short lived.
Emerging evidence has shown that adenoviral vector-mediated nNOS gene transfer into cholinergic intracardiac ganglia in the mouse, rat and guinea-pig (Mohan et al. 2002; Danson et al. 2004; Heaton et al. 2007) or when targeted directly to the cardiac vagus nerve in the pig (Heaton et al. 2005), can increase cardiac baroreflex sensitivity (Mohan et al. 2002; Heaton et al. 2005), facilitate ACh release (Mohan et al. 2002; Danson et al. 2004; Heaton et al. 2007), and bradycardia (Mohan et al. 2002; Danson et al. 2004; Heaton et al. 2005). In the hypertensive rat (Heaton et al. 2007) or infarcted guinea-pig heart (Dawson et al. 2008) this gene transfer strategy can restore parasympathetic responsiveness and improves short-term survivability (Dawson et al. 2008). Moreover, targeted gene transfer of nNOS with a noradrenergic cell-specific adenoviral vector increases the bioactivity of nNOS and cGMP, whereas it decreases cAMP levels in cardiac sympathetic nerves resulting in decreased calcium entry (Wang et al. 2007) and NA release in the normotensive rat (Wang et al. 2006). When nNOS is directly targeted to sympathetic nerves or pacemaking cells in the hypertensive rat, it reduces sympathetic and beta adrenergic hyper-responsiveness back to levels seen in the normotensive WKY rat (Heaton et al. 2006; Li et al. 2007).
Although adenoviral vector-mediated gene transfer is a powerful tool to modulate cardiac neurotransmission (Heaton et al. 2005, 2006), its duration of gene expression is short term and therefore its use is restricted to establishing proof of principle. However, lentiviral vectors are a valuable tool for mediating gene transfer and prolonged gene expression in non-dividing cells such as neurons (Amado & Chen, 1999; Consiglio et al. 2004). Therefore, we developed a lentiviral vector with an elongation factor 1α (EF1α) promoter to drive nNOS or eGFP expression in cardiac neurons in order to evaluate the long-term effect that up-regulation of nNOS had on cyclic nucleotide regulation of cardiac neurotransmission. We measured the release of ACh and NA in intact atrial preparations, and further hypothesised that sustained up-regulation of nNOS could be used to modulate cardiac sympathovagal neurotransmission.
Methods
Generation of recombinant lentivirus expressing nNOS
Lenti.EF1α-nNOS was generated using a self-inactivating lentiviral vector with an EF1α promoter (Kim et al. 1990), the central polypurine tract (cPPT) fragment (Follenzi et al. 2000; Sirven et al. 2000) and a woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) (Donello et al. 1998; Zufferey et al. 1999). nNOS cDNA was amplified by PCR (West et al. 2001). MluI and EcoRI sites were added to the sense and anti-sense primers, respectively. The resulting fragment was cloned into the corresponding sites of the parental vector. The integrity of the resulting vector was confirmed by sequencing the entire length of the insert and both junctions flanking the insert. Recombinant lentiviruses were produced by co-transfecting lentiviral vector with two plasmid second generation packaging plasmids into HEK 293T-17 cells with gag-pol, rev (psPAX2) and VSV-G envelope (pMD2G). A control recombinant lentivirus, lenti.EF1α-GFP, encoding enhanced green fluorescence protein (eGFP) was prepared in parallel. The titre estimates of lentiviruses were determined by p24 ELISA and qPCR as ∼1 × 109 transducing units (TU) ml−1.
Animals
Adult SD rats were purchased from Harlan (Bicester, UK). The investigation conformed to the guidelines laid down by the University of Oxford animal ethics committee and the Animals (Scientific Procedures) 1986 Act (UK) and were performed under British Home Office license requirements (PPL 30/2130). A total of 55 SD rats and one WKY rat was used for this study.
Gene transfer procedure
Targeted percutaneous gene transfer to the right atrium of rats was performed under isofluorane (Isocare, Animalcare Ltd) anaesthesia (4% for induction and 2–3% for maintenance, in 100% O2), using a technique similar to that described previously for the guinea-pig (Mohan et al. 2002). The viral vector was injected through the 4th intercostal space on the right-hand side of the animal, approximately 5 mm lateral to the midline and directed towards the left axilla. SD rats (aged 12–16 weeks, male) received an injection of 1 × 108 TU of Lenti.EF1α-nNOS or Lenti.EF1α-eGFP in 300 μl of sterile phosphate-buffered saline. The rats were allowed to recover and were monitored for 4 h before being transferred to the animal care facility where they were monitored daily until terminal procedure. Animals were terminated with an overdose intraperitoneal injection of pentobarbitone at 4 months following viral transfer for in vitro phenotyping (Western analysis, cyclic nucleotides and measurement of neurotransmitters). Tissue was also harvested from rats at 7–10 days post transduction for immunohistochemistry to establish that gene delivery was targeted to autonomic neurons.
Immunohistochemistry
Cardiac myocyte isolation and transduction was prepared as previously described (Gustafson et al. 1987; Wang et al. 2006). Cultured cardiac myocytes or sliced atria (40 μm) were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 and 1% BSA. Samples were processed for immuno-reactivity with goat anti-choline acetyltransferase (CHAT) (Chemicon) or mouse anti-tyrosine hydroxylase (TH) (Sigma), and then incubated with relevant biotinylated secondary antibodies (Vector Labs). The immunofluoresent signals were detected by Texas Red Streptavidin (SA-5006, Vector Labs) for CHAT or TH.
Western blot analysis of gene expression
Cardiac tissue samples were lysed in CeLLytic MT lysis buffer (Sigma). Protein concentrations were measured using the Bio-Rad DC protein assay kit. Total protein (20 μg per well) was separated on a NuPAGE 3–8% Tris-acetate gel and was transferred to a nitrocellulose membrane. The primary antibodies used were the same as used for immunohistochemistry. The secondary antibodies used were IgG against the primary antibodies conjugated to horseradish peroxidise (IgG-HRP, SantaCruz). Immunoreactivity was detected using luminal based chemiluminescence detection reagents (Western Lightening, Perkin Elmer).
Radioimmuno-assay of atrial cGMP and cAMP levels
Atria from animals were frozen in liquid nitrogen and then were minced in 800 μl of 6% ice-cold trichloroacetic acid and homogenized at 4°C. The homogenate was centrifuged at 2000 g for 10 min at 4°C, and the supernatant was extracted with water-saturated ether three times and then dried in a SpeedVac concentrator (Savant). The dried samples were resuspended and a 125I-cGMP assay kit (GE Healthcare, RPA525) or a 125I- cAMP assay kit (GE Healthcare, RPA509) was used to measure the amount of cGMP or cAMP. Labelled cGMP or cAMP of the bound fraction in the pellet was separated from unbound supernatant by magnetic separation and measured in a scintillation counter. The concentration of cGMP or cAMP in the sample was then determined by interpolation from a standard curve.
[3H] NA or ACh release on the isolated right atrium
Experimental set-up
The right atrium of SD rats was isolated by the method described previously (Oe et al. 1999) with some modification. The right atrium was removed and transferred to a 37°C organ bath containing Tyrode solution. After a 45 min equilibration, the atria were incubated with 10 μCi [methyl3H] choline chloride (Amersham) or 5 μCi l-[7,8-3H] noradrenaline (norepinephrine, NA; Amersham) for 30 min with field stimulations at 5 Hz for 10 s every 30 s to allow tissue incorporation of the compound. Excess radioactive compound was washed off from the preparation by superfusing with Tyrode solution for 60 min at a rate of 3 ml min−1. Following the superfusion, the bath solution was collected every 3 min by replacing with 3 ml Tyrode solution.
Experimental protocols
Experiments were carried out by using two groups of atria to compare the level of neurotransmitter release (Fig. 1). The experiments lasted 72 min. Control cycles were followed by using the nNOS-specific inhibitor N-[(4S)-4-amino-5-[(2-aminoetyl](amino] pentyl]-N′-nitroguanidine (AAAN, 10 μm l). The atrium was stimulated at 5 Hz for 1 min at the beginning of the 15th (S1) and 51st (S2) minutes. At the end of the experiment, the atrium was immersed overnight in 3 ml Tyrode solution containing 4 U ml−1 papain (Sigma) and the radioactivity contained in the extract was determined.
Figure 1. Protocols for [3H]ACh release experiments.
Diagonal bars show the period of sample collections to be compared between groups. S1 and S2 represent the first and second field stimulation, respectively (5 Hz, 1 min).
Measurement of radioactive content
A 0.5 ml aliquot of each collected fraction was transferred to scintillation vials containing 4.5 ml of scintillation fluid (Ecoscint A, National Diagnostics) and the amount of radioactivity in each sample (disintegrations per minute) was measured using a liquid scintillation counter (Tri-carb 2800TR, Packard). [3H] NA/ACh outflow was expressed as a ratio of NA or ACh radioactivity at a particular time point over the total radioactivity.
Statistical analysis
The results are presented as the means ±s.e.m. Analysis was performed using Student's paired or un-paired t test as appropriate. For all experiments, statistical significance was accepted at P < 0.05.
Results
Lentiviral vector-mediated gene expression in the rat hearts
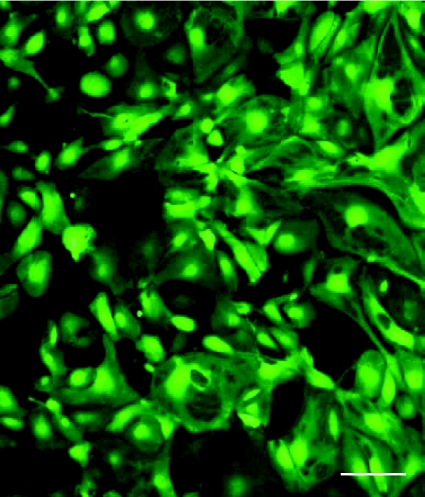
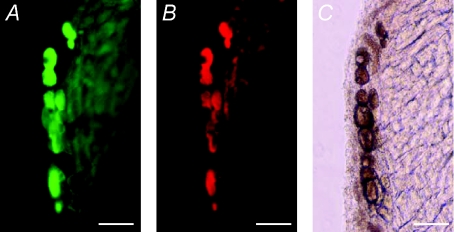
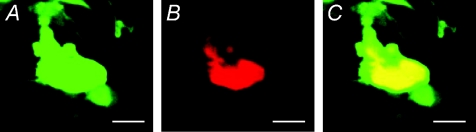
We evaluated the expression pattern of lentiviral gene transfer both in vitro and in vivo (Fig. 2). Since it is difficult to distinguish the endogenous and exogenous NOS signals by nNOS antibody, we analysed the gene expression using a lentiviral vector encoding eGFP driven by a EF1α promoter. Transduction of a lentiviral vector, LentiEF1α-eGFP, into isolated cardiomyocytes resulted in robust GFP expression. We delivered lentiviral vectors into rat heart using an atrial gene delivery technique we previously developed (Mohan et al. 2002). We observed that the GFP expression is not only in cardiomyocytes, but is also located in neural tissue including cholinergic (Fig. 3) and clumps of cardiac intrinsic adrenergic neurons (Fig. 4) as identified by CHAT and TH co-localisation, respectively.
Figure 2. Representative fluorescent images of cardio myocytes after gene transfer with a lentiviral vector Lenti.EF1α-eGFP.
GFP expression is shown in cultured cardio myocytes 7 days after lentiviral transduction. Scale bar, 50 μm.
Figure 3. Lentiviral vector-mediated GFP expression in cardiac cholinergic neurons.
Transgene expression was detected 10 days post-transduction with Lenti.EF1α-eGFP. A, GFP expression. B, same tissue sample stained with cholinergic neuron marker anti-CHAT. C, same tissue sample photographed under phase contract normal light. Note all CHAT-positive neurons (red cells in B) were co-localised with eGFP-expressing cells (green cells in A). Scale bar, 50 μm for all the images.
Figure 4. Co-localization of GFP expression cells and cardiac intrinsic adrenergic neurons 10 days post transduction.
Immunohistochemistry was performed in atria transferred with a lentiviral vector Lenti.EF1α-eGFP. A, GFP expression. B, same tissue sample stained with sympathetic neuron marker anti-TH. C, overlay of GFP and anti-TH (Texas-red streptavitin). Scale bar, 50 μm for all the images.
Persistent nNOS over-expression mediated by Lenti.EF1α-nNOS
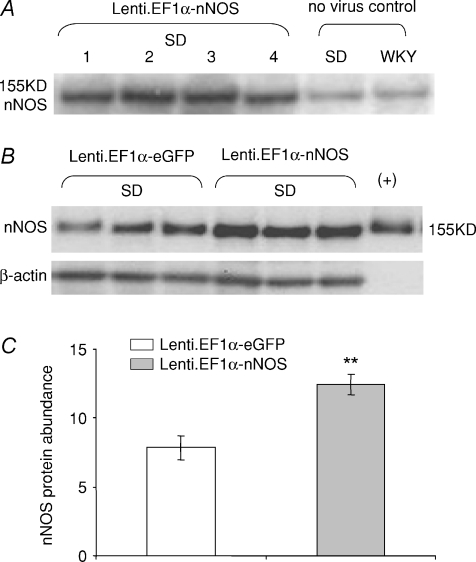
Western blot analysis after 4 month atrial gene transfer to adult SD rats demonstrated that Lenti.EF1α-nNOS gene transfer increased nNOS expression compared to non-transduced SD (Fig. 5). Lenti.EF1α-nNOS transduction caused a 58.8% increase in nNOS gene expression compared to Lenti.EF1α-eGFP-transduced atria (P= 0.002, unpaired t test, n= 7 in each group).
Figure 5. nNos increased 4 months post gene transfer.
A and B, after 4 month gene transfer, Western blots showing higher nNOS protein abundance in Lenti.EF1α-nNOS-transduced SD atria compared with non-viral control or Lenti.EF1α-eGFP-transduced tissue. C, group data demonstrating average 58.8% increase of nNOS abundance in Lenti.EF1α-nNOS- over Lenti.EF1α-eGFP-transduced atria (n= 7 in each group, **P < 0.01, unpaired t test).
Production of cGMP and cAMP in atria following nNOS gene transfer
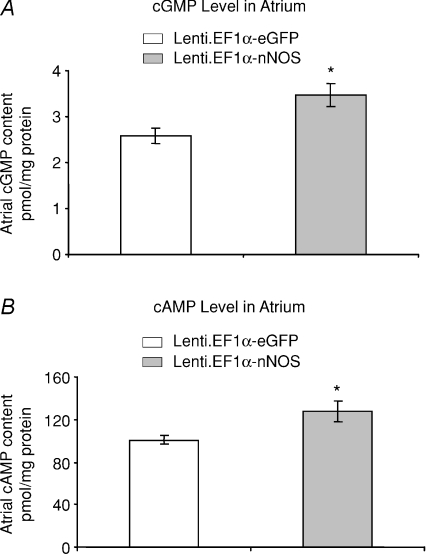
We also observed that the cGMP level was increased 34.4% in Lenti.EF1α-nNOS-transduced atria compared to Lenti.EF1α-eGFP control group (3.47 ± 0.26 vs 2.58 ± 0.17 pmol (mg protein)−1, P= 0.012, unpaired t test, n= 8 in each group). We also observed that the cAMP level in nNOS gene transferred group is increased 26.7% (Fig. 6) compared to the eGFP group (127.70 ± 10.01 vs 100.80 ± 4.02 pmol (mg protein)−1, P= 0.026, unpaired t test, n= 8 in each group).
Figure 6. cGMP and cAMP increased 4 months post gene transfer.
A, tissue levels of cGMP in Lenti.EF1α-nNOS-transduced atria after 4 months were significantly higher than those measured in the Lenti.EF1α-eGFP-treated group (*P < 0.05, unpaired t test, n= 8 in each group). B, tissue cAMP levels were also significantly increased after Lenti.EF1α-nNOS transduction compared to eGFP group (*P < 0.05, unpaired t test) (n= 8 in each group).
Lenti.EF1α-nNOS gene transfer increased cardiac cholinergic neurotransmission
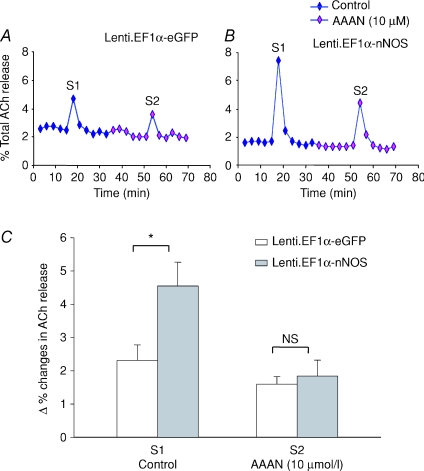
Lenti.EF1α-nNOS (4.56 ± 0.71%, n= 7) caused a 97.1% increase in evoked ACh release compared to Lenti.EF1α-eGFP-transduced atrial tissue (2.31 ± 0.47%, n= 5, P < 0.038, unpaired t test.). Treatment with 10 μm of the nNOS inhibitor AAAN blunted the significance of this difference by preventing the Lenti.EF1α-nNOS-induced augmentation of ACh release, and brought it to a level similar to that in the Lenti.EF1α-eGFP-transduced group (Fig. 7).
Figure 7. nNOS increases ACh release.
A and B, typical data trace showing measurement of [3H]ACh release from isolated atria in response to 5 Hz field stimulation in Lenti.EF1α-nNOS- (A) and Lenti.EF1α-eGFP- (B) transduced group at 4 months. S1 and S2 represent the first and second stimulation. C, statistical data show ACh release in Lenti.EF1α-nNOS-treated tissue (n= 7) was significantly increased at S1 compared to Lenti.EF1α-eGFP group (n= 5, *P < 0.05, unpaired t test). There is no significant difference at S2. AAAN significantly attenuated ACh release from Lenti.EF1α-nNOS-treated tissue. Percentage change in ACh release was expressed as a ratio of increase in ACh radioactivity 3 min following the electrical stimulation over the total radioactivity measured at all time points.
Lenti.EF1α-nNOS gene transfer decreased cardiac sympathetic neurotransmission
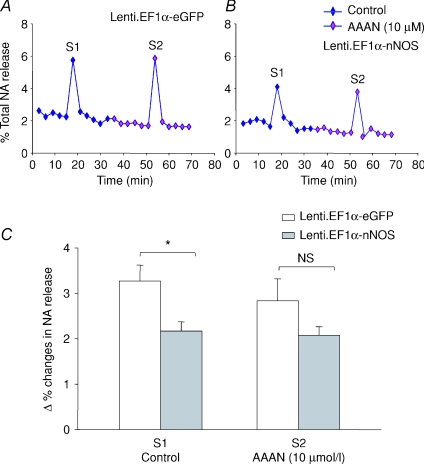
Lenti.EF1α-nNOS (2.20 ± 0.20%, n= 6) caused a 32.8% decrease in evoked NA release compared to Lenti.EF1α-eGFP- (3.27 ± 0.35%, n= 4, P= 0.022, unpaired t test.) transduced atrial tissue. Treatment with 10 μm of the nNOS inhibitor AAAN blunted the significance of this difference in the second stimulation (S2), resulting in an insignificant P value (P= 0.129) (Fig. 8).
Figure 8. nNOS decreases NA release.
A and B, typical data trace showing measurement of [3H]NA release from isolated atria in response to 5 Hz field stimulation in Lenti.EF1α-nNOS- (A) and Lenti.EF1α-eGFP- (B) transduced group at 4 months. S1 and S2 represent the first and second stimulation. C, the data show NA release in Lenti.EF1α-nNOS-treated tissue (n= 7) was significantly decreased at S1 compared to Lenti.EF1α-eGFP group (n= 5, *P < 0.05, unpaired t test). There is no significant difference at S2. Percentage changes in NA release were expressed as a ratio of average increases in NA radioactivity 3 min following the electrical stimulation over the total radioactivity measured at all time points.
Discussion
The novel finding is that sustained nNOS gene expression with a lentiviral vector can modulate sympatho-vagal control of cardiac excitability from the peripheral nervous system when compared to animals treated with eGFP. nNOS-transduced animals displayed much enhanced ACh release and reduced NA release. nNOS-specific inhibition completely reversed the enhanced ACh release, but only attenuated the reduced NA response. nNOS over-expression resulted in significant increases in both cGMP and cAMP levels that were detected in the same atrial tissue. Importantly, gene transfer was successful in tranducing both CHAT- and TH-positive neurons. When taken together, these results suggest that persistent cardiac nNOS up-regulation not only facilitates parasympathetic neurotransmission, but also inhibits sympathetic neurotransmission either directly through varicosities of intrinsic cardiac adrenergic cells or indirectly via vagal inhibition which was enhanced by nNOS over-expression.
Cardiac nNOS gene transfer through lentiviral vector sites of action
The precise sites and intracellular targets of our lentiviral nNOS intervention cannot be firmly established from this study, and therefore remain largely speculative. Nevertheless our data give clues as to the likely major sites of action. Atrial gene transfer of the Lenti.EF1α-nNOS vector resulted in persistent nNOS expression in cardiac cholinergic neurons, as well as in cardiac myocytes. Sympathetic ganglia located outside of the heart tissue and HIV-derived lentiviral vectors pseudotyped with vesicular stomatitis virus (VSV) do not confer retrograde transduction (Wong et al. 2004); nevertheless, we observed decreased NA release on nNOS gene-transferred rat atria. This suggests that neuronal NO acts post-ganglionically on sympathetic fibres that innervate pacemaking tissue. However, given the broad tropism of EF1α promoter, it is likely that Lenti.EF1α-nNOS can also mediate nNOS expression in intrinsic cardiac adrenergic (ICA) neurons since the vector was directly injected into the atrial wall. ICA cells possess a well-developed catecholamine synthetic system capable of release and reuptake of NA (Huang et al. 2005). These cells are very likely to serve as the main targets of neuronal NO in modulating sympathetic neurotransmission in normal and diseased states. Considering that 90% of the intrinsic neurons in supraventricular tissue are cholinergic in origin (Horackova et al. 2000), it is not surprising that our study observed a greater modulation on cholinergic transmission compared to the sympathetic response following atrial nNOS gene transfer. The intracellular pathway responsible for this response has not been established, although data show that increased cAMP levels will predominately act on cholinergic enhancement to a greater degree than sympathetic inhibition given the intracellular signalling pathways responsible for NO-cGMP regulation of intracellular calcium and resulting exocytosis (Heaton et al. 2006, 2007).
It is known that there is significant cross-talk between the cardiac cholinergic and sympathetic system, since noradrenergic and cholinergic nerve terminals lie close together in the heart where the mutual pre-synaptic inhibition between the two opposing effects of autonomic transmitters has been demonstrated (Fuder & Muscholl, 1995). Activation of muscarinic acetylcholine receptors on sympathetic varicosities reduces NA release from peripheral neurons (Fuder & Muscholl, 1995; Boehm & Kubista, 2002), and this may be the major site of sympathetic inhibition in our experimental model that has enhanced parasympathetic neurotransmission. However, we cannot rule out that aspects of the neuromodulation following nNOS over-expression did not result from the paracrine action of NO generated from cardiac myocytes that also over-expressed the enzyme (Paton et al. 2002).
Role of nNOS up-regulation on cardiac sympathovagal neurotransmission
Long-term effects of nNOS over-expression on parasympathetic neurotransmission are consistent with the results published previously in adenoviral vector-mediated gene transfer animals (Heaton et al. 2007). Pharmacological evidence indicates that this results from increased cGMP-mediated inhibition of phosphodiesterase 3 (PDE3), leading to enhanced cAMP–PKA-dependent phosphorylation of calcium channels and calcium-mediated exocytosis of ACh (Herring et al. 2002). The effect of persistent nNOS over-expression on cardiac sympathetic neurotransmission is also consistent with previous findings in the SD rat and spontaneously hypertensive rat (SHR) where nNOS over-expression was mediated by a noradrenergic-specific adenoviral vector (Wang et al. 2006). The signalling pathway responsible for neuronal NO inhibiting sympathetic neurotransmission is not completely understood, but appears to be related to increased cGMP-mediated activation of phosphodiesterase 2 (PDE2), leading to inhibition of cAMP–PKA-dependent phosphorylation of neuronal calcium channel. Noradrenergic-specific transduction with Ad PRS nNOS causes enhanced degradation of cAMP and leads to a decrease in Ca2+ influx-triggered exocytosis of NA (Weng et al. 2007).
Modulating cardiac autonomic balance through long-term nNOS up-regulation
Our study has demonstrated good efficacy that long-term nNOS up-regulation using a lentiviral vector with a human EF1α promoter can modulate cardiac autonomic neurotransmission. As an abundant housekeeping gene promoter, EF1α exhibits much stronger activity than viral promoters such as SV40 and Rous sarcoma virus promoters (Kim et al. 1990), and in comparison to the human cytomegalovirus promoter, yields persistent expression of the transgene in vivo (Guo et al. 1996). As the lentiviral genome can integrate into the host genome, it induces a minimal immune response and produces stable long-term transgene expression compared with other viral vectors (Naldini & Verma, 2000; Kootstra & Verma, 2003). Moreover, to ensure its biosafety, we used a self-inactivating viral promoter with split packaging plasmids. Given the broad tropism and capability to transduce neurons, this further added greater advantage to lentiviral vector-mediated long-term up-regulation of nNOS on both branches of the cardiac autonomic nervous system. Finally, it does, however, remain to be established whether this approach can be used to restore cardiac neural impairment that is a feature and negative prognostic indicator in many primary cardiovascular pathologies.
Acknowledgments
We thank the Lentiviral Vector Production Unit of the Swiss Institute of Technology Lausanne (EPFL), supported by the Association Francaise contre les Myopathies (AFM), for the production of the lentivirus used in the in vivo studies. This work was supported by the British Heart Foundation. There are no author disclosures.
Author contributions
Project conception and design was done by L.W. and D.J.P. L.W. made the Lentiviral vectors, evaluated vector expression, measured cGMP, cAMP, part of ACh and NA release and wrote the paper with D.J.P. D.L. measured neurotransmitter release and analysed the data and reviewed drafts of the manuscript. T.A.D. performed atrial gene delivery and read drafts of the manuscript.
References
- Amado RG, Chen IS. Lentiviral vectors – the promise of gene therapy within reach? Science. 1999;285:674–676. doi: 10.1126/science.285.5428.674. [DOI] [PubMed] [Google Scholar]
- Boehm S, Kubista H. Fine tuning of sympathetic transmitter release via ionotropic and metabotropic presynaptic receptors. Pharmacol Rev. 2002;54:43–99. doi: 10.1124/pr.54.1.43. [DOI] [PubMed] [Google Scholar]
- Choate JK, Paterson DJ. Nitric oxide inhibits the positive chronotropic and inotropic responses to sympathetic nerve stimulation in the isolated guinea-pig atria. J Auton Nerv Syst. 1999;75:100–108. doi: 10.1016/s0165-1838(98)00173-8. [DOI] [PubMed] [Google Scholar]
- Conlon K, Kidd C. Neuronal nitric oxide facilitates vagal chronotropic and dromotropic actions on the heart. J Auton Nerv Syst. 1999;75:136–146. doi: 10.1016/s0165-1838(98)00185-4. [DOI] [PubMed] [Google Scholar]
- Consiglio A, Gritti A, Dolcetta D, Follenzi A, Bordignon C, Gage FH, Vescovi AL, Naldini L. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci U S A. 2004;101:14835–14840. doi: 10.1073/pnas.0404180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson EJ, Li D, Wang L, Dawson TA, Paterson DJ. Targeting cardiac sympatho-vagal imbalance using gene transfer of nitric oxide synthase. J Mol Cell Cardiol. 2009;46:482–489. doi: 10.1016/j.yjmcc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Danson EJ, Mankia KS, Golding S, Dawson T, Everatt L, Cai S, Channon KM, Paterson DJ. Impaired regulation of neuronal nitric oxide synthase and heart rate during exercise in mice lacking one nNOS allele. J Physiol. 2004;558:963–974. doi: 10.1113/jphysiol.2004.062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TA, Li D, Woodward T, Barber Z, Wang L, Paterson DJ. Cardiac cholinergic NO–cGMP signalling following acute myocardial infarction and nNOS gene transfer. Am J Physiol Heart Circ Physiol. 2008;295:H990–H998. doi: 10.1152/ajpheart.00492.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- Fuder H, Muscholl E. Heteroreceptor-mediated modulation of noradrenaline and acetylcholine release from peripheral nerves. Rev Physiol Biochem Pharmacol. 1995;126:265–412. doi: 10.1007/BFb0049778. [DOI] [PubMed] [Google Scholar]
- Guo ZS, Wang LH, Eisensmith RC, Woo SL. Evaluation of promoter strength for hepatic gene expression in vivo following adenovirus-mediated gene transfer. Gene Ther. 1996;3:802–810. [PubMed] [Google Scholar]
- Gustafson TA, Bahl JJ, Markham BE, Roeske WR, Morkin E. Hormonal regulation of myosin heavy chain and α-actin gene expression in cultured fetal rat heart myocytes. J Biol Chem. 1987;262:13316–13322. [PubMed] [Google Scholar]
- Heaton DA, Golding S, Bradley CP, Dawson TA, Cai S, Channon KM, Paterson DJ. Targeted nNOS gene transfer into the cardiac vagus rapidly increases parasympathetic function in the pig. J Mol Cell Cardiol. 2005;39:159–164. doi: 10.1016/j.yjmcc.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Heaton DA, Lei M, Li D, Golding S, Dawson TA, Mohan RM, Paterson DJ. Remodelling of the cardiac pacemaker L-type calcium current and its beta-adrenergic responsiveness in hypertension after neuronal NO synthase gene transfer. Hypertension. 2006;48:443–452. doi: 10.1161/01.HYP.0000233383.04280.3c. [DOI] [PubMed] [Google Scholar]
- Heaton DA, Li D, Almond SC, Dawson TA, Wang L, Channon KM, Paterson DJ. Gene transfer of nNOS into intracardiac cholinergic ganglia reverses vagal impairment in hypertensive rats. Hypertension. 2007;49:380–388. doi: 10.1161/01.HYP.0000255792.97033.f7. [DOI] [PubMed] [Google Scholar]
- Herring N, Danson EJ, Paterson DJ. Cholinergic control of heart rate by nitric oxide is site specific. News Physiol Sci. 2002;17:202–206. doi: 10.1152/nips.01386.2002. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Nitric oxide–cGMP pathway facilitates acetylcholine release and bradycardia during vagal nerve stimulation in the guinea-pig in vitro. J Physiol. 2001;535:507–518. doi: 10.1111/j.1469-7793.2001.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M, Slavikova J, Byczko Z. Postnatal development of the rat intrinsic cardiac nervous system: a confocal laser scanning microscopy study in whole-mount atria. Tissue Cell. 2000;32:377–388. doi: 10.1054/tice.2000.0126. [DOI] [PubMed] [Google Scholar]
- Huang MH, Bahl JJ, Wu Y, Hu F, Larson DF, Roeske WR, Ewy GA. Neuroendocrine properties of intrinsic cardiac adrenergic cells in fetal rat heart. Am J Physiol Heart Circ Physiol. 2005;288:H497–H503. doi: 10.1152/ajpheart.00591.2004. [DOI] [PubMed] [Google Scholar]
- Kim DW, Uetsuki T, Kaziro Y, Yamaguchi N, Sugano S. Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene. 1990;91:217–223. doi: 10.1016/0378-1119(90)90091-5. [DOI] [PubMed] [Google Scholar]
- Kootstra NA, Verma IM. Gene therapy with viral vectors. Annu Rev Pharmacol Toxicol. 2003;43:413–439. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- Li D, Wang L, Lee CW, Dawson TA, Paterson DJ. Noradrenergic cell specific gene transfer with neuronal nitric oxide synthase reduces cardiac sympathetic neurotransmission in hypertensive rats. Hypertension. 2007;50:69–74. doi: 10.1161/HYPERTENSIONAHA.107.088591. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Heaton DA, Danson EJF, Krishnan SPR, Cai S, Channon KM, Paterson DJ. Neuronal nitric oxide synthase gene transfer promotes cardiac vagal gain of function. Circ Res. 2002;91:1089–1091. doi: 10.1161/01.res.0000047531.75030.b5. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Paterson DJ. Activation of sulphonylurea-sensitive channels and the NO-cGMP pathway decreases the heart rate response to sympathetic nerve stimulation. Cardiovasc Res. 2000;47:81–89. doi: 10.1016/s0008-6363(00)00057-2. [DOI] [PubMed] [Google Scholar]
- Naldini L, Verma IM. Lentiviral vectors. Adv Virus Res. 2000;55:599–609. doi: 10.1016/s0065-3527(00)55020-9. [DOI] [PubMed] [Google Scholar]
- Oe K, Sperlagh B, Santha E, Matko I, Nagashima H, Foldes FF, Vizi ES. Modulation of norepinephrine release by ATP-dependent K+-channel activators and inhibitors in guinea-pig and human isolated right atrium. Cardiovasc Res. 1999;43:125–134. doi: 10.1016/s0008-6363(99)00052-8. [DOI] [PubMed] [Google Scholar]
- Paton JF, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- Schwarz P, Diem R, Dun NJ, Forstermann U. Endogenous and exogenous nitric oxide inhibits norepinephrine release from rat heart sympathetic nerves. Circ Res. 1995;77:841–848. doi: 10.1161/01.res.77.4.841. [DOI] [PubMed] [Google Scholar]
- Sears CE, Choate JK, Paterson DJ. Effect of nitric oxide synthase inhibition on the sympatho-vagal contol of heart rate. J Auton Nerv Syst. 1998;73:63–73. doi: 10.1016/s0165-1838(98)00123-4. [DOI] [PubMed] [Google Scholar]
- Sirven A, Pflumio F, Zennou V, Titeux M, Vainchenker W, Coulombel L, Dubart-Kupperschmitt A, Charneau P. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood. 2000;96:4103–4110. [PubMed] [Google Scholar]
- Wang L, Henrich M, Buckler KJ, McMenamin M, Mee CJ, Sattelle DB, Paterson DJ. Neuronal nitric oxide synthase gene transfer decreases [Ca2+]i in cardiac sympathetic neurons. J Mol Cell Cardiol. 2007;43 doi: 10.1016/j.yjmcc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Wang L, Li D, Plested CP, Dawson T, Teschemacher AG, Paterson DJ. Noradrenergic neuron-specific overexpression of nNOS in cardiac sympathetic nerves decreases neurotransmission. J Mol Cell Cardiol. 2006;41:364–370. doi: 10.1016/j.yjmcc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- West NE, Qian H, Guzik TJ, Black E, Cai S, George SE, Channon KM. Nitric oxide synthase (nNOS) gene transfer modifies venous bypass graft remodelling: effects on vascular smooth muscle cell differentiation and superoxide production. Circulation. 2001;104:1526–1532. doi: 10.1161/hc3801.095693. [DOI] [PubMed] [Google Scholar]
- Wong L-F, Azzouz M, Walmsley LE, Askham Z, Wilkes FJ, Mitrophanous KA, Kingsman SM, Mazarakis ND. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol Ther. 2004;9:101–111. doi: 10.1016/j.ymthe.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]